Abstract
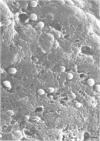
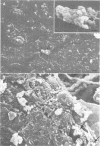
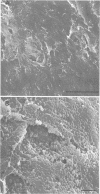
We examined Tenckhoff peritoneal catheters by scanning and transmission electron microscopy to study the morphology of bacterial adherence. Two catheters were removed from uninfected patients, three from patients with exit site infections, four from patients with peritonitis, and one from a patient with both exit site infection and peritonitis. Infecting organisms included three of Staphylococcus aureus and one each of Enterobacter sp., Staphylococcus epidermidis, Achromobacter xylosoxidans, Serratia sp., Klebsiella sp., and Candida albicans. Considerable morphological variation in adherence to the peritoneal dialysis apparatus occurred. No inflammatory cells were ever seen in association with infected cuffs, only two of the five patients with peritonitis had inflammatory cells associated with their catheters. In both instances, these cells tended to occur in clumps and demonstrated no flattening when in contact with the surface. Colonization of the catheter was uneven--bacteria tended to occur in clusters. Extensive matrix formation was evident in several instances, and condensation of this matrix onto the bacteria during the dehydration process rendered clumps of bacterial cells amorphous at times. Bacteria were adherent to the subcutaneous cuff in those patients with exit site infections. Gram-negative bacteria attached to individual dacron fibers of the cuff, often several layers deep. Gram-positive bacteria tended to adhere in clusters.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982 Jul;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duwe A. K., Vas S. I., Weatherhead J. W. Effects of the composition of peritoneal dialysis fluid on chemiluminescence, phagocytosis, and bactericidal activity in vitro. Infect Immun. 1981 Jul;33(1):130–135. doi: 10.1128/iai.33.1.130-135.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén C. S., Eriksson B., Hanson L. A., Jodal U., Kaijser B., Janson G. L., Lindberg U., Olling S. Adhesion to normal human uroepithelial cells of Escherichia coli from children with various forms of urinary tract infection. J Pediatr. 1978 Sep;93(3):398–403. doi: 10.1016/s0022-3476(78)81145-7. [DOI] [PubMed] [Google Scholar]
- Fenton P. Laboratory diagnosis of peritonitis in patients undergoing continuous ambulatory peritoneal dialysis. J Clin Pathol. 1982 Nov;35(11):1181–1184. doi: 10.1136/jcp.35.11.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gokal R. Peritonitis in continuous ambulatory peritoneal dialysis. J Antimicrob Chemother. 1982 Jun;9(6):417–420. doi: 10.1093/jac/9.6.417. [DOI] [PubMed] [Google Scholar]
- Hurley R. M., Muogbo D., Wilson G. W., Ali M. A. Cellular composition of peritoneal effluent: response to bacterial peritonitis. Can Med Assoc J. 1977 Nov 5;117(9):1061–1062. [PMC free article] [PubMed] [Google Scholar]
- Kjelleberg S., Humphrey B. A., Marshall K. C. Effect of interfaces on small, starved marine bacteria. Appl Environ Microbiol. 1982 May;43(5):1166–1172. doi: 10.1128/aem.43.5.1166-1172.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krothapalli R., Duffy W. B., Lacke C., Payne W., Patel H., Perez V., Senekjian H. O. Pseudomonas peritonitis and continuous ambulatory peritoneal dialysis. Arch Intern Med. 1982 Oct;142(10):1862–1863. [PubMed] [Google Scholar]
- Leake E. S., Wright M. J., Gristina A. G. Comparative study of the adherence of alveolar and peritoneal macrophages, and of blood monocytes to methyl methacrylate, polyethylene, stainless steel, and vitallium. J Reticuloendothel Soc. 1981 Nov;30(5):403–414. [PubMed] [Google Scholar]
- Malick L. E., Wilson R. B. Modified thiocarbohydrazide procedure for scanning electron microscopy: routine use for normal, pathological, or experimental tissues. Stain Technol. 1975 Jul;50(4):265–269. doi: 10.3109/10520297509117069. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Costerton J. W. A scanning and transmission electron microscopic study of the surfaces of intrauterine contraceptive devices. Am J Obstet Gynecol. 1983 Jun 15;146(4):384–394. doi: 10.1016/0002-9378(83)90818-9. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Costerton J. W. A scanning electron microscopic study of urine droppers and urine collecting systems. Arch Intern Med. 1983 Jun;143(6):1135–1141. [PubMed] [Google Scholar]
- Marrie T. J., Nelligan J., Costerton J. W. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation. 1982 Dec;66(6):1339–1341. doi: 10.1161/01.cir.66.6.1339. [DOI] [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982 Oct;146(4):479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Microbial colonization of prosthetic devices. II. Scanning electron microscopy of naturally infected intravenous catheters. Zentralbl Bakteriol Mikrobiol Hyg B. 1981;173(5):293–299. [PubMed] [Google Scholar]
- Rubin J., Rogers W. A., Taylor H. M., Everett E. D., Prowant B. F., Fruto L. V., Nolph K. D. Peritonitis during continuous ambulatory peritoneal dialysis. Ann Intern Med. 1980 Jan;92(1):7–13. doi: 10.7326/0003-4819-92-1-7. [DOI] [PubMed] [Google Scholar]
- Rutter J. M., Burrows M. R., Sellwood R., Gibbons R. A. A genetic basis for resistance to enteric disease caused by E. coli. Nature. 1975 Sep 11;257(5522):135–136. doi: 10.1038/257135a0. [DOI] [PubMed] [Google Scholar]
- Sewell C. M., Clarridge J., Lacke C., Weinman E. J., Young E. J. Staphylococcal nasal carriage and subsequent infection in peritoneal dialysis patients. JAMA. 1982 Sep 24;248(12):1493–1495. [PubMed] [Google Scholar]
- Sugarman B. In vitro adherence of bacteria to prosthetic vascular grafts. Infection. 1982 Jan;10(1):9–14. doi: 10.1007/BF01640828. [DOI] [PubMed] [Google Scholar]
- Sugarman B., Musher D. Adherence of bacteria to suture materials. Proc Soc Exp Biol Med. 1981 Jun;167(2):156–160. doi: 10.3181/00379727-167-41141. [DOI] [PubMed] [Google Scholar]
- Tenckhoff H., Schechter H. A bacteriologically safe peritoneal access device. Trans Am Soc Artif Intern Organs. 1968;14:181–187. [PubMed] [Google Scholar]
- Vas S. I. Microbiologic aspects of chronic ambulatory peritoneal dialysis. Kidney Int. 1983 Jan;23(1):83–92. doi: 10.1038/ki.1983.15. [DOI] [PubMed] [Google Scholar]
- Zimmerli W., Waldvogel F. A., Vaudaux P., Nydegger U. E. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982 Oct;146(4):487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]
- Zobell C. E. The Effect of Solid Surfaces upon Bacterial Activity. J Bacteriol. 1943 Jul;46(1):39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]