Abstract
To test the hypothesis that activation of the transient receptor potential vanilloid 4 (TRPV4) channel conveys a hypotensive effect that is enhanced during salt load, male Wistar rats fed a normal (NS, 0.5%) or high sodium (HS, 4%) diet for 3 weeks were given 4α-phorbol 12,13-didecanoate (4α – PDD), a specific TRPV4 activator, in the presence or absence of capsazepine (CAPZ), a selective TRPV1 blocker; ruthenium red (RuR), a TRPV4 blocker; or TRPV4 small hairpin (sh)RNA that selectively knockdowns TRPV4. 4α-PDD (1, 2.5, or 5 mg/kg, iv) dose-dependently decreased mean arterial pressure (MAP, p<0.05). HS enhanced 4α-PDD-induced depressor effects as well as 4α-PDD-mediated release of calcitonin gene related peptide (CGRP) and substance P (SP) (p<0.001). RuR markedly blunted (p<0.001), while CAPZ slightly attenuated (p<0.05), 4α-PDD-induced depressor effects in HS and NS rats. RuR alone increased baseline MAP in both HS and NS rats with a greater magnitude in the former (p< 0.05). Western blot analysis showed that HS increased TRPV4 expression in dorsal root ganglia (DRG) and mesenteric arteries (MA) (p<0.05) but not the renal cortex and medulla. Gene-silencing approach revealed that TRPV4 shRNA down-regulated TRPV4 expression leading to blunted 4α-PDD-induced hypotension (p<0.05). Thus, TRPV4 activation decreases blood pressure in rats given NS. HS enhances TRPV4 expression in sensory nerves/mesenteric arteries and TRPV4-mediated depressor effects and CGRP/SP release, in such that HS causes a greater increase in blood pressure when TRPV4 is blocked. Our data indicate that TRPV4 activation may constitute a compensatory mechanism in preventing salt-induced increases in blood pressure.
Keywords: salt intake, gene-targeting, gene-silencing, sensory nerves, blood pressure, transient receptor potential channel
Introduction
The transient receptor potential vanilloid 4 (TRPV4; also named Osm-9-like TRP channel 4, OTRPC4; vanilloid receptor-related osmotically activated channel, VR-OAC; vanilloid receptor-like channel 2, VRL-2; or TRP 12) receptor is a nonselective cation channel of the vanilloid subfamily of transient receptor potential (TRP) channels. TRPV4 is broadly expressed in the heart, brain, liver, kidney, lung, trachea, placenta, salivary gland, and sensory neurons in the trigeminal ganglion and dorsal root ganglia (DRG).1 TRPV4 is 5~10 times more permeable for Ca2+ than Na+1–3 and can be activated by osmolarity, heat, mechanical stimulation, 4α-phorbol ester derivatives, and lipids including endocannabinoids, arachidonic acid (AA) and their metabolites.4,5 These stimuli promote TRPV4 channel opening by distinct pathways, e.g., cell swelling activates TRPV4 by PLA2-dependent formation of AA and subsequent generation of 5’,6’-epoxyeicosatrienoic acid (EET) via the cytochrome P450 epoxygenase-dependent pathway.4,5 In contrast, activation of TRPV4 evoked by phorbol esters and heat depends on an aromatic residue at the N terminus of the third transmembrane.6
TRPV4 may play a role in a number of physiological responses. As an osmoreceptor, TRPV4 is expressed in neurons of the circumventricular organs, where neurosensory cells are known to be responsive to systemic osmotic pressure.7 TRPV4 gene deletion leads to disturbed osmotic regulation in the face of hyperosmolar.8 Expression of TRPV4 in the rat kidney is restricted to water-impermeant nephon segments, suggesting that TRPV4 is an osmotically responsive cation channel.9 Furthermore, TRPV4 may function in the transduction of osmolarity/hypotonicity stimulation in airways in humans, 10,11 of mechanical or temperature stimulation in C. elegans,12 and of thermal sensation in the hypothalamus in rodents.13,14
We shown that transient receptor potential vanilloid 1 (TRPV1) channels play a key role in preventing salt-induced increases in blood pressure.15,16 However, the role of TRPV4 in modulation of blood pressure in the face of salt challenge is largely unknown. This study tests the hypotheses that TRPV4 activation leads to dose-dependent hypotension and that high salt intake enhances TRPV4-induced depressor effects as a mean to prevent salt-induced increases in blood pressure. Given the lack of selective channel blockers and its co-expression with TRPV1,15,16 the effect of TRPV4 activation or inhibition was examined using the best available pharmacological tools, i.e., 4α-phorbol 12,13-didecanoate (4α–PDD) or ruthenium red (RuR), respectively.17–22 The specificity of 4α–PDD was examined in the presence or absence of TRPV1 or TRPV4 antagonists; and the specificity of RuR was tested in the presence or absence of TRPV1 or TRPV4 agonists. Finally, TRPV4 shRNA was used to specifically knockdown TRPV4 expression and the effect examined.
Methods
Preparation of animals and samples
Male Wistar rats (Charles River laboratory, Wilmington, MA) at age of 6 weeks were randomly assigned to a normal sodium (NS) diet (0.5% of Na+ by weight, Harlan Teklad) or a high sodium (HS) diet (4% of Na+ by weight) group and treated for 3 weeks. All rats drank water ad libitum throughout the experiment.
In addition to rats subject to acute experiments in which various agonists or antagonists were given, a subset of rats fed NS or HS diet was euthanized by decapitation at the end of the 3rd week without subjecting to acute experiments. Blood was collected in EDTA tubes for plasma calcitonin gene-related peptide (CGRP) and substance P (SP) assays. The cervical, thoracic, and lumbar DRG, mesenteric resistance arteries (MA), and the renal cortex and medulla were dissected and collected for Western blot analysis.
Surgical preparation
The rats were anesthetized with ketamine and xylazine (80 and 4 mg/kg, intraperitoneally, respectively) for implantation of vascular catheters, or with urethane (1.5 g/kg, intraperitoneally) when the animals subject to injection of capsaicin (CAP). The left jugular vein and carotid artery were cannulated under anesthesia for administration of drugs and monitoring of mean arterial pressure (MAP) and heart rate (HR) with a Statham 231D pressure transducer coupled to a Gould 2400s recorder (Gould Instruments), respectively. Baseline MAP and its response to various chemicals except for CAP were obtained three hours after surgery with the rats fully awake and unrestrained in their home cages.23
Effects of TRPV4 activation in the presence or absence of the TRPV1 or TRPV4 blockade
To examine whether activation of TRPV4 leads to hypotension, rats fed a NS diet were given various doses (0, 1, 2.5, or 5 mg/kg, intravenous bolus) of 4α-PDD in 4 groups (each group of rats for each dose). After observing a dose-dependent decrease in MAP, rats fed a NS or HS diet were randomly assigned to the following groups for injection of vehicle or 4α-PDD (2.5 mg/kg, iv) alone or in combination with capsazepine (CAPZ, a TRPV1 receptor antagonist, 3 mg/kg, iv), SB 366791 (a TRPV1 receptor antagonist, 2 mg/kg, ip), or ruthenium red (RuR, a TRPV4 channel blocker, 1 or 3 mg/kg, iv). 4α-PDD at 2.5 mg/kg iv was administered 6, 8, 20, or 30 minutes after injection of CAPZ at 3 mg/kg iv, RuR at 1 mg/kg iv, RuR at 3 mg/kg iv, or SB 366791 at 2 mg/kg ip, respectively, and the dose and route of these drug administration were chosen according to previous studies.24–27 MAP was recorded for 30 min starting 10 min pre- and 20 min post-4α-PDD injection in all groups. Two different TRPV1 blockers, CAPZ and SB 366791, were used in light of their distinct potencies and mechanisms of actions.24–27 Additional groups of rats fed a NS or HS diet were given vehicle or RuR (1 or 3 mg/kg, iv) alone to determine MAP responses to blockade of TRPV.
Effects of TRPV1 activation in the presence or absence of the TRPV1 or TRPV4 blockade
To serve as controls of TRPV4 activation, rats fed a NS diet were randomly assigned to the following groups for administration of vehicle or CAP (30 µg/kg, iv, a selective TRPV1 agonist) alone or in combination with SB 366791 (2 mg/kg, ip) or RuR (1 and 3 mg/kg, iv). CAP was injected 5, 8, 20, and 30 min after injection of vehicle; RuR at 1 mg/kg or 3 mg/kg iv, or SD 366791 at 2 mg/kg ip, respectively, and the peak changes in MAP occurred 1–2 min after injection of CAP recorded in all groups. The dose of CAP was chosen based on a previous study.16 Given that CAP is an irritant and causes pain in conscious rats, this protocol was performed in anesthetized rats as previously described.16
Radioimmunoassay
A rabbit anti-rat CGRP radioimmunoassay kit and a rabbit anti-rat SP radioimmunoassay kit (Phoenix Pharmaceuticals) were used to determine CGRP and SP contents in plasma that was collected 6 min after 4α-PDD administration in all groups.
Western blot analysis
Western blot analysis was performed as described previously16 with the use of primary antibody targeted to TRPV4 (1:500, Alomone labs, Jerusalem, Israel) and secondary antibody conjugated with horseradish peroxidase (1:800, Santa Cruz Biotechnology). The membranes were developed using an ECL kit (Amersham Pharmacia Biotech) and exposed to films (Hyperfilm-ECL, Amersham Pharmacia Biotech). The films were scanned and analyzed with the use of the Image Quantity Program (Scion) to obtain integrated densitometric values. β-Actin was used to normalize protein loading on membranes.
Preparation and systemic application of the shRNA/dendrimer complex
A subset of rats fed NS or HS diet was injected via the tail vein of TRPV4 small hairpin (sh)RNA/dendrimer complex or control shRNA/dendrimer complex at the beginning of the 3rd week (i.e. at the end of the 2nd week), once per day, for 7 days (please see http://hyper.ahajournals.org). Plasmid shRNAs (SureSilencing shRNA plasmid for rat TRPV4 with neomycin) were purchased from SuperArray Bioscience Corporation (Frederick, MD). Generation 5 PAMAM dendrimers (Dendritech, Inc. Midland, MI) were used as carriers of the plasmids, and were dissolved in water (27.28%, w/w) and mixed with 1.6 mg/kg shRNA in 0.9% NaCl solution (total injection vol. 1.6 ml) using a charge ratio of 7.
Quantitative real-time PCR
Total tissue RNA was extracted using RNeasy Mini Kit (Qiagen) following the instructions provided by the supplier. Total RNA (0.5 µg) was used for first strand cDNA synthesis with random hexamer primers and Superscript III RNase H− reverse transcriptase (Invitrogen) according to the manufacturer’s protocol. Real time PCR primers for TRPV4, TRPV1, and 18SrRNA were obtained (please see http://hyper.ahajournals.org).28 PCR reactions were performed with QuantiTect SYBR Green PCR Kit (Qiagen), using 3 µl of cDNA as the template in each 20 µl reaction mixture. PCR assays were performed with an Applied Biosystem 7900HT Sequence Detection System (ABI Prism). Data were also normalized with the quantity of 18S rRNA in individual samples to correct for sample variability.
Drugs
4α- PDD (LC Laboratories) or CAP (Sigma) was dissolved in ethanol (5% v/v), Tween-80 (5% v/v), and saline right before administration to animals. CAPZ (Sigma) or SB 366791 (Sigma) was dissolved in dimethyl sulfoxide (DMSO, 5% v/v), Tween-80 (10% v/v), and saline in the same manner as above. RuR (Sigma) was dissolved in saline.
Statistical analysis
All of the values are expressed as means ± SE. Differences between 2 groups were analyzed by using the unpaired Student t test. Differences among groups were analyzed using one-way ANOVA followed by a Bonferroni’s adjustment for multiple comparisons. Differences were considered statistically significant at P<0.05.
Results
MAP responses to intravenous administration of 4α-PDD at various doses
Figure 1 shows that administration of 4α-PDD (1, 2.5, 5 mg/kg, iv) caused a dose-dependent decrease in MAP and an increase in HR in NS rats, indicating that activation of TRPV4 led to a depressor effect on blood pressure and a normal functional baroreflex. The depressor effects reached the peak 6 to 8 minutes after administration. Given that the midrange dose 4α-PDD, 2.5 mg/kg, provided a robust yet not detrimental drop in MAP, it was chosen for the rest studies reported below.
Figure 1.
Time-course responses of mean arterial pressure (MAP, panel A) and heart rate (panel B) to bolus injection of 4α-PDD (1 mg/kg, 2.5 mg/kg, or 5 mg/kg, iv, a TRPV4 activator) in conscious rats fed a normal sodium diet. Vehicle: ethanol (5% v/v), Tween-80 (5% v/v) and saline, iv. Values are mean ± SE (n=5–8). *P<0.05 compared with the corresponding vehicle-treated value.
MAP responses to TRPV4 activation in the presence or absence of TRPV1 or TRPV4 blockade
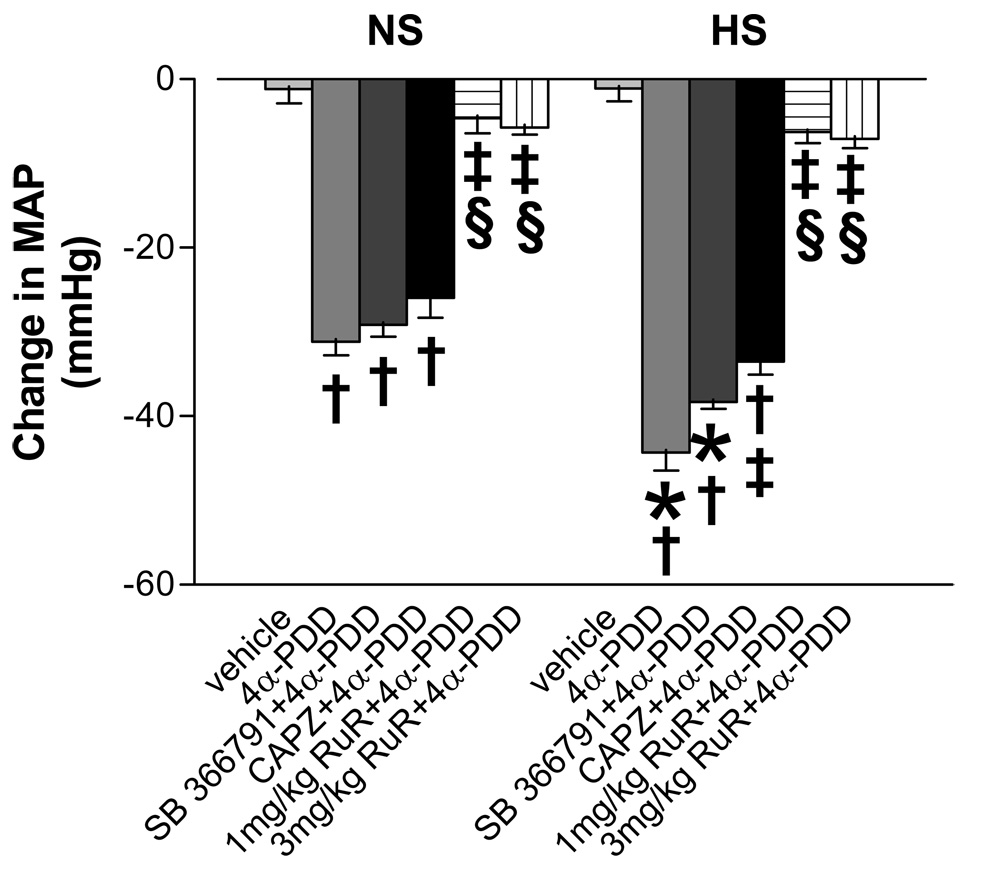
There was no significant difference in baseline MAP between NS and HS groups of rats after 3-week dietary treatment (HS, 103±5 mm Hg vs NS: 94±4 mm Hg, p>0.05). Figure 2 shows the peak changes of MAP that occurred 5–7 min after injection of 4α-PDD with or without other drugs. The magnitude of decreases in MAP induced by 2.5 mg/kg 4α-PDD iv was significantly greater in HS than NS rats, indicating that TRPV4 function is sensitized in rats fed a HS diet. Moreover, blockade of TRPV4 with RuR (1 mg/kg or 3 mg/kg, iv) markedly blunted the depressor effect of 4α-PDD in rats fed a NS or HS diet. In contrast, blockade of TRPV1 with CAPZ but not SB 366791 weakly but significantly attenuated the depressor effect of 4α-PDD in rats fed a HS diet only. These data indicate that the depressor effect induced by 4α-PDD is mainly mediated by activation of TRPV4 but TRPV1 may also contribute to 4α-PDD-induced depressor action in the face of salt load.
Figure 2.
Changes in mean arterial pressure (MAP) 5~7 min after bolus injection of 4α-PDD (2.5 mg/kg, iv, a TRPV4 activator) with or without SB 366791 (2 mg/kg, ip, a selective TRPV1 blocker), capsazepine (CAPZ, 3 mg/kg, iv, a selective TRPV1 blocker) and ruthenium red (RuR, 1 mg/kg and 3 mg/kg, iv, a TRPV4 blocker) in conscious rats fed a NS or HS diet. Vehicle: ethanol (5% v/v), Tween-80 (5% v/v) and saline, iv. Values are mean ± SE (n=5–7). *P<0.05 compared with the corresponding NS group. †P<0.05 compared with the corresponding vehicle-treated group. ‡P<0.05 compared with the corresponding 4α-PDD-treated group. §P<0.05 compared with the SB- and CAPZ-treated group.
Baseline MAP responses to intravenous injection of RuR
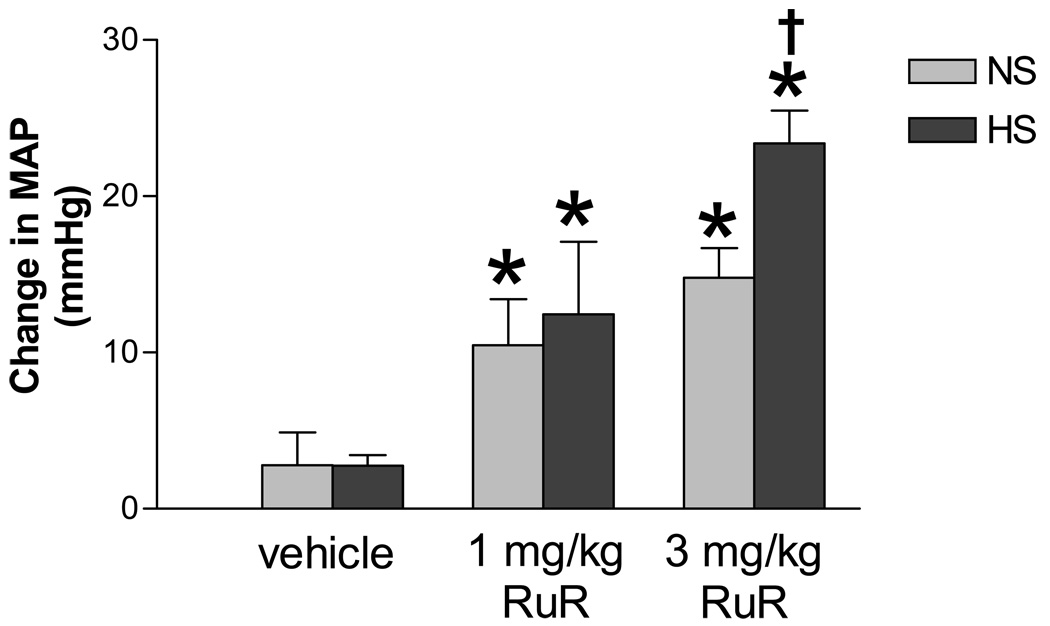
To determine whether blockade of TRPV4 affects baseline blood pressure in HS rats, MAP responses to bolus injection of RuR (1 mg/kg or 3 mg/kg, iv) were examined under the fully awake state of rats. The MAP elevation began immediately after administration of RuR and reached the peak in 3 to 6 minutes in both NS and HS rats. The pressor action of RuR at the doses of 1 mg/kg and 3 mg/kg iv lasted for 6~8 minutes and 15~20 minutes, respectively. The peak MAP responses to RuR at 3 mg/kg iv were significantly elevated in HS compared to NS rats (Figure 3).
Figure 3.
Mean Blood pressure (MAP) responses to intravenous injection of ruthenium red (RuR, 1 mg/kg and 3 mg/kg, iv, a TRPV4 blocker) in conscious rats fed a NS or HS diet. Vehicle:saline, iv. Values are mean ± SE (n=7–10). *P<0.05 compared with the corresponding vehicle-treated group, †P<0.05 compared with the corresponding NS group.
MAP responses to TRPV1 activation in the presence or absence of the TRPV1 or TRPV4 blockade
The peak changes in MAP occurred 1–2 min after injection of CAP in all groups are shown in Table 1. While SB 366791 (2 mg/kg, ip) effectively blocked CAP-induced depressor effects, RuR at 1 or 3 mg/kg was incapable of blockade of CAP-induced depressor effects. These findings are supported by a previous report29 and indicate that the pressor effect of RuR is due to blockade of TRPV4 but not TRPV1 channels.
Table 1.
Effects of capsaicin (CAP) on mean arterial pressure (MAP) in the presence or absence of TRPV1 or TRPV4 blockade in urthane-anesthetized rats fed a normal salt diet
| Treatment Groups | MAP, mmHg |
||
|---|---|---|---|
| Baseline | After CAP | Δ | |
| Vehicle | 115±3 | 113±5 | −2±3 |
| CAP | 112±5 | 86±4* | −26±2* |
| SB 366791+CAP | 110±3 | 108±3† | −3±3† |
| 1mg/kg RuR+CAP | 112±3 | 83±3*‡ | −29±2*‡ |
| 3mg/kg RuR+CAP | 116±4 | 93±5* | −23±1*‡ |
After baseline recording, MAP was recorded 1~2 min after CAP administration. Values are mean ± SE (n=4~6). CAP, 30 µg/kg, iv; SB 366791, 2 mg/kg, ip; RuR, 1 or 3 mg/kg, iv; Vehicle: ethanol (5% v/v), Tween-80 (5% v/v), and saline, iv.
p<0.05 compared with the corresponding vehicle-treated group
p<0.05 compared with the corresponding CAP-treated group
P<0.05 compared with the corresponding SB-treated group
TRPV4 protein expression in DRG, MA, and the kidney in response to HS intake
Figure 4 shows that there was no significant difference in TRPV4 expression in the renal cortex and medulla between NS and HS rats, but HS intake enhanced TRPV4 expression in DRG and MA (p<0.05). Elevated TRPV4 expression in DRG and MA may underlie, at least in part, enhanced depressor effects of TRPV4 observed in HS rats.
Figure 4.
Western blot analysis showing the TRPV4 protein expression in dorsal root ganglia (DRG), mesenteric resistant arteries (MA), the renal cortex and medulla in HS and NS rats. Values are mean ± SE (n=4–5). *P<0.05 compared with the corresponding NS group.
CGRP and SP release induced by 4α-PDD in the presence or absence of TRPV1 or TRPV4 blockade
Plasma CGRP and SP levels 6 min after 4α-PDD administration were measured (Figure 5). Bolus injection of 2.5 mg/kg 4α-PDD significantly increased plasma CGRP but not SP levels in NS and plasma CGRP and SP levels in HS rats, and the increases in these parameters were significantly greater in HS compared to NS rats. SB 366791, CAPZ, or RuR tended to attenuate 4α-PDD-induced SP release in HS rats, and SB 366791 (HS only), CAPZ (NS only), or RuR (both NS and HS) tended to attenuate 4α-PDD-induced CGRP release, but the inhibition did not reach statistically significant difference. These data indicate that 4α-PDD-induced release of CGRP and SP is enhanced in the face of salt load.
Figure 5.
Plasma calcitonin gene-related peptide (CGRP) and substance P (SP) levels 6 min after bolus injection of 4α-PDD (2.5 mg/kg, iv, a TRPV4 activator) with or without SB 366791 (2 mg/kg, ip, a selective TRPV1 blocker), capsazepine (CAPZ, 3 mg/kg, iv, a selective TRPV1 blocker) and ruthenium red (RuR, 1 mg/kg and 3 mg/kg, iv, a TRPV4 blocker) in rats fed a NS or HS diet. Vehicle: ethanol (5% v/v), Tween-80 (5% v/v) and saline, iv. Values are mean ± SE (n=5–8). *P<0.05 compared with the corresponding vehicle-treated group. †P<0.05 compared with the corresponding vehicle-treated group.
Effects of TRPV4 shRNA treatment on expression and function of TRPV4
The results of western blot analysis indicated that TRPV4 shRNA treatment significantly down-regulated TRPV4 protein expression in DRG by 29% in NS rats and 38% in HS rats, MA by 34% in NS rats and 41 % in HS rats, and the renal medulla by 33% in NS rats (albeit p >0.05) and 52% in HS rats but not in the renal cortex in NS or HS rats (Figure 6). To ascertain TRPV4 mRNA expression levels, the renal medulla was used as an example and subjected to quantitative real-time PCR analysis. Consistent with the protein expression, TRPV4 mRNA expression in the renal medulla was significantly reduced in TRPV4 shRNA compared to control shRNA treated rats by 67% and 50% in NS and HS rats, respectively (Figure 7a). In contrast, TRPV4 shRNA treatment had no effect on TRPV1 mRNA expression in NS or HS rats (Figure 7b), confirming the specificity of TRPV4 shRNA in the suppression of TRPV4 but not TRPV1 expression.
Figure 6.
Western blot analysis showing the TRPV4 protein expression in dorsal root ganglia (DRG), mesenteric resistant arteries (MA), the renal cortex and medulla in HS-and NS-treated rats with or without TRPV4 shRNA treatments. Values are mean ± SE (n=4–5). *P<0.05 compared with the control NS group. †P<0.05 compared with the corresponding control shRNA-treated groups.
Figure 7.
Polymerase chain reaction (PCR) analysis showing mRNA expressions of TRPV4 and TRPV1 in the renal medulla in HS- and NS-treated rats with or without TRPV4 shRNA treatments. Values are mean ± SE (n=4–5). *P<0.05 compared with the corresponding control shRNA-treated groups.
There was no significant difference in baseline MAP between control shRNA- and TRPV4 shRNA-treated groups in NS (control shRNA: 87 ± 3 mm Hg vs TRPV4 shRNA: 85 ± 4 mm Hg, p>0.05) or HS (control shRNA: 93 ± 4 mm Hg vs TRPV4 shRNA: 89 ± 5 mm Hg, p>0.05) rats. However, down-regulation of TRPV4 with TRPV4 shRNA significantly attenuated the depressor effects of 4α-PDD (2.5 mg/kg iv) in both NS and HS rats (Figure 8). These data indicate that TRPV4 shRNA specifically and effectively down-regulates mRNA and protein expression of TRPV4 in NS and HS rats, resulting in attenuated depressor effects of TRPV4 observed in these rats.
Figure 8.
MAP responses to intravenous injection of 4α-PDD (2.5 mg/kg, iv, a TRPV4 activator) with or without TRPV4 shRNA treatment in consious rats fed a NS or HS diet. Values are mean ± SE (n=4–6). *P<0.05 compared with the corresponding NS group. †P<0.05 compared with the corresponding control shRNA-treated group.
Discussion
This study examines the role of TRPV4, a newly discovered osmosensor and mechanosensor, in salt-induced regulation of blood pressure. The data presented here show that (1) 4α-PDD induces a dose-dependent decrease in blood pressure by activation of TRPV4 in rats fed a NS diet, and HS intake enhances 4α-PDD-induced depressor effects via mainly activation of TRPV4 as well as possibly TRPV1; (2) blockade of TRPV4 with RuR elevates baseline MAP in rats fed a NS diet, and HS intake sensitizes RuR-induced pressor effects; (3) HS upregulates TRPV4 expression in sensory nerves and mesenteric arteries and enhances TRPV4-mediated CGRP and SP release; and (4) gene delivery of TRPV4 shRNA in vivo down-regulates mRNA and protein expression of TRPV4, leading to attenuated 4α-PDD-induced depressor effects. These data indicate for the first time that salt intake arguments TRPV4-induced depressor effects, which may constitute a compensatory mechanism in preventing salt-induced increases in blood pressure.
It has been reported that, as a TRPV4 channel opener, 4α-PDD also weakly activates TRPV1.30 We therefore examined whether 4α-PDD-induced depressor effects were mediated by TRPV1 activation. Our results showed that blockade of TRPV1 weakly attenuated 4α-PDD-induced hypotension in HS rats only, whereas blockade of TRPV4 markedly blunted the fall of blood pressure induced by 4α-PDD in rats fed a NS or HS diet. These data indicate that 4α-PDD-induced depressor effects are predominantly mediated by TRPV4 activation during NS or HS intake, and that a component of the depressor effect of 4α-PDD is mediated by TRPV1 activation when HS is given, a finding supported by previous in vitro findings.18 Likewise, as a TRPV4 channel blocker, RuR may act on TRPV1 channels to affect its function. However, our data show that RuR had no effect on capsaicin-induced depressor effects, whereas SB 366791 effectively blocked capsaicin action. These data indicate that RuR action is TRPV1-independent, a result consistent with a previous report.29 Moreover, these findings indicate that the effect of RuR on preventing 4α-PDD-induced fall in blood pressure is mediated by blockade of TRPV4.
Our data show that 4α-PDD-induced depressor effects in MAP were augmented by HS intake. The enhanced depressor effects in HS-treated rats could be the result of increased TRPV4 expression observed in mesenteric resistance arteries and sensory nerves in HS-treated rats. Several lines of evidence in vitro and in vivo have shown that TRPV4, expressed abundantly in endothelial cells, may be activated by a variety of physiologically active endogenous lipids including endocannabinoids, arachidonic acid, and their active metabolites to regulate vascular tone.4,5,17,18 Elevated TRPV4 expression by HS intake may augment or sensitize TRPV4 effects induced by these lipids, leading to greater vasodilatation and subsequent fall in blood pressure in these rats.21,22 The mechanisms underlying TRPV4-mediated vasodilatation remain to be defined. However, it has been shown that in response to 5’, 6’-EET, a putative endothelium-derived hyperpolarizing factor (EDHF),19 TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and Ca2+-dependent K+ (BKCa) channels to induce smooth muscle hyperpolarization and arterial dilation via Ca2+-induced Ca2+ release.20,31
In addition, activation of TRPV4 expressed in DRG sensory neurons may cause hypotension via the release of CGRP and SP, the potent vasodilatory neuropeptides.32,33 Indeed, our data show that TRPV4 activation by 4α-PDD increased CGRP release in NS-or HS-treated rats, and that HS intake augmented 4α-PDD-induced increases in CGRP and SP release. Again, elevated TRPV4 expression in DRG sensory neurons of HS-treated rats may underlie sensitized 4α-PDD-induced increases in CGRP and SP release, which may contribute to enhanced depressor effects of 4α-PDD observed during HS intake. On the other hand, given that activation of TRPV1 has been shown to increase CGRP release and that HS intake enhances TRPV1 action,15,23 the participation of TRPV1 especially in the case of HS intake in 4α-PDD-induced increases in CGRP and SP release may not be ruled out. The fact that blockade of TRPV4 or TRPV1 alone tends to, but insignificantly, attenuate 4α-PDD-induced increases in CGRP and SP release supports the notion that each of the two channels may mediate part of the 4α-PDD action.
While TRPV4-mediated depressor effects are augmented by HS intake, it is important to know whether the enhanced depressor effects of TRPV4 convey a functional role in preventing salt-induced elevation in blood pressure. Our data show that blockade of TRPV4 with RuR elevated baseline MAP in both NS and HS-treated rats, and that HS intake augmented pressor effects induced by RuR. Given that RuR effectively blunted 4α-PDD- but not capsaicin-induced hypotension, the pressor effects induced by RuR are likely indeed mediated by blockade of TRPV4 but not TRPV1. These data indicated that TRPV4 possesses an anti-hypertensive effect, especially in the face of salt load. Furthermore, enhanced TRPV4 expression, TRPV4-mediated sensory neuropeptide release, and TRPV4-mediated depressor effects during HS intake may constitute a compensatory effect in offsetting salt-induced increases in blood pressure.
As an effective and specific tool to knockdown the target gene expression,34 TRPV4 shRNA combined with 5 PAMAM, the most used dendrimers for facilitating delivery of intact interfering RNAs into target cells/organs in vivo,35 have been used in the current study to further identify whether the effect induced by 4 α-PDD is mediated by TRPV4 activation. As a result, TRPV4 shRNAs effectively reduced TRPV4 expression in DRG sensory neurons, mesenteric arteries, and the renal medulla, leading to attenuated depressor effects evoked by 4α-PDD. A greater effect may be reached with more suppression of TRPV4 expression, but further studies are necessary to confirm the notion. Nevertheless, these results further support the notion that TRPV4 mediates 4α-PDD-induced depressor effects and activation of TRPV4 conveys an anti-hypertensive effect.
Perspectives
The kidney and central nervous system (CNS) are the two major sites for salt sensing in blood pressure regulation and hypertension.36,37 However, the mechanistic link between dietary salt and hypertension remains poorly understood. Several distinct mechanisms possibly involve in this process, including [Cl−] sensing in renal tubular fluids by Na+-K+-Cl− cotransporters, sensing of [Na+] or osmolality in cerebrospinal fluid (CSF) by TRPV1, and osmolarity sensing in glial cells of supreoptic and paraventricular nuclei by volume-regulated anion channels.15,16,23,24,37,38 TRPV4 has been shown to be expressed in the circumventricular organs, the organum vasculosum of the lamina terminalis (OVLT), and the subfornical organ (SFO), which sense and modulate osmotic pressure by feedback regulation.8 In addition, TRPV4 may affect salt sensitivity of blood pressure by regulating release of antidiuretic hormone (ADH) and the subsequent free-water reabsorption, an action involving both CNS and the kidney.8 Our findings in the present study support the hypothesis that TRPV4 plays a compensatory role in preventing development of salt-sensitive hypertension, an effect mimicking TRPV1.15,16,23,24,38 Thus, TRPV4 may serve as a target for development of therapy treating hypertension, especially salt-sensitive sub-population. Furthermore, in vivo delivery of gene in a format of nanoparticle may be a potential useful tool for down-regulation of expression and function of specific genes and their corresponding proteins, leading to altered functions governed by these proteins to achieve therapeutic purposes.
Acknowledgments
Sources of Funding
This work was supported in part National Institutes of Health (grants HL-57853, HL-73287, and DK67620) and a grant from Michigan Economic Development Corporation.
Footnotes
Disclosures
NONE
References
- 1.Plant TD, Strotmann R. TRPV4. Handb Exp Pharmacol. 2007;179:189–205. doi: 10.1007/978-3-540-34891-7_11. [DOI] [PubMed] [Google Scholar]
- 2.Strotmann R, Schultz G, Plant TD. Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J Biol Chem. 2003;278:26541–26549. doi: 10.1074/jbc.M302590200. [DOI] [PubMed] [Google Scholar]
- 3.Voets T, Prenen J, Vriens J, Watanabe H, Janssens A, Wissenbach U, Bödding M, Droogmans G, Nilius B. Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem. 2002;277:33704–33710. doi: 10.1074/jbc.M204828200. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 5.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97:908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 6.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U S A. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci U S A. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian W, Salanova M, Xu H, Lindsley JN, Oyama TT, Anderson S, Bachmann S, Cohen DM. Renal expression of osmotically responsive cation channel TRPV4 is restricted to water-impermeant nephron segments. Am J Physiol Renal Physiol. 2004;287:F17–F24. doi: 10.1152/ajprenal.00397.2003. [DOI] [PubMed] [Google Scholar]
- 10.Jia Y, Wang X, Varty LA, Rizzo CA, Yang R, Correll CC, Phelps PT, Egan RW, Hey JA. Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L272–L278. doi: 10.1152/ajplung.00393.2003. [DOI] [PubMed] [Google Scholar]
- 11.Liedtke W, Simon SA. A possible role for TRPV4 receptors in asthma. Am J Physiol Lung Cell Mol Physiol. 2004;287:L269–L271. doi: 10.1152/ajplung.00153.2004. [DOI] [PubMed] [Google Scholar]
- 12.Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2003;100:14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Güler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci. 2005;25:1304–1310. doi: 10.1523/JNEUROSCI.4745.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Wang DH. Function and regulation of the vanilloid receptor in rats fed a high salt diet. J Hypertens. 2003;21:1525–1530. doi: 10.1097/00004872-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Wang DH. A novel mechanism contributing to development of Dahl saltsensitive hypertension: role of the transient receptor potential vanilloid type 1. Hypertension. 2006;47:609–614. doi: 10.1161/01.HYP.0000197390.10412.c4. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- 19.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 20.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97:1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 21.Köhler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol. 2006;26:1495–1502. doi: 10.1161/01.ATV.0000225698.36212.6a. [DOI] [PubMed] [Google Scholar]
- 22.Marrelli SP, O'neil RG, Brown RC, Bryan RM., Jr PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am J Physiol Heart Circ Physiol. 2007;292:H1390–H1397. doi: 10.1152/ajpheart.01006.2006. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Kaminski NE, Wang DH. Anandamide-induced depressor effect in spontaneously hypertensive rats: role of the vanilloid receptor. Hypertension. 2003;41:757–762. doi: 10.1161/01.HYP.0000051641.58674.F7. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Kaminski NE, Wang DH. VR1-mediated depressor effects during high-salt intake: role of anandamide. Hypertension. 2005;46:986–991. doi: 10.1161/01.HYP.0000174596.95607.fd. [DOI] [PubMed] [Google Scholar]
- 25.Rawls SM, Ding Z, Cowan A. Role of TRPV1 and cannabinoid CB1 receptors in AM 404-evoked hypothermia in rats. Pharmacol Biochem Behav. 2006;83:508–516. doi: 10.1016/j.pbb.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Lin YS, Ho CY, Chang SY, Kou YR. Laryngeal C-fiber afferents are not involved in the apneic response to laryngeal wood smoke in anesthetized rats. Life Sci. 2000;66:1695–1704. doi: 10.1016/s0024-3205(00)00492-6. [DOI] [PubMed] [Google Scholar]
- 27.Naida AM, Ghosh TK, Mathew OP. Airway protective reflexes elicited by laryngeal ammonia: role of C-fiber afferents. Respir Physiol. 1996;103:11–17. doi: 10.1016/0034-5687(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 28.Yang XR, Lin MJ, McIntosh LS, Sham JS. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1267–L1276. doi: 10.1152/ajplung.00515.2005. [DOI] [PubMed] [Google Scholar]
- 29.Mathison R, Davison JS. Capsaicin sensitive nerves in the jejunum of Nippostrongylus brasiliensis-sensitized rats participate in a cardiovascular depressor reflex. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:638–642. doi: 10.1007/BF00167241. [DOI] [PubMed] [Google Scholar]
- 30.Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW., 4th Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci U S A. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006;99:119–131. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- 32.Kawasaki H, Takasaki K, Saito A, Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988;335:164–167. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- 33.Oh-hashi Y, Shindo T, Kurihara Y, Imai T, Wang Y, Morita H, Imai Y, Kayaba Y, Nishimatsu H, Suematsu Y, Hirata Y, Yazaki Y, Nagai R, Kuwaki T, Kurihara H. Elevated sympathetic nervous activity in mice deficient in α-CGRP. Circ Res. 2001;89:983–990. doi: 10.1161/hh2301.100812. [DOI] [PubMed] [Google Scholar]
- 34.Siolas D, Lerner C, Burchard J, Ge W, Linsley PS, Paddison PJ, Hannon GJ, Cleary MA. Synthetic shRNAs as potent RNAi triggers. Nat Biotechnol. 2005;23:227–231. doi: 10.1038/nbt1052. [DOI] [PubMed] [Google Scholar]
- 35.Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener JW, Meijer EW, Paulus W, Duncan R. Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J Control Release. 2000;65:133–148. doi: 10.1016/s0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Iturbe B, Romero F, Johnson RJ. Pathophysiological mechanisms of salt-dependent hypertension. Am J Kidney Dis. 2007;50:655–672. doi: 10.1053/j.ajkd.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Orlov SN, Mongin AA. Salt-sensing mechanisms in blood pressure regulation and hypertension. Am J Physiol Heart Circ Physiol. 2007;293:H2039–H2053. doi: 10.1152/ajpheart.00325.2007. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Kaminski NE, Wang DH. Endocannabinoid regulates blood pressure via activation of the transient receptor potential vanilloid type 1 in Wistar rats fed a highsalt diet. J Pharmacol Exp Ther. 2007;321:763–769. doi: 10.1124/jpet.106.112904. [DOI] [PubMed] [Google Scholar]