Abstract
Vaccinia virus (VACV) affords long lasting protection against variola virus, the agent of smallpox. VACV-reactive CD8 T cells contribute to protection but their molecular control is unknown. We show that the TNFR molecule OX40 (CD134) controls primary VACV-specific CD8 T cell expansion and anti-viral cytokine production and dictates development of strong memory to both dominant and subdominant VACV epitopes. Using adoptive transfer of OX40-deficient CD8 TCR transgenic T cells responding to antigen in the context of VACV infection, we found this reflects a direct action of OX40 expressed by CD8 T cells. Furthermore, CD8 T cells that can protect against lethal VACV challenge do not develop in mice deficient in OX40. Thus OX40, which has been found to play little if any role in the generation of CD8 T cells to several viruses, including LCMV and influenza, plays a dominant role in shaping the CD8 T cell response to vaccinia virus. These data suggest that unique co-stimulatory pathways might control alternate anti-viral CD8 responses, demonstrating the plasticity of the immune response in utilizing different mechanisms to achieve similar ultimate goals.
Keywords: OX40, Costimulation, Vaccinia Virus, Poxviruses, CD8 T cells
Introduction
CD8 T cells play an important role in controlling many viral infections and are elicited by live viral vaccines. As such it is important to understand how CD8 cells reactive to different antigenic viral peptides become primed. Although a brief encounter (7–20 hrs) with antigen is sufficient to lead to proliferation of CD8 cells and a level of differentiation, increasing the duration of antigenic stimulation is necessary for strong clonal expansion, survival, and full reactivity (1–4). This suggests an important role for signals other than peptide recognition.
Two types of costimulatory signals might be considered as potentially contributing to the development of virus-specific CD8 T cells. One, the interaction of receptors on the surface of T cells with membrane bound ligands on APCs. The other, signals from pro-inflammatory cytokines elicited in response to infection. The importance of membrane bound receptor-ligand interactions to T cell priming has been strongly documented in studies of CD4 cells, examining the requirement for Ig superfamily members such as CD28-B7, and TNFR/TNF superfamily members such as OX40-OX40L (5, 6). More recent studies in simple model systems have also suggested such interactions can control aspects of the response of CD8 T cells (7–11). However, in terms of anti-viral responses, an argument has been put forward that pro-inflammatory cytokines (12), typified by type I interferons (IFN-I) might represent a dominant stimulus controlling development of virus-specific CD8 populations (13, 14). In this regard, reports have shown that IFNα/β receptor-deficient CD8 T cells specific for lymphocytic choriomeningitis virus (LCMV) exhibit a severe defect in their ability to expand and generate functional memory populations after infection (13, 14). Moreover, extensive data with LCMV, as well as several other model viruses such as influenza, vesicular stomatitis virus (VSV), and mouse cytomegalovirus (MCMV), have revealed lesser or no roles for molecules like CD28 (15–17) and OX40 (7, 18, 19) in controlling initial priming of naïve virus-specific CD8 cells. This has contributed to the conclusion that there are times where the latter more classical costimulatory molecules are not strong determinants of primary immunity, and raises the issue of whether all viruses-specific immune responses are controlled by similar molecular mechanisms.
Vaccinia virus (VACV) is a large DNA virus and is a member of the genus Orthopoxvirus, which includes variola, monkeypox, buffalopox, and cowpox. Variola, the etiological agent of smallpox, was responsible for significant morbidity and mortality in humans (20). Large-scale vaccination with live VACV proved extremely effective at protecting humans against variola, and this led to the worldwide eradication of smallpox disease (20). In humans, immunization with VACV elicits a robust CD8 T cell response (21). Notably, recent analysis of cohorts of smallpox vaccine recipients demonstrated that the VACV-specific memory CD8 T cell pool is long-lived, with a half-life of 8–12 years (21–23). In mice, the Western Reserve strain of VACV (VACV-WR) results in an acute infection that also elicits strong development of CD8 T cells (24, 25). At the peak of the effector phase, more than 20% of CD8 T cells are specific for vaccinia virus (24, 25). These then contract in number, stabilize by day 30 as a memory population, and are maintained for more than 300 days post-infection (24). Thus, in many respects VACV elicits CD8 T cells highly analogous to other viruses such as LCMV and influenza. However, there is little information on the molecules that generate protective pools of anti-VACV CD8 T cells. Further, the lack of defined peptide epitopes recognized by VACV has hampered in depth studies of this virus. The recent identification of epitopes that account for nearly the entire anti-VACV CD8 pool (26, 27) has provided a unique opportunity to examine for the costimulatory requirement of anti-VACV CD8 T cells with different specificities.
Here we show that the TNFR family member OX40 is critical for the magnitude of primary CD8 T cell responses to both dominant and subdominant VACV epitopes, including expansion and anti-viral cytokine production, and OX40 also strongly impacts the generation of memory cells. Moreover, CD8 T cells that can protect against lethal VACV challenge do not develop in mice deficient in OX40. Thus OX40, which has been found to play little if any role in the generation of CD8 T cells to several viruses, including LCMV and influenza, plays a critical role in shaping the CD8 T cell response to vaccinia virus.
Materials and Methods
Mice
The studies reported here conform to the animal Welfare Act and the NIH guidelines for the care and use of animals in biomedical research. All experiments were done in compliance with the regulations of the La Jolla Institute Animal care committee in accordance with the guidelines by the Association for assessment and Accreditation of laboratory Animal Care. 8–12 wk-old female and male C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). OT I TCR-transgenic mice were used as a source of Vβ5/Vα2 CD8+ T cells responsive to OVA-derived SIINFEKL peptide. OX40-deficient OT-I TCR transgenic mice were generated in house by crossing OT-I mice with OX40−/− mice (9).
Peptides and Tetramers
Vaccinia virus peptide epitopes used in this study were predicted and synthesized as described previously (26, 27). B8R (20–27; TSYKFESV), A3L (270–227; KSYNYMLL), A8R (189–196; ITYRFYLI), B2R (54–62; YSQVNKRYI), A23R (297–305; IGMFNLTFI). MHC/peptide tetramers for the VACV-WR epitope B8R (20–27; TSYKFESV)/H-2Kb, which were conjugated to allophycocyanin, were obtained from the National Institutes of Health Tetramer Core facility (Emory University, Atlanta, GA).
Viruses
The VACV Western Reserve (VACVWR) strain was purchased from the American Type Culture Collection (Manassas, VA), grown in HeLa cells, and titered on VeroE6 cells (28).
Immunization protocols
For most experiments, mice were infected intraperitonealy (i.p.) with 2×105 PFU of VACV. For dermal scarification, virus (10 µl) was deposited at the base of the tail, and the skin at the site of the droplet was scarified 25 to 30 times with a 25-gauge needle. After 3 to 4 days, pustules or scabs were observed at the scarification site, indicating a localized vaccinia virus infection. Effector responses were analyzed between days 4 and 15 post-infection, while memory responses were analyzed 30 or more days after infection, after restimulating in vitro with VACV peptides.
For adoptive transfer experiments, 1 × 105 naive wt or OX40−/− OT-I CD8 T cells were transferred into wt non-transgenic B6 or OX40−/− mice. One day later, mice were infected i.p. with recombinant VACV expressing full-length OVA protein (VACV-OVA; 2 × 106 PFU/mouse) or PBS as indicated. OT-I expansion and memory formation were detected by FACS staining of transgenic TCR α and β chains after gating on CD8 T cells and in some cases after restimulating in vitro with OVA (SINFEKL) peptide.
Vaccinia virus intranasal challenge
Mice were anesthetized by inhalation of isoflurane and inoculated by the intranasal (i.n.) route with 3.5×106 of VACV-WR. Mice were weighed daily for 2 weeks following challenge and were euthanized when they lost 25% of their initial body weight. For protection experiments, mice were immunized subcutaneously (s.c.) at the base of the tail once with either 10 or 2 µg/mouse of CD8 T cell peptide epitopes emulsified in IFA with HBV core 128–140 (TPPAYRPPNAPIL) epitope. Tissues were collected into 10% neutral-buffered Formalin and H&E-stained sections prepared using standard procedures (29). Body weight was calculated as percentage of the mean weight for each group on the day of challenge.
CD4 and CD8 depletion
Groups of peptide-immunized mice were depleted of CD4+ or CD8+ T cells with anti-CD4 (clone GK1.5; 200 µg/mouse) or anti-CD8 (clone 2.43; 200 µg/mouse) given in one i.v injection 3 days prior to, and one i.p. injection 2 days after, intranasal challenge with VACV-WR. CD4 and CD8 depletion was confirmed by flow cytometry of spleens and lungs of treated mice.
Flow cytometry
Cytokine production in T cells was done as previously described (30), with some modifications. Briefly, after lysing red blood cells (RBCs), splenocytes from infected mice were resuspended in RPMI-1640 medium (Gibco) supplemented with 10% FCS (Omega Scientific), 1% L-glutamine (Invitrogen), 100 µg/ml streptomycin, 100 U/ml penicillin and 50 µM 2-mercaptoethanol (Sigma). 1–2 × 106 cells were plated in round-bottomed 96-well microtiter plates in 200 µl with medium or the indicated VACV peptides at 1 µg/ml for 1 hr at 37°C. GolgiPlug (BD Biosciences) was then added to the cultures according to the manufacture’s instructions and the incubation continued for 7 hrs. Cells were stained with anti-CD8 (PerCP; 53–6.7) and CD62L (PE; MEL-14), followed by fixation with cytofix-cytosperm (BD Biosciences) for 20 min at 4°C. Fixed cells were subjected to intracellular cytokine staining in BD Perm/Wash buffer for 30 min at 4°C. Anti-TNF (FITC; MP6-XT22) and IFN-γ (APC; XMG1.2) were obtained from e-Biosience and used at a 1:100 dilution. Samples were analyzed for their proportion of cytoplasmic cytokines after gating on CD8+CD62Llow T cells by FACSCalibur™ flow cytometer using CellQuest (BD Biosciences) and FlowJo software (Tree Star, san Carlos, CA). In some experiments phenotypic analysis was carried out on B8R-tetramer positive CD8 T cells by co-staining with CD25 (PC61), CD43 (S7), CD44 (IM7), CD69 (H1.2F3), CD127 (SB/199), and OX40 (OX86) all of which were purchased from BD Biosciences.
VACV-titer assay
Tissues from individual mice were homogenized, and sonicated for 0.5 min with a pause every half minute using an ultrasonic cleaner 1210 Branson (Danbury, CT). Serial dilutions were made and the virus titers were then determined by plaque assay on confluent VeroE6 cells.
Statistics
Statistical significance was analyzed by Student’s t test. Unless otherwise indicated, data represent the mean ± SEM, with p < 0.05 considered statistically significant.
Results
OX40 controls the magnitude of expansion of CD8 T cells to VACV
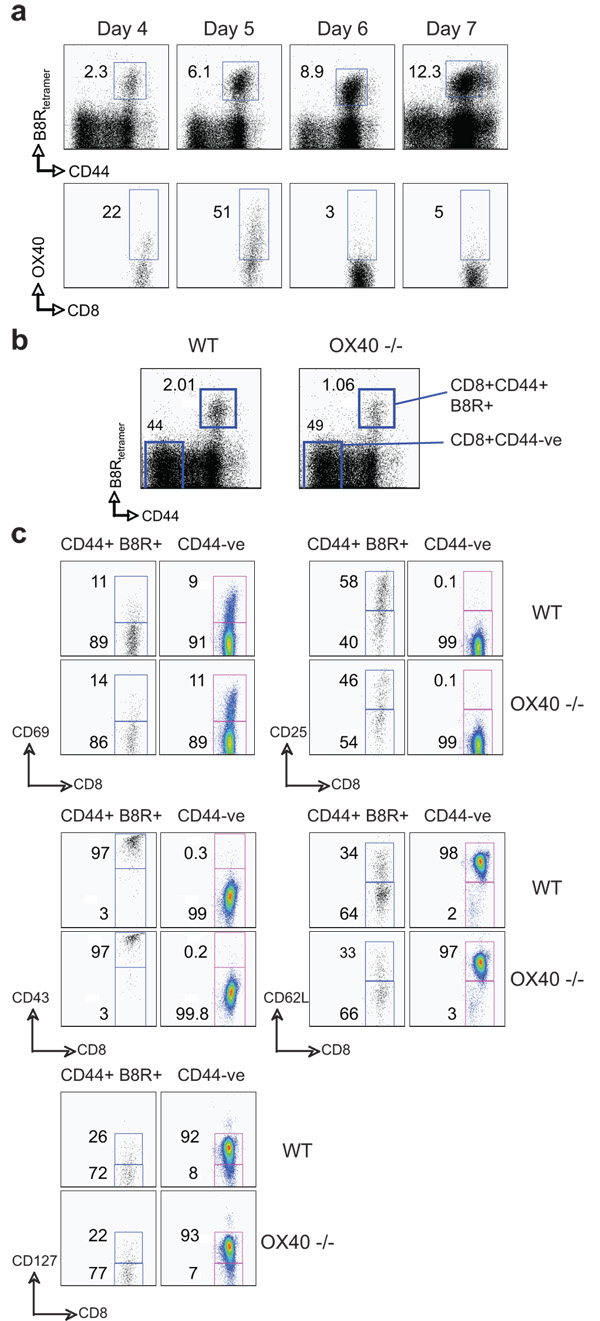
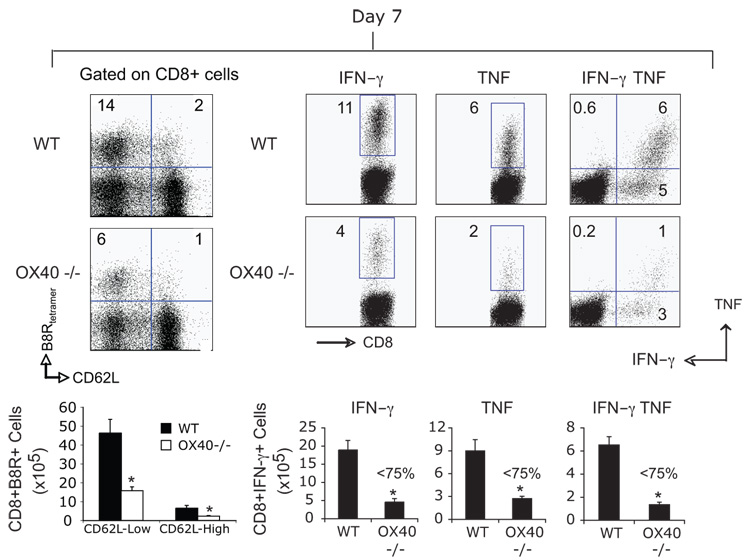
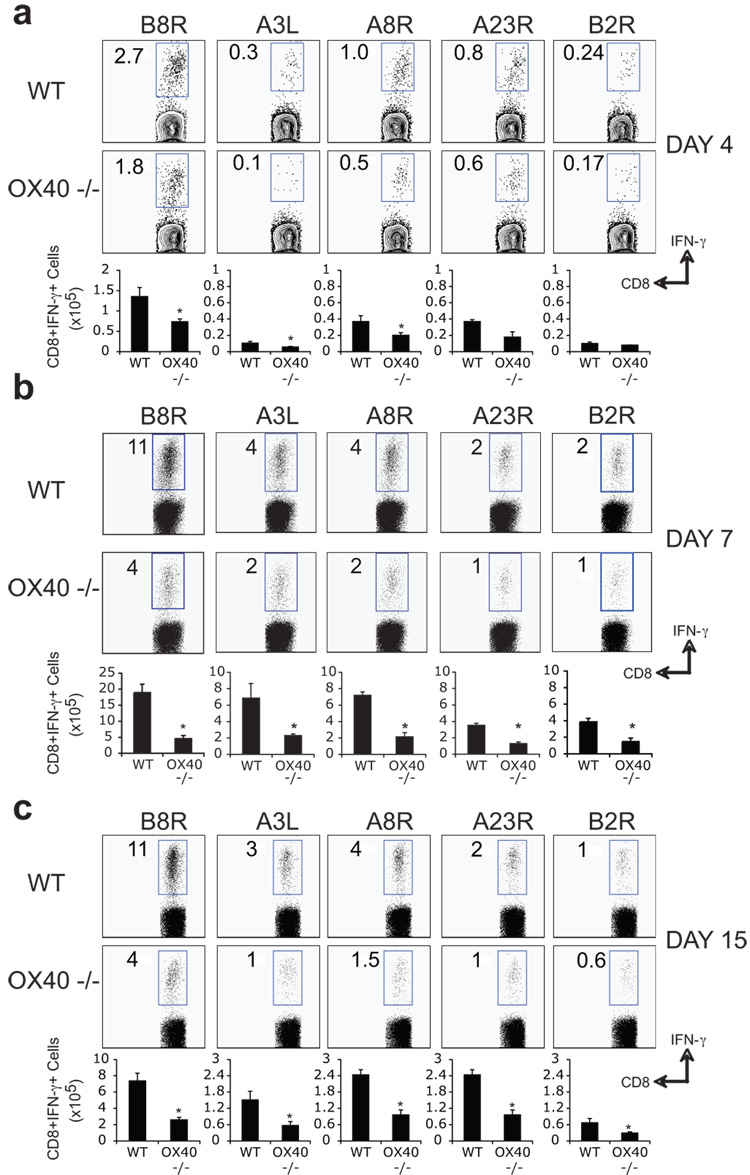
Our recent data assessing reactivity to a tumor-derived antigen or a replication defective adenovirus have highlighted that OX40/OX40L interactions can play significant roles in certain CD8 responses (9, 10). Therefore, we examined the requirement for OX40 in primary expansion and effector function of VACV-specific CD8 T cells. Initially, the immunodominant VACV-reactive CD8 T cell population was tracked with a tetramer of a peptide of B8R (26, 27). OX40 was seen on a proportion of B8R-tetramer reactive CD8 T cells at day 4 post-infection with VACV-WR, and peaked at day 5 (Fig. 1a). Whereas B8R-specific CD8 T cells expanded well over 4 days in wt mice, defective accumulation was already evident in mice deficient in OX40 (Fig. 1b). This was not due to impaired activation of CD8 T cells in that CD69, CD25, and CD43 were similarly elevated in OX40−/− mice (Fig. 1c). Down regulation of CD62L and CD127 were also not different (Fig. 1c). Both percentages and total numbers of B8R-reactive cells (CD62Lhigh and low) were strongly reduced at the peak of the primary response at day 7 in OX40−/− mice (Fig. 2), and B8R-specific CD8 T cells that made IFN-γ, or both IFN-γ and TNF, were reduced by 60–80% (Fig. 2), supporting our prior data that OX40 regulates division and survival of T cells (9–11, 19). Similar results were found for CD8 T cells responding to a range of subdominant VACV epitopes, which was not explained by delayed kinetics of expansion (Fig. 3). Impaired CD8 priming in the absence of OX40 was also observed with VACV given via dermal scarification, mimicking the route of vaccination against smallpox (not shown). Thus, OX40 plays an important role in generating large pools of primary VACV-specific effector CD8 T cells.
Figure 1. Intact activation but reduced early accumulation of VACV-specific CD8 T cells in OX40-deficient mice.
(a) Wt mice were infected i.p with VACV-WR (2 × 105 PFU/mouse). On indicated days post-infection splenocytes were harvested and stained for CD8, CD44, B8R-tetramer, and OX40. Top panel, Percentage of CD44-high expressing B8R-specific CD8 T cells. Bottom panel, Percentage of OX40+ cells gating on CD8+ CD44-high B8R-tetramer positive cells. Quadrant settings based on isotype controls. (b) WT or OX40-deficient (OX40−/−) mice were infected i.p with VACV-WR (2 × 105 PFU/mouse). At day 4, splenocytes were stained with CD8 plus CD44 and B8R-tetramer. Representative plots of tetramer staining, gating on CD8 cells. Percentages of activated B8R-tetramer positive CD8 T cells (CD8+CD44+B8R+) and naïve cells (CD8+CD44−) are indicated. (c) Mice were infected as above. At day 4, CD8 T cell activation was assessed by up-regulation of CD69, CD25, CD43, and down-regulation of CD62L and CD127 on B8R-tetramer positive CD44-high cells (left panel). Naïve (CD44-low B8R-tetramer negative) CD8 T cells were used as controls. Percentages that stained positive for each marker are indicated. Similar results were obtained in 3 separate experiments.
Figure 2. CD8 T cells lacking OX40 are defective in expanding and anti-viral cytokine production after infection with VACV.
(a) WT or OX40-deficient (OX40−/−) mice were infected i.p with VACV-WR (2 × 105 PFU/mouse). At day 7, splenocytes were stained for B8R-tetramer, or stimulated with B8R peptide for intracellular IFN-γ and TNF staining. Top, left: Representative plots of B8R-tetramer staining, gating on CD8 T cells. Percentages of CD8+ CD62L-high and CD62L-low B8R-tetramer positive cells are indicated. Top, right: Representative plots for cytokine-staining, gating on CD8+CD62Llow cells. Percentages that stained positive for IFN-γ alone, or TNF and IFN-γ/TNF are indicated. Quadrant settings were based on controls, using infected splenocytes that were not stimulated with peptide, and uninfected splenocytes stimulated with each peptide (data not shown). Bottom: Total numbers of B8R-tetramer positive CD8+CD62L-high and CD62L-low T cells, CD8+IFN-γ+ cells, or CD8+TNF+, and CD8+IFN-γ+TNF+ cells per spleen. Results are mean number ± SEM (n=6 mice/group) from one experiment. *, p < 0.05 (wt mice vs knockout). Similar results were obtained in 3 separate experiments.
Figure 3. OX40 is required for optimal generation of effector CD8 T cells directed against dominant and subdominant VACV epitopes.
WT or OX40−/− mice were infected i.p. with VACV-WR (2 × 105 PFU/mouse). On days 4 (a), 7 (b), and 15 (c) post infection IFN-γ-secreting CD8 cells were assessed by intracellular cytokine staining after stimulation with VACV peptides as indicated. Data are either representative plots of IFN-γ staining in gated CD8+CD62Llow T cells, with percent positive indicated, or total numbers ± SEM of CD8+IFN-γ+ T cells per spleen from four individual mice. *, p < 0.05 (WT vs OX40−/−). Similar results were obtained in 3 separate experiments.
Impaired generation of memory CD8 T cells in the absence of OX40
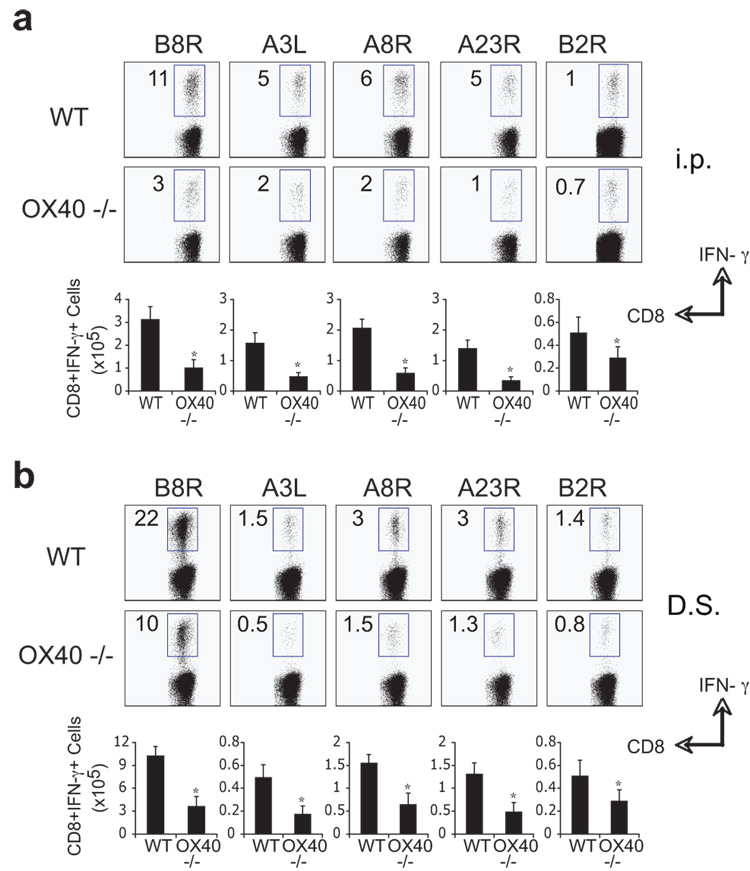
Next we assessed the impact of OX40 deficiency on the generation of memory. 40 days post-infection, VACV-infected wt mice contained high frequencies of memory CD8 T cells specific for all epitopes examined, regardless of whether infection was i.p (Fig. 4A) or via scarification (Fig. 4B), though scarification resulted in a more dominant B8R-reactive population. In contrast, and irrespective of epitope specificity and route of infection, the accumulation of VACV-specific memory CD8 cells in OX40−/− mice was reduced by an average of 60 to 80% (Fig. 4). After the peak of the primary response, the loss of VACV-specific cells in OX40−/− mice was comparable to that in wt mice (75–80%; compare Fig. 2 and Fig. 3), implying that OX40 largely acted during the phase of primary expansion. Together, these results show that OX40 controls the ability of VACV-specific CD8 cells to accumulate in order to form a large cytokine-competent memory pool.
Figure 4. Impaired generation of CD8 memory cells to both dominant and subdominant VACV epitopes in OX40-deficient mice.
Groups of C57BL/6 wild type or OX40-deficient (OX40−/−) mice were infected i.p (a) or by dermal scarification (b) with VACV-WR (2 × 105 PFU/mouse). At day 40, splenocytes were stimulated with VACV peptides as indicated and CD8 T cell priming assessed by intracellular IFN-γ staining. Top: Representative plots of IFN-γ staining in gated CD8 T cells. Percent positive indicated. Bottom: Total numbers of CD8+IFN-γ+ cells per spleen. Results are mean number ± SEM (n=4 mice/group) from one experiment. *, p < 0.05 (wt mice vs knockout) as determined by Student’s t test. Similar results were obtained in 3 separate experiments.
Defective priming of OX40-deficient CD8 T cells to VACV
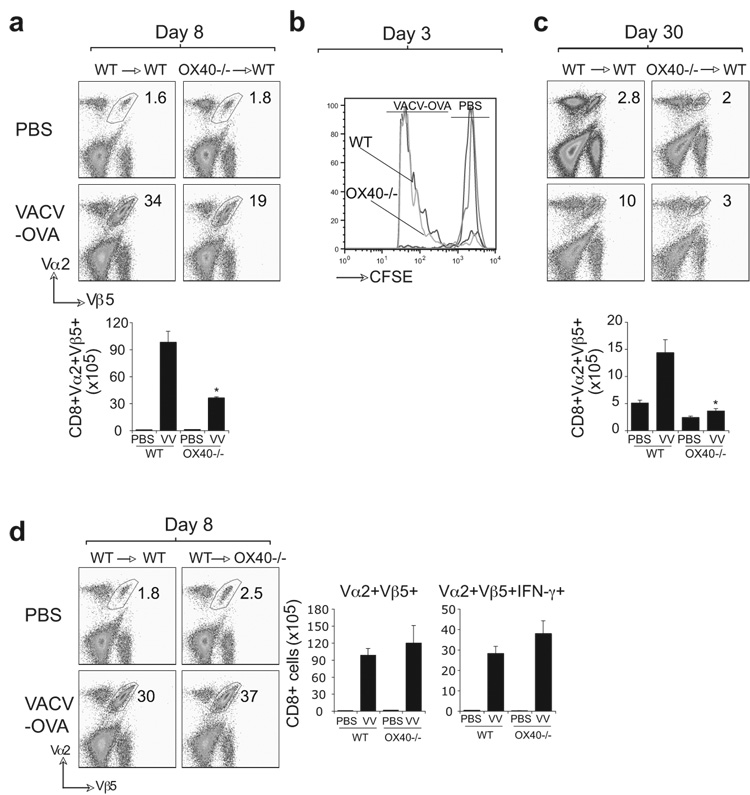
As OX40 is expressed on multiple cell types, we sought to show it was directly required by CD8 cells responding to VACV infection. OVA-specific OX40-deficient CD8 cells from OT-I TCR transgenic mice were transferred into naïve wt recipients subsequently infected with VACV-OVA. Strong expansion of wt OVA-specific CD8 cells was observed, similar to endogenous VACV-specific CD8 cells. In contrast, OX40-deficient CD8 cells poorly expanded to VACV-OVA (Fig. 5A). Next, we compared the ability of wt and OX40−/− OT-1 cells to divide early after VACV-OVA infection. CFSE-labeled wt or OX40−/− T cells were transferred into B6 mice and then one day later mice were infected with VACV-OVA or PBS as control. Without infection, similar CFSE-high Vα2Vβ5 populations of wt and OX40−/− T cells were detected (Fig. 5B). 72 hr after infection, both wt and OX40−/− T cells had undergone comparable division as indicated by a reduction in CFSE staining intensity. Thus, direct OX40 signaling in CD8 T cells was not essential for induction of T cell division but was crucial for T cell survival after VACV infection. Consistent with this, in mice receiving OX40−/− T cells, significantly fewer memory cells were generated after the resolution of infection (Fig. 5C). This closely mimicked the data analyzing endogenous VACV-specific CD8 T cells in OX40−/−mice infected with VACV-WR (Fig. 3 and 4). To exclude that OX40 expressed on a non-T cell population contributed to the defect observed in OX40−/− mice, we performed the reverse experiment. VACV-OVA induced strong expansion of wt OVA-reactive IFN-γ producing CD8 cells regardless of whether they were transferred into OX40−/− or wt mice (Fig. 5D). Thus, OX40 expressed on a CD8 T cell is required for expansion of effector cells and formation of a large population of memory cells during infection with VACV.
Figure 5. OX40 is required directly by CD8 T cells responding to VACV infection.
CFSE labeled naive WT or OX40−/− OT-I CD8 T cells were adoptively transferred into WT B6 (a-c) or OX40−/− (d) mice. One day later, mice were infected i.p. with recombinant VACV expressing full-length OVA (VACV-OVA; 2 × 106 PFU/mouse) or PBS as indicated. After 8 (a, d) 3 (b) or 30 (c) days, CD8 T cell expansion (a, d), division as measured by CFSE-dilution (b) and memory formation (c) were analyzed by tracking the transgenic TCR. Dot plots: Representative co-staining for Vα2 and Vβ5 after gating on CD8 cells. Percent positive indicated. Bottom: Total numbers of CD8+Vα2+Vβ5+ cells (a-d) or CD8+Vα2+Vβ5+IFN-γ+ cells (d) per spleen. Histogram: cell division of wt and OX40−/− CD8 T cells was analyzed on gated CD8+Vα2+Vβ5+ cells 72 h after infection with VACV-OVA. Results are mean number ± SEM (n=4 mice/group) from one experiment. *, p < 0.05 (wt mice vs knockout) as determined by Student’s t test. Similar results were obtained in 1 additional experiment.
OX40 controls development of CD8 cells that protect against lethal VACV infection
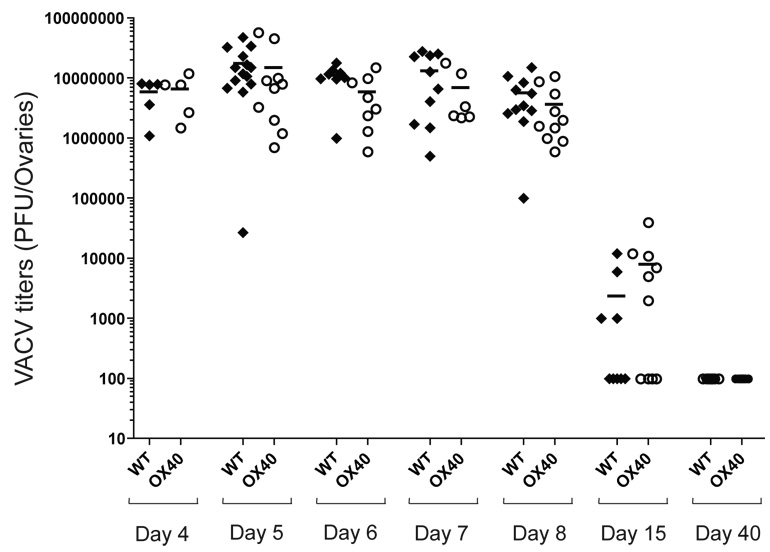
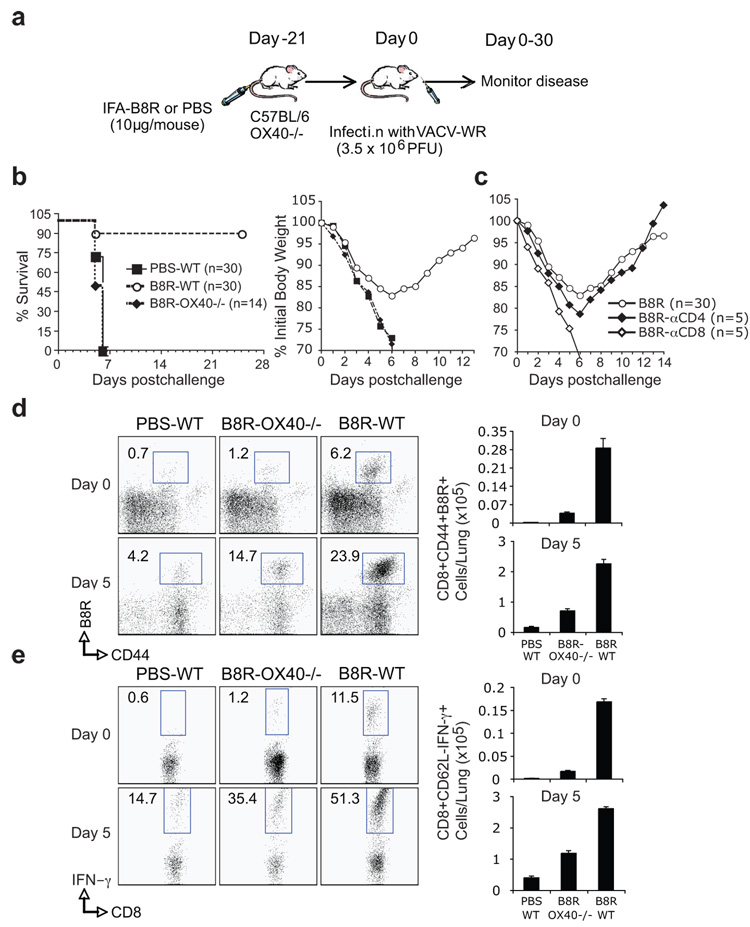
Analysis of VACV-WR titers in the ovaries and spleen did not reveal any significant difference in the kinetics of primary clearance in wt versus OX40−/− mice (Fig. 6). Together with results indicating depletion of CD8 T cells has no major effect on initial viral titers (25), this suggested that enhanced development of VACV-reactive CD8 T cell populations controlled by OX40 might be relevant for protection against subsequent exposure to virus. Because antibody can protect against VACV, we choose a model where CD8 T cell activity can be separated from antibody-mediated protection. After intranasal infection with VACV-WR, naive mice exhibit weight loss and death within 6–9 days (31, 32), and memory CD8 T cells induced by peptide vaccination can afford protection in this model (26, 33). Mice were therefore immunized with a high dose of the immunodominant peptide, B8R20–27, given in IFA, and challenged 3 weeks later with a lethal intranasal dose of VACV-WR (Fig. 7A). An average of 90% of immunized wt mice survived the infection (Fig. 7B). Weight loss (15% to 20%) was seen in these mice, suggesting that they were not fully immune (Fig. 7B), but protection was dependent on CD8 T cells as their depletion prior to challenge resulted in 100% mortality (Fig. 7C). Most significantly, when OX40−/− mice were immunized no protection was evident, and all succumbed to the infection (Fig. 7B). Far fewer B8R-specific memory CD8 T cells were present in the lungs of OX40−/− mice after immunization (Fig. 7D and 7E; Day 0) and far fewer accumulated after VACV infection (Fig. 7D and 7E; Day 5) suggesting the extent of protection was directly related to the number of VACV-specific CD8 T cells. In wt mice, immunization with a low dose of B8R20–27 (2 µg) peptide resulted in significantly fewer memory CD8 T cells (0.061 × 105 cells/lung) that were generated in the lungs compared with immunization with 10 µg (0.168 × 105 cells/lung; Table 1), but comparable to that seen when OX40−/− mice (0.016 ×105) were immunized with 10 µg of B8R peptide (Table 1). Again, the extent of protection (20% vs. 90%) directly correlated with the number of IFN-γ producing B8R-specific memory CD8 cells that were generated prior to challenge (Table 1). Thus, the use of OX40 by naive VACV-specific CD8 T cells dictates the frequency of protective VACV-specific memory CD8 T cells that are elicited.
Figure 6. OX40-deficient mice clear primary vaccinia infection with similar kinetics compared with wild type mice.
WT or OX40−/− mice were infected i.p. with VACV-WR (2 × 105 PFU/mouse). On indicated days post infection, ovaries were removed and VACV-titers determined as described in methods.
Figure 7. OX40 signals control development of CD8 T cell responses that protect against lethal VACV infection.
(a) WT or OX40−/− mice were immunized s.c. at the base of the tail with 10 µg of B8R peptide in IFA. Control groups received adjuvant but no peptide (PBS). 3 weeks post-vaccination, mice were infected i.n. with a lethal dose of VACV-WR (3.5 × 106 PFU/mouse [300 × LD50]). Animals were weighed daily and euthanized if weight loss was greater than 25% body weight. (b) Mean % survival and % of initial body weight from indicated numbers of mice. Mean weight data in some cases were not plotted beyond the point at which mice died and beyond day 7 reflected only mice that survived infection. (c) As indicated, groups of WT mice were depleted of CD4 (αCD4) or CD8 (αCD8) T cells prior to intranasal challenge with VACV. (d-e) % and total numbers of CD8+CD44+ B8R-tetramer+ cells (d) and B8R-reactive IFN-γ producing CD8+CD62L- cells (e) in the lungs before intranasal VACV challenge (d0) and after challenge (d5). Results are mean number ± SEM (n=4 mice/group) from one experiment.
Table 1.
Frequency of B8R-specific CD8 T cells in the Lung prior to challenge corrolates with degree of protection against lethal VACV infection
| Mice | B8R-peptide (µg/mouse) | # of CD8+IFN-γ+ (×105 cells/lung) |
%Survival | |
|---|---|---|---|---|
| Day 0 | Day 5 | |||
| Wt (n=30) |
10 | 0.168 (±0.006) | 2.61 (±0.06) | 90 |
| Wt (n=10) |
2 | 0.061 (±0.008) | 0.88 (±0.32) | 20 |
| OX40−/− (n=14) |
10 | 0.016 (±0.002) | 1.184 (±0.08) | 0 |
WT or OX40−/− mice were immunized s.c. at the base of the tail with 10 µg or 2 µg of B8R peptide in IFA as indicated. 3 weeks post-vaccination, mice were infected i.n. with a lethal dose of VACV-WR (3.5 × 106 PFU/mouse [300 × LD50]). Animals were weighed daily and euthanized if weight loss was greater than 25% body weight.
Total numbers of B8R-reactive IFN-γ producing CD8+CD62L- cells in the lungs before intranasal VACV challenge (d0) and after challenge (d5). Mean % survival from indicated numbers of mice are shown.
Discussion
Numerous spatially and temporally regulated interactions might exist between receptors on the surface of CD8 cells and their soluble or membrane-bound ligands. Defining the precise nature of these molecular interactions during different viral infections is of great interest, and may allow us to understand how to augment anti-viral immunity. Here we provide evidence of the importance of OX40-OX40L interactions to the generation of protective CD8 T cells reactive with VACV. Data from OX40−/− and OX40 ligand (OX40L)−/− mice has shown that these molecules play little to no role in primary CD8 T cell responses to LCMV, VSV, influenza, and MCMV (7, 18, 19, 34). With VACV, our results highlight a previously unappreciated role for OX40 in initial anti-viral immunity. We show that OX40 can strongly influence the response of VACV-specific CD8 T cells and dictates the absolute numbers of effector T cells that accumulate. Furthermore, OX40 is necessary for the generation of large populations of memory cells to dominant and subdominant VACV MHC class-I epitopes. Thus, the capacity of naïve CD8 T cells to bypass a requirement for OX40 signaling is not a property of all viruses, and OX40-dependence likely reflects differences related to the rate of viral replication, antigenic load, cell tropism, and perhaps the specific cytokine milieu induced in response to each virus.
Targets of OX40 are the anti-apoptotic proteins of the Bcl-2 family, such as Bcl-xL, Bfl-1, and Bcl-2, which are increased after OX40 ligation and correspondingly decreased in T cells that cannot express OX40 (6, 9, 35, 36). Additionally, OX40 might simultaneously exert suppressive effects on expression or activity of pro-apoptotic proteins such as Bad and Bim (36). More recently, survivin (an Inhibitor of Apoptosis (IAP) family protein) was shown to be weakly expressed in the absence of OX40, and to control cell cycle progression and coincident apoptosis (37). Therefore, the simplest model, which is supported by our results, is that OX40 signals are required for late proliferation and survival of CD8 T cells when VACV antigen is encountered. Without these signals many of the responding T cells will die, rather than expand and survive to form the high frequency pools of effector and memory cells. Interestingly, this exact function was also proposed for IFN-I during LCMV responses (13, 14), further substantiating the idea of molecular plasticity in using alternate receptors for similar functions in different situations. Like OX40 (36), IFN-I has been reported to promote cell survival by activating phosphatidylinositol 3-kinase and Akt (38). In contrast, IFN-I-induced survival in T cells was suggested to be independent of Bcl-2 and Bcl-xL anti-apoptotic proteins (39), raising the intriguing idea that similar functional outcomes in CD8 T cells could be mediated through alternate signaling pathways.
An important observation is that OX40 is strongly active in the development of CD8 T cells that protect against lethal VACV challenge. Extensive studies in the intranasal model of vaccinia infection have shown that passive immunotherapy with immune serum or monoclonal antibodies are protective (28, 40–42). However, a role for CD8 T cells in protection has been less recognized (25, 32, 33, 43–45). β2m−/− mice, which lack CD8 cells, are able to recover from VACV infection, suggesting that CD8 responses are not essential as long as humoral immunity is intact (44). In line, anti-viral antibody can protect mice efficiently even if CD4 or CD8 T cells are depleted prior to VACV challenge (32, 42, 45). However, this does not address whether CD8 cells can be protective. Consistent with the latter, B cell- or MHC Class II-deficient mice, which are unable to elicit effective antibody responses, were found dependent on anti-viral CD8 cells in protecting against weight loss after primary VACV challenge (25, 45). Our data now complement these studies and highlight that OX40 is a major component regulating the development of high frequencies of protective CD8 T cells. The degree of protection induced by immunization with the B8R CD8 epitope was equivalent to that seen with other strategies, such as antibody therapy (28, 41). In addition, neither CD4 T cells nor antibody were required, again highlighting the capacity of CD8 T cells to protect. Most significantly, when OX40−/− mice were immunized with peptide, relatively few VACV-specific CD8 T cells could be detected in the lung and coordinately no protection was evident.
Taken together we provide new insight into the molecular control of the development of protective CD8 T cells in immunity to vaccinia virus, and demonstrate that molecular plasticity occurs during anti-viral responses with alternate molecules likely providing similar functional activity to control initial priming of CD8 cells. Of interest is whether primary CD8 responses to other orthopoxviruses are also dependent on OX40, and how the extent of plasticity and redundancy in use of costimulatory receptors might be influenced by the virus.
Acknowledgments
This work was supported by NIH grants CA91837 and AI67341 to M.C., AI77079 to S.S.A., AI56268 and HHSN266200400124C to A.S., and Pew Scholar Award and NIH AI63107 to S.C. This is publication #864 from the La Jolla Institute for Allergy and Immunology.
Contributor Information
Shahram Salek-Ardakani, La Jolla Institute for Allergy and Immunology, Division of Molecular Immunology, San Diego, CA 92037..
Magdalini Moutaftsi, La Jolla Institute for Allergy and Immunology, Division Vaccine Discovery, San Diego, CA 92037..
Shane Crotty, La Jolla Institute for Allergy and Immunology, Division Vaccine Discovery, San Diego, CA 92037..
Alessandro Sette, La Jolla Institute for Allergy and Immunology, Division Vaccine Discovery, San Diego, CA 92037..
Michael Croft, La Jolla Institute for Allergy and Immunology, Division of Molecular Immunology, San Diego, CA 92037..
References
- 1.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 4.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 6.Salek-Ardakani S, Croft M. Regulation of CD4 T cell memory by OX40 (CD134) Vaccine. 2006;24:872–883. doi: 10.1016/j.vaccine.2005.07.108. [DOI] [PubMed] [Google Scholar]
- 7.Chen AI, McAdam AJ, Buhlmann JE, Scott S, Lupher ML, Jr, Greenfield EA, Baum PR, Fanslow WC, Calderhead DM, Freeman GJ, Sharpe AH. Ox40-ligand has a critical costimulatory role in dendritic cell:T cell interactions. Immunity. 1999;11:689–698. doi: 10.1016/s1074-7613(00)80143-0. [DOI] [PubMed] [Google Scholar]
- 8.Murata K, Ishii N, Takano H, Miura S, Ndhlovu LC, Nose M, Noda T, Sugamura K. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J Exp Med. 2000;191:365–374. doi: 10.1084/jem.191.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song A, Tang X, Harms KM, Croft M. OX40 and Bcl-xL promote the persistence of CD8 T cells to recall tumor-associated antigen. J Immunol. 2005;175:3534–3541. doi: 10.4049/jimmunol.175.6.3534. [DOI] [PubMed] [Google Scholar]
- 10.Lee SW, Park Y, Song A, Cheroutre H, Kwon BS, Croft M. Functional dichotomy between OX40 and 4-1BB in modulating effector CD8 T cell responses. J Immunol. 2006;177:4464–4472. doi: 10.4049/jimmunol.177.7.4464. [DOI] [PubMed] [Google Scholar]
- 11.Bansal-Pakala P, Halteman BS, Cheng MH, Croft M. Costimulation of CD8 T cell responses by OX40. J Immunol. 2004;172:4821–4825. doi: 10.4049/jimmunol.172.8.4821. [DOI] [PubMed] [Google Scholar]
- 12.Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aichele P, Unsoeld H, Koschella M, Schweier O, Kalinke U, Vucikuja S. CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J Immunol. 2006;176:4525–4529. doi: 10.4049/jimmunol.176.8.4525. [DOI] [PubMed] [Google Scholar]
- 15.Bachmann MF, McKall-Faienza K, Schmits R, Bouchard D, Beach J, Speiser DE, Mak TW, Ohashi PS. Distinct roles for LFA-1 and CD28 during activation of naive T cells: adhesion versus costimulation. Immunity. 1997;7:549–557. doi: 10.1016/s1074-7613(00)80376-3. [DOI] [PubMed] [Google Scholar]
- 16.Kundig TM, Shahinian A, Kawai K, Mittrucker HW, Sebzda E, Bachmann MF, Mak TW, Ohashi PS. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity. 1996;5:41–52. doi: 10.1016/s1074-7613(00)80308-8. [DOI] [PubMed] [Google Scholar]
- 17.Suresh M, Whitmire JK, Harrington LE, Larsen CP, Pearson TC, Altman JD, Ahmed R. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J Immunol. 2001;167:5565–5573. doi: 10.4049/jimmunol.167.10.5565. [DOI] [PubMed] [Google Scholar]
- 18.Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecabert B, Odermatt B, Bachmann MF. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL Responses after virus infection. Immunity. 1999;11:699–708. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- 19.Humphreys IR, Loewendorf A, de Trez C, Schneider K, Benedict CA, Munks MW, Ware CF, Croft M. OX40 costimulation promotes persistence of cytomegalovirus-specific CD8 T Cells: A CD4-dependent mechanism. J Immunol. 2007;179:2195–2202. doi: 10.4049/jimmunol.179.4.2195. [DOI] [PubMed] [Google Scholar]
- 20.Smith GL, McFadden G. Smallpox: anything to declare? Nat Rev Immunol. 2002;2:521–527. doi: 10.1038/nri845. [DOI] [PubMed] [Google Scholar]
- 21.Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol Rev. 2006;211:320–337. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 22.Amara RR, Nigam P, Sharma S, Liu J, Bostik V. Long-lived poxvirus immunity, robust CD4 help, and better persistence of CD4 than CD8 T cells. J Virol. 2004;78:3811–3816. doi: 10.1128/JVI.78.8.3811-3816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 24.Harrington LE, Most Rv R, Whitton JL, Ahmed R. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J Virol. 2002;76:3329–3337. doi: 10.1128/JVI.76.7.3329-3337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004;172:6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 26.Tscharke DC, Karupiah G, Zhou J, Palmore T, Irvine KR, Haeryfar SM, Williams S, Sidney J, Sette A, Bennink JR, Yewdell JW. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J Exp Med. 2005;201:95–104. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moutaftsi M, Peters B, Pasquetto V, Tscharke DC, Sidney J, Bui HH, Grey H, Sette A. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat Biotechnol. 2006;24:817–819. doi: 10.1038/nbt1215. [DOI] [PubMed] [Google Scholar]
- 28.Davies DH, McCausland MM, Valdez C, Huynh D, Hernandez JE, Mu Y, Hirst S, Villarreal L, Felgner PL, Crotty S. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J Virol. 2005;79:11724–11733. doi: 10.1128/JVI.79.18.11724-11733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salek-Ardakani S, Song J, Halteman BS, Jember AG, Akiba H, Yagita H, Croft M. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J Exp Med. 2003;198:315–324. doi: 10.1084/jem.20021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salek-Ardakani S, So T, Halteman BS, Altman A, Croft M. Differential regulation of Th2 and Th1 lung inflammatory responses by protein kinase C theta. J Immunol. 2004;173:6440–6447. doi: 10.4049/jimmunol.173.10.6440. [DOI] [PubMed] [Google Scholar]
- 31.Williamson JD, Reith RW, Jeffrey LJ, Arrand JR, Mackett M. Biological characterization of recombinant vaccinia viruses in mice infected by the respiratory route. J Gen Virol. 1990;71(Pt 11):2761–2767. doi: 10.1099/0022-1317-71-11-2761. [DOI] [PubMed] [Google Scholar]
- 32.Belyakov IM, Earl P, Dzutsev A, Kuznetsov VA, Lemon M, Wyatt LS, Snyder JT, Ahlers JD, Franchini G, Moss B, Berzofsky JA. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc Natl Acad Sci U S A. 2003;100:9458–9463. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snyder JT, Belyakov IM, Dzutsev A, Lemonnier F, Berzofsky JA. Protection against lethal vaccinia virus challenge in HLA-A2 transgenic mice by immunization with a single CD8+ T-cell peptide epitope of vaccinia and variola viruses. J Virol. 2004;78:7052–7060. doi: 10.1128/JVI.78.13.7052-7060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hendriks J, Xiao Y, Rossen JW, van der Sluijs KF, Sugamura K, Ishii N, Borst J. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J Immunol. 2005;175:1665–1676. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 35.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–455. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 36.Song J, Salek-Ardakani S, Rogers PR, Cheng M, Van Parijs L, Croft M. The costimulation-regulated duration of PKB activation controls T cell longevity. Nat Immunol. 2004;5:150–158. doi: 10.1038/ni1030. [DOI] [PubMed] [Google Scholar]
- 37.Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity. 2005;22:621–631. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Yang CH, Murti A, Pfeffer SR, Kim JG, Donner DB, Pfeffer LM. Interferon alpha /beta promotes cell survival by activating nuclear factor kappa B through phosphatidylinositol 3-kinase and Akt. J Biol Chem. 2001;276:13756–13761. doi: 10.1074/jbc.M011006200. [DOI] [PubMed] [Google Scholar]
- 39.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kempe CH, Bowles C, Meiklejohn G, Berge TO, St Vincent L, Babu BV, Govindarajan S, Ratnakannan NR, Downie AW, Murthy VR. The use of vaccinia hyperimmune gamma-globulin in the prophylaxis of smallpox. Bull World Health Organ. 1961;25:41–48. [PMC free article] [PubMed] [Google Scholar]
- 41.Lustig S, Fogg C, Whitbeck JC, Eisenberg RJ, Cohen GH, Moss B. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J Virol. 2005;79:13454–13462. doi: 10.1128/JVI.79.21.13454-13462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edghill-Smith Y, Golding H, Manischewitz J, King LR, Scott D, Bray M, Nalca A, Hooper JW, Whitehouse CA, Schmitz JE, Reimann KA, Franchini G. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 43.Drexler I, Staib C, Kastenmuller W, Stevanovic S, Schmidt B, Lemonnier FA, Rammensee HG, Busch DH, Bernhard H, Erfle V, Sutter G. Identification of vaccinia virus epitope-specific HLA-A*0201-restricted T cells and comparative analysis of smallpox vaccines. Proc Natl Acad Sci U S A. 2003;100:217–222. doi: 10.1073/pnas.262668999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spriggs MK, Koller BH, Sato T, Morrissey PJ, Fanslow WC, Smithies O, Voice RF, Widmer MB, Maliszewski CR. Beta 2-microglobulin-, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc Natl Acad Sci U S A. 1992;89:6070–6074. doi: 10.1073/pnas.89.13.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wyatt LS, Earl PL, Eller LA, Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Natl Acad Sci U S A. 2004;101:4590–4595. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]