Abstract
Context: There are limited and controversial data concerning the relationships between retinol-binding protein 4 (RBP4), weight status, and insulin resistance in obese humans and especially in children.
Objective: Our objective was to study the longitudinal relationships among RBP4, insulin resistance and weight status in obese children.
Design, Setting, and Patients: We conducted a 1-yr longitudinal follow-up study in a primary-care setting with 43 obese children (median age 10.8 yr) and 19 lean children of same the age and gender.
Intervention: Our outpatient 1-yr intervention program was based on exercise, behavior, and nutrition therapy.
Main Outcomes Measures: Changes of weight status (body mass index sd score), RBP4, molar RBP4/serum retinol (SR) ratio, insulin resistance index homeostasis model assessment (HOMA), and quantitative insulin sensitivity check index (QUICKI).
Results: Obese children had significantly (P < 0.01) higher RBP4 concentrations and a higher RBP4/SR ratio compared with lean children. In multiple linear regression analyses adjusted to age, gender, and pubertal stage, RBP4 was significantly correlated to insulin and body mass index. Pubertal children demonstrated significantly decreased QUICKI and significantly increased HOMA index, insulin, and RBP4 concentrations compared with prepubertal children. Changes of RBP4 correlated significantly to changes of insulin (r = 0.29), HOMA index (r = 0.29), QUICKI (r = 0.22), and weight status (r = 0.31). Substantial weight loss in 25 children led to a significant (P < 0.001) decrease of RBP4, RBP4/SR, blood pressure, triglycerides, insulin, and HOMA index and an increase in QUICKI in contrast to the 18 children without substantial weight loss.
Conclusion: RBP4 levels were related to weight status and insulin resistance in both cross-sectional and longitudinal analyses, suggesting a relationship between RBP4, obesity, and insulin resistance in children.
A 1-year longitudinal study of 43 obese children finds that retinol-binding protein 4 levels were related to weight status and insulin resistance, with levels normalizing after weight loss.
Obesity, characterized by excess accumulation of adipose tissue, is the most common risk factor for the metabolic syndrome, a cluster of dyslipidemia, insulin resistance, hypertension, and atherosclerosis (1). Although the molecular pathways that link obesity to such a wide spectrum of metabolic and cardiovascular defects are not yet fully understood, recent studies indicated a central role of adipose tissue in the development of these syndromes (2,3). Moreover, adipose tissue has been demonstrated to secrete various adipokines such as leptin and adiponectin, implicating their involvement in the development of metabolic syndrome and influencing insulin resistance (4). Retinol-binding protein 4 (RBP4), secreted primarily from the liver and adipose tissue, was recently proposed as another link between obesity and insulin resistance (5). Circulating RBP4 concentrations are elevated in several mouse models of obesity and insulin resistance, and deleting the RBP4 gene in mice has been shown to increase insulin sensitivity (5).
The link between RBP4, obesity, and insulin resistance in humans is less clear. Some previous studies in adults have reported significant associations between RBP4, obesity, and insulin resistance (6,7,8,9). In contrast, other studies have found no link between RBP and obesity and/or insulin resistance in adults (10,11,12,13,14). Studies in adults done before RBP4 was identified as an adipokine also reported equivocal results (15,16,17). Due to these controversial findings in cross-sectional studies, it would appear that longitudinal studies are preferable in clarifying these metabolic relationships. However, the few recent weight loss studies in obese humans have reported controversial findings, some demonstrating decreased RBP4 concentrations (7,9,18,19) and some reporting stable RBP4 concentrations (11).
Because insulin resistance as well as metabolic syndrome often begin in childhood or young adulthood (20), studies in this age group are important. One additional advantage of examining children is that a diminished potential confusion exists with adult-onset complications such as coronary disease, medications such as birth-control pills, active tobacco smoking, alcohol use, etc.
Given the inconsistency of the findings concerning RBP4 in obesity and insulin resistance as well as the limited data regarding obese children, we studied RBP4 levels and their changes in obese children as well as their relationships to insulin, insulin resistance, and other markers of the metabolic syndrome in the course of 1 yr in a lifestyle intervention.
Subjects and Methods
Written informed consent was obtained from all children and their parents. The study was approved by the local ethics committee of the University of Witten/Herdecke in Germany.
We examined anthropometrical markers, fasting serum RBP4, serum retinol (SR), prealbumin, glucose, insulin, blood pressure, triglycerides, and high-density lipoprotein (HDL)- and low-density lipoprotein (LDL)-cholesterol concentrations in 43 obese Caucasian children and in 19 lean healthy Caucasian children of similar age, gender, and pubertal stage. The obese children were studied before and after participating in the 1-yr lifestyle intervention Obeldicks, which has been described in detail elsewhere (21,22). Briefly, this outpatient intervention program for obese children is based on physical exercise, nutrition education, and behavior therapy including individual psychological care of the child and his or her family. The nutritional course is based on a fat- and sugar-reduced diet compared with the everyday nutrition of German children.
None of the children in the cohort of the current study suffered from endocrine disorders, premature adrenarche, or syndromal obesity. Obesity was defined according to the definition of the International Task Force of Obesity using population-specific data (23).
Height was measured to the nearest centimeter using a rigid stadiometer. Weight was measured in underwear to the nearest 0.1 kg using a calibrated balance scale. Because distribution of body mass index (BMI) is not comparable in children and adults, not even among the various childhood age groups, we used the LMS method to calculate BMI sd score (SDS) as a measurement for the degree of overweight. The LMS method was chosen because it summarizes the data in terms of three smooth age-specific curves called L (λ), M (μ), and S (σ) based on German population-specific data (24,25). The M and S curves correspond to the median and coefficient of variations of BMI for German children at each age and gender, whereas the L curve allows for the substantial age-dependent skewness in the distribution of BMI (24,25). The assumption underlying the LMS method is that after Box-Cox power transformation, the data at each age are normally distributed (24).
Triceps and subscapular skinfold thicknesses were measured in duplicate using a caliper and averaged to calculate the percentage of body fat using a skinfold thickness equation with the following formulas (26): for boys, body fat percent = 0.783 × (subscapular skinfold thickness + triceps skinfold thickness in millimeters) + 1.6; for girls, body fat percent = 0.546 × (subscapular skinfold thickness + triceps skinfold thickness in millimeters) + 9.7.
The pubertal developmental stage was determined according to Marshall and Tanner and categorized into two groups (prepubertal: boys with pubic hair and gonadal stage I and girls with pubic hair stage and breast stage I; pubertal: boys with pubic hair or gonadal stage ≥ II and girls with pubic hair stage or breast stage ≥ II).
Blood pressure was measured according to the guidelines of the National High Blood Pressure Education Program (27). Systolic and diastolic blood pressure were measured twice at the right arm after a 10-min rest in the supine position using a calibrated sphygmomanometer and averaged. The cuff size of the sphygmomanometer used, based on the length and circumference of the upper arm, was as large as possible without having the elbow skin crease obstruct the stethoscope (27).
Blood sampling was performed in the fasting state at 0800 h. All serum probes were frozen opaque at −81 C and thawed only once. Serum RBP4 concentrations were measured by a high-specific ELISA (human RBP-4; Phoenix Pharmaceuticals, Burlingame, CA). The antibody did not cross-react with insulin, leptin, TNF-α, adiponectin, resistin, apelin, or visfatin. The sensitivity was 2 ng/ml; the inter- and intraassay coefficients of variation were less than 14% and less than 5%, respectively. SR was measured using an Agilent Series 1100 HPLC and a commercially available reversed-phase HPLC assay (Bio-Rad vitamin A by HPLC, catalog no. 195-5869; Bio-Rad, Munich, Germany) coupled with subsequent UV detection and quantitative determination with the help of an Internal Standard (Bio-Rad UV-detection model 1801 at 340 and 295 nm) according to the manufacturer’s instructions. The mobile phase was set at a flow rate of 0.6 ml/min. The intra- and interassay coefficients of variation were 4.6 and 4.9%, respectively. Serum prealbumin was measured using a commercially available nephelometric assay (Dade Behring, Marburg, Germany) with a Behring nephelometer II. In brief, in an immunochemical reaction, the proteins in the sample of human serum form immune complexes with specific antibodies. These complexes scatter a beam of light passed through the sample. The intensity of the scattered light is proportional to the concentration of the relevant protein in the sample. The result is evaluated by comparison with a standard of known concentration. The intra- and interassay coefficients of variation were 1.4 and 1.9%, respectively. The molar RBP4 to SR ratio was calculated by dividing serum RBP4 concentrations (micromoles per liter) by the SR concentrations (micromoles per liter). Insulin concentrations were measured by microparticle-enhanced immunometric assay (MEIA; Abbott, Wiesbaden, Germany). Glucose levels were determined by colorimetric test using a Vitros analyzer (Ortho Clinical Diagnostics, Neckargmuend, Germany). HDL- and LDL-cholesterol concentrations were measured by an enzymatic test (HDL-C-Plus and LDL-C-Plus; Roche Diagnostics, Mannheim, Germany) and triglyceride concentrations by a colorimetric assay using a Vitros analyzer (Ortho Clinical Diagnostics). Intra- and interassay CVs were less than 5% in all these methods. Homeostasis model assessment (HOMA) was calculated by the following formula: resistance (HOMA) = [insulin (milliunits per liter) × glucose (millimoles per liter)]/22.5 (28). The quantitative insulin sensitivity check index (QUICKI) was calculated as follows: QUICKI = 1/[log(fasting insulin in milliunits per liter) + log(fasting glucose milligrams per deciliter)] (29).
Using the LMS calculation method described above, substantial weight loss over the course of the 1 yr was defined by a reduction of SDS-BMI of at least 0.5, because with a reduction of less than 0.5 SDS-BMI, no improvement of insulin resistance and cardiovascular risk factors could be measured in obese children (30,31).
Statistical analyses were performed using the Winstat software package. All variables normally distributed were tested by the Kolmogorov-Smirnov test. Student’s t test for paired and unpaired observations, ANOVA, and χ2 were used as appropriate. Correlations between RBP4, lipids, insulin, and insulin resistance index HOMA at baseline, as well as correlations between changes of weight status, RBP4, lipids, and insulin in the course of 1 yr, were calculated by Pearson’s correlation. Partial regressions analyses adjusted to SDS-BMI or change of SDS-BMI were also performed. Changes were expressed as δ variable calculated by variable at baseline minus variable measured 1 yr later. Direct multiple linear regression analyses were conducted for the dependent variable RBP4, including age, gender, pubertal stage, weight status (BMI), glucose, and insulin as independent variables in the collective of normal-weight and obese children. Gender and pubertal stage were used as classified variables in this model. A P value < 0.05 was considered as significant. Data are presented as mean and sd.
Results
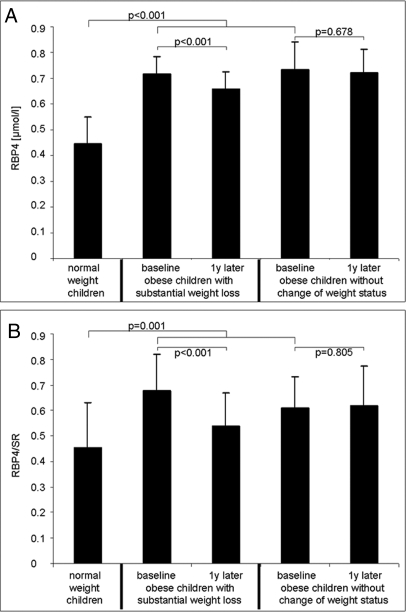
Age, stage of puberty, gender, and degree of overweight (BMI and SDS-BMI) of the 43 obese and 19 lean children are shown in Table 1. The lean and obese children did not differ significantly in terms of age, gender, or pubertal status. RBP4 concentrations and RBP4/SR were significantly (P < 0.001) increased in obese children compared with normal-weight children (see Fig. 1).
Table 1.
Age, gender, pubertal stage, and weight status in obese and normal-weight children
| Obese | Normal weight | P value | |
|---|---|---|---|
| n | 43 | 19 | |
| Age (yr) | 10.8 ± 2.6 | 10.3 ± 2.9 | 0.873 |
| Male (%) | 39 | 42 | 0.824 |
| Prepubertal (%) | 42 | 58 | 0.244 |
| BMI (kg/m²) | 27.3 ± 4.2 | 15.9 ± 2.1 | <0.001 |
| SDS-BMI | 2.3 ± 0.4 | −0.9 ± 0.8 | <0.001 |
Data are shown as mean ± sd.
Figure 1.
A, RBP4 concentrations in 19 normal-weight children, 25 obese children with substantial weight loss, and 18 obese children without change of weight status in the course of 1 yr; B, molar ratio of RBP4/SR in 19 normal-weight children, 25 obese children with substantial weight loss, and 18 obese children without change of weight status in the course of 1 yr (data as mean and sd).
In the collective of obese and nonobese children, RBP4 correlated significantly to SDS-BMI (r = 0.75), percentage of body fat (r = 0.65), and SR (r = 0.31) but not to age at baseline. The molar ratio RBP4/SRo correlated significantly to SDS-BMI (r = 0.47) and percentage of body fat (r = 0.36) but not to age. In partial regression analysis adjusted to SDS-BMI, RBP4 correlated significantly to systolic (r = 0.25) and diastolic (r = 0.32) blood pressure, triglycerides (r = 0.25), insulin (r = 0.38), insulin resistance index HOMA (r = 0.37), and QUICKI (r = −0.53), but not to glucose or HDL- or LDL-cholesterol. The molar ratio RBP4/SR correlated significantly to triglycerides (r = 0.26) in partial regression analysis adjusted for SDS-BMI but not to blood pressure, glucose, insulin, HDL- or LDL-cholesterol, HOMA, or QUICKI. In a direct multivariate linear regression analysis adjusted for age, gender, and stage of puberty (r2 = 0.58), RBP4 was significantly correlated to insulin (coefficient 0.10, 95% confidence interval 0.04–0.16; P = 0.003) and BMI (coefficient 0.30, 95% confidence interval 0.18–0.42; P < 0.001), whereas RBP4 demonstrated no significant correlation to glucose.
In the 43 obese children, changes of RBP4 concentrations (RBP4 at baseline minus RBP4 1 yr later) in the course of 1 yr correlated significantly to changes of SDS-BMI (r = 0.31) but not to changes of prealbumin. Furthermore, changes of RBP4 correlated to changes of insulin (r = 0.28), HOMA (r = 0.29), and QUICKI (r = −0.22) in a partial regression analysis adjusted for changes of SDS-BMI. The changes of the molar ratio RBP4/SR concentrations in the course of 1 yr correlated significantly to changes of SDS-BMI (r = 0.36) but not to changes of prealbumin, insulin, HOMA, or QUICKI in a partial regression analysis adjusted for changes of SDS-BMI.
RBP4 concentrations and the molar ratio RBP4/SR decreased significantly (P < 0.001) in the 25 obese children with substantial weight loss, whereas RBP4 levels and the molar ratio RBP4/SR did not change significantly in the 18 without change of weight status (see Fig. 1). The changes of HOMA and QUICKI, insulin, glucose, lipids, and prealbumin concentrations in the course of 1 yr in the 25 obese children with substantial weight loss and the 18 obese children without substantial weight loss are shown in Table 2. Substantial weight loss led to a significant decrease of skinfold thicknesses, percent body fat, blood pressure, triglycerides, LDL-cholesterol, and insulin concentration as well as to a decrease of insulin resistance index HOMA and an increase of QUICKI. In the obese children without substantial weight loss, there were no significant changes apart from a decrease of LDL-cholesterol levels. The number of children entering into puberty during the lifestyle intervention period did not differ significantly between the children with and without substantial weight loss (12 vs. 6%, respectively).
Table 2.
Changes of weight status, skinfold thicknesses, percent body fat, blood pressure, insulin resistance index HOMA, insulin sensitivity check index QUICKI, fasting serum insulin, glucose, lipids, prealbumin, and retinol concentrations in obese children with substantial weight loss and obese children with stable weight status in the course of 1 yr
| Substantial weight loss (n = 25)
|
No change of weight status (n = 18)
|
|||
|---|---|---|---|---|
| At baseline | 1 yr later | At baseline | 1 yr later | |
| BMI (kg/m²) | 27.1 ± 3.8 | 24.1 ± 2.6a | 27.6 ± 4.8 | 27.8 ± 5.3 |
| SDS-BMI | 2.3 ± 0.4 | 1.7 ± 0.3a | 2.4 ± 0.5 | 2.3 ± 0.6 |
| Subscapular ST (mm) | 30 ± 5 | 23 ± 6a | 31 ± 7 | 30 ± 9 |
| Triceps ST (mm) | 31 ± 8 | 21 ± 7a | 33 ± 7 | 32 ± 9 |
| % body fat | 45 ± 8 | 37 ± 20a | 46 ± 15 | 46 ± 16 |
| Systolic blood pressure (mm Hg) | 119 ± 11 | 108 ± 6a | 119 ± 14 | 118 ± 11 |
| Diastolic blood pressure (mm Hg) | 64 ± 12 | 57 ± 8a | 66 ± 15 | 64 ± 10 |
| Triglycerides (mmol/liter) | 1.14 ± 0.50 | 0.98 ± 0.51a | 1.11 ± 0.38 | 1.11 ± 0.49 |
| LDL-cholesterol (mmol/liter) | 2.89 ± 0.72 | 2.46 ± 0.67a | 2.81 ± 0.72 | 2.35 ± 0.57a |
| HDL-cholesterol (mmol/liter) | 1.27 ± 0.26 | 1.34 ± 0.26 | 1.34 ± 0.44 | 1.34 ± 0.31 |
| Insulin (mU/liter) | 17 ± 11 | 11 ± 6a | 17 ± 9 | 18 ± 8 |
| Glucose (mmol/liter) | 4.71 ± 0.39 | 4.67 ± 0.33 | 4.71 ± 0.33 | 4.67 ± 0.44 |
| HOMA | 3.6 ± 2.8 | 2.4 ± 1.4a | 3.4 ± 1.6 | 3.4 ± 1.6 |
| QUICKI | 0.33 ± 0.03 | 0.35 ± 0.04a | 0.33 ± 0.03 | 0.33 ± 0.02 |
| Prealbumin (g/liter) | 0.19 ± 0.03 | 0.20 ± 0.04 | 0.19 ± 0.03 | 0.20 ± 0.06 |
| Retinol (μmol/liter) | 1.3 ± 0.2 | 1.3 ± 0.4 | 1.2 ± 0.2 | 1.2 ± 0.4 |
In the group with substantial weight loss, age was 10.6 ± 2.6 yr, 32% were male, 40% were prepubertal, and change in SDS-BMI was −0.7 ± 0.2. In the group with no change of weight status, age was 11.0 ± 2.6 yr, 50% were male, 44% were prepubertal, and change in SDS-BMI was 0.0 ± 0.2. Data are shown as mean ± sd. ST, Skinfold thickness.
P < 0.05 baseline vs. 1 yr later.
At baseline, we found no significant differences in age, gender, pubertal stage, percent body fat, and SDS-BMI between the obese children with and without substantial weight loss. Furthermore, glucose, insulin, HOMA, QUICKI, lipids, prealbumin, RBP4 concentrations, and the molar ratio RBP4/SR did not differ significantly at baseline between the children with and without substantial weight loss. One year later, percentage of body fat, blood pressure, triglycerides, insulin, insulin resistance index HOMA, RBP4 concentrations, and the molar ratio RBP4/SR were significantly lower and QUICKI was significantly higher in the children with substantial weight loss compared with the children without substantial weight loss.
At baseline, we found a significant difference between the RBP4 levels of the 28 prepubertal children and the 34 pubertal children (see Table 3). Furthermore, the insulin concentrations and the insulin resistance index HOMA were significantly increased in the pubertal children compared with the prepubertal children, whereas the QUICKI was significantly decreased. The prepubertal and pubertal children did not differ significantly with respect to gender and SDS-BMI. The RBP4 levels of the 26 boys (mean 0.63 ± 0.15 μmol/liter) did not differ significantly (P = 0.681) from those of the 36 girls (mean 0.65 ± 0.16 μmol/liter). Boys and girls did not differ significantly with respect to age, pubertal stage, and SDS-BMI.
Table 3.
Fasting RBP4, insulin, insulin resistance index HOMA, and insulin sensitivity check index QUICKI separated by pubertal stage
| Prepubertal | Pubertal | P value | |
|---|---|---|---|
| n | 28 | 34 | |
| RBP4 (μmol/liter) | 0.60 ± 0.14 | 0.68 ± 0.17 | 0.043 |
| Insulin (mU/liter) | 9.1 ± 7.7 | 15.9 ± 12.7 | 0.013 |
| HOMA | 2.6 ± 1.4 | 4.1 ± 2.7 | 0.023 |
| QUICKI | 0.38 ± 0.07 | 0.35 ± 0.06 | 0.034 |
Data are shown as mean ± sd.
Discussion
To the best of our knowledge, this is the first study analyzing the cross-sectional and longitudinal relationships between RBP4, obesity, insulin resistance, and other markers of the metabolic syndrome in childhood. We were able to demonstrate that obese children had significantly higher RBP4 levels and RBP4/SR ratios compared with lean children in concordance with previous smaller pediatric studies (18,32). We also found that the RBP4 levels of the children we studied were similar to those of adults (6). Most importantly, RBP4 levels and RBP4/SR ratios decreased significantly and in a parallel manner to a decrease of insulin resistance index HOMA and an increase of QUICKI in obese children who reduced their overweight substantially (reduction of SDS-BMI ≥ 0.5) in contrast to obese children without substantial weight loss in the course of 1 yr.
The cross-sectional and longitudinal significant relationships between serum RBP4 levels, insulin levels, HOMA, and QUICKI suggest that RBP4 is likely to be involved in the pathogenesis of insulin resistance in humans. According to an increase of insulin levels and the insulin resistance index HOMA and a decrease of the QUICKI in puberty, RBP4 concentrations were higher in pubertal than in prepubertal children, also supporting a relationship between RBP4 and insulin resistance. Furthermore, the significant association between RBP4 and markers of the metabolic syndrome, such as blood pressure and triglycerides, agrees with the hypothesis that RBP4 influences insulin resistance.
Rodent studies have also demonstrated a strong relationship between RBP4 and insulin resistance (5). Circulating RBP4 concentrations are elevated in several mouse models of insulin resistance, and deleting the RBP4 gene in mice has been shown to increase insulin sensitivity (5). Injection of purified RBP4 into mice or transgenic overexpression of RBP4 in mice impairs insulin signaling in muscle tissue and induces the expression of the gluconeogenic enzyme phosphoenolpyruvate carboxykinase (PEPCK) in the liver (5). In humans, there are also functional studies supporting a role of RBP4 in the pathogenesis of insulin resistance. In adipose tissue from lean, overweight, and obese menopausal women, RBP4 mRNA has been shown to be closely associated with the mRNA levels of the principal insulin-stimulated glucose transporter, glucose transporter 4 (GLUT4) (7,11,33). In one of these studies, decreased expression of GLUT4 in adipocytes predicted an increase in both serum RBP4 levels and insulin resistance (7). Although the mechanism by which a decrease in adipocyte GLUT4 results in an increase in RBP4 expression remains unknown, it is suspected to involve the sensing of glucose by adipocytes (34). It has been demonstrated that in insulin-resistant states, the expression of GLUT4 is selectively down-regulated in adipocytes (35). However, the consequences of decreased GLUT4 expression in adipocytes remain unclear because adipose tissue contributes little to whole-body glucose disposal (36). Other mechanisms whereby RBP4 might modulate insulin sensitivity in muscle and liver have been suggested. In skeletal muscle, RBP4 appears to reduce insulin sensitivity by inhibiting both insulin receptor substrate-1 phosphorylation and phosphatidylinositol 3-kinase activation, while increasing hepatic glucose production by increasing PEPCK expression (5). Additionally, increased delivery of retinol by RBP4 might explain its effects on insulin metabolism. However, the link between retinoids and insulin action is complex because some retinoids increase insulin sensitivity (37), whereas others produce insulin resistance in humans (38,39). It bears mentioning that retinoids can stimulate PEPCK expression in the liver and hepatic gluconeogenesis. In addition, retinol has been shown to be a precursor for the synthesis of ligands of the peroxisome-proliferator-activated receptor family that regulate genes central to fatty acid metabolism (40). RBP4 could thus be linked to insulin resistance through impaired fatty acid metabolism (37).
RBP4 concentrations were increased in our obese children and decreased when substantial weight loss occurred, both of which are in accordance with most of the previous smaller studies (9,18,19,41). In the report with stable RBP4 concentrations after weight loss, the weight loss was much lower (5%) than in our study (11). This is a likely explanation of the different findings, especially because we defined substantial weight loss in our study as SDS-BMI of at least 0.5 based on the findings that with a reduction of less than 0.5 SDS-BMI, no improvement of insulin resistance and cardiovascular risk factors can be measured in obese children (30,31). Therefore, one could conclude that the increase of RBP4 in obesity seems to be a reversible consequence of obesity. Nevertheless, it remains unclear as to how obesity leads to increased fasting RBP4 levels in humans. In a very recent study by Janke et al. (11), RBP4 was highly expressed in isolated mature human adipocytes and secreted by differentiating human adipocytes. However, the authors also found that RBP4 mRNA was down-regulated in the adipose tissue of obese women. Yet, in a rodent study (42), only 20% of systemic RBP4 was shown to be produced by adipocytes, and RBP4 gene expression was 20% compared with expression in the liver, which is the major source for RBP4 in rodents and most likely also in humans (11,43). It therefore appears possible that the increase in systemic RBP4 concentrations in insulin-resistant subjects (5,7,15,44), who are frequently obese, is not explained by increased RBP4 production in adipose tissue, and the relationship between RBP4 and obesity is just an epiphenomenon of insulin resistance. This might explain, apart from differences in age, gender, ethnicities, and degrees of overweight, why some studies have found a relationship between obesity and RBP4 (5,6,7,8,9,45), whereas other studies have not reported this correspondence in their obese cohorts (6,10,11,12,13,14,15,16,17). Indeed, studies demonstrating a relationship between obesity and RBP4 have consisted predominantly of diabetic or severely insulin-resistant individuals (7,9,45). Also, different types of fat distribution may offer other explanations for the controversial findings because RPB4 levels are influenced by adipose tissue distribution (31,32,46,47). Additionally, the discrepancies observed between different studies may be due, at least in part, to different assays used to measure RPB4 (48).
Similar to the study of Aeberli et al. (32), we found a significant correlation between RBP4 and SR. In contrast to this study, we did not find a stronger correlation between the molar ratio RBP4/SR and markers of obesity, triglycerides, and insulin resistance compared with the relationships between RBP4 levels and these parameters.
The strengths of this study are the longitudinal design of the analyses of both prepubertal and pubertal children and the determination of RBP4 in relation to SR concentrations. However, this study has a few potential limitations. First, BMI percentiles and skinfold measurements were used to classify overweight. Although BMI and skinfold measurements are a good measure for overweight, one needs to be aware of their limitations as an indirect measure of fat mass, especially with the phenomenon of increased RBP4 production in adipose tissue. Second, the HOMA model and QUICKI are only assessments of insulin resistance and insulin sensitivity (49). Clamp studies are actually the gold standard for analyzing insulin resistance and sensitivity (49). Furthermore, the HOMA model is more representative of liver insulin resistance than muscle insulin resistance (50). Third, we were not able to differentiate the effect of diet, increased physical exercise, or weight loss on RBP4 concentrations due to our study protocol. However, changes of RBP4 and RBP4/SR were not related to changes of other markers of nutritional status such as prealbumin. For example, in another study, exercise training without weight loss was associated with a reduction in serum RBP4 levels (7). Finally, we did not analyze waist and hip circumferences, although waist-to-hip ratio has been reported to be associated with RBP4 levels (32,33,46,47). Our study lacked these factors due to the fact that evaluated and standardized waist and hip percentiles do not exist for German children and adolescents.
In summary, RBP4 concentrations were higher in pubertal than in prepubertal children and were independent of age and gender. The increase of RBP4 levels in obese children tended to normalize after weight loss. Because RBP4 concentrations were significantly related to insulin resistance both in cross-sectional and longitudinal analyses, these findings support our hypothesis of a functional relevant relationship between RBP4 and insulin resistance in obesity. Further prospective research is necessary to clarify the role of RBP4 in the pathogenesis of insulin resistance in humans as well as related molecular pathways leading to the development of metabolic syndrome.
Acknowledgments
We thank Ms. R. Maslak and Ms. K. Schark-Zimmer, Children’s Hospital University of Bonn, for their kind support in the laboratory.
Footnotes
This work has been funded by the Bonfor Research Foundation, University of Bonn, Germany and by NIH RR0163 and DK 62202 and by Sandoz Pharmaceuticals GmbH.
This study is registered at clinicaltrials.gov (NCT00435734).
Disclosure Statement: T.R. received grant support (2007–2008) from Sandoz Pharmaceuticals GmbH. C.R. received grant support (2005–2008) from Bonfor Research Foundation, University of Bonn, Germany, and from NIH RR0163 and DK 62202. B.S. has nothing to declare.
First Published Online April 8, 2008
Abbreviations: BMI, Body mass index; GLUT4, glucose transporter 4; HDL, high-density lipoprotein; HOMA, homeostasis model assessment; LDL, low-density lipoprotein; PEPCK, phosphoenolpyruvate carboxykinase; QUICKI, quantitative insulin sensitivity check index; RBP4, retinol-binding protein 4; SDS, sd score; SR, serum retinol.
References
- Grundy SM 2004 Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 89:2595–2600 [DOI] [PubMed] [Google Scholar]
- Moller DE, Kaufman KD 2005 Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med 56:45–62 [DOI] [PubMed] [Google Scholar]
- Ferroni P, Basili S, Falco A, Davi G 2004 Inflammation, insulin resistance, and obesity. Curr Atheroscler Rep 6:424–431 [DOI] [PubMed] [Google Scholar]
- Fu Y, Luo N, Klein RL, Garvey WT 2005 Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res 46:1369–1379 [DOI] [PubMed] [Google Scholar]
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB 2005 Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436:356–362 [DOI] [PubMed] [Google Scholar]
- Cho YM, Youn BS, Lee H, Lee N, Min SS, Kwak SH, Lee HK, Park KS 2006 Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care 29:2457–2461 [DOI] [PubMed] [Google Scholar]
- Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB 2006 Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 354:2552–2563 [DOI] [PubMed] [Google Scholar]
- Yoshida A, Matsutani Y, Fukuchi Y, Saito K, Naito M 2006 Analysis of the factors contributing to serum retinol binding protein and transthyretin levels in Japanese adults. J Atheroscler Thromb 13:209–215 [DOI] [PubMed] [Google Scholar]
- Haider GD, Schindler K, Prager G, Bohdjalian A, Luger A, Woltz M, Ludvik B 2007 Serum retinol-binding protein-4 is reduced after weight loss in morbidly obese subjects. J Clin Endocrinol Metab 92:1168–1171 [DOI] [PubMed] [Google Scholar]
- Erikstrup C, Mortensen OH, Pedersen BK 2006 Retinol-binding protein 4 and insulin resistance. N Engl J Med 355:1393–1394 [PubMed] [Google Scholar]
- Janke J, Engeli S, Boschmann M, Adams F, Bohnke J, Luft FC, Sharma AM, Jordan J 2006 Retinol-binding protein 4 in human obesity. Diabetes 55:2805–2810 [DOI] [PubMed] [Google Scholar]
- Takashima N, Tomoike H, Iwai N 2006 Retinol-binding protein 4 and insulin resistance. N Engl J Med 355:1392 [DOI] [PubMed] [Google Scholar]
- Broch M, Vendrell J, Ricart W, Richart C, Fernández-Real JM 2007 Circulating retinol-binding protein-4, insulin sensitivity, insulin secretion, and insulin disposition index in obese and nonobese subjects. Diabetes Care 30:1802–1806 [DOI] [PubMed] [Google Scholar]
- Gavi S, Stuart LM Kelly P, Melendez, MM, Mynarik DC, Gelato MC, McNurlan MA 2007 Retinol-binding protein 4 is associated with insulin resistance and body fat distribution in nonobese subjects without type 2 diabetes. J Clin Endocrinol Metab 92:1886–1890 [DOI] [PubMed] [Google Scholar]
- Abahusain MA, Wright J, Dickerson JW, de Vol EB 1999 Retinol, α-tocopherol and carotenoids in diabetes. Eur J Clin Nutr 53:630–635 [DOI] [PubMed] [Google Scholar]
- Sasaki H, Iwasaki T, Kato S, Tada N 1995 High retinol retinol-binding protein ratio in noninsulin-dependent diabetes-mellitus. Am J Med Sci 310:177–182 [DOI] [PubMed] [Google Scholar]
- Basu TK, Basualdo C 1997 Vitamin A homeostasis and diabetes mellitus. Nutrition 13:804–806 [DOI] [PubMed] [Google Scholar]
- Balagopal P, Graham TE, Kahn BB, Altomare A, Funanage V, George D 2007 Reduction of elevated serum retinol binding protein in obese children by lifestyle intervention: association with subclinical inflammation. J Clin Endocrinol Metab 92:1971–1974 [DOI] [PubMed] [Google Scholar]
- Vitkova M, Klimcakova E, Kovacikova M, Valle C, Moro C, Polak J, Hanacek J, Capel F, Viguerie N, Richterova B, Bajzova M, Hejnova J, Stich V, Langin D 2007 Plasma levels and adipose tissue messenger ribonucleic acid expression of retinol-binding protein 4 are reduced during calorie restriction in obese subjects but are not related to diet-induced changes in insulin sensitivity. J Clin Endocrinol Metab 92:2330–2335 [DOI] [PubMed] [Google Scholar]
- Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA 1994 Association between multiple cardiovascular risk factors and arteriosclerosis in children and young adults. N Eng J Med 338:1650–1656 [DOI] [PubMed] [Google Scholar]
- Reinehr T, de Sousa G, Toschke M, Andler W 2006 Long-term follow-up of cardiovascular disease risk factors in obese children after intervention. Am J Clin Nutr 84:490–496 [DOI] [PubMed] [Google Scholar]
- Reinehr T, Kersting M, Alexy U, Andler W 2003 Long-term follow-up of overweight children: after training, after a single consultation session and without treatment. J Pediatr Gastroenterol Nutr 37:72–74 [DOI] [PubMed] [Google Scholar]
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH 2000 Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ 1990 The LMS method for constructing normalized growth standards. Eur J Clin Nutr 44:45–60 [PubMed] [Google Scholar]
- Kromeyer-Hauschild K, Wabitsch M, Geller F, Ziegler A, Geiss HC, Hesse V, Hippel V, Jaeger U, Johnsen D, Kiess W, Korte W, Kunze D, Menner K, Müller G, Müller M, Niemann-Pilatus A, Remer T, Schaefer F, Wittchen HU, Zabransky S, Zellner K, Hebebrand J 2001 Percentiles of body mass index in children and adolescents evaluated from different regional German studies. Monatsschr Kinderheilkd 149:807–818 [Google Scholar]
- Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, Bemben DA 1988 Skinfold equations for estimation of body fatness in children and youth. Hum Biol 60:709–723 [PubMed] [Google Scholar]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents 2004 The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114(Suppl 4th Report):555–576 [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ 2000 Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85:2402–2410 [DOI] [PubMed] [Google Scholar]
- Reinehr T, Andler W 2004 Changes in the atherogenic risk-factor profile according to degree of reduction of overweight. Arch Dis Child 89:419–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr T, de Sousa G, Andler W 2005 Longitudinal analyses between overweight, insulin resistance, and cardiovascular risk factors in children. Obes Res 13:1824–1833 [DOI] [PubMed] [Google Scholar]
- Aeberli I, Biebinger R, Lehmann R, L'allemand D, Spinas GA, Zimmermann MB 2007 Serum retinol-binding protein 4 concentration and its ratio to serum retinol are associated with obesity and metabolic syndrome components in children. J Clin Endocrinol Metab 92:4359–4365 [DOI] [PubMed] [Google Scholar]
- Yao-Borengasser A, Varma V, Bodles AM, Rasouli N, Phanavanh B, Lee MJ, Starks T, Kern LM, Spencer 3rd HJ, Rashidi AA, McGehee Jr RE, Fried SK, Kern PA 2007 Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab 92:2590–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamori Y, Sakaue H, Kasuga M 2006 RBP4, an unexpected adipokine. Nat Med 12:30–31 [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Gnudi L, Tozzo E, Yang H, Leach F, Kahn BB 1993 Adipose cell hyperplasia and enhanced glucose disposal in transgenic mice overexpressing GLUT4 selectively in adipose tissue. J Biol Chem 268:22243–22246 [PubMed] [Google Scholar]
- Shepherd PR, Kahn BB 1999 Glucose transporters and insulin action: implications for insulin resistance and diabetes mellitus. N Engl J Med 341:248–257 [DOI] [PubMed] [Google Scholar]
- Sivitz WI, Desautel SL, Kayano T, Bell GI, Pessin JE 1989 Regulation of glucose transporter messenger-RNA in insulin-deficient states. Nature 340:72–74 [DOI] [PubMed] [Google Scholar]
- Koistinen HA, Remitz A, Gylling H, Miettinen TA, Koivisto VA, Ebeling P 2001 Dyslipidemia and a reversible decrease in insulin sensitivity induced by therapy with 13-cis-retinoic acid. Diabetes Metab Res Rev 17:391–395 [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Xu HE, Lambert MH, Willson TM 2001 Peroxisome proliferator-activated receptors: from genes to physiology. Recent Prog Horm Res 56:239–263 [DOI] [PubMed] [Google Scholar]
- Ferre P 2004 The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes 53:S43–S50 [DOI] [PubMed] [Google Scholar]
- Merritt RJ, Blackburn GL, Bistrian BR, Palombo J, Suskind RM 1981 Consequences of modified fasting in obese pediatric and adolescent patients: effect of a carbohydrate-free diet on serum proteins. Am J Clin Nutr 34:2752–2755 [DOI] [PubMed] [Google Scholar]
- Tsutsumi C, Okuno M, Tannous L, Piantedosi R, Allan M, Goodman DS, Blaner WS 1992 Retinoids and retinoid-binding protein expression in rat adipocytes. J Biol Chem 267:1805–1810 [PubMed] [Google Scholar]
- Blaner WS 1989 Retinol-binding protein: the serum transport protein for vitamin A. Endocr Rev 10:308–316 [DOI] [PubMed] [Google Scholar]
- Basualdo CG, Wein EE, Basu TK 1997 Vitamin A (retinol) status of first nation adults with non-insulin-dependent diabetes mellitus. J Am Coll Nutr 16:39–45 [DOI] [PubMed] [Google Scholar]
- Hahn S, Backhaus M, Broecker-Preuss M, Tan S, Dietz T, Kimmig R, Schmidt M, Mann K, Janssen OE 2007 Retinol-binding protein 4 levels are elevated in polycystic ovary syndrome women with obesity and impaired glucose metabolism. Eur J Endocrinol 157:201–207 [DOI] [PubMed] [Google Scholar]
- Lee JW, Im JA, Lee HR, Shim JY, Youn BS, Lee DC 2007 Visceral adiposity is associated with serum retinol binding protein-4 levels in healthy women. Obesity (Silver Spring) 15:2225–2232 [DOI] [PubMed] [Google Scholar]
- Jia W, Wu H, Bao Y, Wang C, Lu J, Zhu J, Xiang K 2007 Association of serum retinol-binding protein 4 and visceral adiposity in Chinese subjects with and without type 2 diabetes. J Clin Endocrinol Metab 92:3224–3229 [DOI] [PubMed] [Google Scholar]
- Graham TE, Wason CJ, Blüher M, Kahn BB 2007 Shortcomings in methodology complicate measurements of serum retinol binding protein (RBP4) in insulin-resistant human subjects. Diabetologia 50:814–823 [DOI] [PubMed] [Google Scholar]
- Uwaifo GI, Fallon EM, Chin J 2002 Indices of insulin action, disposal, and secretion derived from fasting samples and clamps in normal glucose-tolerant black and white children. Diabetes Care 25:2081–2087 [DOI] [PubMed] [Google Scholar]
- Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA 2006 Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 55:1430–1435 [DOI] [PubMed] [Google Scholar]