Abstract
Context: Apolipoprotein (apo) C-III is associated with hypertriglyceridemia and progression of cardiovascular disease. Plasma apoC-III is elevated in centrally obese men, and we hypothesized that the kinetics of apoC-III are disturbed in these subjects.
Objective: We developed a compartmental model to determine very low-density lipoprotein (VLDL) and high-density lipoprotein (HDL) apoC-III metabolic parameters in centrally obese men and investigated the associations with VLDL-apoB and HDL-apoA-I kinetics.
Study Design: Apolipoprotein kinetics was determined using stable isotope techniques and compartmental modelling in 39 centrally obese and 12 nonobese men.
Results: Compared with nonobese subjects, centrally obese subjects had increased plasma apoC-III concentration (160 ± 5 mg/liter vs. 103 ± 9 mg/liter, P < 0.001), reflecting increased concentrations of both VLDL-apoC-III and HDL-apoC-III. These related to increased production rate (PR) of VLDL-apoC-III (2.12 ± 0.14 vs. 1.56 ± 0.29 mg/kg·d, P < 0.05) and reduced fractional catabolic rate (FCR) of both VLDL- and HDL-apoC-III (0.70 ± 0.02 pools/d vs. 0.82 ± 0.05 pools/d, P < 0.05). In centrally obese men, VLDL-apoC-III concentration was significantly (P < 0.05) associated with VLDL-apoB concentration and PR as well as HDL-apoA-I FCR and PR and inversely with VLDL-apoB FCR. HDL-apoC-III concentration was significantly (P < 0.05) associated with the concentrations of both VLDL-apoB and HDL-apoA-I, the FCR, and the PR of HDL-apoA-I and inversely with the VLDL-apoB FCR. In multiple regression analysis, both VLDL-apoC-III and HDL-apoC-III concentrations were significantly associated with HDL-apoA-I FCR.
Conclusions: In centrally obese men, elevated VLDL-apoC-III and HDL-apoC-III concentrations are a consequence of elevated production and decreased catabolism of VLDL-apoC-III and reduced catabolism of HDL-apoC-III, respectively. These defects are associated with disturbances in VLDL-apoB and HDL-apoA-I metabolism.
A study of insulin-resistant and centrally obese men defines the disrupted kinetics of apolipoprotein (apo) C-III, a major risk factor for cardiovascular disease, including elevated plasma apoC-III, due to elevated VLDL-apoC-III production and reduced VLDL- and HDL-apoC-III catabolism, as well as disturbances in VLDL-apoB and HDL-apoA-I metabolism.
Dyslipidemia is a major independent risk factor for cardiovascular disease (CVD) in individuals with obesity and/or type 2 diabetes (1). Apolipoprotein (apo)C-III, an important regulator of lipoprotein metabolism, is strongly associated with hypertriglyceridemia (HTG) and progression of CVD (2,3). ApoC-III impairs the lipolysis of triglyceride-rich lipoproteins (TRLs) by inhibiting lipoprotein lipase (4) and the hepatic uptake of TRLs by the low-density lipoprotein (LDL) receptor (5). In the circulation, apoC-III is associated with TRL and high-density lipoproteins (HDL). We and others have previously reported that increased apoC-III concentrations, resulting from an overproduction of very low-density lipoprotein (VLDL)-apoC-III (6), are strongly associated with delayed catabolism of triglycerides and apoB in VLDL (7,8). Compared with TRLs, little is known about the role of apoC-III in HDL metabolism. There is some evidence that HDL-apoC-III concentration is positively correlated with HDL-cholesterol and HDL-apoA-I levels (9).
ApoC-III exchanges between TRLs and HDL. During hydrolysis of VLDL-triglycerides by lipoprotein lipase, apoC-III redistributes from VLDL to HDL (10) and is then transferred back to newly synthesized VLDL (11). However, the mechanism responsible for regulating apoC-III exchange remains unknown. In vitro studies and in vivo radiotracer studies have demonstrated rapid exchange and equilibration between VLDL and HDL particles (12,13,14). Other radioisotope studies have suggested nonequilibrating pools of apoC-III that do not exchange between VLDL and HDL (15,16,17). More recently endogenous stable isotope tracer studies suggested nonequilibrating pools of VLDL- and HDL-apoC-III (18,19). The discrepancies in these findings might be related to methodological limitations inherent in the isolation of apoC-III from HDL. Using a new method for isolation of HDL-apoC-III, we demonstrated that the kinetics of apoC-III in VLDL and HDL are similar, supporting the concept of a single kinetically homogeneous pool of apoC-III in plasma (20).
In the present study, we examined the kinetics of apoC-III in VLDL and HDL in centrally obese and nonobese men. Using the apoC-III tracer data, we developed a simple compartmental model that best describes apoC-III kinetics in plasma. Given that plasma concentration of apoC-III is elevated in men with visceral obesity as we previously reported (21), we also aimed to test the hypothesis that the kinetics of VLDL- and HDL-apoC-III are disturbed in these subjects. We also examined the associations of these defects with the kinetics of VLDL-apoB and HDL-apoA-I.
Subjects and Methods
Subjects
Thirty-nine centrally obese (waist circumference > 100 cm) and 12 nonobese men (waist circumference < 100 cm, plasma triglyceride < 1.7 mmol/liter and LDL-cholesterol < 2.60 mmol/liter) were recruited from the community. None of subjects had diabetes mellitus (excluded by oral glucose tolerance test), apoE2/E2 genotype, macroproteinuria, creatinemia (>120 μmol/liter), hypothyroidism, abnormal liver enzymes, a history of CVD; were on any medication or agents known to affect lipid metabolism; or consumed more than 30 g alcohol per day. The study was approved by the Ethics Committee of the Royal Perth Hospital.
Clinical protocols
All subjects were admitted to the metabolic ward in the morning after a 14-h fast and studied in a semirecumbent position. Baseline venous blood collected from all participants for measurement of biochemical analytes. A single bolus of d3-leucine (5 mg/kg body weight) was administered iv. Blood samples were taken at baseline; at 5, 10, 20, 30, and 40 min; and 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, and 10 h after isotope injection and additional fasting blood was collected in the morning on the following 4 d of the same week (24, 48, 72, 96 h).
Biochemical measurements
Laboratory methods for measurements of lipids, lipoproteins, and other biochemical analytes have been previously detailed (21,22). Insulin resistance was calculated using a homeostasis model assessment (HOMA) score. HDL-apoC-III and VLDL-apoC-III were determined by electroimmunodiffusion using a Hydragel LP CIII electroimmunodiffusion kit (Sebia, Moulineaux, France) (23).
Isolation of apolipoproteins and measurement of leucine enrichment
ApoB
The laboratory methods for the isolation and measurement of the isotopic enrichment apoB have been previously fully described by Chan et al. (21). Briefly, apoB in the VLDL fraction was separated by ultracentrifugation, precipitated by isopropanol, delipidated, hydrolyzed, and derivatized with acetonitrile/N-methyl-N-(tert-butyldimethylsilyl)-trifluoroacetamide. Isotopic enrichment was then determined by selected ion monitoring of derivatized samples at a mass to charge ratio of 305 and 302. Tracer to tracee ratios were derived from isotopic ratios for each sample.
ApoA-I
Laboratory methods for isolation and measurement of isotopic enrichment have been previously detailed (24). Briefly, apoB was precipitated from 250 μl plasma using heparin (25 μl) and 12.5 μl of 2.0 m MnCl2. Sixty microliters of 64% CsCl were added to 200 μl of heparin/manganese-treated plasma to adjust the density to 1.21 g/ml. HDL was subsequently isolated from 230 μl of this sample by ultracentrifugation (Optima XL-100K, Beckman Coulter, Fullerton, Australia). ApoA-I was isolated using PAGE and transferred to polyvinylidene fluoride (PVDF) membrane. The apoA-I band was excised from the membrane, hydrolyzed with 200 μl 6 m HCL at 110 C for 16 h, and dried for derivatization using the oxazolinone method as previously described (25).
ApoC-III
The methods used for the isolation of lipoproteins and apoC-III have been described previously (20). Briefly, 3 ml plasma was used for isolation of 1 ml VLDL (<1.006 kg/liter) and 1 ml HDL (1.063–1.21 kg/liter) fractions by sequential ultracentrifugation at 40,000 rpm in a Ti 50.4 rotor (Optima LE-80K; Beckman Coulter). The VLDL and HDL samples were then prepared for isoelectric focusing (IEF) gel electrophoresis. VLDL (200 μl) from each time point was delipidated and reconstituted in 50 μl of IEF sample buffer [8 m urea; 0.001% (wt/vol) bromphenol blue]. HDL (1 ml) from each time point was prepared using an Intralipid method to isolate HDL-apoC-III (20). Briefly, 1 ml of sodium chloride solution (1.006 kg/liter) was added to 1 ml of HDL from each time point. Twenty percent IL emulsion (20% triglyceride, 1.2% phospholipid, and 2.2% glycerol; Fresenius Kabi AB, Uppsala, Sweden) was diluted to 1% using saline solution, and 1 ml was added to each tube. The suspensions were mixed by inversion and ultracentrifuged for 24 h at 40,000 rpm. After centrifugation, the IL layer (0.5 ml) was aspirated into 15-ml disposable polypropylene tubes (Sarstedt, Newton, NC). Ten milliliters of acetone-ethanol (1:1) solution were then added to each sample, mixed at 4 C for 30 min, and delipidated overnight at −20 C. The resulting protein precipitates were reconstituted in 100 μl IEF sample buffer. apoC-III was isolated by preparative IEF gel electrophoresis [8 m urea; 7.5% acrylamide; 1.5% ampholytes (pH 4–6); 16 h; 200 V; 4 C], transferred to PVDF membranes (Immobilon; Millipore, Bedford, MA), and stained with Coomassie Brilliant Blue R250. IEF resolves apoC-III into three isoforms, apoC-III0, apoC-III1, and apoC-III2. ApoC-III1 is the most abundant isoform, and radiotracer studies support that the kinetics of apoC-III isoforms are similar (12,26,27). apoC-III in the present study refers to the apoC-III1 isoform. The apoC-III1 protein bands were excised from the PVDF membranes, hydrolyzed and derivatized using a modified oxazolinone method (25), and analyzed by negative ion chemical ionization gas chromatography-mass spectrometry. The isotopic enrichment was determined as the tracer to tracee ratio of monitored selected ions at a mass to charge ratio of 212 (derived from d3-leucine) and 209 (derived from unlabeled leucine).
Model of apolipoprotein metabolism and calculation of kinetic parameters
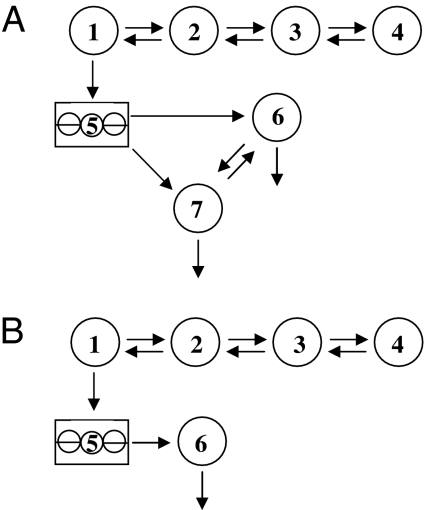
Models of apoB and apoA-I and calculation of kinetic parameters have been previously described (21,24). Figure 1 shows two compartment models developed, using the SAAMII program (Resource Facility for Population Kinetics, University of Washington, Seattle, WA), to describe apoC-III tracer data. Both models have a four-compartment leucine subsystem (compartments 1–4), as described previously for the apoB model (21), that describes the plasma leucine tracer data. Compartment 1 is connected to compartment 5, a compartment that accounts for the intrahepatic delay associated with synthesis and secretion of apoC-III into plasma. In model A, compartments 6 and 7 represent apoC-III associated with the VLDL and HDL particles, respectively. In model B, compartment 6 represents apoC-III associated with either VLDL or HDL particles. The compartment models were fitted to the apoC-III tracer data to derive the fractional catabolic rates (FCR) for apoC-III and expressed as pools/day.
Figure 1.
Compartmental models describing apoC-III tracer kinetics. Compartments 1–4 represent the leucine subsystem (compartment 2 is the plasma pool), and compartment 5 represents the intrahepatic delay compartment. In model A, compartments 6 and 7 represent the VLDL- and HDL-apoC-III, respectively. In model B, compartment 6 represents VLDL-, HDL-, or plasma-apoC-III.
Calculation of kinetic parameters
Given that VLDL- and HDL-apoC-III FCRs are equivalent, as we previously reported (20), the production rates (PRs) for apoC-III in VLDL and HDL were derived as the product of VLDL-apoC-III FCR and their corresponding pool sizes and expressed as milligrams per kilogram per day. Pool size was calculated as the product of apoC-III concentration (milligrams per liter) and plasma volume (0.045 liters/kg), with a correction factor to adjust for the decrease in relative plasma volume associated with an increase in body weight (28).
Statistical analysis
All analyses were carried out using SPSS software version 11.5 (SPSS, Inc., Chicago, IL). Skewed variables were logarithmically transformed. Comparisons between centrally obese and nonobese control subjects were performed using unpaired t test. Associations were examined by univariate and multiple regression modeling including covariates [i.e. body mass index (BMI) and insulin resistance] that are known to influence HDL-apoA-I kinetics. Significance was defined at the 5% level using a two-tailed test.
Results
Table 1 shows the clinical and biochemical characteristics of the centrally obese and nonobese men. As expected, centrally obese men had significantly higher body weight, BMI, waist circumference, and waist to hip ratio, compared with the nonobese group (P < 0.001). Plasma glucose (P < 0.05), insulin, and HOMA scores were significantly higher in the centrally obese group (P < 0.001). The centrally obese group had significantly (P < 0.001) higher plasma cholesterol, triglycerides, LDL cholesterol, apoB, and plasma apoC-III but lower HDL cholesterol (P < 0.05), compared with nonobese men.
Table 1.
Clinical and biochemical characteristics of the nonobese and centrally obese men
| Nonobese (n = 12) | Centrally obese (n = 39) | |
|---|---|---|
| Age, yr | 52.3 ± 3.5 | 54.3 ± 1.4 |
| Body weight, kg | 76.2 ± 3.5 | 103.8 ± 2.3a |
| BMI, kg/m2 | 24.4 ± 1.0 | 33.4 ± 0.6a |
| Waist circumference, cm | 89.2 ± 2.3 | 112.7 ± 1.5a |
| Waist to hip ratio | 0.93 ± 0.01 | 1.01 ± 0.01a |
| Nonesterified fatty acids, mmol/liter | 0.30 ± 0.04 | 0.29 ± 0.02 |
| Glucose, mmol/liter | 4.97 ± 0.16 | 5.49 ± 0.12b |
| Insulin, mU/liter | 20.0 ± 2.3 | 34.4 ± 1.6a |
| Insulin resistance, HOMA score | 4.56 ± 0.62 | 8.51 ± 0.55a |
| Total cholesterol, mmol/liter | 4.49 ± 0.18 | 5.91 ± 0.10a |
| Total triglycerides, mmol/liter | 0.75 ± 0.07 | 1.89 ± 0.13a |
| HDL-cholesterol, mmol/liter | 1.34 ± 0.13 | 1.01 ± 0.03b |
| LDL cholesterol, mmol/liter | 2.80 ± 0.10 | 3.89 ± 0.10a |
| ApoB, g/liter | 0.81 ± 0.03 | 1.28 ± 0.03a |
| ApoA-I, g/liter | 1.31 ± 0.07 | 1.21 ± 0.03 |
| ApoC-III, mg/liter | 103 ± 9 | 160 ± 5a |
Data are presented as mean ± sem.
P < 0.001, compared with nonobese controls.
P < 0.05, compared with nonobese controls.
ApoC-III enrichment and model development
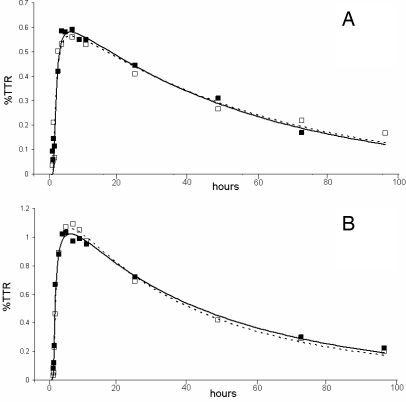
Figure 2 shows the isotopic enrichment of apoC-III with d3-leucine in the VLDL and HDL fractions in centrally obese and nonobese subjects. Within each subject group, the VLDL- and HDL-apoC-III isotopic enrichment curves were identical. VLDL- and HDL-apoC-III tracer curves are also identical when fresh plasma is used for the isolation and measurement of apoC-III kinetics (data not shown). To further corroborate our earlier finding that the FCR of VLDL- and HDL-apoC-III were similar (20), we show, using the model in Figure 1B, that there were no significant differences between the FCRs of VLDL- and HDL-apoC-III within the nonobese (0.81 ± 0.05 vs. 0.83 ± 0.04 pools/d) and obese (0.70 ± 0.04 vs. 0.71 ± 0.04 pools/d) groups. VLDL-apoC-III FCR was also significantly (P < 0.001) correlated with HDL-apoC-III FCR in both nonobese and centrally obese subjects (r = 0.998 and r = 0.931, respectively).
Figure 2.
Isotopic enrichment of apoC-III with deuterium-labeled leucine of VLDL (black squares) and HDL (white squares) expressed as a tracer to tracee ratio (TTR; percent) for centrally obese (A) and nonobese men (B). The solid and dashed lines show the model fits to the apoC-III enrichment data in VLDL and HDL fractions, respectively.
As described above, the apoC-III model shown in Fig. 1A includes the secretion of apoC-III into the VLDL and HDL fractions, the exchange of apoC-III between VLDL and HDL, and the removal of apoC-III from plasma via both VLDL and HDL. It is the most general compartment model of apoC-III that is consistent with our understanding of apoC-III metabolism. From a theoretical standpoint, there is a unique solution or set of model parameters that can be determined for this model. Although the compartment model fits the apoC-III tracer data, the model parameters cannot be estimated with any degree of precision because of the rapid exchange of apoC-III between the VLDL and HDL fractions. We also investigated several alternative compartment models to describe the apoC-III tracer data. The model shown in Fig. 1B was the simplest model that described apoC-III kinetics in the VLDL and HDL fractions. This model describes apoC-III kinetics using a single, homogeneous plasma compartment that fits the VLDL- and HDL-apoC-III tracer data separately and estimates FCR with high precision.
VLDL- and HDL-ApoC-III metabolism in centrally obese and nonobese men
Table 2 shows the VLDL- and HDL-apoC-III concentrations and corresponding kinetic parameters in the subject studied. Compared with nonobese subjects, VLDL-apoC-III concentrations were significantly (P < 0.001) increased in centrally obese subjects, owing to elevated apoC-III PR (P < 0.05) and reduced apoC-III FCR (P < 0.05). Centrally obese men had significantly (P < 0.001) elevated HDL-apoC-III concentration and reduced apoC-III FCR, compared with nonobese men. Centrally obese men also had significantly elevated VLDL-apoB concentration (95.7 ± 5.8 vs. 41.6 ± 6.5 mg/liter, P < 0.001), related to both reduced VLDL-apoB FCR (4.1 ± 0.2 vs. 6.2 ± 0.9 pools/d, P < 0.05) and elevated VLDL-apoB PR (14.6 ± 1.1 vs. 9.6 ± 1.0 mg/kg·d, P < 0.05). Compared with controls, centrally obese subjects had significantly elevated HDL-apoA-I FCR (0.28 ± 0.01 vs. 0.20 ± 0.01 pools/d, P < 0.01) and a trend toward increased HDL-apoA-I PR (13.6 ± 0.6 vs. 11.8 ± 0.9 mg/kg·d, P = 0.140) that accounted for no significant difference in HDL-apoA-I concentrations (1.2 ± 0.03 vs. 1.3 ± 0.07 g/liter, P = 0.236). Nonobese men had significantly higher concentration and PR of HDL-apoC-III than VLDL-apoC-III.
Table 2.
Plasma concentrations, FCRs, and PRs of VLDL-apoC-III and HDL-apoC-III in nonobese and centrally obese men
| Nonobese (n = 12) | Centrally obese (n = 39) | |
|---|---|---|
| VLDL-apoCIII | ||
| Concentrations, mg/liter | 41.6 ± 6.6 | 79.6 ± 3.8a |
| FCR, pools/d | 0.82 ± 0.05 | 0.70 ± 0.02b |
| PR, mg/kg·d | 1.56 ± 0.29 | 2.12 ± 0.14b |
| HDL-apoC-III | ||
| Concentrations, mg/liter | 61.3 ± 5.3c | 79.9 ± 1.5a |
| FCR, pools/d | 0.82 ± 0.05 | 0.70 ± 0.02b |
| PR, mg/kg·d | 2.31 ± 0.26c | 2.13 ± 0.09 |
Data are presented as mean ± sem.
P < 0.001, compared with nonobese controls.
P < 0.05, compared with nonobese controls.
P < 0.05, within-group comparison with corresponding VLDL-apoC-III parameters.
In a pooled analysis, VLDL-apoC-III and HDL-apoC-III concentrations were significantly (P < 0.01) associated with waist circumference (r = 0.447 and r = 0.505, respectively), BMI (r = 0.482 and r = 0.482, respectively), insulin (r = 0.435 and r = 0.416, respectively), and HOMA score (r = 0.380 and r = 0.375, respectively). Waist circumference and BMI were significantly (P < 0.05 for both) associated with VLDL-apoC-III PR (r = 0.326 and r = 0.342, respectively). In a multiple regression model including age and HOMA score, waist circumference was an independent and significant predictor (P < 0.05) of VLDL-apoC-III (model adjusted R2 = 21%, P < 0.01), HDL-apoC-III concentrations (model adjusted R2 = 30%, P < 0.01), and VLDL-apoC-III PR (model adjusted R2 = 11%, P < 0.05).
Correlations
Table 3 shows the association of VLDL-apoC-III and HDL-apoC-III kinetics in centrally obese men. VLDL-apoC-III concentrations were significantly and positively associated with HDL-apoC-III concentrations, and both were also significantly associated with the PR of VLDL-apoC-III and HDL-apoC-III. The FCRs of VLDL and HDL-apoC-III were both positively associated with the PRs of VLDL-apoC-III and HDL-apoC-III.
Table 3.
Associations of VLDL-apoC-III and HDL-apoC-III kinetics in centrally obese men
| VLDL-apoC-III
|
HDL-apoC-III
|
|||||
|---|---|---|---|---|---|---|
| Concentration | FCR | PR | Concentration | FCRa | PR | |
| VLDL-apoC-III | ||||||
| Concentration | −0.016 | 0.788b | 0.842b | −0.016 | 0.378c | |
| FCR | −0.016 | 0.497b | −0.133 | 0.777b | ||
| PR | 0.788b | 0.497b | 0.587b | 0.497b | 0.831b | |
| HDL-apoA-I PR | ||||||
| HDL-apoC-III | ||||||
| Concentration | 0.842b | −0.133 | 0.587b | −0.133 | 0.344c | |
| FCRa | −0.016 | 0.497b | −0.133 | 0.777b | ||
| PR | 0.378c | 0.777b | 0.831b | 0.344c | 0.777b | |
| HDL-apoA-I PR | ||||||
Data in boldface are statistically significant.
HDL-apoC-III FCR is equivalent to VLDL-apoC-III FCR (see text).
P < 0.01.
P < 0.05.
Table 4 shows the associations of VLDL-apoC-III and HDL-apoC-III kinetics with VLDL-apoB and HDL-apoA-I kinetics in centrally obese men. VLDL-apoC-III and HDL-apoC-III concentrations were both significantly and positively associated with VLDL-apoB concentration, the FCR and PR of HDL-apoA-I and inversely with VLDL-apoB FCR. Similar associations were found between plasma total apoC-III concentration and the corresponding kinetic parameters. VLDL-apoC-III concentration was significantly associated with the PR of VLDL-apoB (r = 0.348, P < 0.05). The FCRs of VLDL- and HDL-apoC-III were also significantly and positively associated with VLDL-apoB FCR (P < 0.05). The PRs of VLDL and HDL-apoC-III were both positively associated with VLDL-apoB concentration and the PRs of VLDL-apoB and HDL-apoA-I. Plasma triglyceride concentration was also significantly (P < 0.01) associated with the concentrations of plasma apoC-III (r = 0.735), VLDL-apoC-III (r = 0.736), and HDL-apoC-III (r = 0.477) as well as VLDL-apoC-III PR (r = 0.575) and HDL-apoA-I FCR (r = 0.438). Correlations from pooled analyses of centrally obese and nonobese subjects were consistent with those observed in the centrally obese men (data not shown). In stepwise multiple regression analysis including age, BMI, HOMA score, plasma triglyceride, and apoC-III, plasma triglyceride was the best predictor of HDL-apoA-I FCR (β-coefficient = 0.438, P < 0.01).
Table 4.
Associations of VLDL-apoC-III and HDL-apoC-III kinetics with VLDL-apoB and HDL-apoA-I kinetics in centrally obese men
| VLDL-apoC-III
|
HDL-apoC-III
|
|||||
|---|---|---|---|---|---|---|
| Concentration | FCR | PR | Concentration | FCRa | PR | |
| VLDL-apoB | ||||||
| Concentration | 0.688b | −0.024 | 0.606b | 0.643b | −0.024 | 0.338c |
| FCR | −0.320c | 0.343c | −0.109 | −0.348c | 0.343c | 0.095 |
| PR | 0.348b | 0.200 | 0.454b | 0.293 | 0.200 | 0.373c |
| HDL-apoA-I PR | ||||||
| HDL-apoA-I | ||||||
| Concentration | 0.256 | −0.064 | 0.196 | 0.334c | −0.064 | 0.108 |
| FCR | 0.327c | 0.073 | 0.199 | 0.439b | 0.073 | 0.122 |
| PR | 0.350c | 0.215 | 0.452b | 0.457b | 0.215 | 0.444b |
| HDL-apoA-I PR | ||||||
Data in boldface are statistically significant.
HDL-apoC-III FCR is equivalent to VLDL-apoC-III FCR (see text).
P < 0.01.
P <<0.0.
In multiple regression models including BMI and HOMA score, VLDL-apoC-III concentration was an independent and significant predictor of HDL-apoA-I FCR (model 1 β-coefficient = 0.326, adjusted R2 = 14%, P = 0.04). HDL-apoC-III (model 2 β-coefficient = 0.404, adjusted R2 = 20%, P = 0.01), total apoC-III (model 3 β-coefficient = 0.360, adjusted R2 = 16%, P = 0.02), or plasma triglyceride (model 4 β-coefficient = 0.400, adjusted R2 = 18%, P = 0.02) concentrations were also independent predictors in the same regression models. The corresponding β-coefficients of BMI in models 1–4 were 0.232, 0.232, 0.231, and 0.230, respectively (P > 0.05), whereas HOMA scores were 0.114, 0.077, 0.102, and 0.024, respectively (P > 0.05). The addition of VLDL-apoB FCR did not alter the findings (data not shown).
In nonobese subjects, there were no significant associations between the apoC-III kinetic parameters and VLDL-apoB or HDL-apoA-I kinetic parameters. VLDL-apoC-III concentration was significantly correlated with VLDL-apoC-III PR and plasma triglycerides (r = 0.94 and r = 0.76, respectively, P < 0.01 for both). Plasma apoC-III concentration was also significantly (P < 0.01) correlated with plasma triglycerides (r = 0.88, P < 0.01).
Discussion
We have developed a simple compartmental model, based on our previous findings supporting rapid exchange of apoC-III between VLDL and HDL particles, which best describes the kinetics of apoC-III in plasma. Our major findings were that, compared with nonobese men, centrally obese men had significantly elevated VLDL- and HDL-apoC-III concentrations, as a consequence of elevated production of VLDL-apoC-III and reduced catabolism of both VLDL- and HDL-apoC-III. Central obesity as reflected by waist circumference is an independent predictor of elevated VLDL-apoC-III production that leads to elevations in apoC-III concentration and consequently higher plasma triglycerides. Another new finding was that both VLDL- and HDL-apoC-III concentrations were positively associated with HDL-apoA-I hypercatabolism, independent of BMI and insulin resistance. Given that hypercatabolism of apoA-I is a major determinant of low HDL-cholesterol, our findings are important because they highlight not only the principal role of apoC-III in regulating triglyceride-rich lipoprotein metabolism but also consequently its effect on the kinetics of HDL-apoA-I in obesity. Collectively, these results provide a mechanistic explanation for raised plasma apoC-III concentrations in obesity that may lead to hypertriglyceridemia and low HDL-cholesterol and contribute to the increased cardiovascular risk observed in such subjects.
ApoC-III model
Early radioisotope studies demonstrated rapid exchange of apoC-III between VLDL and HDL fractions in both hyperlipidemic and normolipidemic subjects (12,14,29). Several stable isotope studies, using endogenous labeling and the primed constant infusion protocol, have shown divergence of the VLDL and HDL apoC-III enrichment curves, suggesting the presence of kinetically distinct pools of VLDL- and HDL-apoC-III (18,19). However, we previously demonstrated that IEF separation of HDL apoC-III and the subsequent measurement of tracer enrichment could be inaccurate owing to amino acid or protein contamination from background amino acids accumulated during isolation procedures, and proteins in the sample that are unresolved on the gel. To overcome this, we used Intralipid extraction before IEF protein separation to isolate apoC-III from HDL free of other protein contamination. Using this IL method (20), we found that the isotopic enrichments of apoC-III in VLDL and HDL are identical in both obese and normolipidemic subjects (Fig. 2). This is consistent with a rapid exchange of apoC-III between VLDL and HDL fractions. The kinetic parameters of our apoC-III model (Fig. 1A) could not therefore be determined with any degree of precision, given the multiple sets of model parameters that can fit the apoC-III tracer data. Because the kinetics of apoC-III in the VLDL and HDL fractions are indistinguishable and hence the exchange between VLDL and HDL cannot be measured, we developed a simple model to describe the kinetics of apoC-III in VLDL and HDL fractions (Fig. 1B). This model fitted the tracer data well, allowing the FCRs to be determined with acceptable level of precision. We propose that future studies could use this simple model to determine the kinetics of apoC-III in the individual VLDL and HDL fractions or possibly even in total plasma.
Instead of using primed constant infusion method, we used a bolus infusion protocol to endogenously label apolipoproteins. The bolus administration of tracer is better than the primed constant infusion because the apoprotein tracer data display greater dynamics by virtue of the pulse administration tracer. It is more suitable to the study of lipoprotein metabolism, including lipoproteins with a slow rate of turnover, such as apoA-I in HDL. The bolus dose also better facilitates the determination of newly synthesized particles because the intracellular precursor enrichment is greater at the start of the study. Moreover, the clinical protocol of the bolus approach is more convenient for both subjects and researchers.
ApoC-III kinetics in centrally obese and nonobese men
Elevated concentrations of plasma apoC-III have been associated with HTG and the progression of CVD. We and others had previously examined apoC-III kinetics in men with wide-ranging BMI and patients with HTG (types IIb and III) (6,8). Our present data extend previous studies by using a larger sample size and examining the association of VLDL- and HDL-apoC-III kinetics with VLDL-apoB and HDL-apoA-I kinetics in centrally obese and nonobese subjects. Previous studies demonstrated that central obesity plays an important role in regulating lipoprotein metabolism. Consistent with this, we found using a pooled analysis that waist circumference was a significant predictor of plasma concentrations of VLDL-apoC-III and HDL-apoC-III as well as the VLDL-apoC-III production. Furthermore, our results demonstrate that in centrally obese subjects, VLDL-apoC-III concentration is significantly correlated with its PR as well as VLDL-apoB concentration and its corresponding FCR, consistent with previous studies (6,7). We also observed that the production rate of VLDL-apoC-III and VLDL-apoB was significantly associated. The precise reason for this remains unclear, although a recent study by Sundaram et al. (30) demonstrated, in vitro, that oversecretion of apoC-III stimulated apoB synthesis and VLDL assembly and secretion. However, it may also relate to the metabolic effect of insulin resistance and/or increased visceral fat by driving the production of these lipoproteins, given that the above-mentioned associations were not observed in the nonobese group.
Unlike VLDL-apoC-III, little is known about HDL-apoC-III metabolism and its associations with other lipoproteins. Previous studies using radioisotopes and stable isotopes examined HDL-apoC-III metabolism but not in obese subjects. In radiotracer studies, Le et al. (9) observed in nonobese subjects with a wide range of triglyceride and HDL-cholesterol concentrations that HDL-apoC-III concentration was positively associated with HDL-apoA-I concentration and inversely with HDL-apoA-I-FCR. More recently Cohn et al. (18) demonstrated, using endogenous labeling methodology, that HDL apoC-III PR was correlated with HDL apoA-I concentration and residence time. Using an improved isolation method for HDL-apoC-III with a larger sample size, we found that HDL-apoC-III concentration was significantly elevated in centrally obese men, compared with nonobese men, owing to reduced catabolism. However, HDL-apoC-III concentration was significantly and directly correlated with both FCR and PR of HDL-apoA-I in centrally obese men, inconsistent with previous studies. Several factors could account for the discrepancies among different studies. Other studies did not specifically examine kinetics in obese subjects in whom obesity and insulin resistance markedly impact on HDL-apoA-I metabolism. Compared with the study by Le et al. (9), our nonobese subjects (n = 12) had much lower triglyceride concentrations (0.3–1.2 mmol/liter, mean value of 0.75 mmol/liter). As discussed below, triglyceride content in VLDL and HDL could be a key factor modulating the catabolism of HDL-apoA-I. That we did not confirm the findings by Cohn et al. (18) was probably due to the differences in laboratory methods used to isolate HDL-apoC-III, and as discussed earlier, our Intralipid method results in improved purity of HDL-apoC-III isolated from plasma. We also found that HDL-apoC-III PR was strongly associated with its FCR.
An important finding was that in centrally obese subjects, the significant association between VLDL- or HDL-apoC-III concentration and HDL-apoA-I kinetics was independent of BMI and insulin resistance. Radiotracer studies demonstrated that enhanced HDL-apoA-I clearance is dependent on triglyceride enrichment of HDL (31). Triglyceride-enriched HDL, generated by increased neutral lipid exchange with VLDL, is a preferred substrate for hepatic lipase, which accelerates the catabolism of these thermodynamically unstable HDL particles (32). Consistent with the direct role of triglycerides in regulating apoA-I catabolism, we found that plasma triglyceride was the best predictor of HDL-apoA-I FCR. Given the functional role of apoC-III in inhibiting the hydrolysis of triglycerides, it is conceivable that accumulation of apoC-III in plasma will favor the formation of unstable triglyceride-rich HDL particles, thereby increasing the FCR of HDL-apoA-I. This notion is consistent with our present findings that both elevated VLDL- and HDL-apoC-III and triglyceride concentrations are independent determinants of hypercatabolism of HDL-apoA-I. Whether these associations simply reflect an overall accumulation of apoC-III in plasma or an independent effect of VLDL-apoC-III and HDL-apoC-III on HDL catabolism merits further investigation. As shown in Table 4, VLDL apoC-III concentration was associated with HDL-apoA-I FCR. In contrast, apoC-III kinetic parameters were only weakly, and nonsignificantly, associated with HDL-apoA-I FCR. These findings would tend to support the concept that the relationship between apoC-III and HDL metabolism is secondary to the primary function of apoCIII and, furthermore, that the plasma concentration of apoC-III is a key determinant of lipoprotein metabolism rather than its rate of production or catabolism. Moreover, the correlations of apoC-III with kinetic variables in Tables 3 and 4 suggest that its association with VLDL-apoB and HDL-apoA-I kinetics could be accounted for by additional factors, including genetic variation or dietary intake (33,34,35,36). Consistent with this, we previously reported that the hepatic secretion of apoB was dependent on apoB signal peptide, apoE, cholesteryl ester transfer protein, microsomal triglyceride transfer protein, and ATP binding cassette transporter G8 gene polymorphisms (33,34).
Limitations
We did not study LDL-apoC-III metabolism. Given that LDL-apoC-III may play an important role in mediating atherogenesis, further studies should examine LDL-apoC-III kinetics in these subjects. The lack of significant associations between apoC-III and apoB and apoA-I kinetics in nonobese men may reflect lack of statistical power. Hence, the findings need to be confirmed in a larger sample size. Although ultracentrifugation is a standard method to isolate lipoproteins in plasma, it could introduce artifacts in apolipoprotein distribution and affect the interpretation of the results. Isolation of apoC-III from frozen plasma may also affect the distribution of apolipoproteins. However, we previously found no significant differences between kinetic data derived from fresh and frozen samples. We did not measure triglyceride content in HDL particles, which could otherwise provide new information data on its association with HDL-apoA-I FCR and apoC-III kinetics. Further work is required to confirm whether our findings also apply to women and patients with type 2 diabetes mellitus as well as subjects of other ethnic groups.
The recognition of hypertriglyceridemia and low HDL-cholesterol as independent predictors of CVD necessitates the identification of factors involved in the regulation of apoB and apoA-I metabolism. The results of large clinical studies have indicated that apoC-III concentrations are a better predictor of risk for the development and progression of CVD than triglycerides (2,3).
Elevated apoC-III concentrations are a common feature of lipid disorders seen in insulin-resistant and centrally obese subjects. Our kinetic studies of apoC-III metabolism provide new knowledge of the underlying mechanisms responsible for these related disorders in central obesity. Men with central obesity and insulin resistance have elevated plasma apoC-III, a product of elevated VLDL-apoC-III production and reduced VLDL- and HDL-apoC-III catabolism, which is associated with elevated VLDL-apoB concentration and increased HDL-apoA-I catabolism. This, together with new data supporting the role of apoC-III driving VLDL-apoB secretion, highlights the importance of therapies that might regulate apoC-III metabolism to manage dyslipidemia and CVD risk. Weight loss improves dyslipidemia in insulin-resistant, obese subjects (37) as is accompanied by reduction in apoC-III concentrations (Chan, D. C., T. W. K. Ng, P. H. R. Barrett, and G. F. Watts, unpublished observation). It is conceivable that improvement in insulin resistance with weight reduction may account for reduced apoC-III gene expression and plasma concentration, although this remains to be confirmed. Consistent with this hypothesis, Nagashima et al. (38) demonstrated that the peroxisomal proliferator-activated receptor-γ agonist pioglitazone improved insulin sensitivity and reduced apoC-III secretion in dyslipidemic diabetic subjects. Future kinetic studies should also explore the mechanisms by which peroxisomal proliferator-activated receptor-α agonists reduce apoC-III concentrations and improve dyslipidemia.
Footnotes
This work was supported by a project grant from the National Health and Medical Research Foundation (NHMRC) of Australia. D.C.C. is an NHMRC Career Development Fellow. P.H.R.B. is an NHMRC research fellow and is supported in part by National Institutes of Health Grant NIBIB P41 EB-001975.
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 13, 2007
Abbreviations: apo, Apolipoprotein; BMI, body mass index; CVD, cardiovascular disease; FCR, fractional catabolic rate; HDL, high-density lipoproteins; HOMA, homeostasis model assessment; HTG, hypertriglyceridemia; IEF, isoelectric focusing; LDL, low-density lipoprotein; PR, production rate; PVDF, polyvinylidene fluoride; TRL, triglyceride-rich lipoprotein; VLDL, very low-density lipoprotein.
References
- Ginsberg HN 2000 Insulin resistance and cardiovascular disease. J Clin Invest 106:453–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onat A, Hergenc G, Sansoy V, Fobker M, Ceyhan K, Toprak S, Assmann G 2003 Apolipoprotein C-III, a strong discriminant of coronary risk in men and a determinant of the metabolic syndrome in both genders. Atherosclerosis 168:81–89 [DOI] [PubMed] [Google Scholar]
- Sacks FM, Alaupovic P, Moye LA, Cole TG, Sussex B, Stampfer MJ, Pfeffer MA, Braunwald E 2000 VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation 102:1886–1892 [DOI] [PubMed] [Google Scholar]
- Brown VW, Baginsky ML 1972 Inhibition of lipoprotein lipase by an apoprotein of human very low density lipoprotein. Biochem Biophys Res Commun 46:375–382 [DOI] [PubMed] [Google Scholar]
- Sehayek E, Eisenberg S 1991 Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J Biol Chem 266:18259–18267 [PubMed] [Google Scholar]
- Cohn JS, Tremblay M, Batal R, Jacques H, Rodriguez C, Steiner G, Mamer O, Davignon J 2004 Increased apoC-III production is a characteristic feature of patients with hypertriglyceridemia. Atherosclerosis 177:137–145 [DOI] [PubMed] [Google Scholar]
- Chan DC, Watts GF, Nguyen MN, Barrett PHR 2006 Apolipoproteins C-III and A-V as predictors of very-low-density lipoprotein triglyceride and apolipoprotein B-100 kinetics. Arterioscler Thromb Vasc Biol 26:590–596 [DOI] [PubMed] [Google Scholar]
- Cohn JS, Patterson BW, Uffelman KD, Davignon J, Steiner G 2004 Rate of production of plasma and very-low-density lipoprotein (VLDL) apolipoprotein C-III is strongly related to the concentration and level of production of VLDL triglyceride in male subjects with different body weights and levels of insulin sensitivity. J Clin Endocrinol Metab 89:3949–3955 [DOI] [PubMed] [Google Scholar]
- Le NA, Gibson JC, Ginsberg HN 1988 Independent regulation of plasma apolipoprotein C-II and C-III concentrations in very low density and high density lipoproteins: implications for the regulation of the catabolism of these lipoproteins. J Lipid Res 29:669–677 [PubMed] [Google Scholar]
- Glangeaud MC, Eisenberg S, Olivecrona T 1976 Very low density lipoprotein: dissociation of apolipoprotein C during lipoprotein lipase induced lipolysis. Biochim Biophys Acta 486:23–35 [PubMed] [Google Scholar]
- Wu AL, Windmueller HG 1979 Relative contributions by liver and intestine to individual plasma apolipoproteins in the rat. J Biol Chem 254:7316–7322 [PubMed] [Google Scholar]
- Huff MW, Fidge NH, Nestel PJ, Billington T, Watson B 1981 Metabolism of C-apolipoproteins: kinetics of C-II, C-III1 and C-III2, and VLDL-apolipoprotein B in normal and hyperlipoproteinemic subjects. J Lipid Res 22:1235–1246 [PubMed] [Google Scholar]
- Eisenberg S, Bilheimer DW, Levy RI 1972 The metabolism of very low density lipoprotein proteins: II. Studies on the transfer of apoproteins between plasma lipoproteins. Biochim Biophys Acta 280:94–104 [PubMed] [Google Scholar]
- Malmendier CL, Lontie JF, Grutman GA, Delcroix C 1988 Metabolism of apolipoprotein C-III in normolipemic human subjects. Atherosclerosis 69:51–59 [DOI] [PubMed] [Google Scholar]
- Bukberg PR, Le NA, Ginsberg HN, Gibson JC, Rubinstein A, Brown WV 1985 Evidence for non-equilibrating pools of apolipoprotein C-III in plasma lipoproteins. J Lipid Res 26:1047–1057 [PubMed] [Google Scholar]
- Tornoci L, Scheraldi CA, Li X, Ide H, Goldberg IJ, Le NA 1993 Abnormal activation of lipoprotein lipase by non-equilibrating apoC-II: further evidence for the presence of non-equilibrating pools of apolipoproteins C-II and C-III in plasma lipoproteins. J Lipid Res 34:1793–1803 [PubMed] [Google Scholar]
- Le NA, Bukberg PR, Ginsberg HN, Gibson JC, Brown VW 1996 Direct determination of apolipoprotein C-III specific activity using immunoaffinity chromatography. Methods Enzymol 129:457–469 [DOI] [PubMed] [Google Scholar]
- Cohn JS, Batal R, Tremblay M, Jacques H, Veilleux L, Rodriguez C, Mamer O, Davignon J 2003 Plasma turnover of HDL apoC-I, apoC-III, and apoE in humans: in vivo evidence for a link between HDL apoC-III and apoA-I metabolism. J Lipid Res 44:1976–1983 [DOI] [PubMed] [Google Scholar]
- Batal R, Tremblay M, Barrett PHR, Jacques H, Fredenrich A, Mamer O, Davignon J, Cohn JS 2000 Plasma kinetics of apoC-III and apoE in normolipidemic and hypertriglyceridemic subjects. J Lipid Res 41:706–718 [PubMed] [Google Scholar]
- Nguyen MN, Chan DC, Dwyer KP, Bolitho P, Watts GF, Barrett PHR 2006 Use of Intralipid for kinetic analysis of HDL apoC-III: evidence for a homogeneous kinetic pool of apoC-III in plasma. J Lipid Res 47:1274–1280 [DOI] [PubMed] [Google Scholar]
- Chan DC, Watts GF, Redgrave TG, Mori TA, Barrett PHR 2002 Apolipoprotein B-100 kinetics in visceral obesity: associations with plasma apolipoprotein C-III concentration. Metabolism 51:1041–1046 [DOI] [PubMed] [Google Scholar]
- Chan DC, Watts GF, Barrett PHR, Beilin LJ, Redgrave TG, Mori TA 2002 Regulatory Effects of HMG CoA reductase inhibitor and fish oils on apolipoprotein B-100 kinetics in insulin-resistant obese male subjects with dyslipidemia. Diabetes 51:2377–2386 [DOI] [PubMed] [Google Scholar]
- Brites FD, Bonavita CD, Cloes M, Yael MJ, Fruchart JC, Castro GR, Wikinski RW 1998 VLDL compositional changes and plasma levels of triglycerides and high density lipoprotein. Clin Chim Acta 269:107–124 [DOI] [PubMed] [Google Scholar]
- Watts GF, Barrett PHR, Ji J, Serone AP, Chan DC, Croft KD, Loehrer F, Johnson AG 2003 Differential regulation of lipoprotein kinetics by atorvastatin and fenofibrate in subjects with the metabolic syndrome. Diabetes 52:803–811 [DOI] [PubMed] [Google Scholar]
- Dwyer KP, Barrett PHR, Chan DC, Foo JI, Watts GF, Croft KD 2002 Oxazolinone derivative of leucine for GC-MS: a sensitive and robust method for stable isotope kinetic studies of lipoproteins. J Lipid Res 43:344–349 [PubMed] [Google Scholar]
- Fidge NH, Nestel PJ 1986 Metabolism of apolipoprotein C. Methods Enzymol 129:443–457 [DOI] [PubMed] [Google Scholar]
- Mauger JF, Couture P, Bergeron N, Lamarche B 2006 Apolipoprotein C-III isoforms: kinetics and relative implication in lipid metabolism. J Lipid Res 47:1212–1218 [DOI] [PubMed] [Google Scholar]
- Riches FM, Watts GF, Hua J, Stewart GR, Naoumova RP, Barrett PHR 1999 Reduction in visceral adipose tissue is associated with improvement in apolipoprotein B-100 metabolism in obese men. J Clin Endocrinol Metab 84:2854–2861 [DOI] [PubMed] [Google Scholar]
- Malmendier CL, Lontie JF, Delcroix C, Dubois DY, Magot T, De Roy L 1989 Apolipoproteins C-II and C-III metabolism in hypertriglyceridemic patients: effect of a drastic triglyceride reduction by combined diet restriction and fenofibrate administration. Atherosclerosis 77:139–149 [DOI] [PubMed] [Google Scholar]
- Sundaram M, Links P, Khalil MB, Zhong S, Yao Z 2007 New insights into the roles of apolipoprotein C-III in stimulating the production of hepatic VLDL. Arterioscler Thromb Vasc Biol 27:e62 [Google Scholar]
- Lamarche B, Uffelman KD, Carpentier A, Cohn JS, Steiner G, Barrett PH, Lewis GF 1999 Triglyceride enrichment of HDL enhances in vivo metabolic clearance of HDL apo A-I in healthy men. J Clin Invest 103:1191–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid S, Barrett PHR, Uffelman KD, Watanabe T, Adeli K, Lewis GF 2002 Lipolytically modified triglyceride-enriched HDLs are rapidly cleared from the circulation. Arterioscler Thromb Vasc Biol 22:483–487 [DOI] [PubMed] [Google Scholar]
- Watts GF, Riches FM, Humphries SE, Talmud PJ, van Bockxmeer FM 2000 Genotypic associations of the hepatic secretion of VLDL apolipoprotein B-100 in obesity. J Lipid Res 41:481–488 [PubMed] [Google Scholar]
- Chan DC, Watts GF, Barrett PHR, Whitfield AJ, van Bockxmeer FM 2004 ATP-binding cassette transporter G8 gene as a determinant of apolipoprotein B-100 kinetics in overweight men. Arterioscler Thromb Vasc Biol 24:2188–2191 [DOI] [PubMed] [Google Scholar]
- Watts GF, Moroz P, Barrett PH 2000 Kinetics of very-low-density lipoprotein apolipoprotein B-100 in normolipidemic subjects: pooled analysis of stable-isotope studies. Metabolism 49:1204–1210 [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Haas MJ, Wong NCW 2006 The effect of select nutrients on serum high-density lipoprotein cholesterol and apolipoprotein A-I levels. Endocr Rev 27:2–16 [DOI] [PubMed] [Google Scholar]
- Ng TW, Watts GF, Barrett PHR, Rye KA, Chan DC 2007 Effect of weight loss on LDL and HDL kinetics in the metabolic syndrome: associations with changes in plasma retinol-binding protein-4 and adiponectin levels. Diabetes Care 30:2945–2950 [DOI] [PubMed] [Google Scholar]
- Nagashima K, Lopez C, Donovan D, Ngai C, Fontanez N, Bendadoun A, Fruchart-Najib J, Holleran S, Cohn JS, Ramakrishnan R, Ginsberg HN 2006 Effects of the PPARγ agonist pioglitazone on lipoprotein metabolism in patients with type 2 diabetes mellitus. J Clin Invest 115:1323–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]