Abstract
Background
Adherence to inhaled anti-inflammatory therapy and self-management skills are essential parts of the asthma treatment plan to improve asthma control and prevent exacerbations. Whether self-management education improves long-term medication adherence is less clear.
Objective
A 24-week prospective, randomized controlled trial was performed to study the impact of self-management education on long-term adherence to inhaled corticosteroid (ICS) therapy and markers of asthma control.
Methods
After stabilization on ICS medication during a run-in phase, 95 adults with moderate to severe asthma were recruited from a large metropolitan community and 84 were randomized to individualized self-management education including self-monitoring of symptoms and peak flow or usual care with self-monitoring alone. The key components of the 30-minute intervention were asthma information, assessment and correction of inhaler technique, an individualized action plan based on self-monitoring data, and environmental control strategies for relevant allergen and irritant exposures. The intervention was personalized based on pulmonary function, allergen skin test reactivity, and inhaler technique and reinforced at 2 week intervals.
Results
Participants randomized to the self-management intervention maintained consistently higher ICS adherence levels and showed a nine-fold greater odds of more than 60% adherence to prescribed dose compared to controls at the end of the intervention (p=.02) and maintained a three-fold greater odds of higher than 60% adherence at the end of the study. Perceived control of asthma improved (p=.006), nighttime awakenings decreased (p=.03), and inhaled beta-agonist use decreased (p=.01) in intervention participants compared to controls.
Conclusion
Our results show that individualized asthma self-management education attenuates the usual decline in medication adherence and improves clinical markers of asthma control.
Keywords: Asthma, Self-Management, Adherence, Asthma Control
Asthma affects approximately 21 million people in the United States, causing over 1.5 million emergency department visits1, 2. To prevent serious exacerbations, daily self-management is necessary. Effective self-management requires mastery of specific knowledge and skills.3–7 Research published over the last two decades shows that instruction in these skills improves asthma health outcomes8, 9.
Self-management education that incorporates behavioral strategies also can improve adherence to inhaled corticosteroids (ICS) 10–14, suggesting that adherence to treatment may be the mechanism by which self-management education improves asthma control. More recent evidence has shown that tailored educational interventions have greater efficacy than standardized interventions because patients believe the instruction is personally relevant15,16. What remains unclear is which elements of self-management education account for improvement in adherence to treatment. It is thought that one important element is self-monitoring of symptoms and/or peak flow. By heightening the patient’s awareness of symptoms and airflow obstruction, monitoring alone may be sufficient to enhance adherence by showing the patient that asthma control deteriorates when treatment is ignored and improves when it is taken regularly. We undertook to determine whether instruction in self-management adds significantly to the effects of self-monitoring alone on adherence to ICS treatment.
METHODS
Participants
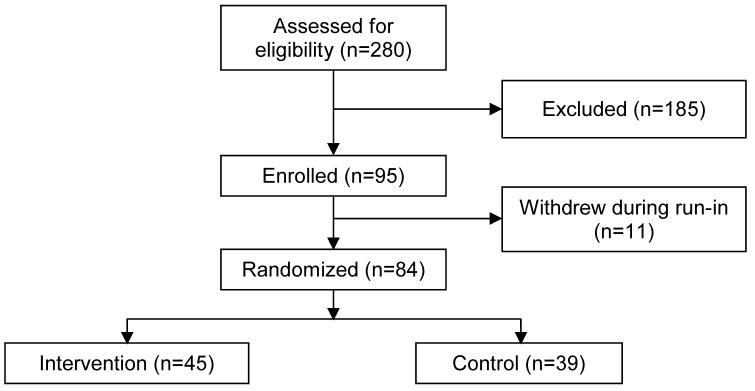
Adults with asthma (N=280) were recruited from private and public community clinics in the San Francisco Bay Area using posted flyers and advertisements. Patients telephoned to volunteer and were screened for eligibility. Participants included in the trial were 18–55 years of age with moderate to severe persistent asthma (i.e. FEV1< 80% predicted, daily symptoms, and ≥ 1 nighttime awakening per week)17, non-smoking with ≤5 pack-years of smoking history, and demonstrated spirometric evidence of reversible airflow obstruction or bronchial reactivity to inhaled methacholine18. Those receiving systemic steroids within four weeks of study enrollment; with upper respiratory tract infection within 6 weeks of enrollment, pregnancy, cardiac, gastrointestinal, psychiatric, or other lung disease; or prior participation in a formal asthma education program were excluded. Of those screened, 100 were ineligible because of mild or intermittent asthma, current smoking status, or nonreversible airflow obstruction, and another 85 were not interested (Figure 1). Ninety-five participants gave written consent and were enrolled in the 6 month trial. During the run-in phase prior to randomization, 11 participants voluntarily withdrew reporting inability to continue study visits due to time constraints. Participants were reimbursed for their time by hourly fee, received parking vouchers and received fluticasone without cost.
Figure 1.
Enrollment Flowchart
Study Design
The study was a randomized, controlled trial with run-in, intervention, and observation phases. The 4-week run-in with biweekly visits was used to stabilize ICS therapy (fluticasone) by adjusting the dose to the level recommended in the NHLBI Guidelines17 before introducing the intervention and to familiarize participants with self-monitoring. At the end of run-in, participants (n=84) were randomized by computer generated method to individualized asthma self-management education with self-monitoring of symptoms, peak flow, and nighttime awakenings (intervention) or self-monitoring alone (control). The 4-week intervention period of biweekly visits was followed by 14 weeks of observation with visits held at 4-week intervals. Except for the study coordinator, who had no role in data management or assessment, the investigators were blinded to group assignment.
Protocol
During each phase of the trial, all participants measured morning peak flow on an electronic peak flow meter (Airwatch™ iMetrikus, Carlsbad, California) and also recorded their daily values in a diary. All participants were told that higher peak flow numbers meant their airways were more open; lower numbers meant their airways were more closed. An electronic medication monitor, which concealed readings from the subject (Doser CT™, MediTrack, Hudson, MA), was placed on each ICS inhaler. Participants also monitored daily symptoms, nighttime awakenings, and tabulated ICS and IBA use in the diary. Data from the electronic monitors and diary pages were collected at each study visit.
The intervention was delivered in three identical 30-minute visits following randomization. Control participants attended the same number of visits, focusing on data collection only. Lung function, sputum markers, QOL and perceived control of asthma were measured at the end of run-in, end of intervention, and end of study. At the last study visit all participants were asked to report in writing what, if any, changes in asthma self-management they had made as a result of being in the study.
Individualized Self-Management Educational Intervention
The theoretically-based self-management intervention used for this study was described and validated previously10, 11. Tailored individualized components were added to maximize relevance19. The self-management intervention sessions were designed to be delivered in 30 minutes to simulate a clinical encounter. The first of the identical scripted sessions was delivered by a trained advanced practice nurse who was a certified asthma educator; the second and third were repeat reinforcements delivered by a respiratory therapist, also a certified asthma educator, who attended the first session. The education consisted of standardized components regarding asthma facts and medication actions, as well as individualized components. Personalized components included verbal and graphic interpretation of spirometry, peak flow trends, metered dose inhaler technique errors, and results of allergen skin testing along with specific strategies for control of personally relevant environmental exposures. This last component has been used previously in children20 but not in adults. Lastly, the peak flow monitor of the intervention participants was adjusted to reveal how daily readings compared with individual personal best. Zones based on a “traffic light” analogy were displayed on the monitor face and correlated to a simple written action plan. The action plan was not personalized to include increased doses of ICS or individualized prednisone as subjects remained under the care of their own personal physicians. No information about medication adherence was included in the intervention.
Outcomes
ICS adherence was calculated as the percentage of prescribed doses taken each week as measured by the electronic device validated for monitoring metered-dose inhaler use21. To avoid overestimation of adherence greater than 100% per day, the numerator was capped at the prescribed doses per day. Pulmonary function was assessed by spirometry pre-bronchodilator, after withholding short-acting beta-agonists for ≥ 6 hours and long-acting beta-agonist for ≥ 24hours. Forced expiratory volume in one second (FEV1) % predicted was used as a proxy variable for overall lung function22. QOL and perceived control of asthma were assessed using validated, self-completed questionnaires23, 24. Peak flow was measured by the electronic peak flow meter.
Asthma symptoms were rated daily by participants on validated 10-point numerical rating scales, where 0 equaled “none” and 10 equaled “very severe” from subjects’ diaries 25–28. Scores were averaged weekly for analysis. Symptom-free days (symptom score = 0), frequency of nighttime awakenings, and IBA use (# of puffs) were recorded daily and summed weekly for analysis.
Induced sputum samples were collected at end of run-in, end of intervention, and end of study to assess the degree of airway inflammation. Markers of inflammation included eosinophils, neutrophils, eosinophil cationic protein (ECP) and typtase. Processing and analysis were performed as previously described 11.
Statistical Analysis
The a priori power analysis showed a sample size of 80 was necessary to provide 80% power to detect a 10% change in adherence at α = .05; we enrolled 95 and randomized 84. Intention-to-treat analyses included all participants randomized, 78 with complete data and 6 with incomplete data.
The effect of the intervention on adherence was analyzed as mean adherence and also by categorizing adherence dichotomously as ≥60% or <60% adherence to prescribed dose12. Research has shown that typical ICS adherence is no greater than 50% in adults with asthma29. We chose 60% as an important cut-off point to determine whether average ICS adherence could be improved to and sustained above this level, i.e. 10% higher than reported norms. Effects of the intervention were assessed using linear mixed models analysis when variables were continuous; linear mixed models with a Poisson distribution were used with count variables. A mixed logistic model was used to assess binary variables (Stata Corp., College Station, Texas). These analyses were chosen as they account for missing data in the calculation of outcome variables. Non-skewed data were reported as mean change over time. Skewed data were log-transformed and presented as odds ratios, as were binary data. Count data was presented as incidence rate ratios (IRR).
We compared within group change rates from end of run-in (T0) to the end of intervention (T1), end of intervention to the end of study (T2), and end of run-in (T0) to end of study (T2) to assess the within group effects for the intervention and control groups. We then compared the change rates between the groups during those same time intervals to assess the effect of the individualized self-management intervention.
RESULTS
Table 1 shows the baseline characteristics for subjects randomized. There were no significant differences between groups except in peak flow. We adjusted the analysis of lung function for peak flow and there were no differences between the adjusted and unadjusted analyses. There were no significant differences between the participants that were randomized and those that were not (n=11, data not shown). Electronic and diary adherence and peak flow data were compared to look for concurrence, but only the electronic data for both were used in the analysis. Retrospective analysis showed that adherence to diary keeping and peak flow measuring over time declined by 0.02% for intervention subjects and 0.12% for controls (p=0.10).
Table 1.
Baseline Sample Characteristics
| Characteristic | Intervention (n = 45) |
Control (n = 39) |
P Value* |
|---|---|---|---|
| Number (%) or Mean ± SD | |||
| Age (years) | 36.8 ± 9.4 | 39.7 ± 9.3 | 0.17 |
| Female Gender | 24 (53) | 21 (54) | 0.96 |
| Race | |||
| Asian | 10 (22) | 6 (15) | 0.45 |
| Black/African-American | 1 (2) | 4 (10) | |
| Caucasian | 28 (62) | 26 (67) | |
| Other | 6 (14) | 3 (8) | |
| Ethnicity (Hispanic) | 3 (7) | 10 (26) | 0.02 |
| Education (years) | 16.1 ± 2.1 | 15.2 ± 2.3 | 0.06 |
| Employed | 41 (91) | 36 (92) | 0.84 |
| Health Care | |||
| Primary Care | 28 (62) | 22 (56) | 0.16 |
| Specialist | 12 (27) | 7 (18) | |
| Other | 5 (11) | 10 (26) | |
| Insured | 37 (82) | 27 (69) | 0.16 |
| Asthma Duration (years) | 22.0 ± 13.1 | 25.3 ± 14.7 | 0.29 |
| Rhinitis (yes) | 28 (62) | 29 (74) | 0.24 |
| Sinusitis (yes) | 7 (16) | 6 (15) | 0.98 |
| Severity by FEV1 Criteria (% participants)† | |||
| Severe (≤60% predicted) | 22 (49) | 18 (46) | 0.37 |
| Moderate (61–79% predicted) | 21 (47) | 21 (54) | |
| Mild (≥80% predicted) | 2 (4) | 0 | |
| FEV1 – Post-Bronchodilator (% Predicted) | 82.7 ± 14.1 | 78.5 ± 12.8 | 0.16 |
| Perceived Asthma Control Score (26–53) | 41.8 ± 6.1 | 40.2 ± 4.2 | 0.14 |
| Asthma Quality of Life Score (0–54) | 16.0 ± 11.0 | 15.8 ± 11.1 | 0.94 |
| Adherence (%) | 82 ± 18 | 81 ± 18 | 0.71 |
| Peak Flow (AM Only) | 427.4 ± 91.1 | 381.8 ± 110.2 | 0.04 |
| Mean Weekly Puffs of Beta-Agonist Used | 1.5 ± 1.9 | 1.7 ± 2.2 | 0.71 |
| Mean Weekly Symptom Score | 4.5 ± 4.4 | 5.1 ± 5.1 | 0.55 |
| Mean Percent of Symptom-Free Days per Week | 34.1 ± 37.1 | 31.0 ± 37.2 | 0.70 |
| Mean Weekly Number of Night Time Awakenings | 0.29 ± 0.69 | 0.35 ± 0.97 | 0.75 |
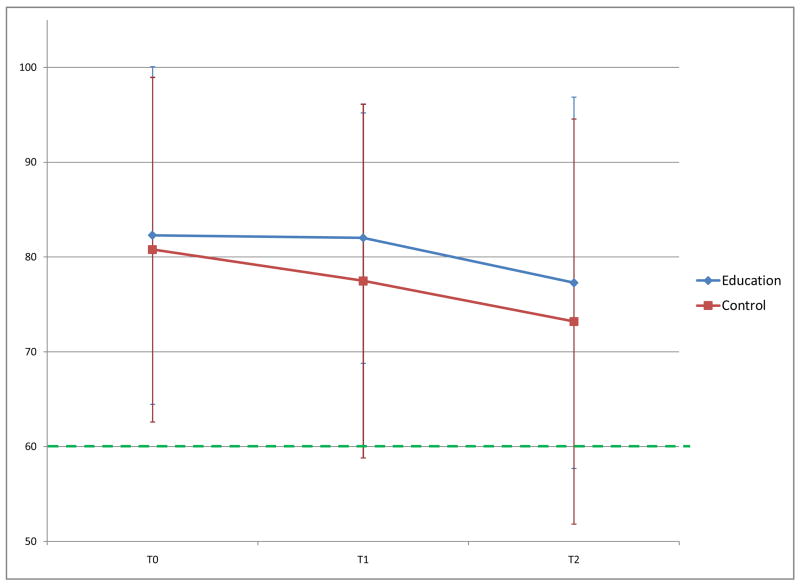
Mean (±SD) adherence for the intervention and control groups at T0 was 82% vs. 80%, at T1 it was 82% vs. 77% and at T2, 77% vs. 73% respectively. Mean adherence did not differ significantly between groups but stayed consistently higher over time in the intervention group compared to controls. At the end of the study, median adherence was 86% for intervention and 76% for controls. Mean change in adherence decreased in both groups over time, less so in the intervention group, but the differences were not statistically significant (Table 3). Odds of maintaining ≥60% adherence over the intervention period increased 9-fold for the intervention group and no change in the control group (OR 9.2 vs. 0.4, p=0.02; Table 2). At T2 the intervention group maintained 3-fold greater odds of ≥60% adherence.
Table 3.
Mean Changes in Outcomes
| Outcome | Time Interval* | Group | Mean Change | Within Group Significance | Between Group Significance |
|---|---|---|---|---|---|
| Adherence (%) | T0 – T1 | I | −0.18 | 0.94 | 0.72 |
| C | −1.40 | 0.58 | |||
|
| |||||
| T1 – T2 | I | −4.28 | 0.06 | 0.97 | |
| C | −4.41 | 0.09 | |||
|
| |||||
| T0 – T2 | I | −4.46 | 0.13 | 0.79 | |
| C | −5.81 | 0.08 | |||
|
| |||||
| FEV1 % predicted (pre-bronchodilator) | T0 – T1 | I | 1.47 | 0.08 | 0.32 |
| C | 2.72 | 0.003 | |||
|
| |||||
| T1 – T2 | I | 1.13 | 0.19 | 0.25 | |
| C | −0.37 | 0.70 | |||
|
| |||||
| T0 – T2 | I | 2.60 | 0.003 | 0.85 | |
| C | 2.35 | 0.02 | |||
|
| |||||
| Morning Peak Flow | T0 – T1 | I | 20.9 | <0.0005 | 0.24 |
| C | 11.5 | 0.05 | |||
|
| |||||
| T1 – T2 | I | 8.7 | 0.13 | 0.62 | |
| C | 13.0 | 0.05 | |||
|
| |||||
| T0 – T2 | I | 29.6 | <0.0005 | 0.65 | |
| C | 24.5 | 0.004 | |||
|
| |||||
| Perceived Control of Asthma | T0 – T1 | I | 1.89 | <0.0005 | 0.07 |
| C | 0.53 | 0.33 | |||
|
| |||||
| T1 – T2 | I | 0.98 | 0.06 | 0.28 | |
| C | 0.14 | 0.80 | |||
|
| |||||
| T0 – T2 | I | 2.87 | <0.0005 | 0.006 | |
| C | 0.68 | 0.25 | |||
|
| |||||
| Quality of Life | T0 – T1 | I | −2.71 | 0.01 | 0.36 |
| C | −1.39 | 0.20 | |||
|
| |||||
| T1 – T2 | I | −1.11 | 0.28 | 0.27 | |
| C | 0.58 | 0.61 | |||
|
| |||||
| T0 – T2 | I | −3.82 | <0.0005 | 0.06 | |
| C | −0.80 | 0.49 | |||
|
| |||||
| Symptom Score | T0 – T1 | I | −1.28 | 0.003 | 0.84 |
| C | −1.41 | 0.002 | |||
| T1 – T2 | I | −0.97 | 0.01 | 0.06 | |
| C | 0.11 | 0.80 | |||
| T0 – T2 | I | −2.25 | <0.0005 | 0.19 | |
| C | −1.30 | 0.02 | |||
|
| |||||
| Neutrophils | T0 – T1 | I | 2.7 | 0.46 | 0.41 |
| C | −1.7 | 0.67 | |||
| T1 – T2 | I | 2.6 | 0.50 | 0.18 | |
| C | −5.2 | 0.23 | |||
| T0 – T2 | I | 5.3 | 0.18 | 0.04 | |
| C | −6.7 | 0.13 | |||
|
| |||||
| Eosinophilic Cationic Protein (ECP) | T0 – T1 | I | 0.88 | 0.53 | 0.55 |
| C | 1.05 | 0.82 | |||
|
| |||||
| T1 – T2 | I | 0.88 | 0.52 | 0.44 | |
| C | 1.11 | 0.65 | |||
|
| |||||
| T0 – T2 | I | 0.77 | 0.21 | 0.18 | |
| C | 1.17 | 0.49 | |||
T0 = end of run-in phase, T1 = end of intervention phase, T2 = end of observation phase
Quality of Life Score
Table 2.
Odds Ratios for Outcomes
| Outcome | Time Interval* | Group | Odds Ratio | Within Group Significance | Between Group Significance |
|---|---|---|---|---|---|
| Adherence (≥60%) | T0 – T1 | I | 9.2 | 0.02 | 0.02 |
| C | 0.4 | 0.37 | |||
|
| |||||
| T1 – T2 | I | 0.3 | 0.32 | 0.31 | |
| C | 1.1 | 0.90 | |||
|
| |||||
| T0 – T2 | I | 3.0 | 0.38 | 0.22 | |
| C | 0.5 | 0.58 | |||
|
| |||||
| Symptom Free Days | T0 – T1 | I | 2.2 | 0.01 | 0.48 |
| C | 1.6 | 0.23 | |||
|
| |||||
| T1 – T2 | I | 2.7 | 0.07 | 0.63 | |
| C | 1.8 | 0.36 | |||
|
| |||||
| T0 – T2 | I | 5.9 | 0.02 | 0.51 | |
| C | 2.8 | 0.23 | |||
|
| |||||
| Night Time Awakenings | T0 – T1 | I | 0.2 | 0.001 | 0.13 |
| C | 0.7 | 0.37 | |||
|
| |||||
| T1 – T2 | I | 0.7 | 0.49 | 0.45 | |
| C | 1.2 | 0.71 | |||
|
| |||||
| T0 – T2 | I | 0.2 | <0.0005 | 0.03 | |
| C | 0.8 | 0.59 | |||
|
| |||||
| Beta-Agonist Use† | T0 – T1 | I | 0.6 | <0.0005 | 0.01 |
| C | 0.8 | 0.057 | |||
|
| |||||
| T1 – T2 | I | 0.5 | 0.001 | 0.98 | |
| C | 0.5 | 0.002 | |||
|
| |||||
| T0 – T2 | I | 0.3 | <0.0005 | 0.30 | |
| C | 0.4 | 0.004 | |||
|
| |||||
| Eosinophils (>0%) | T0 – T1 | I | 0.5 | 0.24 | 0.40 |
| C | 1.0 | 0.97 | |||
| T1 – T2 | I | 3.1 | 0.12 | 0.09 | |
| C | 0.6 | 0.38 | |||
| T0 – T2 | I | 1.7 | 0.49 | 0.29 | |
| C | 0.6 | 0.41 | |||
|
| |||||
| Tryptase (>0) | T0 – T1 | I | 0.1 | 0.02 | 0.29 |
| C | 0.2 | 0.22 | |||
| T1 – T2 | I | 0.1 | 0.10 | 0.24 | |
| C | 0.4 | 0.54 | |||
| T0 – T2 | I | 0.0 | 0.03 | 0.08 | |
| C | 0.1 | 0.27 | |||
T0 = end of run-in phase, T1 = end of intervention phase, T2 = end of observation phase
Reported values are in incidence rate ratios.
The odds of being symptom free were significantly higher during the intervention and at end of study for the intervention group (T0 –T1 OR 2.2, p=0.01; T0–T2 OR 5.9, p=0.02) but the change rates were not significantly different between groups (Table 2). Mean change in symptom scores also decreased significantly for both groups over time and the change rates were not significantly different between groups (Table 3).
The odds of nighttime awakenings decreased significantly over time in the intervention group (T0–T1 OR 0.24, p=0.001; T0–T2 OR 0.17, p<.001) compared to no significant change in the control group (Table 2). The odds of experiencing nighttime awakenings due to asthma over the entire study decreased significantly in the intervention group vs. controls (T0–T2 OR 0.17 vs. 0.77, p=0.03).
The incidence of rescue beta agonist use decreased significantly during the intervention period in intervention vs. control subjects (T0–T1 IRR 0.56, p<0.001; Table 2). Both groups decreased beta agonist use over time with no significant differences between groups by T2.
Pre-bronchodilator FEV1 % predicted improved in both groups during the intervention period and over the period of the entire study with no significant differences between groups (Table 3). Post-bronchodilator FEV1% predicted improved in the intervention group at all time points and declined in the control group with no significant differences between groups. Morning peak flow improved significantly for the intervention group compared to controls during the intervention period (T0–T1 20.9 L/min., p<0.001 vs. 11.5 L/min., p=0.052) and both groups improved by end of study (T0–T2 29.6 L/min., p<0.001 vs. 24.5 L/min., p=0.004) with no significant differences in the change rates between groups (Table 3).
In the intervention group perceived control of asthma improved significantly during the intervention period (T0–T1 1.89, p<0.001 vs. 0.53, p=0.37) and during the entire study (T0–T2 2.87, p<0.001 vs. 0.68, p=0.25; between group difference, p=0.006, Table 3). Quality of life scores improved significantly in the intervention group from T0–T1 (−2.71, p=0.01) and T0–T2 (−3.82, p<0.001) compared to no change in the control group; between group difference for T0–T2 showed a trend favoring the intervention (p=0.07).
Eosinophils (%) and tryptase (μg/L) were highly skewed and converted to binary variables; positive for eosinophils if total cell count was above the lower limit of detection (positive >1/500cells) and positive for tryptase if total concentration was above the lower limit of detection (positive > 1μg/L). Neutrophils (%) and ECP (ng/mL) were reported as means. There were no significant differences in the odds of eosinophils being positive within groups or between groups. Likewise, there were no significant differences within or between groups in ECP. There was a significant decrease in the odds of tryptase being positive for the intervention group T0 to T1 (OR 0.06, p=0.02) and an overall decrease from T0 to T2 (OR 0.003, p=0.03) but no significant differences between groups (Table 2). There were no significant within group changes in neutrophils in either group. However, over the entire study period there was a increase in mean neutrophils of 5.3% for the intervention group and a decrease in neutrophils of −6.7% for the control group; with a significant between group difference (p=0.04).
Intervention subjects reported significantly more changes in self-management behavior during the study than controls (mean per person changes 1.82 versus 0.87, p<0.0005). Overall, 98% of intervention subjects reported one or more changes vs. 64% of controls. Intervention subjects reported improving inhaler technique (p=0.03), reducing outdoor allergen exposure (p=0.02) and reducing indoor dust exposure (p<0.0005) significantly more frequently than controls as shown in table 4.
Table 4.
Self-Described Changes in Self-Management Behavior after Study Participation
| Change | Intervention | Control | P Value |
|---|---|---|---|
| Number (%) or Mean ± SD | |||
| Improved Adherence | 21 (47) | 17 (44) | 0.78 |
| Changed Medication | 2 (4) | 2 (5) | 0.88 |
| Improved Inhaler Technique | 10 (22) | 2 (5) | 0.03 |
| Increased Peak Flow Meter Use | 8 (18) | 6 (15) | 0.77 |
| Started Using Spacer | 4 (9) | 0 | 0.06 |
| Reduced Dust Exposure | 19 (42) | 2 (5) | <0.0005 |
| Reduced Pet Exposure | 4 (9) | 0 | 0.06 |
| Mould Remediation | 1 (2) | 0 | 0.35 |
| Increased In-Home Ventilation | 2 (4) | 1 (3) | 0.64 |
| Avoided Outdoor Air Pollution | 1 (2) | 2 (5) | 0.47 |
| Reduced Household Chemical Exposure | 2 (4) | 0 | 0.18 |
| Reduced Occupational Trigger Exposure | 0 | 1 (3) | 0.28 |
| Avoided Food Triggers | 1 (2) | 1 (3) | 0.92 |
| Avoided Alcohol Triggers | 1 (2) | 0 | 0.35 |
| Avoided Outdoor Allergens | 6 (7) | 0 | 0.02 |
|
| |||
| Average Number of Changes per Person | 1.82 ± 0.91 | 0.87 ± 0.89 | <0.0005 |
DISCUSSION
Meta-analysis of published randomized trials of asthma self-management has shown improved health outcomes, especially reduction in ED visits and hospitalizations8. Fewer trials have evaluated the impact of self-management education on adherence to ICS treatment with mixed results12, 30. In our study, individualized asthma self-management training that incorporated behavioral strategies and self-monitoring was more effective in maintaining adherence to ICS, decreasing nighttime awakenings and rescue IBA use, and increasing perceived control of asthma than self-monitoring alone. Although participants in the control group self-monitored and had research visits as often as the intervention group, they did not maintain ICS adherence, decrease nighttime awakenings or IBA use, or improve their perception of asthma control. Mean ICS adherence in the control group declined over the study to levels similar to those reported in usual care31. Rates of adherence for the control group were higher than those reported by other published adherence studies32 but this finding was consistent with our expectation of the influence of continuous monitoring and free ICS medication (fluticasone). The effect of the intervention was more pronounced during the intervention period, producing a 9-fold increase in the odds of ≥60% adherence to ICS in the intervention group compared to controls, p=0.02. The odds of ≥60% adherence were preserved in the intervention group at a ratio of 3:1 to the end of the 24 week trial. There was waning of adherence over time in both groups but less so in those who had been trained in asthma self-management.
Key indicators of clinical asthma control improved over time in the intervention group compared to controls with the odds of experiencing nighttime awakenings decreasing significantly by the end of the intervention and by the end of the study. Rescue IBA use also decreased significantly in the intervention group compared to controls during the intervention period. Eventually during the observation period, incidence of IBA use decreased significantly in the control group as well, suggesting that personalized self-management has a more immediate impact but that self-monitoring alone also decrease IBA use over time. Perceived control of asthma also improved significantly among intervention participants compared to controls, showing a parallel change with other clinical markers of asthma control. Symptoms decreased in both groups suggesting this outcome is sensitive to the placebo effect of being enrolled in a research trial. The intervention had little impact on pulmonary function, as others have found 8 and a small statistically significant effect on mean peak flow of 30 L/min in the intervention group.
Self-management education had mixed effects on sputum markers of inflammation with no effects on the eosinophils or their primary constituent, ECP. Intervention participants had a significant decrease in tryptase. A possible explanation is the significant reduction in allergen exposures made by the intervention participants (table 4). A pattern emerged with respect to neutrophilic changes between the two groups. While neutrophils decreased 6.7% in the control group, they increased 5.7% in the intervention group, possibly reflecting the neutrophilic effects of greater cumulative corticosteroid inhalation as a function of greater adherence. These findings were inconsistent with those found previously11 and difficult to assess for true significance due to the highly dispersed nature of the data. The role of monitoring inflammatory biological markers in asthma remains unclear.
Limitations
Although our adherence results are strengthened by the use and analysis of electronic monitoring, no device is infallible. The Doser CT™ has a date stamp that tallied the number of puffs for each day. Data was capped at prescribed dose/frequency to avoid overestimation of adherence at greater than 100% per day. The phenomenon of “data dumping” just before a research visit could not be ruled out up to the prescribed daily dose. Loss of data can occur if the device is lost or the battery fails between research visits. We used analytic methods that allowed for missing data. Diary forms were necessary to collect information about symptoms and nighttime awakenings and these were subject to the limitations of self-report, which include forgetting to record information, loss of diary booklets, recording incorrect information, and nonadherence with recording data overtime. Similarly, changes in self-management behavior during the trial were also subject to the limitations of self-report. Additionally, our findings may not be generalizable to populations with low levels of education and high levels of unemployment, given the relatively high level of employment and education in our study population.
Conclusions
Notably self-monitoring alone did not prevent a decline in medication adherence. These results are similar to results reported by others who found self-monitoring did not improve medication adherence33–35. Our results show that self-management education coupled with self-monitoring attenuated the often observed decline in medication adherence and improved asthma clinical outcomes. With the modest time and resources required to achieve these outcomes it appears to be worth the effort to include self-management education with self-monitoring in clinical practice settings where adults with asthma are seen. Our study was not designed to analyze the cost effectiveness of the intervention. However, a trained and certified asthma educator may be cost-effective if the goal is to improve asthma control and reduce urgent care visits. Alternatively, if the clinical goal is to reduce overuse of IBA, then self-monitoring of symptoms and peak flow may be an adequate and cost-effective approach.
Including the novel feature of personalizing allergen exposure control based on skin test results in adult asthma self-management education is unique to this study. It is now recommended by EPR-3 asthma guidelines36 and likely increased the power of our intervention. The improvements observed indicate the positive value of personalizing asthma self-management training.
Figure 2.
Mean Adherence to Inhaled Corticosteroid Over 6 Months.
Acknowledgments
Funding Sources: This research was supported by a grant from the National Institutes of Health (NIH/NHLBI R01HL64586) and unrestricted donations from GlaxoSmithKline (Donation of Flovent® Inhalation Aerosol)
ABREVIATIONS
- ICS
Inhaled Corticosteroid
- IBA
Inhaled Beta-agonist
- FEV1
Forced Expiratory Volume in One Second
- QOL
Quality of Life
- PB
Personal Best
- ECP
Eosinophil Cationic Protein
- OR
Odds Ratio
Footnotes
Conflict of Interest: None of the authors of this article have any conflict of interest in the sense of the ICMJE requirements for manuscripts.
CLINICAL IMPLICATIONS
Individualized self-management education coupled with self-monitoring of asthma symptoms, nighttime awakenings, and peak flow confers additional benefits in adults with asthma beyond self-monitoring alone and should be considered in clinical settings where adults with asthma are seen.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lethbridge-Sejku MSJ, Bernadel L. Summary Health Statistics for U.S. Adults: National Health Interview Survey, 2002. National Center for Health Statistics Vital Health Stat. 2004;10(222) [PubMed] [Google Scholar]
- 2.Kochanek KDSL, Murphy BS, Anderson RN, Scott C. Deaths: Final Data for 2002. National Center for Health Statistics Vital Health Stat. 2004;53(5) [PubMed] [Google Scholar]
- 3.Ignacio-Garcia JM, Gonzalez-Santos P. Asthma self-management education program by home monitoring of peak expiratory flow. Am J Respir Crit Care Med. 1995;151(2 Pt 1):353–9. doi: 10.1164/ajrccm.151.2.7842191. [DOI] [PubMed] [Google Scholar]
- 4.Wilson SR, Scamagas P, German DF, Hughes GW, Lulla S, Coss S, et al. A controlled trial of two forms of self-management education for adults with asthma. Am J Med. 1993;94(6):564–76. doi: 10.1016/0002-9343(93)90206-5. [DOI] [PubMed] [Google Scholar]
- 5.Bailey WC, Richards JM, Jr, Brooks CM, Soong SJ, Windsor RA, Manzella BA. A randomized trial to improve self-management practices of adults with asthma. Arch Intern Med. 1990;150(8):1664–8. [PubMed] [Google Scholar]
- 6.Kotses H, Bernstein IL, Bernstein DI, Reynolds RV, Korbee L, Wigal JKE, et al. A self-management program for adult asthma.Part I: Development and evaluation. J Allergy Clin Immunol. 1995;95(2):529–40. doi: 10.1016/s0091-6749(95)70315-2. [DOI] [PubMed] [Google Scholar]
- 7.Kotses H, Stout C, McConnaughy K, Winder JA, Creer TL. Evaluation of individualized asthma self-management programs. J Asthma. 1996;33(2):113–8. doi: 10.3109/02770909609054539. [DOI] [PubMed] [Google Scholar]
- 8.Gibson PG, Powell H, Coughlan J, Wilson AJ, Abramson M, Haywood P, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev. 2003;(1):CD001117. doi: 10.1002/14651858.CD001117. [DOI] [PubMed] [Google Scholar]
- 9.Powell H, Gibson PG. Options for self-management education for adults with asthma. Cochrane Database Syst Rev. 2003;(1):CD004107. doi: 10.1002/14651858.CD004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janson SL, Fahy JV, Covington JK, Paul SM, Gold WM, Boushey HA. Effects of individual self-management education on clinical, biological, and adherence outcomes in asthma. Am J Med. 2003;115(8):620–6. doi: 10.1016/j.amjmed.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Janson SL, Hardie G, Fahy JV, Boushey HA. Use of biological markers of airway inflammation to detect the efficacy of nurse-delivered asthma education. Heart & Lung. 2001;30:39–46. doi: 10.1067/mhl.2001.110290. [DOI] [PubMed] [Google Scholar]
- 12.Gallefoss F, Bakke PS. How does patient education and self-management among asthmatics and patients with chronic obstructive pulmonary disease affect medication? Am J Respir Crit Care Med. 1999;160(6):2000–5. doi: 10.1164/ajrccm.160.6.9901028. [DOI] [PubMed] [Google Scholar]
- 13.Schaffer SD, Tian L. Promoting adherence: effects of theory-based asthma education. Clin Nurs Res. 2004;13(1):69–89. doi: 10.1177/1054773803259300. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Vina A, del Castillo-Arevalo E. Influence of peak expiratory flow monitoring on an asthma self-management education programme. Respir Med. 2000;94(8):760–6. doi: 10.1053/rmed.2000.0815. [DOI] [PubMed] [Google Scholar]
- 15.Ryan P, Lauver DR. The efficacy of tailored interventions. J Nurs Scholarsh. 2002;34(4):331–7. doi: 10.1111/j.1547-5069.2002.00331.x. [DOI] [PubMed] [Google Scholar]
- 16.Paasche-Orlow MK, Riekert KA, Bilderback A, Chanmugam A, Hill P, Rand CS, et al. Tailored education may reduce health literacy disparities in asthma self-management. Am J Respir Crit Care Med. 2005;172(8):980–6. doi: 10.1164/rccm.200409-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NAEPP. L. National Heart, and Blood Institute. National Institutes of Health; Bethesda, MD: 2002. NAEPP Expert Panel Report Guideline for the Diagnosis and Management of Asthma - Update on Selected Topics 2002. [Google Scholar]
- 18.Popa V. ATS guidelines for methacholine and exercise challenge testing. Am J Respir Crit Care Med. 2001;163(1):292–3. doi: 10.1164/ajrccm.163.1.16310b. [DOI] [PubMed] [Google Scholar]
- 19.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 20.Bonner S, Zimmerman BJ, Evans D, Irigoyen M, Resnick D, Mellins RB. An individualized intervention to improve asthma management among urban Latino and African-American families. J Asthma. 2002;39(2):167–79. doi: 10.1081/jas-120002198. [DOI] [PubMed] [Google Scholar]
- 21.Simmons MS, Nides MA, Kleerup EC, Chapman KR, Milgrom H, Rand CS, et al. Validation of the Doser, a new device for monitoring metered-dose inhaler use. J Allergy Clin Immunol. 1998;102(3):409–412. doi: 10.1016/s0091-6749(98)70128-9. [DOI] [PubMed] [Google Scholar]
- 22.De Smet BD, Erickson SR, Kirking DM. Self-reported adherence in patients with asthma. Ann Pharmacother. 2006;40(3):414–20. doi: 10.1345/aph.1G475. [DOI] [PubMed] [Google Scholar]
- 23.Marks GB, Dunn SM, Woolcock AJ. A scale for the measurement of quality of life in adults with asthma. J Clin Epidemiol. 1992;45(5):461–72. doi: 10.1016/0895-4356(92)90095-5. [DOI] [PubMed] [Google Scholar]
- 24.Katz PP, Yelin EH, Eisner MD, Blanc PD. Perceived control of asthma and quality of life among adults with asthma. Ann Allergy Asthma Immunol. 2002;89(3):251–8. doi: 10.1016/S1081-1206(10)61951-5. [DOI] [PubMed] [Google Scholar]
- 25.Haahtela T, Jarvinen M, Kava T, Kiviranta K, Koskinen S, Lehtonen K, et al. Comparison of a beta 2-agonist, terbutaline, with an inhaled corticosteroid, budesonide, in newly detected asthma. N Engl J Med. 1991;325(6):388–92. doi: 10.1056/NEJM199108083250603. [DOI] [PubMed] [Google Scholar]
- 26.Janson-Bjerklie S, Shnell S. Effect of peak flow information on patterns of self-care in adult asthma. Heart Lung. 1988;17(5):543–9. [PubMed] [Google Scholar]
- 27.Janson-Bjerklie S, Ferketich S, Benner P. Predicting the outcomes of living with asthma. Res Nurs Health. 1993;16(4):241–50. doi: 10.1002/nur.4770160403. [DOI] [PubMed] [Google Scholar]
- 28.Beasley R, Cushley M, Holgate ST. A self management plan in the treatment of adult asthma. Thorax. 1989;44(3):200–4. doi: 10.1136/thx.44.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillisen A. Patient’s adherence in asthma. J Physiol Pharmacol. 2007;58(Suppl 5 Pt 1):205–22. [PubMed] [Google Scholar]
- 30.Couturand F, Proust A, Frachon I, Dewitte JD, Oger E, Quiot JJ, et al. Education and self-management: a one-year randomized trial in stable asthmatic patients. J Asthma. 2002;39(6):493–500. doi: 10.1081/jas-120004913. [DOI] [PubMed] [Google Scholar]
- 31.Williams LK, Pladevall M, Xi H, Peterson EL, Joseph C, Lafata JE, Ownby DR, Johnson CC. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol. 2004;114(6):1288–93. doi: 10.1016/j.jaci.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 32.Cochrane MG, Bala MV, Downs KE, Mauskopf J, Ben-Joseph RH. Inhaled corticosteroids for asthma therapy: patient compliance, devices, and inhalation technique. Chest. 2000;117(2):542–50. doi: 10.1378/chest.117.2.542. [DOI] [PubMed] [Google Scholar]
- 33.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews 2008. (2):CD000011. doi: 10.1002/14651858.CD000011.pub3. Art. No. [DOI] [PubMed] [Google Scholar]
- 34.Cote J, Cartier A, Robichaud P, Boutin H, Malo JL, Rouleau M, Fillion A, Lavallee M, Krusky M, Boulet LP. Influence on asthma morbidity of asthma education programs based on self-management plans following treatment optimization. Am J Respir Crit Care Med. 1997;155:1509–1514. doi: 10.1164/ajrccm.155.5.9154850. [DOI] [PubMed] [Google Scholar]
- 35.Morice AH, Wrench C. The role of the asthma nurse in treatment compliance and self-management following hospital admission. Respiratory Medicine. 2001;95:851–856. doi: 10.1053/rmed.2001.1166. [DOI] [PubMed] [Google Scholar]
- 36.NAEPP. National Heart, Lung, and Blood Institute, editor. National Institutes of Health; Bethesda, MD: 2007. NAEPP Expert Panel Report Guideline for the Diagnosis and Management of Asthma. [Google Scholar]