Abstract
Strategies aimed at primary prevention provide an outstanding opportunity for reducing the onset and burden of cardiovascular (CV) disease. Lipid abnormalities, including high levels of low-density lipoprotein cholesterol (LDL-C), elevated triglycerides and low levels of high-density lipoprotein cholesterol (HDL-C), are associated with an increased risk of CV events, thereby serving as contributors to this process. By consensus, lowering LDL-C, generally with statin therapy, is the primary target of lipid-lowering therapy. However, statin therapy may be insufficient for patients with mixed dyslipidemia, especially those with insulin resistance syndromes. While the addition of niacin, fibrate or omega-3 fatty acids may be useful in this setting, outcomes data are lacking. Therefore, data from ongoing prospective studies will hopefully resolve this issue and facilitate identification of optimal strategies to augment CV risk reduction.
Introduction
Dyslipidemia is recognized as a prominent risk factor for cardiovascular (CV) disease.1 Current guidelines focus on lowering low-density lipoprotein cholesterol (LDL-C) with a statin in both primary and secondary intervention settings.2–4 This approach is supported by extensive evidence from large, prospective studies. In a recent meta-analysis of 14 statin studies including 90 056 patients, lowering LDL-C by 39 mg/dl (1 mmol/l) was associated with about one-fifth reduction in the 5-year incidence of major CV events.5 However, the residual risk of vascular events remained high; 14.1% of statin-treated patients compared with 17.8% of control subjects experienced vascular events representing a residual relative risk of 79%. While high-dose statin therapy may provide some incremental benefit of between 10% and 20%,6,7 statin-treated patients remain at high residual risk for future CV events.
Beyond LDL-C
Other important lipid abnormalities include low levels of high-density lipoprotein cholesterol (HDL-C) and elevated triglycerides, both of which are independent predictors of CV disease (Table 1).8–10 However, it is important to recognize that much of the published HDL-C and triglyceride data has been largely driven by epidemiologic studies and the National Cholesterol Education Program has not endorsed a specific goal for managing either low HDL-C or high triglycerides. Moreover, a recent meta-regression analysis of intervention trials failed to demonstrate that increasing HDL-C levels improved CV outcome.11 In regard to triglycerides, observational data from the National Health and Nutrition Examination Survey (NHANES) found that the percentage of adults aged 60 years or more with elevated triglycerides (⩾150 mg/dl) increased more than 5-fold over the period 1976–2006.12 This is largely due to increasing rates of diabetes and obesity.13 Among individuals with type 2 diabetes or metabolic syndrome mixed dyslipidemia characterized by low levels of HDL-C, elevated triglycerides and atherogenic apolipoprotein B (apoB)-containing lipoproteins [including very low-density lipoproteins (VLDL) and LDL] and VLDL-triglycerides, and an increase in small, dense LDL particles is typical. Although levels of LDL-C may be normal or only modestly elevated, this measure may be misleading, given the increased number of atherogenic LDL and cholesterol-enriched remnant particles.14 Insulin resistance is a key driver of this abnormal lipid profile.15–17 Acting together, these metabolic abnormalities promote increased deposition of cholesterol within the artery wall, resulting in an increased risk of atherosclerotic disease. Thus, it stands to reason that the combination of elevated LDL-C and triglycerides may promote atherosclerosis to a greater degree than LDL-C alone. In fact, data from the Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22 (PROVE IT-TIMI 22) trial not only demonstrated the highest risk of death, myocardial infarction (MI) and recurrent acute coronary syndrome in patients with the combination of elevated LDL-C and triglycerides, but also the most favourable prognosis in patients in whom the lowest on-treatment LDL-C and triglycerides (<70 and 150 mg/dl, respectively) were obtained.18
Table 1.
HDL-C and triglycerides are independent predictors of CV disease
| HDLC-C |
| Meta-analysis of four major prospective studies showed that for every 1 mg/dl increase in HDL-C there was a decrease in coronary risk by 2–3%, independent of LDL-C.8 |
| Triglycerides |
| Meta-analysis of 29 prospective studies (262 525 subjects of whom 10 158 were CHD cases) showed that coronary risk was 72% higher in subjects with triglycerides in the top-third vs. bottom-third; odds ratio of 1.72 (95% CI 1.56–1.90).9 |
Despite the elevated risk associated with high triglycerides and LDL-C that is often accompanied by low HDL-C, there are currently no outcome data demonstrating that lowering elevated triglycerides improves CV risk beyond LDL-C goal attainment. Consequently, mixed dyslipidemia is often inadequately treated. Data from NHANES III showed that in individuals with metabolic syndrome (of whom ∼75% had low HDL-C and/or elevated triglycerides as defined by National Cholesterol Education Program Adult Treatment Panel III criteria), over-one-third did not receive appropriate intervention presumably because LDL-C levels were not substantially elevated.19 Moreover, recent 30-year data from NHANES highlight a need for renewed focus in addressing lipid and lipoprotein components other than LDL-C, in view of the 2-fold increased prevalence of mixed dyslipidemia (from 2.1% to 4.8%) between 1976 and 2006.12
Therapeutic intervention: lifestyle and pharmacotherapy
Lifestyle interventions that include reduction in total calories as well as intake of saturated and trans fats that are coupled to increased physical activity with associated weight loss continue to play an important a role in controlling mixed dyslipidemia.2,20 However, lifestyle intervention may insufficiently address LDL-C lowering, the primary target of therapy2,3 and long-term compliance may also serve as an impediment21 thereby facilitating the addition of pharmacologic therapy. Because many patients with elevated triglycerides and low HDL-C have elevated levels of apolipoprotein B, non-HDL-C serves as a better surrogate of residual risk than LDL-C. To this end, the National Cholesterol Education Program recommends non-HDL-C as a secondary target of therapy if triglyceride levels exceed 200 mg/dl2,3 and a recent ADA/ACC consensus statement also endorsed apolipoprotein B treatment goals in addition to LDL-C and non-HDL-C.4
Statins are efficacious in reducing CV risk in both primary and secondary prevention with these benefits extending to patients with diabetes and/or the metabolic syndrome.5,22 If patients cannot tolerate statins or fail to achieve LDL-C goals, the addition of ezetimibe may be useful. However, even with reductions in LDL-C levels (and hence apoB levels) of at least 30–40%, many patients with mixed dyslipidemia remain at high risk for coronary events23 raising the possibility that while attaining low LDL-C is a necessary goal, it may be insufficient to reduce the excess CV risk attributable to low HDL-C and/or elevated triglycerides. For example, in the Treating to New Targets (TNT) study, statin-treated patients with HDL-C levels <39 mg/dl had a 39% increase in risk for major CV events compared with those with levels >55 mg/dl.24 Additionally, data from the PROVE IT-TIMI 22 study showed that the incidence of death, MI or recurrent acute coronary syndrome in statin-treated patients who achieved LDL-C levels <70 mg/dl was 36% lower in those with triglycerides <200 mg/dl than those with triglycerides ⩾200 mg/dl.18 Thus, targeting multiple lipid/lipoprotein abnormalities, including low HDL-C levels and elevated serum triglycerides with the addition of a fibrate, niacin or omega-3 fatty acids may potentially provide greater improvement in the overall lipid profile (Table 2), which in turn may translate into greater CV risk reduction.
Table 2.
Lipid-modifying effects of fibrates, niacin and omega-3 fatty acids
| LDL-C (%) | HDL-C (%) | Triglycerides (%) | |
|---|---|---|---|
| Fibrates | ↑↓ 10–30 | ↑ 5–15 | ↓ 30–50a |
| Niacin | ↓ 15–20 | ↑ 20–25 | ↓ Up to 35 |
| Omega-3 fatty acids | ↑↓ 10–30 | ↑ 9 | ↓ ∼45 |
aDepending on baseline levels.
Fibrates
Fibrates lower plasma levels of triglycerides by 30–50%, typically increase levels of HDL-C by 5–15%, and may lower LDL-C 5–20%, although the magnitude of these effects is variable, depending on patient characteristics, the potency and specificity of the individual fibrate, and baseline lipid levels.25,26 In patients with elevated triglycerides (>200 mg/dl), the magnitude of the reduction in triglycerides may exceed 50%.25 Fibrates also promote a shift in particle size from small, dense LDL to larger and more buoyant particles, with increased binding affinity for the LDL receptor, as demonstrated in clinical studies with fenofibrate.27 Furthermore, there is evidence to suggest that fibrates may exert important pleiotropic effects in the artery wall, including attenuation of production of pro-inflammatory stimuli such as interleukin-6, as well as expression of inflammatory proteins of the acute phase including fibrinogen and C-reactive protein,28,29 and have favourable effects on the coagulation and fibrinolytic systems, increasing fibrinolysis and attenuating platelet hyperaggregability in hypercholesterolemic subjects.25,30
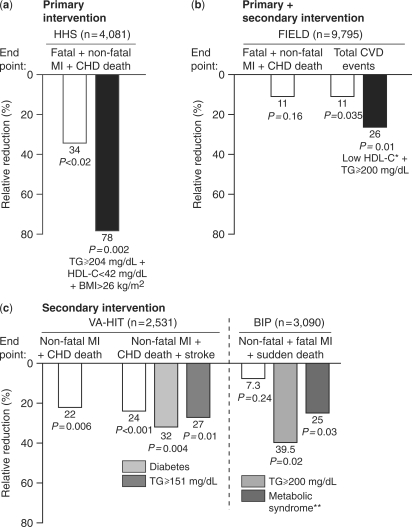
Clinical studies have demonstrated outcome benefits with fibrate monotherapy in primary and secondary prevention settings (Figure 1).31–36 In the Helsinki Heart Study including 4081 men at moderate risk of CHD (non-HDL-C ⩾ 200 mg/dl), treatment with the fibrate gemfibrozil (1200 mg/day) resulted in an 11% decrease in LDL-C, a 35% decrease in triglycerides and an 11% increase in HDL-C, compared with placebo. These lipid changes were associated with a 34% reduction in major coronary events at 5 years (P < 0.02), as well as a 37% reduction in non-fatal MI (P < 0.05), although no significant effect on overall mortality was observed.31 Subgroup analysis showed that the benefits of treatment were significantly greater among patients with mixed dyslipidemia, especially those in the highest tertile for both body mass and baseline triglycerides.32 Findings from the BIP (Bezafibrate Infarction Prevention) study and Veterans Affairs HDL Intervention Trial, both secondary prevention studies, also show that patients with mixed dyslipidemia associated with insulin resistance syndromes such as type 2 diabetes or metabolic syndrome, derive greatest benefit from fibrate therapy (Figure 1).34,36 In the BIP study, post hoc analysis revealed that patients with baseline triglycerides ⩾200 mg/dl had 39.5% reduction in risk for the primary endpoint of fatal or non-fatal MI or sudden death (P = 0.002).33
Figure 1.
Effect of fibrate treatment on clinical outcomes. Results from four major studies with (a) gemfibrozil (b) fenofibrate and (c) gemfibrozil or bezafibrate. HHS,31,32 FIELD,37 VA-HIT: Veterans Affairs HDL Intervention Trial;35,36 BIP,33,34 CHD: coronary heart disease; CVD: CV disease; TG: triglycerides.
The FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) study (n = 9795)37 evaluated patients with type 2 diabetes who were generally considered to be at relatively low CV risk. This was based upon the low prevalence of prior CV disease (22%), relatively short duration of diabetes, well controlled glycemia and only mildly elevated baseline lipids (Table 3). While the reduction in the primary endpoint (coronary heart disease death and non-fatal MI) was not significant (relative risk reduction 11%, P = 0.16), there was an 11% reduction (P = 0.035) in the secondary endpoint of total CV events. Recent exploratory analyses from FIELD show that type 2 diabetes patients with the combination of markedly elevated triglycerides ⩾200 mg/dl and low HDL-C (<40 mg/dl in men and <50 mg/dl in women) not only were at highest risk for CV events (event rate 17.8% over 5 years) but also obtained greatest benefit from fenofibrate treatment (relative risk reduction 27%, P = 0.005) (Figure 1).38
Table 3.
The FIELD patient population was at low CV risk
| FIELD (n = 9795) | |
|---|---|
| Median duration of diabetes | 5 years |
| Median HbA1C | 6.9% |
| Blood glucose medication | |
| Diet alone | 26% |
| Oral antidiabetic alone | 34% |
| CV disease | 22% |
| Microvascular complications | 21% |
| Mean baseline lipids (mg/dl) | |
| LDL-C | 119 |
| HDL-C | 42 |
| Triglycerides | 153 |
FIELD.37
FIELD also provided encouraging data suggesting that fenofibrate treatment is associated with preventive effects on diabetes-related microvascular disease. These benefits included reduction in the need for first retinal laser therapy (from 4.9% on placebo to 3.4% on fenofibrate, RRR 31%, P = 0.0002), with similar benefits in macular edema and proliferative retinopathy39 an effect that was evident within 8 months of treatment. In a substudy of the FIELD population including 1012 patients who underwent serial standardized retinal photography, 2-step progression of Early Treatment Diabetic Retinopathy Study retinopathy grade (the primary endpoint), was reduced with fenofibrate treatment in patients with pre-existing retinopathy (P = 0.004), although not in all patients (P = 0.10). There was also benefit with fenofibrate on a post hoc exploratory composite outcome representative of retinal pathology (primary endpoint plus occurrence of macular edema or laser treatment, P = 0.022). Additionally, fenofibrate treatment was associated with reduction in albuminuria (15% increase in regression and 14% decrease in progression P = 0.0018)37 and a 38% reduction in the number of non-traumatic amputations (P = 0.011).40 These findings are noteworthy in view of the impairment in patient quality of life that often accompanies microvascular complications of diabetes. The associated benefit in microvascular disease progression may offer pharmacoeconomic advantages.
Nevertheless, we await clinical endpoint data demonstrating clinical superiority of fibrate–statin combination compared with statin monotherapy (see below).
Niacin
Niacin (nicotinic acid) is another well-established agent for the treatment of mixed dyslipidemia, particularly in the management of low HDL-C.2 Niacin treatment is associated with increases in HDL-C of up to 26% at recommended clinical doses of 1–2 g/day of the extended-release formulation, together with decreases in LDL-C and triglycerides of up to 16 and 35%, respectively.41,42 In the secondary prevention Coronary Drug Project of 8341 men with previous MI, niacin use was not associated with a statistically significant effect on the primary endpoint, total mortality. However, patients experienced reduction in secondary endpoints, notably the incidence of non-fatal MI by 26% and cerebrovascular events by 24% compared with placebo at the end of the 6-year study. Niacin treatment was also associated with significant reduction in mortality 9 years after the study was completed (11% vs. placebo, P < 0.001).43,44 Recent subgroup analysis demonstrated greater reduction in non-fatal MI (by 57%) in patients with the highest fasting blood glucose (⩾126 mg/dl).45
While niacin is effective in raising HDL-C, higher doses may also raise plasma glucose levels. This issue has been addressed in clinical studies which showed that changes in glycemic control may be effectively treated with adjustment of antidiabetic medication,46 supporting recent recommendations by the American Diabetes Association.47
Flushing remains the principal side effect associated with niacin treatment. Recent approaches to alleviate this problem have investigated the use of laropiprant, an inhibitor of the prostaglandin D2 receptor, which in turn has been implicated in the flushing response.48 However, while clinical studies have shown that the combination of niacin plus laropiprant significantly reduces the extent of flushing,49 long-term safety is currently under investigation (see below).
Omega-3 fatty acids
Treatment with prescription formulation omega-3 fatty acids (each 1 g capsule containing at least 900 mg of the ethyl esters of omega-3 fatty acids, 20-carbon eicosapentaenoic acid, EPA and 22-carbon docosahexaenoic acid, DHA) is indicated as monotherapy for patients with very high triglycerides (⩾500 mg/dl). In this setting, treatment with omega-3 fatty acids (4 g/day) has been associated with decreases in triglycerides of 37% and VLDL-C of 33%, as well as increases in HDL-C (11%).50 Compared with simvastatin monotherapy (40 mg/day), addition of omega-3 fatty acids (4 g/day) to simvastatin in patients with triglycerides ⩾200 mg/dl and <500 mg/dl and mean LDL-C levels ⩽10% above the NCEP ATPIII goal resulted in significantly greater decreases in triglycerides (−29.5% vs. −6.3% with simvastatin monotherapy, P < 0.0001) and VLDL-C (−27.5% vs. −6.3%, P < 0.0001) and significant increase in HDL-C (+3.4% vs. −1.2%) (all P < 0.001). The combination treatment also had minimal effect on LDL-C levels (+0.7% vs. −2.8% with simvastatin monotherapy).51
The prescription formulation of omega-3 fatty acids is generally well tolerated with eructation, infection, flu syndrome and dyspepsia the most common adverse events reported in clinical trials. Some studies demonstrated prolongation of bleeding time, although this did not exceed normal limits and did not produce significant bleeding episodes. However, patients receiving anticoagulants concomitantly should be monitored periodically.50 Additionally, patients may complain of a ‘fishy’ after taste, although this is less problematic than with omega-3 supplements.
Outcome benefits have been demonstrated with supplementation with omega-3 fatty acids, either as part of a Mediterranean diet52 or at a dose of 1 g/day (850–882 mg EPA and DHA in the ratio of 1:1.2).53 Recently, the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico (GISSI) study showed that in patients with heart failure supplementation with 1 g daily n-3 polyunsaturated fatty acids (provided a survival benefit compared with placebo (adjusted hazard ratio 0.91, 95.5% CI 0.833–0.998, P = 0.041) and fewer CV admissions (adjusted hazard ratio 0.92, 99% CI 0.849–0.999, P = 0.009).54 However, this may relate to more to favourable effects on the atherothrombotic CV disease including arrhythmias, rather than on blood lipids.55 Patients were also receiving adjunctive CV medication at admission including blockers of the renin angiotensin system (94%), diuretics (90%), beta-blockers (65%), aspirin (48%) and spironolactone (39%). Open statin therapy was received by about 23% of patients. The Japan EPA lipid intervention study (JELIS) demonstrated support for outcome benefits associated with combination treatment with omega-3 fatty acids (EPA 1800 mg daily) and low-dose statin therapy (pravastatin 10 mg or simvastatin 5 mg daily) compared with statin monotherapy.56 Importantly, however, these observed CV benefits were unlikely to be the consequence of triglyceride lowering (decrease by 9% vs. 4% in control patients) owing to the relatively low doses employed in the omega-3 treatment arm.
Potential of combination therapy
Combination lipid-modifying therapy is a potentially useful strategy for achieving lipid targets in patients with mixed dyslipidemia. Clinical studies demonstrate that the combination of a low to moderate dose of statin and a fibrate57,58 or fenofibric acid,59,60 ezetimibe,61 omega-3 fatty acids62,63 or niacin64–66 provides enhanced overall lipid control in patients with mixed dyslipidemia. Additionally, treatment with the combination of fenofibrate plus simvastatin (160/20 mg daily),67 ezetimibe plus fenofibrate68 or simvastatin plus ezetimibe plus fenofibrate69 has been shown to favourably reduce both LDL-C and triglycerides. Clinicians may even consider triple combination therapy such as the combination of ezetimibe/simvastatin plus extended-release niacin to provide an effective, broad, lipid-modifying therapy with improvements beyond LDL-C.70 However, the use of multiple lipid therapies should be reserved for patients based upon recommendations of national committees (e.g. NCEP, ADA) due to lack of data demonstrating clinical outcome benefit.
Moreover, while national treatment guidelines recommend combination therapy in patients needing treatment for elevated LDL-C and triglycerides and low HDL-C, there may be concerns relating to tolerability.2,71 In respect of the combination of a fibrate and statin, consensus highlights a risk for myopathy with both statins and fibrate monotherapy (Table 4),71,72 which may be enhanced when these agents are co-administered. There is, however, evidence that fibrates differ in their interaction potential. Safety surveillance data from the US Food and Drug Administration's Adverse Events Reporting System database (1998–2002) showed that the incidence of rhabdomyolysis and myopathy was 15- and 33-fold higher, respectively, with the combination of gemfibrozil and a statin (excluding the discontinued cerivastatin) than fenofibrate plus statin.73 The reason for this is thought to be due to competition between gemfibrozil and the statin for specific glucuronidases responsible for drug biotransformation, supported by in vitro evidence.74 In contrast, fenofibrate appears to be metabolized via different enzymes suggestive of a low interaction potential in combination with a statin. Clinical interaction studies are supportive of these findings. Neither fenofibrate75–77 nor fenofibric acid78 significantly influenced the pharmacokinetics of commonly prescribed statins. Furthermore, in the FIELD study, only three patients (<1%) in the fenofibrate arm and none of the 890 subjects who also received a statin, developed rhabdomyolysis (Table 4).37 The FIELD study also showed that fenofibrate increased serum creatinine levels, although this effect was reversible within 8 weeks of stopping study treatment, suggestive of no permanent impairment of renal function.37 Recently, The FDA approved fenofibric acid for combination with a statin.79
Table 4.
Incidence of myopathy in the fibrate trials
| Trial | Treatment (mg/day) | Muscle symptoms | Fibrate | Placebo |
|---|---|---|---|---|
| HHS | Gemfibrozil 1200 | Myopathy | 0 | 0 |
| DAIS | Fenofibrate 200 | Muscle serious adverse events | 0 | 0.5% |
| Myositis | 2 (<0.001%) | 1 (<0.001%) | ||
| FIELD | Fenofibrate 200 | Rhabdomyolysis | 3 (<0.001%)a | 1 (<0.001%) |
| CK>10 × ULN | 8 (<0.001%)a | 3 (<0.001%) | ||
| BIP | Bezafibrate 400 | Muscle pain | 5 (0.32%) | 7 (0.45%) |
| VA-HIT | Gemfibrozil 1200 | Myopathy | 0 | 0 |
Adapted from Davidson et al. (2007).71 aNone of these patients were also receiving statin.37 DAIS: Diabetes Atherosclerosis Intervention Study.
As mentioned above, clinical outcomes data are needed to determine the most effective combination therapies. Data from HATS (HDL-Atherosclerosis Treatment Study)80 and the ARBITER 2 (ARterial Biology for the Investigation of the Treatment Effects of Reducing cholesterol) study81 showed that the combination of simvastatin plus niacin halted progression of atherosclerosis, as assessed by serial coronary angiography or measurement of carotid intima-medial thickness. However, while these findings are encouraging with respect to surrogate CV endpoints, large prospective outcomes studies with hard clinical endpoints are required.
To this end, four major studies are ongoing, two evaluating the combination of niacin plus simvastatin, one evaluating the combination of fenofibrate and simvastatin and one evaluating the combination of ezetimibe plus simvastatin. AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL-C/High Triglyceride and Impact on Global Health Outcomes),82 is comparing extended-release niacin combined with simvastatin versus simvastatin monotherapy in about 3300 patients with established vascular disease and mixed dyslipidemia and HPS2-Thrive (Heart Protection Study-Treatment of HDL to Reduce the Incidence of Vascular Events)83 is comparing the combination of extended-release niacin/laropiprant with placebo in about 20 000 patients with a history of MI, stroke or peripheral arterial disease and LDL-C levels optimized with statin therapy. However, results are not expected until 2011 or 2012. The lipid-modifying arm of the ACCORD (Action to Control Cardiovascular Risk in Diabetes)84 is comparing the combination of simvastatin plus a fibrate (fenofibrate) versus simvastatin monotherapy in a cohort of about 5000 patients with type 2 diabetes mellitus with defined glycemic control and existing clinical or subclinical CVD or CVD risk factors. Results are expected in 2009. Finally, IMPROVE IT (Examining Outcomes in Subjects with Acute Coronary Syndrome: Vytorin (ezetimibe/simvastatin) vs. Simvastatin) is evaluating the combination of simvastatin plus ezetimibe versus simvastatin monotherapy, with data expected in 2012 or 2013.85
Conclusions
Efforts aimed at primary prevention offer the greatest opportunity for reducing the onset and burden of CV disease. While lowering LDL-C with a statin remains the cornerstone in the management of dyslipidemia, this approach may be insufficient in patients with other lipid abnormalities including low HDL-C and elevated triglycerides, both of which are independently associated with CV risk.
Adjunctive approaches to the management of mixed dyslipidemia include fibrates, omega-3 fatty acids and/or niacin. The combination of lipid-modifying therapies has been proposed by national treatment guidelines. While there is evidence to support enhanced lipid-modifying efficacy with the addition of ezetimibe, a fibrate, niacin or omega-3 fatty acids to statin therapy, data are eagerly awaited from IMPROVE IT, ACCORD, AIM-HIGH and HPS2-THRIVE to determine which (if any) of these combinations may be clinically superior to statin therapy alone to reduce residual CV risk.
Clinical significance
Primary prevention provides the greatest opportunity for reducing the burden of vascular disease.
Statins incompletely address CV risk due to mixed dyslipidemia.
Combination therapy, with addition of a fibrate, ezetimibe, omega-3 fatty acids or niacin can provide greater lipid-modifying efficacy.
Ongoing clinical trials will determine whether combination therapy is clinically superior to statin monotherapy on CV outcomes.
Funding
National Institutes of Health (HL-42663); Veterans Affairs Merit Award; American Heart Association Grant-In-Aid; unrestricted educational grant from Abbott Laboratories.
Conflict of interest: Dr Miller has been a consultant for Glaxo Smith Kline and Roche and receives research funding from Merck-Schering Plough and Roche.
Acknowledgements
The author acknowledges research assistance provided by Jane Stock PhD.
References
- 1.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with MI in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 2.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final report. Circulation. 2002:106–421. [PubMed] [Google Scholar]
- 3.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, et al. National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 4.Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, et al. American Diabetes Association; American College of Cardiology Foundation. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008;31:811–22. doi: 10.2337/dc08-9018. [DOI] [PubMed] [Google Scholar]
- 5.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Cholesterol Treatment Trialists' (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 6.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–35. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 7.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 8.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 9.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–8. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 10.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–16. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 11.Briel M, Ferreira-Gonzalez I, You JJ, Karanicolas PJ, Akl EA, Wu P, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. Br Med J. 2009;338:b92. doi: 10.1136/bmj.b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen JD, Cziraky MJ, Jacobson TA, Wallace A, Cai C. Changes in the prevalence of abnormal lipid fractions among US adults: results from the National Health and Nutrition Examination Survey II, III and 1999–2006. Circulation. 2008;118:S_1081–2. [Abstract 1198] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) National Diabetes Surveillance System. [Accessed June 2007];Diabetes data and trends. CDC Web site. [ http://www.cdc.gov/diabetes/statistics/prev/national/figpersons.htm]
- 14.Miller M. Disorders of hypertriglyceridemia. In: Kwiterovich PO, editor. The Johns Hopkins Textbook of Dyslipidemia. Baltimore, MD, Lippincott, Williams & Wilkins: 2009. [Google Scholar]
- 15.Lamarche B, Tchernof A, Mauriège P, Cantin B, Dagenais GR, Lupien PJ, et al. Fasting insulin and apoplipoprotein B levels and low-density lipoprotein particle size as risk factors for ischemic heart disease. JAMA. 1998;279:1955–61. doi: 10.1001/jama.279.24.1955. [DOI] [PubMed] [Google Scholar]
- 16.Sattar N, Williams K, Sniderman AD, D'Agostino R, Jr, Haffner SM. Comparison of the associations of apolipoprotein B and non-high-density lipoprotein cholesterol with other cardiovascular risk factors in patients with metabolic syndrome. Circulation. 2004;110:2687–93. doi: 10.1161/01.CIR.0000145660.60487.94. [DOI] [PubMed] [Google Scholar]
- 17.Sierra-Johnson J, Somers VK, Kuniyoshi FH, Garza CA, Isley WL, Gami AS, et al. Comparison of apoliprotein B-apolipoprotein-AI in subjects with versus without the metabolic syndrome. Am J Cardiol. 2006;98:1369–73. doi: 10.1016/j.amjcard.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 18.Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E. PROVE-IT TIMI 22 Investigators. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE-IT TIMI 22 trial. J Am Coll Cardiol. 2008;51:724–30. doi: 10.1016/j.jacc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 19.Jacobson TA, Case CC, Roberts S, Buckley A, Murtaugh KM, Sung JC, et al. Characteristics of US adults with the metabolic syndrome and therapeutic implications. Diabetes Obes Metab. 2004;6:353–62. doi: 10.1111/j.1462-8902.2004.00354.x. [DOI] [PubMed] [Google Scholar]
- 20.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. American Heart Association Nutrition Committee. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 21.Chiuve SE, McCullough ML, Sacks FM, Rimm EB. Healthy lifestyle factors in the primary prevention of coronary heart disease among men: benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation. 2006;114:160–7. doi: 10.1161/CIRCULATIONAHA.106.621417. [DOI] [PubMed] [Google Scholar]
- 22.Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, et al. Cholesterol Treatment Trialists' (CTT) Collaborators. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–25. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 23.Girman CJ, Rhodes T, Mercuri M, Pyörälä K, Kjekshus J, Pedersen TR, et al. 4S Group; AFCAPS/TexCAPS Research Group. The metabolic syndrome and risk of major coronary events in the Scandinavian Simvastatin Survival Study (4S) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) Am J Cardiol. 2004;93:136–41. doi: 10.1016/j.amjcard.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 24.Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, et al. Treating to New Targets Investigators. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–10. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 25.Chapman MJ. Fibrates in 2003: therapeutic action in atherogenic dyslipidaemia and future perspectives. Atherosclerosis. 2003;171:1–13. doi: 10.1016/s0021-9150(03)00156-4. [DOI] [PubMed] [Google Scholar]
- 26.Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from receptors to drug discovery. J Med Chem. 2000;22:717–26. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 27.Vakkilainen J, Steiner G, Ansquer JC, Aubin F, Rattier S, Foucher C, et al. DAIS Group. Relationships between low-density lipoprotein particle size, plasma lipoproteins, and progression of coronary artery disease: the Diabetes Atherosclerosis Study (DAIS) Circulation. 2003;107:1733–7. doi: 10.1161/01.CIR.0000057982.50167.6E. [DOI] [PubMed] [Google Scholar]
- 28.Barbier O, Torra IP, Duguay Y, Blanquart C, Fruchart JC, Glineur C, et al. Pleiotrophic actions of peroxisome proliferator-activated receptors in lipid metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol. 2002;22:717–26. doi: 10.1161/01.atv.0000015598.86369.04. [DOI] [PubMed] [Google Scholar]
- 29.Devchand P, Keller H, Peters J, Vazquez M, Gonzalez F, Wahli W. The PPARα-leucotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 30.Simpson IA, Lorimer AR, Walker ID, Davidson JF. Effect of ciprofibrate on platelet aggregation and fibrinolysis in patients with hypercholesterolaemia. Thromb Haemostat. 1989;54:442–4. [PubMed] [Google Scholar]
- 31.Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, et al. Helsinki Heart study: primary prevention trial with gemfibrozil in middle—aged men with dyslipidemia. N Engl J Med. 1987;317:1237–45. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 32.Manninen V, Tenkanen L, Koskinen P, Huttunen JK, Mänttäri M, Heinonen OP, et al. Joint effects of serum triglycerides and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study: implications for treatment. Circulation. 1992;85:37–45. doi: 10.1161/01.cir.85.1.37. [DOI] [PubMed] [Google Scholar]
- 33.The Bezafibrate Infarction Prevention (BIP) study group. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. The Bezafibrate Infarction Prevention (BIP) study. Circulation. 2000:102–7. doi: 10.1161/01.cir.102.1.21. [DOI] [PubMed] [Google Scholar]
- 34.Tenenbaum A, Motro M, Fisman EZ, Tanne D, Boyko V, Behar S. Bezafibrate for the secondary prevention myocardial infarction in patients with metabolic syndrome. Arch Intern Med. 2005;165:1154–60. doi: 10.1001/archinte.165.10.1154. [DOI] [PubMed] [Google Scholar]
- 35.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–8. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 36.Rubins HB, Robins SJ, Collins D, Nelson DB, Elam MB, Schaefer EJ, et al. Diabetes, plasma insulin, and cardiovascular disease. Subgroup analysis from the Department of Veterans Affairs High-density Lipoprotein Intervention Trial (VA-HIT) Arch Intern Med. 2002;162:2597–604. doi: 10.1001/archinte.162.22.2597. [DOI] [PubMed] [Google Scholar]
- 37.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, et al. FIELD study investigators. The FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–61. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 38.Scott R, O'Brien R, Fulcher G, Pardy C, d'Emden M, Tse D, et al. on behalf of the FIELD Study Investigators. The effects of fenofibrate treatment on cardiovascular disease risk in 9795 people with type 2 diabetes and various components of the metabolic syndrome: the FIELD study. Diabetes Care. 2008;32:493–8. doi: 10.2337/dc08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, et al. FIELD study investigators. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–97. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 40.Burgess D, Hunt D, Li LP, Zhang J, Sy R, Laakso M, et al. on behalf of the FIELD Investigators. Effects of fenofibrate on silent myocardial infarction, hospitalization for acute coronary syndromes and amputation in type 2 diabetes: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Circulation. 2007;116 II_838 [abstract] [Google Scholar]
- 41.Morgan JM, Capuzzi DM, Guyton JR, Centor RM, Goldberg R, Robbins DC, et al. Treatment effect of NIASPAN, a controlled-release nicotinic acid, in patients with hypercholesterolemia: a placebo-controlled trial. J Cardiovasc Pharmacol Ther. 1996;1:195–202. doi: 10.1177/107424849600100302. [DOI] [PubMed] [Google Scholar]
- 42.Goldberg A, Alagona P, Jr, Capuzzi DM, Guyton J, Morgan JM, Rodgers J, et al. Multiple-dose efficacy and safety of an extended-release form of nicotinic acid in the management of hyperlipidemia. Am J Cardiol. 2000;85:1100–5. doi: 10.1016/s0002-9149(00)00703-7. [DOI] [PubMed] [Google Scholar]
- 43.Coronary Drug Project Research Group. Clofibrate and niacin in coronary heart disease. JAMA. 1975:231–81. [Google Scholar]
- 44.Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245–55. doi: 10.1016/s0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- 45.Canner PL, Furberg CD, Terrin ML, McGovern ME. Benefits of niacin by glycemic status in patients with healed myocardial infarction (from the Coronary Drug Project) Am J Cardiol. 2005;95:254–7. doi: 10.1016/j.amjcard.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Grundy SM, Vega GL, McGovern ME, Tulloch BR, Kendall DM, Fitz-Patrick D, et al. Diabetes Multicenter Research Group. Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes. Results of the assessment of diabetes control and evaluation of the efficacy of Niaspan trial. Arch Intern Med. 2002;162:1568–76. doi: 10.1001/archinte.162.14.1568. [DOI] [PubMed] [Google Scholar]
- 47.American Diabetes Association. Standards of medical care in diabetes-2008. Diabetes Care. 2008;31(Suppl. 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 48.Lai E, De Lepeleire I, Crumley TM, Liu F, Wenning LA, Michiels N, et al. Suppression of niacin-induced vasodilation with an antagonist to prostaglandin D2 receptor subtype1. Clin Pharmacol Ther. 2007;81:49–57. doi: 10.1038/sj.clpt.6100180. [DOI] [PubMed] [Google Scholar]
- 49.Paolini JF, Mitchel YB, Reyes R, Kher U, Lai E, Watson DJ, et al. Effects of laropiprant on nicotinic acid-induced flushing in patients with dyslipidemia. Am J Cardiol. 2008;101:625–30. doi: 10.1016/j.amjcard.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 50.Stalenhoef AF, de Graaf J, Wittekoek ME, Bredie SJ, Demacker PN, Kastelein JJ. The effect of concentrated n-3 fatty acids versus gemfibrozil on plasma lipoproteins, low density lipoprotein heterogeneity and oxidizability in patients with hypertriglyceridemia. Atherosclerosis. 2000;153:129–38. doi: 10.1016/s0021-9150(00)00381-6. [DOI] [PubMed] [Google Scholar]
- 51.Davidson MH, Stein EA, Bays HE, Maki KC, Doyle RT, Shalwitz RA, et al. COMBination of prescription Omega-3 with Simvastatin (COMBOS) Investigators. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2007;29:1354–67. doi: 10.1016/j.clinthera.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 52.de Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I, et al. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994;343:1454–9. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- 53.Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction. results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 54.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, et al. Gissi-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–30. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 55.Leaf A. Omega-3 fatty acids and prevention of arrhythmias. Curr Opin Lipidol. 2007;18:31–4. doi: 10.1097/MOL.0b013e328012d61b. [DOI] [PubMed] [Google Scholar]
- 56.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–8. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 57.Vega GL, Ma PT, Cater NB, Filipchuk N, Meguro S, Garcia-Garcia AB, et al. Effects of adding fenofibrate (200 mg/day) to simvastatin (10 mg/day) in patients with combined hyperlipidemia and metabolic syndrome. Am J Cardiol. 2003;91:956–60. doi: 10.1016/s0002-9149(03)00111-5. [DOI] [PubMed] [Google Scholar]
- 58.Grundy SM, Vega GL, Yuan Z, Battisti WP, Brady WE, Palmisano J. Effectiveness and tolerability of simvastatin plus fenofibrate for combined hyperlipidemia (the SAFARI trial) Am J Cardiol. 2005;95:462–8. doi: 10.1016/j.amjcard.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Mohiuddin SM, Pepine CJ, Kelly MT, Buttler SM, Setze CM, Sleep DJ, et al. Efficacy and safety of ABT-335 (fenofibric acid) in combination with simvastatin in patients with mixed dyslipidemia: a phase 3, randomized, controlled study. Am Heart J. 2009;157:195–203. doi: 10.1016/j.ahj.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 60.Jones PH, Davidson MH, Kashyap ML, Kelly MT, Buttler SM, Setze CM, et al. Efficacy and safety of ABT-335 (fenofibric acid) in combination with rosuvastatin in patients with mixed dyslipidemia: a phase 3 study. Atherosclerosis. 2008;32:493–8. doi: 10.1016/j.atherosclerosis.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 61.McKenney JM, Farnier M, Lo KW, Bays HE, Perevozkaya I, Carlson G, et al. Safety and efficacy of long-term co-administration of fenofibrate and ezetimibe in patients with mixed hyperlipidemia. J Am Coll Cardiol. 2006;47:1584–7. doi: 10.1016/j.jacc.2005.11.072. [DOI] [PubMed] [Google Scholar]
- 62.Davidson MH, Stein EA, Bays HE, Maki KC, Doyle RT, Shalwitz RA, et al. COMBination of prescription Omega-3 with Simvastatin (COMBOS) Investigators. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2007;29:1354–67. doi: 10.1016/j.clinthera.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 63.Maki KC, McKenney JM, Reeves MS, Lubin BC, Dicklin MR. Effects of adding prescription omega-3 acid ethyl esters to simvastatin (20 mg/day) on lipids and lipoprotein particles in men and women with mixed dyslipidemia. Am J Cardiol. 2008;102:429–33. doi: 10.1016/j.amjcard.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 64.McKenney JM, Jones PH, Bays HE, Knopp RH, Kashyap ML, Ruoff GE, et al. Comparative effects on lipid levels of combination therapy with a statin and extended-release niacin or ezetimibe versus a statin alone (the COMPELL study) Atherosclerosis. 2007;192:432–7. doi: 10.1016/j.atherosclerosis.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 65.Ballantyne CM, Davidson MH, McKenney J, Keller LH, Bajorunas DR, Karas RH. Comparison of the safety and efficacy of a combination tablet of niacin extended release and simvastatin vs simvastatin monotherapy in patients with increased non-HDL cholesterol (from the SEACOAST I study) Am J Cardiol. 2008;101:1428–36. doi: 10.1016/j.amjcard.2008.02.092. [DOI] [PubMed] [Google Scholar]
- 66.Karas RH, Kashyap ML, Knopp RH, Keller LH, Bajorunas DR, Davidson MH. Long-term safety and efficacy of a combination of niacin extended release and simvastatin in patients with dyslipidemia: the OCEANS study. Am J Cardiovasc Drugs. 2008;8:69–81. doi: 10.2165/00129784-200808020-00001. [DOI] [PubMed] [Google Scholar]
- 67.Grundy SM, Vega GL, Yuan Z, Battisti WP, Brady WE, Palmisano J. Effectiveness and tolerability of simvastatin plus fenofibrate for combined hyperlipidemia (the SAFARI trial) Am J Cardiol. 2005;95:462–8. doi: 10.1016/j.amjcard.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 68.Farnier M, Freeman MW, Macdonell G, Perevozskaya I, Davies MJ, Mitchel YB, et al. The Ezetimibe Study Group. Efficacy and safety of the coadministration of ezetimibe with fenofibrate in patients with mixed hyperlipidaemia. Eur Heart J. 2005;26:897–905. doi: 10.1093/eurheartj/ehi231. [DOI] [PubMed] [Google Scholar]
- 69.Farnier M, Roth E, Gil-Extremera B, Mendez GF, Macdonell G, Hamlin C, et al. Ezetimibe/Simvastatin + Fenofibrate Study Group. Efficacy and safety of the coadministration of ezetimibe/simvastatin with fenofibrate in patients with mixed hyperlipidemia. Am Heart J. 2007;153:1–8. doi: 10.1016/j.ahj.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 70.Guyton JR, Brown BG, Fazio S, Polis A, Tomassini JE, Tershakovec AM. Lipid-altering efficacy and safety of ezetimibe/simvastatin coadministered with extended-release niacin in patients with type IIa or type IIb hyperlipidemia. J Am Coll Cardiol. 2008;51:1564–72. doi: 10.1016/j.jacc.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 71.Davidson MH, Armani A, McKenney JM, Jacobson TA. Safety considerations with fibrate therapy. Am J Cardiol. 2007;99(Suppl.):3C–18C. doi: 10.1016/j.amjcard.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 72.Rosenson RS. Current overview of statin-induced myopathy. Am J Med. 2004;116:408–16. doi: 10.1016/j.amjmed.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 73.Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin. Am J Cardiol. 2005;95:120–2. doi: 10.1016/j.amjcard.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 74.Prueksaritanont T, Tang C, Qiu Y, Mu L, Subramanian R, Lin JH. Effects of fibrates on metabolism of statins in human hepatocytes. Drug Metab Dispos. 2002;30:1280–7. doi: 10.1124/dmd.30.11.1280. [DOI] [PubMed] [Google Scholar]
- 75.Pan WJ, Gustavson LE, Achari R, Rieser MJ, Ye X, Gutterman C, et al. Lack of a clinically significant pharmacokinetic interaction between fenofibrate and pravastatin in healthy volunteers. J Clin Pharmacol. 2000;40:316–23. doi: 10.1177/00912700022008874. [DOI] [PubMed] [Google Scholar]
- 76.Martin PD, Dane AL, Schneck DW, Warwick MJ. An open-label, randomized, three-way crossover trial of the effects of coadministration of rosuvastatin and fenofibrate on the pharmacokinetic properties of rosuvastatin and fenofibric acid in healthy male volunteers. Clin Ther. 2003;25:459–71. doi: 10.1016/s0149-2918(03)80089-9. [DOI] [PubMed] [Google Scholar]
- 77.Bergman AJ, Murphy G, Burke J, Zhao JJ, Valesky R, Liu L, et al. Simvastatin does not have a clinically significant pharmacokinetic interaction with fenofibrate in humans. J Clin Pharmacol. 2004;44:1054–62. doi: 10.1177/0091270004268044. [DOI] [PubMed] [Google Scholar]
- 78.Zhu T, Awni WM, Hosmane B, Kelly MT, Sleep DJ, Stolzenbach JC, et al. ABT-335, the choline salt of fenofibric acid, does not have a clinically significant pharmacokinetic interaction with rosuvastatin in humans. J Clin Pharmacol. 2009;49:63–71. doi: 10.1177/0091270008325671. [DOI] [PubMed] [Google Scholar]
- 79.FDA approves fenofibric acid for lipid target controls. [Accessed January 20, 2009]; [ http://www.endocrinetoday.com/view.aspx?rid=35718]
- 80.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–92. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 81.Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing cholesterol (ARBITER) 2. A double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110:3512–7. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 82.Clinical Trial: AIM HIGH. Niacin plus statin to prevent vascular events. [Accessed 24 April 2008];Heart Protection Study-Treatment of HDL to Reduce the Incidence of Vascular Events. [ www.clinicaltrials.gov.ct/show/NCT00120289]
- 83.Cinical Trial. HPS2-Thrive Study. Heart Protection Study-Treatment of HDL to Reduce the Incidence of Vascular Events. [Accessed 7 April 2008]; [ http://www.ctsu.ox.ac.uk/pressreleases/2006-05-31/hps2-thrive-press-release]
- 84.Clinical Trial. ACCORD. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. [Accessed 20 April 2008]; [ http://www.clinicaltrials.gov/ct/show/NCT00000620]
- 85.Clinical Trial. IMPROVE-IT. Examining Outcomes in Subjects With Acute Coronary Syndrome:Vytorin (Ezetimibe/Simvastatin) vs Simvastatin. [Accessed 19 December 2008]; NCT00202878. [ http://clinicaltrials.gov/show/NCT00202878]