Abstract
Context: The initial diagnosis of pheochromocytoma relies on plasma fractionated metanephrines levels. Normal levels exclude pheochromocytoma, but positive tests have a low positive predictive value due to the disease’s rarity.
Objectives: The objective of the study was to evaluate three approaches to distinguish between true-positive and false-positive tests: 1) increased cutoff for plasma fractionated metanephrines, 2) measurement of serum/plasma chromogranin A (CGA), and 3) urine fractionated metanephrine testing.
Design: We studied retrospectively all Mayo Clinic patients with positive plasma fractionated metanephrine tests over a 15-month period and determined their final diagnosis based on histology, imaging, additional biochemical tests, and more than 1 yr follow-up. For a subgroup, urine fractionated metanephrine results were available. All original plasma samples were retested for CGA.
Results: Of 140 patients, 40 had a chromaffin tumor confirmed and 100 excluded, indicating a positive predictive value of plasma fractionated metanephrines of 28.6%. Increasing the threshold for a positive test improved specificity to 98% but missed eight cases (20%). Incorporation of urine fractionated metanephrine testing as follow-up test achieved 80% specificity and 91% sensitivity. The corresponding figures for CGA were 71 and 87% for all patients and 89 and 87% when patients taking proton pump inhibitors were excluded.
Conclusions: Unless plasma fractionated metanephrines levels are elevated more than 4-fold above the upper limit of normal, patients with a positive plasma fractionated metanephrines test should be evaluated with urine fractionated metanephrines and serum/plasma CGA assays before being subjected to imaging or invasive diagnostic tests.
Pheochromocytoma is frequently considered in the differential diagnosis of severe hypertension. It can usually be excluded by normal plasma free metanephrine levels. However, modestly elevated free plasma metanephrine levels are often false positive. To reduce false positives, this study recommends patients with a less than 4-fold elevation of plasma fractionated free metanephrines undergo follow-up tests of plasma chromogranin A or urine fractionated metanephrines.
Pheochromocytoma and paraganglioma occur in up to 2–5% of patients with hypertension or adrenal incidentalomas, respectively (1,2). They are often considered in the differential diagnosis of severe or atypical hypertension and can prove fatal when diagnosis is delayed. The recommended case detection test, measurement of free plasma fractionated metanephrines, achieves 82–97% specificity with 96–99% sensitivity (3,4,5). However, this level of specificity in a rare tumor results in more false-positive tests than true positives, often leading to unnecessary tumor-localization attempts.
Strategies to reduce false positives have included the following: 1) retesting plasma fractionated metanephrines from asupine venipuncture setting or after removal of potentially interfering medications, 2) higher free plasma fractionated metanephrines diagnostic cutoffs, 3) additional tests, such as urine fractionated metanephrines or clonidine suppression, and 4) age-adjusted plasma fractionated metanephrine reference ranges (3,4,6,7,8,9).
Alternatively, testing for catecholamines or their metabolites might be combined with assessment of other facets of neuroendocrine secretion. Chromogranin A (CGA) is a promising candidate for such an approach. CGA is oversecreted in patients with carcinoid tumors, pheochromocytomas, neuroblastomas, medullary thyroid carcinomas, and some pituitary tumors (10,11). We therefore evaluated the utility of CGA follow-up testing in patients with a positive free plasma fractionated metanephrine test and compared CGA’s diagnostic performance with that of an alternative cutoff level for plasma fractionated metanephrines and with urine fractionated metanephrine testing.
Subjects and Methods
Experimental subjects
This study was approved by the Mayo Clinic Institutional Review Board. Specimens were selected from all plasma fractionated metanephrine tests performed over 15 months if all of the following were met: 1) a free plasma fractionated metanephrines test was ordered to confirm or exclude a chromaffin tumor and the test result was positive (defined below), 2) the patient had been seen by a Mayo Clinic physician, 3) absence of comorbidities known to affect plasma fractionated metanephrines or CGA levels, and 4) residual specimen available for further testing. All clinical, laboratory, and imaging data on these patients were reviewed and subjects were classified as either pheochromocytoma/paraganglioma confirmed (Pheo-positive) or excluded (Pheo-excluded). Confirmed cases had a definitive histological diagnosis or, in cases of inoperable lesions, diagnostic 123I-metaiodobenzylguanidine scintigraphy or 18F-fluorodeoxyglucose positron emission tomography. We considered chromaffin tumors excluded when none of these criteria were met and subsequent clinical follow-up, further biochemical work-up, and imaging studies had ruled out the diagnosis.
Biochemical assays
Free plasma fractionated metanephrines were measured at the Mayo Clinic Rochester using liquid chromatography-tandem mass spectrometry as previously described (12). A plasma metanephrine value of 0.5 nmol/liter or greater or a plasma normetanephrine value of 0.9 nmol/liter or greater was considered positive based on in-house established reference ranges (12). All samples were frozen after testing until determination of CGA concentrations.
Serum CGA was measured with an in-house developed chemiluminometric assay, described in detail in the supplemental appendix, published as supplemental data on The Endocrine Society’s Journals Online Web site at http://jcem.endojournals.org. A value of greater than 225 ng/ml was considered positive based on in-house developed reference ranges.
Fifty-nine patients had also undergone 24-h urine fractionated metanephrine testing by liquid chromatography-tandem mass spectrometry (13). For this method, we established clinically validated cutoffs (14): 302 μg per 24 h for metanephrine, 733 μg per 24 h for normetanephrine, or 1000 μg per 24 h for total metanephrines (sum of both fractions).
Data analysis
Data analysis was performed using JMP version 6 (SAS Institute, Inc., Cary, NC). Receiver-operating characteristics (ROC) curves were constructed using mROC (15). The likelihood ratios for positive tests and negative tests were calculated by dividing sensitivity by (1 − specificity) and (1 − sensitivity) by specificity, respectively.
Results
Patients
Of 24,204 plasma fractionated metanephrine tests performed during the study period, 1,034 had either positive metanephrine or normetanephrine levels. Of these, 140 fulfilled all other selection criteria. The median age was 62.5 yr, 90 were female, and 50 were male. The mean metanephrine concentration was 0.48 nmol/liter (median 0.20 nmol/liter, range 0.20–11.1 nmol/liter), and the mean normetanephrine concentration was 4.12 nmol/liter (median 1.33 nmol/liter, range 0.9–131 nmol/liter). Forty individuals were Pheo-positive; the remaining 100 patients were Pheo-excluded. Among the Pheo-excluded patients, three were taking tricyclic antidepressants, and none were taking phenoxybenzamine, medications most frequently associated with false-positive plasma fractionated metanephrine results (6).
Performance of fractionated free plasma metanephrines
A negative predictive value could not be calculated because the study cohort was limited to patients with positive plasma fractionated metanephrine levels. The positive predictive value of a positive result was 28.6%.
Increasing the cutoff levels of plasma fractionated metanephrines to limits proposed to reduce false positives (1.20 and 2.19 nmol/liter for plasma metanephrine and normetanephrine, respectively) caused a marked decrease in false positives (3). Only two of 100 Pheo-excluded patients were positive, for a specificity of 98%. However, the sensitivity of the adjusted cutoffs decreased to 80% because eight of 40 Pheo-positive patients were missed.
Neither antihypertensive medications nor proton pump inhibitors (PPIs) affected the levels of plasma metanephrine or normetanephrine significantly (P > 0.6).
Performance of CGA as a follow-up test
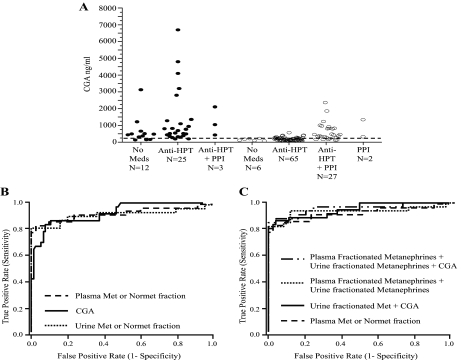
Patients taking PPIs alone or in combination with antihypertensive medications had significantly higher CGA levels (mean 635 ng/ml) than patients not taking these medications (mean 174 ng/ml; P < 0.001) (Fig. 1A). For antihypertensive medications alone, the mean serum CGA level was 178 ng/ml, not significantly different from no medications (P = 0.41). These data indicate that PPIs elevate CGA concentrations.
Figure 1.
Performance of CGA and urine fractionated metanephrines as follow-up tests in pheochromocytoma. A, Serum CGA levels in Pheo-positive (solid circles) and Pheo-excluded (open circles) patients. The patient population was divided based on the medication profile: no medications (no meds), patients taking antihypertensive medications (anti-HPT), patients taking PPIs, or both. There were no Pheo-positive patients who were taking only PPIs. The dashed horizontal line represents the upper limit of the population reference range. B, ROC curves of CGA, plasma fractionated metanephrines, and urine total metanephrines (sum of metanephrine and normetanephrine fractions). C, ROC curves incorporating the various assay combinations in 59 patients who had all three assays performed (excluding patients on PPI medications).
When patients on PPIs were excluded, a serum CGA cutoff greater than 225 ng/ml was 87% sensitive and 73% specific for catecholamine-secreting tumors (Table 1). Using an optimized cutoff of greater than 270 ng/ml, based on ROC curve analysis, resulted in 89% specificity with identical assay sensitivity (Table 1).
Table 1.
Comparison of the diagnostic efficacy of CGA and urine fractionated metanephrines assays for the detection of pheochromocytoma in patients with a positive test for plasma fractionated metanephrines
| Test | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Positive plasma fractionated metanephrines and positive CGA (cutoff > 225 ng/ml) | 32/37 (87) | 52/71 (73) |
| Positive plasma fractionated metanephrines and positive CGA (cutoff > 270 ng/ml) | 32/37 (87) | 63/71 (89) |
| Positive plasma fractionated metanephrines and a positive urine metanephrine or normetanephrine fraction | 31/34 (91) | 20/25 (80) |
Patients taking PPIs were excluded from the analysis.
Performance of urine fractionated metanephrines as a follow-up test
Of the 59 patients who also underwent urine fractionated metanephrines testing, 34 were Pheo-positive and 25 Pheo-excluded (Table 1). The sensitivity and specificity in this group were 26 and 100% for urine metanephrine, 88 and 80% for urine normetanephrine, 82 and 88% for total urine metanephrines, and 91 and 80% for either a positive metanephrine or normetanephrine.
Comparison of all three assays
The performance of all three biochemical assays was compared using ROC analysis. The ROC curve for CGA including all patients in the study showed an area under the curve (AUC) of 0.83 [95% confidence interval (CI) 0.76–0.90]; when patients taking PPIs are excluded, CGA and plasma fractionated metanephrines showed a similar diagnostic performance, AUC: 0.92 (95% CI 0.85–0.96) and 0.93 (95% CI 0.83–0.97), respectively (Fig. 1B). AUC of urine fractionated metanephrines was 0.91 (95% CI 0.79–0.96) (Fig. 1B). The ROC curves generated by incorporation of multiple variables show an AUC of 0.95 (95% CI 0.88–0.98) for the combined plasma fractionated metanephrine and CGA assays; 0.94 (95% CI 0.83–0.98) for the combined plasma fractionated metanephrine and urine fractionated metanephrine assays; and 0.95 (95% CI 0.85–0.98) for the combination of all three assays (Fig. 1C).
The likelihood ratio for having a pheochromocytoma/paraganglioma was 7.9 with CGA levels greater than 270 ng/ml, whereas for CGA levels less than 270 ng/ml, it was 0.15, i.e. a patient in whom both fractionated plasma metanephrines and CGA are positive is greater than 50 times more likely to have a chromaffin tumor than one with only positive plasma fractionated metanephrines. For the combination of plasma and urine fractionated metanephrines, the corresponding likelihood ratios were 4.6 and 0.11. Sequential testing for CGA or urine fractionated metanephrines in patients with a positive plasma fractionated metanephrine result increases the positive predictive value to 80 and 86%, compared with a 28.6% positive predictive value for plasma fractionated metanephrines alone.
The three Pheo-confirmed patients missed by urine fractionated metanephrine testing were positive for CGA, and the five Pheo-confirmed cases with a CGA less than 270 ng/ml had positive urine fractionated metanephrines results. Likewise, of the eight Pheo-confirmed cases missed by the higher plasma fractionated metanephrine cutoffs, three were positive by CGA alone, three were positive by urine fractionated metanephrines alone, and the remaining two were positive for both assays. This indicates that CGA and urine fractionated metanephrines, when used in combination as follow-up tests, will reduce the number of patients who need further work-up by almost 10-fold and still detect all true positive cases (see supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online Web site at http://jcem.endojournals.org).
Discussion
Higher diagnostic cutoffs for plasma fractionated metanephrines eliminate most false positives, but a significant number of true cases are missed, whereas CGA or urine fractionated metanephrine follow-up testing avoids this problem. The First International Symposium on Pheochromocytoma recommended to maintain the upper population reference range limit as the diagnostic cutoff for plasma fractionated metanephrine testing to avoid, potentially deadly, missed diagnoses (16). However, for cases with slightly increased concentrations, additional biochemical testing was advised. Unfortunately, there were no specific recommendations concerning the best biochemical test for follow-up.
CGA is coreleased with amines/peptides from neuroendocrine tumor cells, and CGA levels correlate with tumor mass and secretory activity (17). The reported sensitivity of CGA for detection of pheochromocytoma ranges from 65 to 95% (17,18,19,20). We did not assess CGA testing in the initial case detection for pheochromocytoma but rather as a follow-up assay to decrease the number of false positives. Based on our results, combining plasma fractionated metanephrine and CGA analysis will result in an 89% reduction in the number of false positives requiring further work-up.
Comparison of CGA and urine fractionated metanephrines as follow-up tests showed similar diagnostic efficiency. CGA had a slightly higher follow-up testing specificity than urine fractionated metanephrines (89 vs. 80%) but somewhat lower sensitivity (87 vs. 91%). Some true-positive cases will therefore still be missed if a single follow-up test is used. This can be overcome by performing both CGA and urine fractionated metanephrine testing in a step-wise fashion and reviewing the results in the context of the clinical presentation. Optimal test performance was achieved when the recommended, definitively diagnostic, 4-fold elevation criterion for plasma fractionated metanephrines was supplemented with both urine fractionated metanephrines and CGA analyses for those cases with lesser plasma fractionated metanephrines elevations.
However, no testing approach is infallible. Timed urine tests have a significant rate of inaccurate collections (∼15%), and incorrect urine preservatives can lead to invalid results. CGA, in turn, can be secreted by nonchromaffin neuroendocrine tumors (11) and can be elevated in liver or kidney failure or due to PPI therapy. Finally, measurements of plasma or urinary fractionated metanephrines may fail to detect rare tumors that synthesize exclusively dopamine (4). No studies so far have addressed the utility of CGA in these cases.
In summary, we have shown that serum/plasma CGA and urine fractionated metanephrine measurements show great promise as follow-up tests in cases with mild elevations of plasma fractionated metanephrines. Follow-up testing with these assays improves the positive predictive value of plasma fractionated metanephrines and maintains diagnostic sensitivity. Because negative plasma fractionated metanephrines is highly predictive of the absence of pheochromocytoma, it is uncertain whether additional CGA or urine fractionated metanephrine testing should be added to the initial work-up. However, our data indicate that these assays should be used to clarify any intermediate elevations of plasma fractionated metanephrines. Here the savings in unnecessary procedures and costs can be substantial. These promising findings should be expanded and validated in multicenter prospective studies to determine the optimal approach to the biochemical diagnosis of chromaffin tumors.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grant CA80117 (to S.K.G.G., co-principal investigator), Mayo Graduate School of Medicine (to A.A.-S.), and Mayo Clinic Departments of Medicine and Laboratory Medicine and Pathology funds.
Disclosure Statement: S.K.G.G. consults for Diagnostic Hybrids, Inc. No other authors have any disclosures.
First Published Online October 16, 2007
Abbreviations: AUC, Area under the curve; CGA, chromogranin A; CI, confidence interval; PPI, proton pump inhibitor; ROC, receiver-operating characteristic.
References
- Vaclavik J, Stejskal D, Lacnak B, Lazarova M, Jedelsky L, Kadalova L, Janosova M, Frysak Z, Vlcek P 2007 Free plasma metanephrines as a screening test for pheochromocytoma in low-risk patients. J Hypertens 25:1427–1431 [DOI] [PubMed] [Google Scholar]
- Young Jr WF 2000 Management approaches to adrenal incidentalomas. A view from Rochester, Minnesota. Endocrinol Metab Clin North Am 29:159–185 [DOI] [PubMed] [Google Scholar]
- Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, Keiser HR, Goldstein DS, Eisenhofer G 2002 Biochemical diagnosis of pheochromocytoma: which test is best? JAMA 287:1427–1434 [DOI] [PubMed] [Google Scholar]
- Sawka AM, Jaeschke R, Singh RJ, Young Jr WF 2003 A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 88:553–558 [DOI] [PubMed] [Google Scholar]
- Raffesberg W, Bischof M, Scheuba C, Niederle B, Gasic S, Waldhausl W, Roden M 2000 Diagnostic efficacy of unconjugated plasma metanephrines for the detection of pheochromocytoma. Arch Intern Med 160:2957–2963 [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Goldstein DS, Walther MM, Friberg P, Lenders JW, Keiser HR, Pacak K 2003 Biochemical diagnosis of pheochromocytoma: how to distinguish true- from false-positive test results. J Clin Endocrinol Metab 88:2656–2666 [DOI] [PubMed] [Google Scholar]
- Kudva YC, Sawka AM, Young Jr WF 2003 Clinical review 164: the laboratory diagnosis of adrenal pheochromocytoma: the Mayo Clinic experience. J Clin Endocrinol Metab 88:4533–4539 [DOI] [PubMed] [Google Scholar]
- Lenders JWM, Willemsen JJ, Eisenhofer G, Ross HA, Pacak K, Timmers HJLM, Sweep CGJ 2007 Is supine rest necessary before blood sampling for plasma metanephrines? Clin Chem 53:352–354 [DOI] [PubMed] [Google Scholar]
- Sawka AM, Thabane L, Gafni A, Levine M, Young Jr WF 2005 Measurement of fractionated plasma metanephrines for exclusion of pheochromocytoma: can specificity be improved by adjustment for age? BMC Endocr Disord 5:1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg K, Stridsberg M 2000 Chromogranins as diagnostic and prognostic markers in neuroendocrine tumours. Adv Exp Med Biol 482:329–337 [DOI] [PubMed] [Google Scholar]
- Taupenot L, Harper KL, O’Connor DT 2003 The chromogranin-secretogranin family. N Engl J Med 348:1134–1149 [DOI] [PubMed] [Google Scholar]
- Lagerstedt SA, O’Kane DJ, Singh RJ 2004 Measurement of plasma free metanephrine and normetanephrine by liquid chromatography-tandem mass spectrometry for diagnosis of pheochromocytoma. Clin Chem 50:603–611 [DOI] [PubMed] [Google Scholar]
- Taylor RL, Singh RJ 2002 Validation of liquid chromatography-tandem mass spectrometry method for analysis of urinary conjugated metanephrine and normetanephrine for screening of pheochromocytoma. Clin Chem 48:533–539 [PubMed] [Google Scholar]
- Perry CG, Sawka AM, Singh R, Thabane L, Bajnarek J, Young Jr WF 2007 The diagnostic efficacy of urinary fractionated metanephrines measured by tandem mass spectrometry in detection of pheochromocytoma. Clin Endocrinol 66:703–708 [DOI] [PubMed] [Google Scholar]
- Kramar A, Faraggi D, Fortune A, Reiser B 2001 mROC: a computer program for combining tumour markers in predicting disease states. Comput Methods and Programs Biomed 66:199–207 [DOI] [PubMed] [Google Scholar]
- Pacak K, Eisenhofer G, Ahlman H, Bornstein SR, Gimenez-Roqueplo AP, Grossman AB, Kimura N, Mannelli M, McNicol AM, Tischler AS 2007 Pheochromocytoma: recommendations for clinical practice from the First International Symposium, October 2005. Nat Clin Pract Endocrinol Metab 3:92–102 [DOI] [PubMed] [Google Scholar]
- Hsiao RJ, Parmer RJ, Takiyyuddin MA, O’Connor DT 1991 Chromogranin A storage and secretion: sensitivity and specificity for the diagnosis of pheochromocytoma. Medicine 70:33–45 [PubMed] [Google Scholar]
- Bernini GP, Moretti A, Ferdeghini M, Ricci S, Letizia C, D’Erasmo E, Argenio GF, Salvetti A 2001 A new human chromogranin ‘A’ immunoradiometric assay for the diagnosis of neuroendocrine tumours. Br J Cancer 84:636–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanella L, Squin N, Ghelfo A, Ceriani L 2006 Chromogranin A immunoradiometric assay in diagnosis of pheochromocytoma: comparison with plasma metanephrines and 123I-MIBG scan. Q J Nucl Med Mol Imaging 50:344–347 [PubMed] [Google Scholar]
- Grossrubatscher E, Dalino P, Vignati F, Gambacorta M, Pugliese R, Boniardi M, Rossetti O, Marocchi A, Bertuzzi M, Loli P 2006 The role of chromogranin A in the management of patients with phaeochromocytoma. Clin Endocrinol 65:287–293 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.