Crosscampling of the portal vein and inferior vena cava during the anhepatic phase of orthotopic hepatic transplantation and the resulting venous stagnation can lead to hemodynamic instability and congestive damage of the small intestine. These sequelae are more severe and can initiate a cascade of fatal complications in patients who are hemodynamically compromised or lack adequate collateral circulation from the occluded venous trunks, or both. The use of venovenous bypass is particularly helpful in these instances (1, 2). Alternatively, the piggyback technique (3), in which the systemic venous return is preserved throughout the procedure, was proposed for instances in which the use of the venovenous bypass is problematic (4). We report herein the use of a combination of the piggyback technique with a temporary portacaval shunt during the anhepatic phase in four pediatric recipients who were hemodynamically compromised and had no established portosystemic collateral circulation.
PATIENTS
The four patients described herein were children in whom venovenous bypass was thought to be impossible or difficult to maintain and had a marked absence of portal venous hypertension and portosystemic collaterals. In the first patient, the hemodynamic compromise was the result of a severe hypoplasia and stenosis of the pulmonary artery associated with Alagille's syndrome, as well as a patent ductus arteriosus. The second patient, also with Alagille's syndrome, had pulmonary arterial stenosis as well as a history of repaired coarctation of the aorta, aortc valve balloon valvuloplasty and pacemaker placement for sinus bradycardia. The third and fourth patients had fulminant hepatitis and graft versus host disease after bone marrow transplantation, respectively, and were critically ill at the time of hepatic replacement. Immunosuppression was with FK 506 and low dose prednisone. The second patient also required OKT3 treatment.
SURGICAL TECHNIQUE
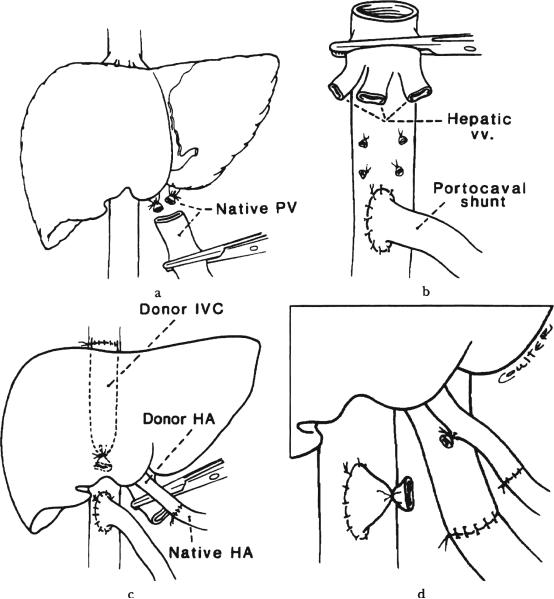
The hilar structures of the liver were identified. The common bile duct and hepatic artery were ligated and transected. The hepatic artery was prepared for later anastomosis. The portal vein was transected just beyond its bifurcation (Fig. 1a). The latter provided unimpaired view of the infrahepatic inferior vena cava onto which a side biting clamp was applied. An end to side portacaval anastomosis was performed using a continuous monofilament suture. In all four patients, congestion of the intestine was notable by the time the anastomosis was completed and was relieved promptly upon unclamping.
Fig. 1.
a, b and c, Stages of temporary end to side portacaval shunt combined with piggyback hepatic transplantation. d, Inset. PV, Portal vein; IVC, inferior vena cava; vv, hepatic veins, and HA, hepatic artery.
The liver was stripped off the inferior vena cava after the hepatic venous tributaries were ligated. The superior hepatic veins were preserved (Fig. 1b). The ostia of all veins (Patient Nos. 1 and 2) or the middle and left (Patient Nos. 3 and 4) were joined to form a venous outflow of the graft.
The grafts were whole livers (Patient Nos. 1, 2 and 4) and the left lobe of a split liver graft (Patient No. 3). (The right lobe of the latter graft was implanted into an adult recipient.) After the suprahepatic venous anastomosis was completed, the arterial anastomosis was performed (Fig. 1c). The portal vein was ligated just proximal to the anastomosis to the inferior vena cava after crossclamping and the native to donor portal vein anastomosis was performed (Fig. 1d). Intestinal congestion was again noted by the time of completion of the portal vein anastomosis. The graft was perfused. Biliary drainage was with a choledochojejunostomy into a Roux-en-Y loop in all patients.
RESULTS
All four patients survived the procedure and all four grafts functioned promptly. Estimated blood loss was 850, 1,200, 4,425 and 690 milliliters, respectively. Hemodynamic changes were minimal in all instances except in Patient No. 3, in whom hypotension occurred after reperfusion because of bleeding from the hepatic surface.
Patient No. 2 died of lymphoproliferative disease three months after transplantation. All other patients are well one week to nine months after transplantation, with normally functioning grafts.
DISCUSSION
The use of portacaval shunts had been a necessary step for successful orthotopic hepatic transplantation in dogs before the advent of motorized extracorporeal venovenous bypass. A side to side portacaval shunt was used in combination with a passive extracorporeal venovenous bypass decompressing the inferior into the superior vena cava (5).
There are two alterations of the originally described technique that were used in the patients reported herein. The inferior vena cava and its flow were preserved throughout the procedure, obviating the need for extracorporeal venous decompression.
The choice of an end to side portacaval shunt was made in favor of the previously described side to side shunt because it allows the total length of the portal vein to be preserved without the need of a proximal venotomy, which could jeopardize its integrity. Completion of the shunt requires little time because all portal structures have been already severed and exposure of the inferior vena cava is unimpaired. The recipient hepatectomy, hemostasis, suprahepatic venous and arterial anastomoses can be completed under hemodynamic stability.
Although speedy completion of all stages is important, urgency imposed by impending hemodynamic instability is only present during the performance of the portal venous connections. In the instances reported herein, all three vascular anastomoses were performed before the graft was perfused. Alternatively, the liver could be perfused after completion of the arterial anastomosis and before ligation of the shunt, if because of unusual circumstances the anastomotic time of the suprahepatic vena cava or the hepatic artery is prolonged.
The technique may be of use in orthotopic hepatic transplantation of critically ill patients, principally pediatric, with compromised hemodynamic status and poor collateral portosystemic circulation in whom venovenous bypass is difficult or impossible to use. The technique could also be used as “poor man's” venovenous bypass when the latter is not available. If obstruction of the vena cava is caused inadvertently during the anhepatic period, it has been shown that the splanchnic circulation is less damaged if it is connected to an obstructed vena cava than if it is obstructed in isolation (6).
Acknowledgments
Supported by Research Grants from the Veterans Administration and the National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Denmark SW, Shaw BW, Jr., Starzl TE, Griffith BP. Venovenous bypass without systemic anticoagulation in canine and human liver transplantation. Surg. Forum. 1983;34:380–382. [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw BW, Jr., Martin W, Marquez JM, et al. Venous bypass in clinical liver transplantation. Ann. Surg. 1984;200:524–534. doi: 10.1097/00000658-198410000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calne RY, William R. Liver transplantation in man. I. Observations on technique and organization in five cases. Br. Med. J. 1968;4:535–540. doi: 10.1136/bmj.4.5630.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tzakis AG, Todo S, Starzl TE. Orthotopic liver transplantation with preservation of the inferior vena cava. Ann. Surg. 1989;210:649–652. doi: 10.1097/00000658-198911000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stazl TE, Kaupp HA, Jr., Brock DR, et al. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg. Gynecol. Obstet. 1960;111:733–743. [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Bernhard VM, Benvenuto R, Cortes N. A new method for one-stage hepatectomy in dogs. Surgery. 1959;46:880–886. [PMC free article] [PubMed] [Google Scholar]