Abstract
Context: Dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) are the major circulating adrenal steroids and substrates for peripheral sex hormone biosynthesis. In Addison’s disease, glucocorticoid and mineralocorticoid deficiencies require lifelong replacement, but the associated near-total failure of DHEA synthesis is not typically corrected.
Objective and Design: In a double-blind trial, we randomized 106 subjects (44 males, 62 females) with Addison’s disease to receive either 50 mg daily of micronized DHEA or placebo orally for 12 months to evaluate its longer-term effects on bone mineral density, body composition, and cognitive function together with well-being and fatigue.
Results: Circulating DHEAS and androstenedione rose significantly in both sexes, with testosterone increasing to low normal levels only in females. DHEA reversed ongoing loss of bone mineral density at the femoral neck (P < 0.05) but not at other sites; DHEA enhanced total body (P = 0.02) and truncal (P = 0.017) lean mass significantly with no change in fat mass. At baseline, subscales of psychological well-being in questionnaires (Short Form-36, General Health Questionnaire-30), were significantly worse in Addison’s patients vs. control populations (P < 0.001), and one subscale of SF-36 improved significantly (P = 0.004) after DHEA treatment. There was no significant benefit of DHEA treatment on fatigue or cognitive or sexual function. Supraphysiological DHEAS levels were achieved in some older females who experienced mild androgenic side effects.
Conclusion: Although further long-term studies of DHEA therapy, with dosage adjustment, are desirable, our results support some beneficial effects of prolonged DHEA treatment in Addison’s disease.
A 12-month study of dehydroepiandrosterone replacement for primary adrenal insufficiency finds improved femoral neck bone mineral density and lean body mass but no significant benefit with fatigue and cognitive or sexual function.
Synthesis of dehydroepiandrosterone (DHEA) and its sulfate ester (DHEAS) is by the zona reticularis of the adrenal gland. Levels decline dramatically after birth, gradually increasing again during adrenarche to reach peak levels in young adulthood. Unlike the other major steroids secreted by the adrenal, serum DHEA(S) levels then follow a progressive age-related decline (1,2). A large body of epidemiological data from cross-sectional correlational studies has documented an association between the decline in DHEAS levels and various age-related disorders, including increased risk of cardiovascular events (3), malignancy (4), and osteoporosis (5). In concert with these findings, positive effects of DHEA replacement have been described on psychological well-being (6), body composition (7,8), and bone mineral density (BMD) (9) in older, normal adults.
It is not clear whether DHEA(S) has its effect via direct action on target tissues and/or as a precursor for the biosynthesis of other steroids, including gonadal steroids such as testosterone. In the brain, DHEA(S) may act directly on neural tissue and thus qualify as a neurosteroid (10,11). In the hippocampus, it has been found to be neuroprotective to injury (12,13,14) and to enhance memory and neurogenesis in the adult brain (15). It can also act as an antiglucocorticoid, antagonizing both glucocorticoid-induced thymic involution (16,17) and the suppressive actions of corticosterone on the proliferation of progenitor cells in the dentate gyrus of the hippocampus (15). Although its cellular mode of action is unknown and a specific cell surface or nuclear receptor for DHEA has not been identified hitherto, microarray studies show that it induces a pattern of gene expression distinct from glucocorticoid or testosterone, further supporting a distinct mechanism of action (18).
Deficiencies of glucocorticoid and mineralocorticoid in primary adrenal insufficiency (Addison’s disease) are well recognized and require lifelong replacement. However, the associated deficiency of DHEA(S) has been investigated only recently, and its possible clinical significance remains controversial. Patients with Addison’s disease on optimal glucocorticoid and mineralocorticoid replacement therapy still report a reduced quality of life when compared with normal individuals (19) and score significantly worse than age- and sex-matched population controls on validated psychological tests that measure well-being (20,21).
Several short-term studies of DHEA supplementation in adrenal insufficiency have now been reported: Young et al. (22) validated the efficacy of oral DHEA treatment in restoring physiological circulating levels of DHEA(S) in 10 adults with panhypopituitarism and showed some biotransformation of DHEA into sex steroids. Arlt et al. (23) studied 24 women, 14 of whom had primary adrenal insufficiency, in a randomized, placebo-controlled, double-blind crossover trial for 4 months. In addition to the expected changes in levels of DHEA(S) and its metabolites, the authors reported enhanced well-being and sexuality. Our previous placebo-controlled 3-month crossover trial of 39 patients (including 15 males) with primary adrenal insufficiency showed similar biochemical changes and enhanced psychological well-being, independent of gender (20). Johannsson et al. (24) demonstrated behavioral changes, reported by their partners, in 38 panhypopituitary females after 6 months of DHEA(S) replacement. No changes in body composition, BMD or cognition were demonstrated in any of these short-term studies of DHEA replacement. A 9-month, parallel group trial of DHEA replacement in 39 patients showing no benefit in health status (25) may have been underpowered (26).
We therefore undertook a 12-month trial of DHEA replacement therapy, with primary end points being to determine whether there were positive effects on bone mineral density (BMD), body composition, or effects on cognitive function, which might be related to the neuroprotective action of DHEA. We also wanted to confirm that the changes in biochemistry, well-being, and fatigue observed in our previous short-term trial (20) could be replicated and maintained with more protracted administration of DHEA, and these parameters were designated as secondary end points. A final objective of this longer-term trial was to assess the possible emergence of side effects that might not have been apparent in the shorter-term.
Subjects and Methods
Trial participants
Subjects were recruited from the Endocrine clinics in Cambridge, Oxford, St. Bartholomew’s Hospital, London, UK, and Christchurch, New Zealand, together with individuals from the U.K. Addison’s Disease Patient Self-Help Group. The diagnosis of Addison’s disease was substantiated by documented hypocortisolemia associated with either raised serum ACTH (>100 ng/liter) or hyperpigmentation and, where available, positive adrenal antibodies. Duration of Addison’s disease for at least 1 yr was an inclusion criterion. Exclusion criteria were age younger than 18 yr or older than 65 yr, pregnancy, past personal history of hormone-dependent malignancy, and any intercurrent significant medical or psychiatric condition (e.g. epilepsy, depression) requiring neuroactive medication. All patients took their usual glucocorticoid and mineralocorticoid hormone replacement with dosage and timing of administration being unchanged for 3 months before and throughout the duration of the trial. Patients were also instructed not to alter their diet or exercise habits. The project had local ethical committee approval, and prior informed consent was obtained from all participants.
Study design
One hundred patients were required to power the study to detect changes in BMD, and we aimed to recruit at least this number of subjects who fulfilled the entry criteria, within a 3-month time interval. The trial was a double-blind, placebo-controlled, parallel group design. One hundred subjects (49 DHEA group, 51 placebo group) completed the study, and their results were subsequently analyzed. Twenty-three subjects had taken part in our previous, short-term trial of DHEA (20) but were distributed comparably across DHEA (n = 9) and placebo (n = 14) groups. Furthermore, after completion of this first study, all subjects had been off DHEA for 2 yr. Demographic and clinical characteristics of trial participants are shown in Table 1. Each patient was randomly assigned to a 12-month treatment period of either oral micronized DHEA (50 mg daily) or a lactose-containing placebo tablet of identical appearance (McPherson Labs, Inc., Stafford, TX). The randomization was stratified by age (18–34, 35–49, 50–65 yr) and gender and undertaken by an independent statistician. Treatment allocation details were coded and kept confidential until the trial was completed. Two female patients who were concerned about acne reduced their dose to half a study tablet after 6 months and continued at this dose until the end of the trial. A washout interval of 1 month followed each treatment arm.
Table 1.
Demographics and clinical details of patients recruited into trial
| DHEA | Placebo | |
|---|---|---|
| n | 54 | 52 |
| Males | 24 | 20 |
| Females | 30 | 32 |
| Median age (range), yr | 46 (23–65) | 46 (22–65) |
| Median disease duration (range), yr | 11 (1–46) | 10 (1–49) |
| Hydrocortisone dose, mg per 24 h | 25 | 27 |
| Autoimmune thyroid disease | 20 | 14 |
| Type 1 diabetes mellitus | 4 | 1 |
| Pernicious anaemia | 3 | 1 |
| Vitiligo | 3 | 1 |
| Premature ovarian failure (HRT) | 9 (8) | 3 (2) |
| Post menopausal (HRT) | 9 (4) | 11 (6) |
HRT, Hormone replacement therapy.
Measurements
Major assessments were undertaken at baseline and after 12 months of treatment (DHEA or placebo) at one of the two trial centers (Cambridge, UK, and Christchurch, New Zealand). On each occasion, fasting blood samples were followed by a structured interview, with assessment of cognitive and psychological function. Body composition and BMD were then measured by dual-energy x-ray absorptiometry (DEXA).
Structured interview and cognitive tests
Subjects were asked questions about their general health, mental function, recent life events, sleep, and possible adverse effects of treatment (second visit only). These were followed by a series of cognitive tests, which focused on memory and executive function. Memory is known to be dependent on hippocampal function, which may be compromised in Addison’s disease (27), and there appears to be a particularly strong link between spatial memory and hippocampal function (28,29,30). Accordingly, we included tests of both verbal and spatial memory. The verbal memory tests were recall of a short story from the Wechsler Memory Scale (31) and recall of a 16-item word list from the California Verbal Learning Test (32). Spatial memory was assessed by a recently developed measure of spatial location memory (33), which involved recalling the location of 10 items randomly located on a grid containing 30 squares. Executive function was assessed in three ways: a verbal fluency task, which required the subject to name as many animals as possible in 1 min; a letter cancellation task, which required them to cross out two specified letters on a sheet containing random letters of the alphabet as quickly and accurately as possible in 1 min; and the Stroop Color-Word Test, which comprises a list of color names (the words red, green, blue) printed in ink of a conflicting color. The same tests were used at both interviews, because parallel versions were not available for all tests. Analysis assumes that practice effects would be equal in both study groups. The National Adult Reading Test was used as a measure of verbal ability at baseline only (34).
Psychological symptoms
Aspects of psychological status were assessed using validated self-completion questionnaires at baseline, at 6 and 12 months, and after washout (at 13 months) in each treatment arm. The Short Form-36 (SF-36) questionnaire examined symptoms relating to physical and mental health and is a tool that has been validated in population studies (35,36). The Multidimensional Fatigue Inventory (MFI-20) (37) was also used to quantify physical and mental fatigue, activity, and motivation. It was originally developed to assess symptoms in cancer patients undergoing radiotherapy and is suited to this study because it concentrates on fatigue, a common complaint in Addison’s disease (19). General well-being was assessed by self-completion of the General Health Questionnaire (GHQ-30) by Goldberg (38), which includes five subscales of mental health: anxiety, self-esteem, depression, difficulty coping, and social dysfunction (39), and it was scored using a Likert scale. Sexual function was assessed using a self-completion questionnaire and visual analog scale, which had been used in a previous trial of DHEA in aging (40).
Morphological measurements
Body composition together with lumbar, femoral, and radial BMD were measured at baseline and at 12 months by DEXA using QDR 4500 scanners (Hologic, Bedford, MA) at both trial centers, with individual patients being assessed on the same machine throughout. The precision of BMD measurements was less than 1 or 1% in lumbar spine and hip, respectively.
Biochemical parameters
Serum DHEAS, testosterone, androstenedione, SHBG, lipids, free T4, TSH, IGF-I, testosterone, and estradiol were measured at baseline and 12 months, using previously described specific immunoassays (20) in a single laboratory with all samples from an individual patient being analyzed in the same assay. Estradiol was measured only in males because the hormonal status of females was variable, some being postmenopausal and/or on exogenous estrogen replacement therapy. The intra- and interassay coefficients of variation were less than 10% throughout. Liver function was assessed by measurement of aspartate aminotransferase, γ-glutamyl transferase, and alkaline phosphatase.
Statistical analysis
We aimed to recruit at least 100 patients to have 95% power to detect a 2.5% change in hip BMD and 80% power to detect a 1% change in spine BMD. BMD data were analyzed by comparing changes from baseline to 12 months between using an independent samples t test. For the other primary end points (body composition and cognitive function), values at 12 months in placebo vs. DHEA-treated groups were compared, adjusting for values at baseline. The rationale for this approach was that it enabled conclusions to be drawn about differences in 12-month values using baseline data to explain a large proportion of the between-subject variance, hence improving power. The effect of gender and the possibility of differential effects within genders were investigated using ANOVA.
For secondary end points (biochemical parameters, well-being, fatigue), changes in parameters at intermediate time points after DHEA or placebo treatment were also analyzed, adjusting for values at baseline. For data that were not normally distributed (e.g. cognitive function data) the Mann Whitney U test was substituted. Categorical variables were analyzed using χ2 and Fisher’s exact test. Five and 1% levels of significance were used for primary and secondary end points, respectively.
Results
One hundred six patients were recruited initially. Of the 52 randomized to DHEA, three patients failed to complete the study: one young man did not want to risk being on placebo, one man was lost to follow-up, and one woman started antidepressants and was removed from the trial. Fifty-four patients were randomized to placebo and three individuals in this arm failed to complete the study: one young man was lost to follow-up, one man felt unwell on what he perceived was DHEA, and an older woman was dissuaded by her family practitioner. The data analysis are thus based on 49 subjects receiving DHEA and 51 receiving placebo who completed the study (Table 1).
Hormonal and biochemical changes
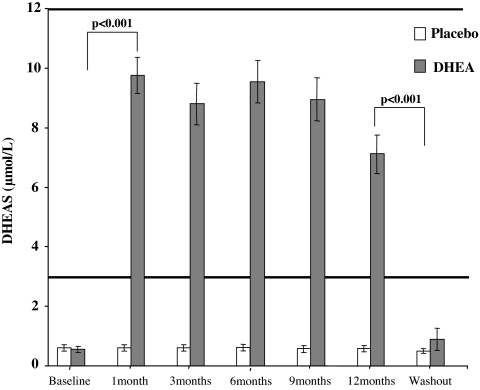
In those receiving 50 mg oral micronized DHEA, serum DHEAS rose markedly within 1 month from grossly subnormal to levels within the physiological range for young adults in both male and female subjects. These levels were maintained throughout the 12-month period, signifying compliance with treatment. One month after discontinuing treatment, DHEAS levels fell back to baseline low levels, confirming satisfactory washout of the active study treatment (Fig. 1).
Figure 1.
Mean (± sem) serum DHEAS levels in DHEA (shaded bars) or placebo-treated (open bars) groups throughout the 12-month trial and after treatment washout. The bold horizontal lines denote the normal laboratory reference range for DHEAS (not adjusted for age or gender).
Table 2 illustrates changes in serum androstenedione, testosterone, SHBG, and lipid profiles in males and females and estradiol in males. As expected, there was a similar rise in androstenedione during DHEA treatment in both sexes. The associated changes in circulating androgens and SHBG were also analyzed according to the patient’s gender. In females taking DHEA, serum total testosterone increased from subnormal to low normal levels (after placebo 0.13 vs. after DHEA 0.41 nmol/liter, P < 0.001), but there was no associated change in circulating SHBG. In males there was no significant change in SHBG, total testosterone, or estradiol during DHEA treatment. Circulating fasting lipid profiles were unchanged as was thyroid function (data not shown), and changes in serum IGF-I in females reached borderline significance (P = 0.01) (Table 2).
Table 2.
Changes in biochemical parameters between baseline and 12 months in placebo and DHEA-treated groups
| Parameter | DHEA
|
Placebo
|
P valuea | ||
|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | ||
| Androstenedione (nmol/liter) | 1.56 (0.2) | 4.2 (0.3) | 1.68 (0.2) | 1.6 (0.3) | <0.001 |
| Testosterone (nmol/liter) | |||||
| Male | 17.8 (1.3) | 16.5 (1.2) | 16.2 (1.1) | 14.2 (0.9) | 0.39 |
| Female | 0.21 (0.03) | 0.41 (0.05) | 0.14 (0.02) | 0.13 (0.02) | <0.001 |
| Estradiol (nmol/liter) | |||||
| Male | 118.9 (6.8) | 109.6 (6.3) | 105.5 (4.3) | 102.9 (4.3) | 0.95 |
| SHBG(U/liter) | |||||
| Male | 35.7 (3.0) | 38.8 (3.2) | 36.0 (3.6) | 38.4 (3.8) | 0.90 |
| Female | 67.9 (7.9) | 63.7 (7.2) | 78.9 (7.7) | 81.7 (7.4) | 0.33 |
| Lipids (nmol/liter) | |||||
| Cholesterol | 4.45 (0.22) | 4.62 (0.16) | 4.63 (0.19) | 4.57 (0.18) | 0.36 |
| Triglycerides | 1.23 (0.14) | 1.30 (0.12) | 1.43 (0.14) | 1.56 (0.15) | 0.38 |
| HDL | 1.27 (0.08) | 1.43 (0.06) | 1.26 (0.07) | 1.36 (0.06) | 0.27 |
| LDL | 2.57 (0.15) | 2.65 (0.12) | 2.78 (0.15) | 2.55 (0.14) | 0.15 |
| IGF-I (nmol/liter) | |||||
| Male | 26.8 (1.8) | 25.2 (1.9) | 25.0 (2.0) | 23.7 (1.7) | 0.64 |
| Female | 23.7 (1.6) | 25.0 (1.9) | 22.5 (1.5) | 20.9 (1.3) | 0.01 |
Data shown are mean (sem).
P value relates to the comparison of 12-month values, adjusted for baseline, between DHEA and placebo groups.
Body composition and BMD
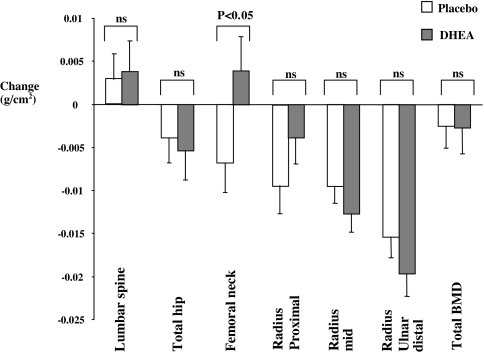
At baseline, mean BMD was generally low in all Addison’s disease patients. Using World Health Organization T-score criteria, in the lumbar spine, 39% of males and females were osteopenic and 7% of males and 5% of females osteoporotic; in the femoral neck, 39% of males and females were osteopenic and 2% of females were osteoporotic. During the 12 months of the study, there was progressive diminution in BMD at most sites in patients. In those receiving DHEA, there was a marked and significant reversal of this trend in the femoral neck [mean (sem) BMD (grams per square centimeter) changes: after DHEA, 0.0039 (0.004) vs. after placebo, −0.0068(0.003) g/cm2, P < 0.046] but not at any other site (Fig. 2). Analyses by gender showed a differential effect only at the proximal radius, with significant enhancement of BMD after DHEA in men but not women [mean (sem) BMD (grams per square centimeter) changes: males: after DHEA, 0.0046 (0.0046) and after placebo, −0.0136 (0.006) P = 0.025; females: after DHEA, −0.0091 (0.0036) and after placebo, −0.0062 (0.0037), P = 0.583].
Figure 2.
Mean (± sem) change in BMD at 12 months in DHEA (shaded bars) or placebo-treated (open bars) groups. The P value (<0.05) relates to comparison of mean absolute changes in BMD between DHEA and placebo-treated groups. ns, Not significant.
Overall there was no effect of DHEA treatment on body mass index (data not shown). However, when body composition in individual compartments was analyzed, DHEA increased lean mass, both total and truncal, with no effect on fat mass (Table 3). Analysis by gender showed that this effect was more marked in females, who showed greater changes in total body lean mass than their male counterparts [mean (sem) lean mass (grams) changes: females, after DHEA, 869 (211) and after placebo, 183 (269), P = 0.034; males, after DHEA, −117(247) and after placebo, −399(258)].
Table 3.
Changes in body composition in DHEA and placebo-treated subjects
| Parameter | DHEA
|
Placebo
|
P valuea | ||
|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | ||
| Total body fat mass (g) | 24,952 (1,484) | 25,050 (1,522) | 22,947 (1,218) | 22,966 (1,250) | 0.79 |
| Truncal fat mass (g) | 11,896 (754) | 11,963 (768) | 10,639 (673) | 10,728 (686) | 0.89 |
| Total body lean mass (g) | 46,021 (1,458) | 46,839 (1,435) | 45,784 (1,459) | 45,850 (1,482) | 0.022 |
| Truncal lean mass (g) | 23,165 (623) | 23,419 (630) | 23,208 (679) | 22,992 (686) | 0.017 |
Data shown are mean (sem).
P value relates to the comparison of 12-month values, adjusted for baseline, between DHEA and placebo groups.
Cognitive function
The National Adult Reading Test, as a measure of verbal IQ, showed no difference at baseline between the two randomized groups. There were no significant differences between the DHEA and placebo groups for any parameter of cognitive function with no differences at its conclusion between those who received DHEA or placebo (Table 4). Data analysis was repeated on subjects (27 placebo, 30 DHEA treated) aged 45 yr or older to determine whether there might have been selective effects of DHEA in this subgroup, but again DHEA had no significant effect on cognitive measures (data not shown).
Table 4.
Changes in cognitive function in DHEA and placebo-treated groups
| Tests | Maximum possible score | Baseline
|
12 months
|
P valuea | ||
|---|---|---|---|---|---|---|
| DHEA | Placebo | DHEA | Placebo | |||
| Memory for story | ||||||
| Immediate recall | 25 | 14 (11, 17) | 13 (9, 15) | 16 (12, 18) | 14 (13, 16) | 0.15 |
| Delayed recall | 25 | 11 (10, 15) | 11 (8, 14) | 13 (11, 17) | 14 (11, 16) | 0.86 |
| Memory for spatial location | ||||||
| Immediate | 10 | 6 (5, 9) | 7 (5, 9) | 7 (5, 9) | 6 (4, 9) | 0.41 |
| Delayed | 10 | 6 (4, 8) | 6 (3, 8) | 6 (4, 9) | 5 (3, 8) | 0.50 |
| California Verbal Learning Test | ||||||
| Trial 1 | 16 | 8 (7, 9) | 8 (7, 10) | 8 (7, 10) | 8 (7, 10) | 0.93 |
| Trial 2 | 16 | 12 (10, 13) | 11 (9, 14) | 11 (10, 13) | 12 (9, 13) | 0.86 |
| Trial 3 | 16 | 13 (10, 14) | 12 (11, 14) | 13 (11, 14) | 12 (11, 14) | 0.80 |
| Short delay | 16 | 11 (9, 12) | 10 (8, 13) | 11 (8, 13) | 10 (8, 14) | 0.73 |
| Long-delay recall | 16 | 10 (8, 12) | 11 (7, 14) | 11 (9, 14) | 11 (9, 14) | 0.94 |
| Prospective memory | 6 | 3 (2, 5) | 5 (2, 5) | 5 (2, 5) | 5 (2, 5) | 0.65 |
| Verbal fluency | 21 (17, 26) | 20 (18, 23) | 21 (19, 26) | 19 (17, 23) | 0.064 | |
| Visual search | ||||||
| Accuracy | 71 | 21 (18, 25) | 22 (17, 25) | 21 (19, 24) | 21 (19, 24) | 0.72 |
| Speed | ||||||
| Stroop effect | ||||||
| Reading time (sec) | 55 (46, 65) | 58 (52, 66) | 55 (48, 66) | 59 (50, 68) | 0.40 | |
| Color naming time (sec) | 118 (104, 130) | 119 (105, 150) | 118 (100, 139) | 119 (104, 138) | 0.65 | |
Data shown are median (interquartile range) values.
P values are for comparison of DHEA vs. placebo at 12 months, and no statistically significant changes were observed.
Well-being, fatigue, and sexual function
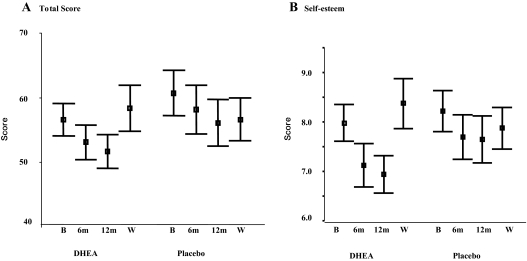
In comparison with normative data from a U.K. population (39), subjects with Addison’s disease showed a higher total GHQ-30 score, denoting worse mental health status at baseline, particularly on the anxiety and self-esteem subscales (Table 5). During DHEA treatment, either total GHQ-30 scores (Fig 3A) or for the self-esteem subscale (Fig 3B) declined (signifying improvement), with a rise in scores after washout, but comparison with the placebo group showed that these changes were not statistically significant (DHEA vs. placebo scores: total GHQ 6 months, P = 0.55, 12 months, P = 0.32, after washout, P = 0.23; self esteem 6 months, P = 0.17, 12 months, P = 0.037; after washout, P = 0.75). As with GHQ status, using the SF-36 questionnaire as a tool to assess a broad range of physical symptomatology as well as psychological morbidity, the Addison’s disease subjects had lower scores at baseline, denoting worse health status than a normal U.K. population (36) (Table 6). Interestingly, they exhibited an identical profile of deficits in particular dimensions of health (role physical, general health, vitality, role emotional) as a Norwegian population of Addison’s disease patients studied independently by another group (21) (Table 6). During the trial, spider plots of the SF-36 scores at baseline, 6 and 12 months and after washout showed remarkable constancy in the placebo group, but some change during DHEA treatment (Fig. 4). In particular, the score for the role emotional dimension of health improved significantly at 12 months (P = 0.004 for DHEA vs. placebo groups). Interestingly, after treatment washout, scores for both role physical and role emotional dimensions fell more markedly (denoting deterioration of health status) in the DHEA group, but the changes did not achieve statistical significance.
Table 5.
Baseline GHQ-30 questionnaire scores in Addison’s patients and comparison with normative data
| GHQ-30 | Total GHQ | Anxiety | Self-esteem | Depression | Coping | Social dysfunction |
|---|---|---|---|---|---|---|
| U.K. normative dataa | 55.1 | 14.4 | 7.6 | 7.3 | 9.8 | 6.0 |
| Mean (sem) | (0.4) | (0.2) | (0.05) | (0.09) | (0.07) | (0.04) |
| U.K. Addison’s patients | 58.7 | 16.3 | 8.1 | 7.2 | 10.3 | 6.0 |
| Mean (sem) | (1.09) | (0.5) | (0.14) | (0.21) | (0.25) | (0.11) |
| P value | <0.001 | <0.001 | 0.002 | 0.69 | 0.02 | 0.60 |
Mean scores are shown with higher values denoting worse health status.
GHQ-30 normative data from U.K. control population (36).
Figure 3.
Comparison of changes in mental health status, as assessed by GHQ-30 scores (mean and 95% confidence interval) at baseline (B), 6 months (6m), 12 months (12m), or after washout (W). Total score (A) and self-esteem subscale score (B) after placebo or DHEA treatment are shown. Higher scores denote worse health status.
Table 6.
Baseline SF-36 questionnaire scores in Addison’s patients and comparison with normative data
| SF-36 | Physical functioning | Role physical | Bodily pain | General health | Vitality | Social function | Role emotional | Mental health |
|---|---|---|---|---|---|---|---|---|
| Norwegian normative dataa | 87.2 | 77.9 | 75.1 | 76.8 | 60 | 85.6 | 81.7 | 78.8 |
| Mean (sem) | (0.39) | (0.76) | (0.54) | (0.46) | (0.43) | (0.46) | (0.69) | (0.34) |
| Norwegian Addison’s patientsa | 84.4 | 60.8 | 76.2 | 56.6 | 51.7 | 79.7 | 70.3 | 78.1 |
| Mean (sem) | (2.09) | (4.86) | (2.84) | (2.93) | (2.93) | (2.79) | (4.19) | (1.58) |
| P valuea | 0.13 | <0.001 | 0.70 | <0.001 | 0.001 | 0.024 | 0.003 | 0.70 |
| U.K. normative datab | 88.0 | 87.2 | 78.8 | 71.1 | 58.0 | 82.8 | 85.8 | 71.9 |
| Mean (sem) | (0.21) | (0.24) | (0.25) | (0.22) | (0.21) | (0.25) | (0.23) | (0.19) |
| U.K. Addison’s patients | 83.8 | 46.2 | 75.7 | 58.8 | 47.4 | 78.3 | 56.5 | 72.7 |
| Mean (sem) | (1.73) | (3.91) | (2.45) | (2.63) | (2.29) | (2.17) | (4.31) | (1.74) |
| P value | 0.03 | <0.001 | 0.17 | <0.001 | <0.001 | 0.049 | <0.001 | 0.65 |
Figure 4.
Spider plots showing the eight dimensions of health assessed by the SF-36 questionnaire at baseline, 6 months, 12 months, and after washout in DHEA- (left panel) or placebo-treated (right panel) groups. Lower scores denote worse health status. The only significant difference between groups was improvement of the role emotional dimension (RE; P = 0.004) at 12 months after DHEA treatment.
The MFI-20 covers dimensions that include physical fatigue, mental fatigue, reduced activity, and reduced motivation. Compared with the placebo group, there was a reduction (improvement) in scores for all dimensions in the DHEA group, but the changes only bordered on statistical significance for mental fatigue at the 6-month time point (P = 0.03). After washout, fatigue scores in the DHEA-treated group rose back to levels comparable with placebo-treated patients (data not shown).
Using a questionnaire that had previously shown effects of DHEA in older normal subjects (40), we observed no significant changes in libido or sexual function in either gender (data not shown).
Adverse events
An equivalent number of individuals in each arm guessed their treatment correctly, discounting the possibility that side effects had unblinded the study subjects. Female subjects receiving DHEA reported increased occurrence of skin spots, greasy skin, and axillary hair growth (Table 7). We measured sebum production using white adherent tapes (Sebutape; CuDerm Corp., Dallas, TX) applied to forehead skin at each assessment as described previously (41), but there was no difference in Sebutape scores between the placebo and DHEA groups (data not shown), despite the reported subjective symptoms.
Table 7.
Percentage of patient-reported side effects at 12 months after DHEA or placebo treatment
| Skin changes
|
Hair increase
|
||||||
|---|---|---|---|---|---|---|---|
| None | Greasy | Spots | None | Pubic | Axillary | Facial | |
| Males (40) | |||||||
| DHEA (18) | 66 | 11 | 17 | 0 | 0 | 0 | 7 |
| Placebo (22) | 68 | 9 | 0 | 0 | 0 | 0 | 5 |
| Females (60) | |||||||
| DHEA (31) | 69 | 45a | 64a | 28 | 13 | 58a | 19 |
| Placebo (29) | 69 | 0 | 14 | 76 | 10 | 7 | 14 |
Patient numbers are in parentheses.
P < 0.02 where indicated for changes between placebo and DHEA-treated groups with other differences not being significant.
Discussion
This 12-month study of DHEA replacement in patients with Addison’s disease both supports the improved well-being documented in our previous short-term study and adds new information on longer-term effects of DHEA. We report novel effects of DHEA on body composition (lean mass), femoral neck BMD, and particular psychological parameters (fatigue and self-esteem). Measures of cognitive function were not altered by DHEA therapy.
As expected, untreated Addison’s patients had grossly subnormal DHEAS levels. Oral replacement with 50 mg micronized DHEA daily restored DHEAS blood levels to within the normal range for young adults. Because circulating DHEA levels decline with age (1,2) and all subjects received the same dose of DHEA, there was a tendency for DHEAS levels to be above age-matched controls in those aged 50 yr or older. The hormone profile of DHEA-treated subjects was similar to that observed in our previous study (20) with significant increases in androstenedione in both sexes and testosterone in women but not in men. These changes are in accordance with DHEA being a sex steroid precursor. In men, higher basal testosterone levels are likely to have obscured the relatively small additional effect of administered DHEA. Although DHEA is converted to estradiol, the magnitude of this effect is insufficient to cornify the vaginal epithelium in rats (a sensitive assay) (42), and by analogy, we suggest that our observed lack of change in circulating estradiol levels in men receiving DHEA is not unexpected.
The low baseline BMD in Addison’s subjects progressed, with diminution in bone density at most sites in placebo-treated subjects during the subsequent 12-month period. In this context, reversal of this trend with an observed increase in femoral neck BMD after DHEA therapy is notable. We suggest that such selective improvement in femoral neck BMD may be significant for two reasons: first, there is a correlation between reduced BMD at this site and low circulating DHEAS in several other contexts including hypopituitarism (43), aging and the postmenopausal state (5,44,45), and steroid-induced osteoporosis (46); second, other studies of DHEA therapy in postmenopausal women (9) or aging (40,47) have also shown selective improvement in BMD at this site. Although the improvement in BMD is modest, compared with conventional therapies, this change may become significant if compounded, as would be the case with long-term DHEA treatment of patients.
DHEA therapy increased both truncal and total body lean mass measured by DEXA. The improvement in lean muscle mass mirrors that seen in previous studies with DHEA supplementation in aging or postmenopausal women (7,8). The mechanism by which increased lean muscle mass occurs is not known, but it is noteworthy that there was no associated diminution in fat mass as has been reported by other groups after DHEA supplementation (48,49). Despite earlier reports suggesting changes in IGF-I with DHEA replacement, both our earlier short-term trial (20) and the present study showed no statistically significant change in circulating IGF-I levels, in agreement with another recent replacement study (50), arguing against a primary role of this hormone in mediating increases in lean muscle mass.
The effects of DHEA on psychological function were assessed both by comparing hormone and placebo-treated groups during 12 months of DHEA treatment and, in addition, determining whether any changes were reversed after washout in the DHEA-treated subjects. Because both the GHQ-30 and SF-36 tests have been validated and used on large populations of normal individuals, we were able to compare baseline scores in our Addison’s disease patients before hormone/placebo treatment with normative data. We recognize that these control subjects were not contemporaneous, making such comparison tentative. There were striking reductions in baseline scores for some subscales of GHQ-30 and dimensions of SF-36, compared with normal subjects drawn from a reference population. Interestingly, similar abnormalities in the self-esteem subscale of GHQ-30 occurred in both of our studies [this one and an earlier shorter-term replacement study (20)], and an identical pattern of abnormalities in baseline SF-36 scores were observed in another Addison’s population from Norway (21), suggesting there may be a disorder-specific profile of psychological deficit in Addison’s disease. During DHEA treatment, scores for the subscales of GHQ-30 and SF-36 improved and worsened more markedly (albeit nonsignificantly) after washout of DHEA. Furthermore, we observed a similar trend with physical and mental fatigue dimensions of the MFI-20 inventory (a prominent complaint in Addison’s patients), with statistically nonsignificant improvement at 6 and 12 months during DHEA treatment, followed by deterioration of scores after washout. This pattern of initial early improvement in well-being and fatigue followed by a rebound in scores after DHEA washout may be noteworthy. In a earlier 6-month study of DHEA treatment in hypopituitarism, beneficial effects on well-being were much more evident to partners of patients than study subjects (24). We speculate that the beneficial psychological effects of DHEA are most perceptible soon after starting treatment or after drug withdrawal, with diminished self-awareness of changes during longer-term continuation of therapy.
In contrast to the effects on well-being and fatigue, there were no significant changes in any of the wide range of cognitive functions assessed during DHEA therapy. This agrees with previous studies of DHEA supplementation in subjects with normal adrenal function (51,52). Furthermore, a large cohort study on aging men failed to show any correlation between cognitive function and decreasing DHEA(S) levels (53). Several explanations are possible: although the study lasted 12 months, this may still be too brief for beneficial effects on cognitive function to become apparent; the possibility that there might have been selective effects on older patients, who would be expected to undergo some degree of cognitive decline under even normal circumstances, was examined by subgroup analysis of patients over 45 yr, but this also failed to show any significant DHEA effect (data not shown); effects on cognition may vary, depending on the duration of DHEA deficit, amount of glucocorticoid replacement, or some combination of these factors with age; there may be other ways of testing cognitive function that might reveal positive effects of DHEA. Finally, despite animal experimental data suggesting that DHEA has a neuroprotective effect, it may have no significant ameliorating effect on cognitive dysfunction in humans. Nevertheless, our data represent the most comprehensive evaluation of the effects of DHEA on cognition in Addison’s disease. We cannot discount the possibility that there may be subgroups of patients (e.g. related to age or gender) in whom DHEA might be particularly beneficial, but given our negative overall results, this remains speculative.
Finally, we observed no changes in sexual function after DHEA treatment, which is in keeping with observations in some short- (20,48,54) and longer-term trials (25) but differs from observations in women with primary or secondary adrenal failure (23,24). Another androgen (testosterone) also improves sexual function in surgically menopausal women (55,56). Accordingly, it is possible that beneficial effects of DHEA on sexual function are primarily androgen mediated, therefore being most evident in women with severe androgen deficiency. Future trials of DHEA in subjects with a combination of both Addison’s disease and ovarian failure could address this possibility.
This trial describes the longest duration of DHEA replacement therapy in a comparatively large number of patients with Addison’s disease and provides important additional information on its effects and tolerability. Our results show that daily oral administration of DHEA in physiological dosage for 12 months normalizes serum DHEAS levels and does have positive psychological effects. Our study also suggests that patients with Addison’s disease may have a disorder-specific psychological deficit. By analogy with GH therapy in adult GH deficiency, it is possible that future development of an Addison’s disease-specific quality of life measure may provide a tool that better detects changes in well-being or better defines patients in whom DHEA therapy is indicated. Beneficial responses to DHEA treatment in lean body mass and femoral BMD were also observed, changes that if sustained in the long term, could reduce morbidity. Overall, DHEA therapy was generally well tolerated, although some females described androgenic side effects. In future trials, the dosage of DHEA may require adjustment in both sexes: older females may need only 25 mg daily; conversely, younger males may require more than 50 mg to restore age-related circulating DHEAS levels.
Acknowledgments
We thank the nursing staff from the endocrine special test center, Christchurch Hospital for facilitating assessment of patients. We also thank Shirley Love for bone density measurements.
Footnotes
This work was supported by the National Osteoporosis Society and by funding from the National Institute for Health Research Biomedical Research Center. V.K.K.C., E.M.G., and S.E.C. were supported by the Wellcome Trust.
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 13, 2007
Abbreviations: BMD, Bone mineral density; DEXA, dual-energy x-ray absorptiometry; DHEA, dehydroepiandrosterone; DHEAS, DHEA sulfate; GHQ-30, General Health Questionnaire; MFI-20, Multidimensional Fatigue Inventory; SF-36, Short Form-36.
References
- Orentreich N, Brind JL, Rizer RL, Vogelman JH 1984 Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab 59:551–555 [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Vogelman JH, Andres R, Baldwin H 1992 Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J Clin Endocrinol Metab 75:1002–1004 [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Khaw KT, Yen SSC 1986 A prospective study of dehydroepiandrosterone sulfate, mortality and cardiovascular disease. N Engl J Med 315:1519–1524 [DOI] [PubMed] [Google Scholar]
- Ebeling P, Kiovisto VA 1994 Physiological importance of dehydroepiandrosterone. Lancet 343:1479–1481 [DOI] [PubMed] [Google Scholar]
- Sambrook P, Birmingham J, Champion D, Kelly P, Kempler S, Freund J, Eisman J 1992 Postmenopausal bone loss in rheumatoid arthritis: effect of estrogens and androgens. J Rheumatol 19:357–361 [PubMed] [Google Scholar]
- Morales AJ, Nolan JJ, Nelson JC, Yen SSC 1994 Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab 78:1360–1367 [DOI] [PubMed] [Google Scholar]
- Morales AJ, Haubrich RH, Hwang JY, Asakura H, Yen SSC 1998 The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol (Oxf) 49:421–432 [DOI] [PubMed] [Google Scholar]
- Diamond P, Cusan L, Gomez J-L, Belanger A, Fabrie F 1996 Metabolic effects of 12-month percutaneous dehydroepiandrosterone replacement therapy in postmenopausal women. J Endocrinol 150:S43–S50 [PubMed] [Google Scholar]
- Labrie F, Diamond P, Cusan L, Gomez JL, Belanger A, Candas B 1997 Effect of 12-month dehydroepiandrosterone replacement therapy on bone, vagina and endometrium in postmenopausal women. J Clin Endocrinol Metab 82:3498–3505 [DOI] [PubMed] [Google Scholar]
- Robel P, Bourreau E, Corpechot C, Dang DC, Halberg F, Clarke C, Haug M, Schlegel ML, Synguelakis M, Vourch C, Baulieu EE 1987 Neuro-steroids: 3β-hydroxy-Δ5-derivatives in rat and monkey brain. J Steroid Biochem 27:649–655 [DOI] [PubMed] [Google Scholar]
- Robel P, Baulieu EE 1995 Dehydroepiandrosterone (DHEA) is a neuroactive neurosteroid. Ann NY Acad Sci 774:82–110 [DOI] [PubMed] [Google Scholar]
- Li H, Klein G, Sun P, Buchan AM 2001 Dehydroepiandrosterone (DHEA) reduces neuronal injury in a rat model of global cerebral ischemia. Brain Res 888:263–266 [DOI] [PubMed] [Google Scholar]
- Kimonides VG, Khatibi NH, Svendsen CN, Sofroniew MV, Herbert J 1998 Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc Natl Acad Sci USA 95:1852–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood JF, Smith GE, Roberts E 1988 Dehydroepiandrosterone and its sulfate enhance memory retention in mice. Brain Res 447:269–278 [DOI] [PubMed] [Google Scholar]
- Karishma KK, Herbert J 2002 Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone-induced suppression. Eur J Neurosci 16:445–453 [DOI] [PubMed] [Google Scholar]
- Kalimi M, Shafagoj Y, Loria R, Padgett D, Regelson W 1994 Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Mol Cell Biochem 131:99–104 [DOI] [PubMed] [Google Scholar]
- Browne ES, Wright BE, Porter JR, Svec F 1992 Dehydroepiandrosterone: antiglucocorticoid action in mice. Am J Med Sci 303:366–371 [DOI] [PubMed] [Google Scholar]
- Maurer M, Trajanoski Z, Frey G, Hiroi N, Galon J, Willenberg HS, Gold PW, Chrousos GP, Scherbaum WA, Bornstein SR 2001 Differential gene expression profile of glucocorticoids, testosterone, and dehydroepiandrosterone in human cells. Horm Metab Res 33:691–695 [DOI] [PubMed] [Google Scholar]
- Baker SJK, Hunt PJ, Wass JAH 1997 Assessing the potential for finetuning the management of Addison’s disease/steroid replacement therapy 188th Society for Endocrinology (UK) meeting, London. J Endocrinol Abstract Suppl 155:P2 [Google Scholar]
- Hunt PJ, Gurnell EM, Huppert FA, Richards C, Prevost AT, Wass JA, Herbert J, Chatterjee VK 2000 Improvement in mood and fatigue after dehydroepiandrosterone replacement in Addison’s disease in a randomized, double blind trial. J Clin Endocrinol Metab 85:4650–4656 [DOI] [PubMed] [Google Scholar]
- Lovas K, Loge JH, Husebye ES 2002 Subjective health status in Norwegian patients with Addison’s disease. Clin Endocrinol (Oxf) 56:581–588 [DOI] [PubMed] [Google Scholar]
- Young J, Couzinet B, Nahoul K, Brailly S, Chanson P, Baulieu EE, Schaison G 1997 Panhypopituitarism as a model to study the metabolism of dehydroepiandrosterone (DHEA) in humans. J Clin Endocrinol Metab 82:2578–2585 [DOI] [PubMed] [Google Scholar]
- Arlt W, Callies F, van Vlijmen JC, Koehler I, Reincke M, Bidlingmaier M, Huebler D, Oettel M, Ernst M, Schulte HM, Allolio B 1999 Dehydroepiandrosterone replacement in women with adrenal insufficiency. N Engl J Med 341:1013–1020 [DOI] [PubMed] [Google Scholar]
- Johannsson G, Burman P, Wiren L, Engstrom BE, Nilsson AG, Ottosson M, Jonsson B, Bengtsson BA, Karlsson FA 2002 Low dose dehydroepiandrosterone affects behavior in hypopituitary androgen-deficient women: a placebo-controlled trial. J Clin Endocrinol Metab 87:2046–2052 [DOI] [PubMed] [Google Scholar]
- Lovas K, Gebre-Medhin G, Trovik TS, Fougner KJ, Uhlving S, Nedrebo BG, Myking OL, Kampe O, Husebye ES 2003 Replacement of dehydroepiandrosterone in adrenal failure: no benefit for subjective health status and sexuality in a 9-month, randomized, parallel group clinical trial. J Clin Endocrinol Metab 88:1112–1118 [DOI] [PubMed] [Google Scholar]
- Arlt W, Allolio B 2003 DHEA replacement in adrenal insufficiency. J Clin Endocrinol Metab 88:4001; author reply 4001–4002 [DOI] [PubMed] [Google Scholar]
- Maehlen J, Torvik A 1990 Necrosis of granule cells of hippocampus in adrenocortical failure. Acta Neuropathol (Berl) 80:85–87 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H 1997 How does the brain organize memories? Science 277:330–332 [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L, Keightley S, Kill D 1975 Fornix lesions selectively abolish place learning in the rat. Exp Neurol 48:152–166 [DOI] [PubMed] [Google Scholar]
- Owen AM, Morris RG, Sahakian BJ, Polkey CE, Robbins TW 1996 Double dissociations of memory and executive functions in working memory tasks following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Brain 119(Pt 5):1597–1615 [DOI] [PubMed] [Google Scholar]
- Zagar R, Arbit J, Stuckey M, Wengel WW 1984 Developmental analysis of the Wechsler Memory Scale. J Clin Psychol 40:1466–1473 [DOI] [PubMed] [Google Scholar]
- Delis DC, Freeland J, Kramer JH, Kaplan E 1988 Integrating clinical assessment with cognitive neuroscience: construct validation of the California Verbal Learning Test. J Consult Clin Psychol 56:123–130 [DOI] [PubMed] [Google Scholar]
- van Niekerk JK, Nielsen TA, Pasu S, Shore S, Huppert FA 2004 The development of new tests of source memory and a new approach to the testing of equivalence of parallel versions. Aging Neuropsychol Cognition 11:416–427 [Google Scholar]
- Nelson HE 1982 National Adult Reading Test (NART): test manual. Windsor, Berkshire, UK: NFER-Nelson [Google Scholar]
- Keller SD, Ware Jr JE, Bentler PM, Aaronson NK, Alonso J, Apolone G, Bjorner JB, Brazier J, Bullinger M, Kaasa S, Leplege A, Sullivan M, Gandek B 1998 Use of structural equation modeling to test the construct validity of the SF-36 Health Survey in ten countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 51:1179–1188 [DOI] [PubMed] [Google Scholar]
- Jenkinson C, Stewart-Brown S, Petersen S, Paice C 1999 Assessment of the SF-36 version 2 in the United Kingdom. J Epidemiol Community Health 53:46–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets EM, Garssen B, Cull A, de Haes JC 1996 Application of the multidimensional fatigue inventory (MFI-20) in cancer patients receiving radiotherapy. Br J Cancer 73:241–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg DP 1978 Manual of the General Health Questionnaire. Windsor, UK: NFER Publishing [Google Scholar]
- Huppert FA, Walters DE, Day N, Elliott BJ 1989 The factor structure of the General Health Questionnaire (GHQ-30): a reliability study on 6317 community residents. Br J Psychiatry 155:178–185 [DOI] [PubMed] [Google Scholar]
- Baulieu EE, Thomas G, Legrain S, Lahlou N, Roger M, Debuire B, Faucounau V, Girard L, Hervy MP, Latour F, Leaud MC, Mokrane A, Pitti-Ferrandi H, Trivalle C, de Lacharriere O, Nouveau S, Rakoto-Arison B, Souberbielle JC, Raison J, Le Bouc Y, Raynaud A, Girerd X, Forette F 2000 Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci USA 97:4279–4284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligman AM, Miller DL, McGinley KJ 1986 Sebutape: a device for visualizing and measuring human sebaceous secretion. J Soc Cosmet Chem 37:369–374 [Google Scholar]
- Sourla A, Flamand M, Belanger A, Labrie F 1998 Effect of dehydroepiandrosterone on vaginal and uterine histomorphology in the rat. J Steroid Biochem Mol Biol 66:137–149 [DOI] [PubMed] [Google Scholar]
- Miller KK, Biller BM, Hier J, Arena E, Klibanski A 2002 Androgens and bone density in women with hypopituitarism. J Clin Endocrinol Metab 87:2770–2776 [DOI] [PubMed] [Google Scholar]
- Osmanagaoglu MA, Okumus B, Osmanagaoglu T, Bozkaya H 2004 The relationship between serum dehydroepiandrosterone sulfate concentration and bone mineral density, lipids, and hormone replacement therapy in premenopausal and postmenopausal women. J Womens Health (Larchmt) 13:993–999 [DOI] [PubMed] [Google Scholar]
- Tok EC, Ertunc D, Oz U, Camdeviren H, Ozdemir G, Dilek S 2004 The effect of circulating androgens on bone mineral density in postmenopausal women. Maturitas 48:235–242 [DOI] [PubMed] [Google Scholar]
- Hampson G, Bhargava N, Cheung J, Vaja S, Seed PT, Fogelman I 2002 Low circulating estradiol and adrenal androgens concentrations in men on glucocorticoids: a potential contributory factor in steroid-induced osteoporosis. Metabolism 51:1458–1462 [DOI] [PubMed] [Google Scholar]
- Nair KS, Rizza RA, O’Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton 3rd LJ, Smith GE, Khosla S, Jensen MD 2006 DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 355:1647–1659 [DOI] [PubMed] [Google Scholar]
- Libe R, Barbetta L, Dall’Asta C, Salvaggio F, Gala C, Beck-Peccoz P, Ambrosi B 2004 Effects of dehydroepiandrosterone (DHEA) supplementation on hormonal, metabolic and behavioral status in patients with hypoadrenalism. J Endocrinol Invest 27:736–741 [DOI] [PubMed] [Google Scholar]
- Villareal DT, Holloszy JO 2004 Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA 292:2243–2248 [DOI] [PubMed] [Google Scholar]
- Christiansen JJ, Gravholt CH, Fisker S, Svenstrup B, Bennett P, Veldhuis J, Andersen M, Christiansen JS, Jorgensen JO 2004 Dehydroepiandrosterone supplementation in women with adrenal failure: impact on twenty-four hour GH secretion and IGF-related parameters. Clin Endocrinol (Oxf) 60:461–469 [DOI] [PubMed] [Google Scholar]
- van Niekerk JK, Huppert FA, Herbert J 2001 Salivary cortisol and DHEA: association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology 26:591–612 [DOI] [PubMed] [Google Scholar]
- Huppert FA, Van Niekerk JK, Herbert J 2000 Dehydroepiandrosterone (DHEA) supplementation for cognition and well-being. Cochrane Database Syst Rev CD000304 [DOI] [PubMed] [Google Scholar]
- Fonda SJ, Bertrand R, O’Donnell A, Longcope C, McKinlay JB 2005 Age, hormones, and cognitive functioning among middle-aged and elderly men: cross-sectional evidence from the Massachusetts Male Aging Study. J Gerontol A Biol Sci Med Sci 60:385–390 [DOI] [PubMed] [Google Scholar]
- van Thiel SW, Romijn JA, Pereira AM, Biermasz NR, Roelfsema F, van Hemert A, Ballieux B, Smit JW 2005 Effects of dehydroepiandrostenedione, superimposed on growth hormone substitution, on quality of life and insulin-like growth factor I in patients with secondary adrenal insufficiency: a randomized, placebo-controlled, cross-over trial. J Clin Endocrinol Metab 90:3295–3303 [DOI] [PubMed] [Google Scholar]
- Shifren JL, Braunstein GD, Simon JA, Casson PR, Buster JE, Redmond GP, Burki RE, Ginsburg ES, Rosen RC, Leiblum SR, Caramelli KE, Mazer NA 2000 Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med 343:682–688 [DOI] [PubMed] [Google Scholar]
- Simon J, Braunstein G, Nachtigall L, Utian W, Katz M, Miller S, Waldbaum A, Bouchard C, Derzko C, Buch A, Rodenberg C, Lucas J, Davis S 2005 Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. J Clin Endocrinol Metab 90:5226–5233 [DOI] [PubMed] [Google Scholar]