Abstract
Objective: The aim of this study was to define the metabolic abnormalities underlying the prediabetic status of isolated impaired fasting glucose (IFG), isolated impaired glucose tolerance (IGT), and combined IFG/IGT in obese youth.
Research Design and Methods: We used state-of-the-art techniques (hyperinsulinemic-euglycemic and hyperglycemic clamps), applying a model of glucose-stimulated insulin secretion to the glucose and C-peptide concentration, in 40 normal glucose tolerance (NGT), 17 IFG, 23 IGT, and 11 IFG/IGT obese adolescents. Percent fat (by dual-energy x-ray absorptiometry), age, gender and ethnicity were comparable among groups.
Results: Peripheral insulin sensitivity was similar between the IFG and NGT groups. In contrast, the IGT and IFG/IGT groups showed marked reductions in peripheral insulin sensitivity (P < 0.002). Basal hepatic insulin resistance index (basal hepatic glucose production × fasting plasma insulin) was significantly increased in IFG, IGT, and IFG/IGT (P < 0.009) compared with NGT. Glucose sensitivity of first-phase insulin secretion was progressively lower in IFG, IGT, and IFG/IGT compared with NGT. Glucose sensitivity of second-phase secretion showed a statistically significant defect only in the IFG/IGT group. In a multivariate regression analysis, glucose sensitivity of first-phase secretion and basal insulin secretion rate were significant independent predictors of FPG (total r2 = 25.9%).
Conclusions: IFG, in obese adolescents, is linked primarily to alterations in glucose sensitivity of first-phase insulin secretion and liver insulin sensitivity. The IGT group is affected by a more severe degree of peripheral insulin resistance and reduction in first-phase secretion. IFG/IGT is hallmarked by a profound insulin resistance and by a new additional defect in second-phase insulin secretion.
Examination of the prediabetic status of obese adolescents reveals that those with isolated impaired fasting glucose (IFG) have alterations in glucose sensitivity of first phase insulin secretion and liver insulin sensitivity, whereas those with isolated impaired glucose tolerance (IGT) have a more severe degree of peripheral insulin resistance and reduction in first phase secretion. Those with IFG and IGT have profound insulin resistance and a new additional defect in second phase insulin secretion.
Prediabetes is fueling the epidemics of type 2 diabetes (T2DM) and cardiovascular disease worldwide (1). Prediabetes is characterized by isolated impaired fasting glucose (IFG), isolated impaired glucose tolerance (IGT), and combined IFG/IGT (2). Epidemiological studies in adults indicate that IFG and IGT are two distinct categories of individuals with only partial overlap (3,4,5,6,7). This would suggest that different abnormalities characterize these two phenotypes. In our multiethnic clinic-based cohort of 761 obese nondiabetic youth, the prevalence of IFG is 10%, whereas for IGT, it is 15% (personal data). Of note, only a small number of subjects meet both criteria, showing that these categories overlap only to a very limited extent in children, as already reported in adults (2,7). Based on data from the 1999–2000 (8) and 2001–2002 National Health and Nutrition Examination Surveys (NHANES), the most recent national estimate for the prevalence of IFG among U.S. adolescents is 11% (9). In contrast, more recent data from the pilot STOP-T2DM, a school-based study, reported a surprisingly high percentage (40.5%) of youth with IFG. Thus, a substantial number of youngsters in the United States have IFG (10).
Clinical physiology studies in adults generally, but not unanimously, found that IFG was a better marker of β-cell dysfunction, whereas IGT was more closely related to insulin-resistant states (7). To date, little information is available regarding the underlying putative metabolic defects in insulin action and β-cell function that might be present in obese adolescents with different prediabetes phenotypes. Earlier studies from our group showed that the IGT phenotype in obese adolescents is a pre-T2DM state with marked peripheral insulin resistance (11) and impaired first-phase secretion (12). We here extend our work in this area by asking the following questions in obese adolescents. Are the underlying pathogeneses of isolated IFG and isolated IGT different? Do certain metabolic features set these two conditions apart?
To answer these questions, we used state-of-art techniques to assess insulin sensitivity and β-cell function in obese adolescents with either IFG or IFG/IGT, and we compared them to obese adolescents with either isolated IGT or NGT, matched for age, gender, ethnicity, and degree of obesity.
Subjects and Methods
Subjects
Subjects were recruited from a multiethnic cohort of obese children and adolescents drawn from the Yale Pediatric Obesity Clinic. Obese youth with marked obesity and positive risk factors for T2DM are screened by using a standard oral glucose tolerance test (OGTT) (11). Ninety-one subjects volunteered to participate in the present study. On the basis of the OGTT results, subjects were classified as normal glucose tolerance (NGT) [n = 40; fasting plasma glucose (FPG) < 100 mg/dl and 2-h glucose (2hG) < 140 mg/dl], IFG (n = 17; 100 mg/dl > FPG < 126 mg/dl and 2hG < 140 mg/dl), IGT (n = 23; FPG < 100 and 140 mg/dl ≥ 2hG ≤ 199 mg/dl), or combined IFG/IGT (n = 11). Given the intra-individual variability of FPG, subjects were classified as IFG on the basis of at least two positive results from three measurements obtained on separate days (3–4 wk apart). The diagnosis of glucose tolerance status was based on a single OGTT. Therefore, there is a possibility of misclassification into IGT and IFG/IGT. In a previous study, we found the results from the OGTT to be fairly reproducible, because the intra-individual variability was rather low in obese children and adolescents (13). Eligible subjects were from 10–18 yr of age, on no medication that may alter glucose metabolism, and otherwise healthy. All underwent a complete physical examination and detailed medical history. Stage of development was assessed on the basis of breast development in girls and genital development in boys according to Tanner criteria. All subjects were in Tanner stage II–IV, with a body mass index (BMI) z-score greater than 2 for age and sex (14). All subjects were negative for autoimmune markers for T1DM (IAA, GAD65, and ICA 512). Data from the hyperglycemic clamp studies on 30 NGT and 22 IGT subjects have been described in a previous publication (12). The study was approved by the Yale Human Investigational Committee. Written informed consent was obtained from the participants.
Metabolic studies
All participants were instructed by a registered dietitian to follow a weight-maintenance diet consisting of at least 250 g carbohydrate for 7 d before the studies and to refrain from physical activity the day before. After an overnight fast of 10–12 h, the subject underwent the metabolic studies. The interval between studies was no longer than 3–4 wk.
Assessment of insulin sensitivity of glucose metabolism and lipolysis
Hyperinsulinemic-euglycemic clamp
The children arrived at Yale Clinical Research Center at 0730 h. Two iv catheters, one for blood sampling and one for infusion of glucose, insulin, and tracers, were inserted in the antecubital vein of each arm after local infiltration with lidocaine. The arm used for blood sampling was kept in a heated box for arterializations of blood. Whole-body insulin sensitivity was measured by 2-h one-step euglycemic clamp (15) by infusing insulin as a primed continuous infusion at 80 mU/m2·min. A primed-continuous infusion of 6,6-deuterium-labeled glucose was given at a rate of 11.1 mmol/min·m2. A continuous infusion of [2H5]glycerol at a rate of 0.21 mmol/min·m2 was used to quantify insulin's effects on glycerol turnover (11). Arterialized blood samples were collected every 5–10 min during the last 30 min of the baseline period for measurement of glucose enrichments, hormones, and substrates. The glucose infusion rates were calculated during the last 30 min of the clamp and expressed as micromoles of glucose per minute per kilogram of lean body mass (LBM).
The glucose tracer data were used to compute basal glucose turnover rate and residual endogenous glucose production (EGP) during hyperinsulinemia, as previously described (11). Basal glucose clearance was computed as the ratio of basal glucose turnover rate to fasting plasma glucose. Peripheral glucose disposal rate was calculated as the sum of residual EGP plus the glucose infusion rates corrected for the changes in the size of the glucose pool. Glycerol turnover rate was used as an index of whole-body lipolytic activity in the basal state and during hyperinsulinemia.
Assessment of insulin secretion
Hyperglycemic clamp
To quantify insulin secretion, blood glucose concentration was rapidly raised to 11 mmol/liter by infusion of 20% dextrose at variables rates and kept at that value for 120 min (12). Samples were drawn at 2, 4, 6, 8, 10, and every 5 min afterward for glucose, insulin, and C-peptide concentrations. Incremental first-phase concentration of insulin and C-peptide was calculated as the mean value in 2, 4, 6, 8, and 10 min minus the fasting levels. Mean second-phase concentration of insulin and C-peptide was calculated as the mean value between 60 and 120 min.
Analysis of the hyperglycemic clamp data
The analyses of the glucose and C-peptide curves during the hyperglycemic clamp follow the general strategy proposed by several laboratories (16) with some slight modifications, which are detailed in the Appendix (published as supplemental data on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). In this study, insulin secretion during the hyperglycemic clamp is described as the sum of three components: 1) basal (postabsorptive) secretion rates, 2) insulin secretion in response to the rate of increase in plasma glucose [dynamic secretion component (17,18), known as first-phase secretion], and 3) insulin secretion in response to the actual glucose levels above the postabsorptive glucose concentration [static or proportional secretion component (19), known as second-phase secretion]. The proportional component is further boosted by a gain factor, which comes into play in the last part of the clamp and is proportional to the integral of the hyperglycemic stimulus (19,20). Parameters were estimated by implementing this minimal model of C-peptide secretion in the SAM-II 1.2 software (SAAM Institute, Seattle, WA). Numerical values of the unknown parameters were estimated by using nonlinear least squares. Weights were chosen optimally, i.e. equal to the inverse of the variance of the measurement errors, which were assumed to be additive, uncorrelated, with zero mean, and a constant coefficient of variation (CV) of 13%.
The main outputs of this model are basal insulin secretion rate (BSR, picomoles per minute per kilogram LBM); glucose sensitivity of first-phase secretion (σ1, dynamic secretion component), expressed as the amount of insulin secreted in response to a rate of increase in glucose concentration rate of 1 mmol/liter between time 0 and 1 min of the study (picomoles per kilogram LBM per millimole per liter per minute) (the CV of glucose sensitivity of first-phase secretion, as estimated by the model, was 13.8 ± 1.2%); and glucose sensitivity of second-phase secretion (σ2, static secretion component), expressed as the steady-state insulin secretion rate in response to a step increase in glucose concentration of 1 mmol/liter (picomoles per minute per kilogram LBM per millimole per liter) (the CV of glucose sensitivity of second-phase secretion, as estimated by the model, was 19.6 ± 2.2%). We also calculated an index of basal insulin clearance (milliliters per minute per kilogram LBM) as the ratio of BSR divided by fasting plasma insulin (FPI).
Abdominal magnetic resonance imaging and total body composition
Abdominal magnetic resonance imaging studies were performed on a Siemens Sonata 1.5-T system, as previously reported (11). Total body composition was measured by dual-energy x-ray absorptiometry with a Hologic scanner (Boston, MA) (11).
Analytical methods
Plasma glucose was determined with a YSI 2700 Analyzer (Yellow Springs Instrument, Yellow Springs, OH). Plasma free fatty acids were assayed by a colorimetric method. Plasma insulin was measured with a RIA (Millipore, Bedford, MA), and plasma C-peptide was measured with a Diagnostic Products Corp. (Los Angeles, CA) assay. The intraassay variation was 5.4% for insulin and 11.6% for C-peptide, and the interassay variation was 6.2% for insulin and 8.47% for C-peptide. Plasma adiponectin was measured by a double-antibody RIA from Millipore. The intra- and interassay CV are 7.1 and 9.5%, respectively. Plasma glucagon was measured using a RIA kit from Millipore. The intra- and interassay CV are 9 and 4.7%, respectively. Plasma leptin was measured using a RIA assay from Millipore. The intra- and interassay CV are 6.5 and 8.0%, respectively.
Statistical analysis
All data are presented as means ± sem. Parameters that were not normally distributed were log transformed before analyses. Parameters regarding the β-cell function were normally distributed, and therefore, no log-transformation was needed. Variables of interest were first analyzed by one-way ANOVA with the glucose homeostasis status (NGT, IFG, IGT, or IFG/IGT) as the classifying variable. When statistical significance was found, between-group contrasts were performed taking the NGT subjects as the reference group. Other between-group contrasts, when needed, were performed with the post hoc Bonferroni correction. Simple correlations were sought by computing Pearson's r correlation coefficients. Spearman correlation coefficients were estimated to describe association between continuous variables. To further examine the independent association between FPG and glucose sensitivity of σ1 phase and σ2 phase secretion, we used a stepwise forward multiple regression analysis. To do this, we used five steps in the total cohort and then stratified by race. In step 1, we entered age, sex, and race; in step 2, percent fat was added; in step 3, whole-body insulin sensitivity (M/LBM) was added; in step 4, glucose sensitivity of σ2 phase was added; and finally, in step 5, glucose sensitivity of σ1 phase insulin secretion was added to the model. To test the independent association between 2hG and M/LBM, we used a second stepwise forward multiple regression analysis. Basically, we used the same model using the 2hG as the dependent variable. To examine whether the combined effect of IFG and IGT was beyond what would be expected from their additive effect, we assessed the combined effect of IFG and IGT by models using main effects for IFG and IGT along with their interaction. These models were similarly adjusted for age, gender, ethnicity, and percent fat. We observed a significant interaction of IFG and IGT only for the hepatic glucose production (HGP) during the euglycemic-hyperinsulinemic clamp. Because we were primarily interested in describing the phenotype for these four groups and comparisons with NGT, only results from the pairwise comparisons of groups were presented. All analyses were performed using SPSS (14.0 for Windows; SPSS Inc., Chicago, IL).
Results
Anthropometric and fasting biochemical parameters (Tables 1 and 2)
Table 1.
Anthropometric parameters of participants
| NGT | IFG | IGT | IFG/IGT | |
|---|---|---|---|---|
| n | 40 | 17 | 23 | 11 |
| Gender (male/female) | 16/24 | 8/9 | 8/15 | 5/6 |
| Age (yr) | 14.8 ± 0.57 | 13.7 ± 0.6 | 13.7 ± 0.76 | 15.8 ± 0.9 |
| Ethnicity (White/African-American/Hispanic) | 13/7/20 | 3/6/8 | 12/5/6 | 3/2/6 |
| Height (m) | 1.62 ± 1.52 | 1.59 ± 3.1 | 1.58 ± 2.4 | 1.67 ± 3.45 |
| Weight (kg) | 101.3 ± 3.6 | 93.9 ± 6.5 | 93.9 ± 6.3 | 109 ± 7.8 |
| BMI (kg/m2) | 38.3 ± 0.92 | 36.5 ± 1.8 | 36.7 ± 1.7 | 38.5 ± 1.6 |
| BMI z-score | 2.5 ± 0.05 | 2.44 ± 0.77 | 2.42 ± 0.07 | 2.5 ± 0.95 |
| Percent fat (%) | 42.1 ± 0.82 | 40.3 ± 1.47 | 41.2 ± 1.22 | 42.1 ± 1.8 |
| Visceral fat (cm2) | 61.1 ± 6.1 | 58.5 ± 7.6 | 59.6 ± 5.5 | 81 ± 17.7 |
| Subcutaneous fat (cm2) | 600.3 ± 27.3 | 519.8 ± 39.8 | 476.3 ± 38.8 | 500.1 ± 36a |
| Visceral/sc fat | 0.10 ± 0.009 | 0.11 ± 0.01 | 0.13 ± 0.014 | 0.14 ± 0.027 |
Data are means ± sem.
P < 0.018 (IGT vs. NGT).
Table 2.
Metabolic parameters of participants
| NGT | IFG | IGT | IFG-IGT | |
|---|---|---|---|---|
| n | 40 | 17 | 23 | 11 |
| FPG (mmol/liter) | 5.09 ± 0.05 | 5.84 ± 0.05a | 5.01 ± 0.05 | 5.96 ± 0.1a |
| 2hG (mmol/liter) | 6.26 ± 0.15 | 6.47 ± 0.21 | 8.5 ± 0.139a | 8.9 ± 0.32a |
| FPI (μU/ml) | 31.8 ± 2.1 | 39.2 ± 4.2 | 38.9 ± 3.5 | 37.8 ± 5.2 |
| 2-h plasma insulin (μU/ml) | 145.8 ± 16.3 | 136.5 ± 20.7 | 175.2 ± 18.3 | 263.5 ± 55.8a |
| Fasting C-peptide (pmol/liter) | 1101.6 ± 53.5 | 1198.2 ± 94.7 | 1090.5 ± 79.9 | 1477.9 ± 132.9 |
| 2-h C-peptide (pmol/liter) | 3302.4 ± 200.2 | 2973.6 ± 198.5 | 5290.6 ± 629.5a | 4146.4 ± 378.6 |
| First-phase insulin (μU/ml) | 150.4 ± 13.6 | 135.1 ± 18.5 | 151.2 ± 14.3 | 80.1 ± 9.8a |
| First-phase C-peptide (pmol/liter) | 2458.5 ± 129.3 | 2316 ± 224.4 | 2369 ± 112.1 | 1745 ± 163.5 |
| Second-phase insulin (μU/ml) | 265.6 ± 24.9 | 292.6 ± 43.7 | 335.3 ± 65 | 206.3 ± 35.2 |
| Second-phase C-peptide (pmol/liter) | 4407 ± 203 | 4252 ± 255.2 | 4079 ± 270.6 | 4153.2 ± 497a |
| HOMA-IR | 7.26 ± 0.5 | 10.1 ± 1.13a | 8.7 ± 0.8 | 10.2 ± 1.5a |
| Glucose disposal rate (mg/kg·LBM·min) | 8.5 ± 0.67 | 9.06 ± 0.58 | 6.5 ± 0.52a | 5.47 ± 0.97a |
| HGP (mg/kg·LBM·min) | 3.08 ± 0.16 | 3.87 ± 0.432 | 4.27 ± 0.364 | 3.69 ± 0.65 |
| Hepatic IR (FPI × HGP) | 93.7 ± 6.5 | 136.9 ± 20.31a | 156.3 ± 17.2a | 164.5 ± 74.2a |
| Hepatic IR (BSR × HGP) | 19.4 ± 1.3 | 27 ± 0.42a | 29.5 ± 3.1a | 29.1 ± 6.4a |
| Glucagon (pg/ml) | 75.5 ± 3.53 | 75.7 ± 9.2 | 68.8 ± 6.1 | 62.8 ± 5.6 |
| Leptin (ng/ml) | 33.5 ± 2.8 | 29.8 ± 3.5 | 28.9 ± 2.98 | 33.2 ± 3.25 |
| Adiponectin (μg/ml) | 6.64 ± 0.52 | 8.6 ± 0.88 | 6.17 ± 0.77 | 6.8 ± 0.83 |
| FFA (μmol/liter) | 536.7 ± 23.7 | 619.2 ± 48.2 | 613.5 ± 32.5 | 594.4 ± 32.9 |
Data are means ± sem. Mean absolute values are given for first- and second-phase insulin and C-peptide concentrations. IR, Insulin resistance.
P < 0.05.
Age, gender, and ethnicity were equally represented across categories of glucose tolerance. The distribution in Tanner stage among the NGT, IFG, and IGT groups was similar (Tanner 2–4), whereas in the IFG/IGT group, most of the adolescents were in Tanner stage 4. BMI, BMI z-score, percent fat, and visceral fat were similar across all groups, whereas sc fat volume was lower in both IGT and IFG/IGT compared with NGT subjects. This resulted in a significantly higher visceral to sc fat ratio in the subjects with IGT and IFG/IGT.
As expected, there were statistically significant differences in FPG and 2hG levels. FPI, free fatty acids, leptin, glucagon, and adiponectin were not significantly different across groups.
Peripheral and hepatic insulin sensitivity and clearance (Table 2 and Fig. 1)
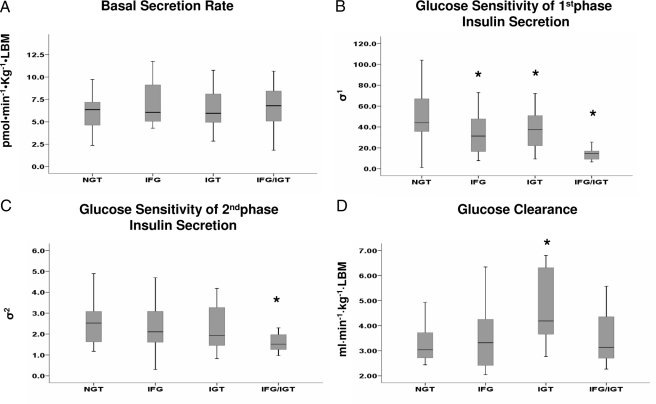
Figure 1.
A, BSR. B, Glucose sensitivity of first-phase insulin secretion (σ1); *, P = 0.004 IFG vs. NGT; *, P = 0.04 IGT vs. NGT; *, P = 0.0001 IFG/IGT vs. NGT adolescents. C, Glucose sensitivity of second-phase insulin secretion (σ2). D, Glucose clearance; *, P = 0.02 IGT vs. NGT.
Homeostasis model assessment of insulin resistance (HOMA-IR) score was higher in subjects with elevated FPG (IFG and IFG/IGT) compared with subjects with normal FPG (NGT and IGT, P < 0.057). However, peripheral insulin sensitivity (M/LBM) measured during the insulin clamp was similar in the IFG and in the NGT group. In contrast, both the IGT and IFG/IGT groups showed marked reductions in peripheral insulin sensitivity (P < 0.002).
Basal HGP was somewhat higher in all three prediabetic groups compared with the NGT individuals, but the differences were not statistically significant. However, this was so in the face of increased fasting insulin levels. Because the latter may be influenced also by insulin clearance, we computed an insulin resistance index of basal HGP as the product of BSR (prehepatic) multiplied by HGP. This modified index of insulin resistance of basal HGP was greater in each prediabetic state (P < 0.01–0.05) when compared with the NGT group. During the insulin clamp, EGP was totally suppressed in all four groups, and a similar steady-state plasma concentration of insulin was achieved in the prediabetes groups or NGT subjects during the last 60 min of the study (data not shown).
Glycerol turnover, which reflects whole-body lipolysis, was similar in all groups, both in the basal state and during the euglycemic insulin clamp (data not shown).
Model-derived parameters of insulin secretion and glucose clearance (Table 2 and Fig. 1)
Absolute values for the mean first- and second-phase insulin and C-peptide concentrations in the IFG and IGT groups were not significantly different from the NGT group (Table 2).
Figure 1 displays the differences in model-derived insulin secretion parameters among the four groups. BSR was not different among the four groups (P = 0.44 by ANOVA), and σ1 was reduced by about 38% in the IFG group (P = 0.004 vs. NGT), by about 23% in the IGT group (P = 0.04 vs. NGT), and by about 61% in the IFG/IGT group (P = 0.0001 vs. NGT).
The σ2 value showed a nonsignificant reduction of 14–16% in the IFG and in the IGT group but was reduced by about 38% (P = 0.015 vs. NGT) in the IFG/IGT group.
The booster component of second-phase secretion was not significantly different across all groups (data not shown). This additional component of insulin secretion is a glucose/time-dependent amplifier of second-phase secretion, which significantly improves the description of insulin secretion dynamics in the final part of the study (last 20–40 min) when compared with the previous model (supplemental data in the Appendix online) (21,11,22).
Basal glucose clearance showed a nonsignificant approximately 9% reduction in the IFG group (P = 0.51 vs. the NGT group). In contrast, it was significantly increased by about 30% in the IGT group (P < 0.02 vs. the NGT group).
Relationships
When combining all four groups, FPG was significantly correlated to BSR (r = +0.28; P < 0.01), σ1 (r = −0.37; P < 0.001), and the booster of second-phase secretion (r= +0.32; P < 0.01). In contrast, no significant correlations were found between 2hG and any model-derived parameter of β-cell function. However, 2hG was significantly correlated to HOMA-IR (r = +0.384; P < 0.000).
To identify independent association between model-derived β-cell function and glucose levels, we performed stepwise forward multiple regression analysis in the whole cohort. σ1 (r2 change = 14.4%) and BSR (r2 change = 11.5%) were significant independent predictors of FPG (total r2 = 25.9%). Other variables, including age, BMI, waist, percent body fat, and peripheral insulin sensitivity, showed no additional predictive power.
As for the 2hG, insulin resistance of EGP (r2 = 19.9%) and peripheral insulin sensitivity were significant independent predictors (total r2 = 28.6%). Including other variables, such as age, BMI, waist, percent body fat, glucose sensitivity of σ1 and σ2 phase secretion, and insulin clearance, brought no additional predictive power.
Discussion
Our current study, performed with state-of-the-art techniques, is a cross-sectional analysis of β-cell function and tissue insulin sensitivity in a multiethnic cohort of obese adolescents with a wide range of disturbances in glucose homeostasis. We divided the subjects according to prediabetic IFG, IGT, or IFG/IGT phenotypes. Interestingly, prediabetic glycemias describe separate populations with only partial overlap (only 11 with IFG/IGT of 51 adolescents with prediabetes), suggesting that different metabolic abnormalities characterize IFG and IGT in youth. In our obese adolescents, this lack of concordance cannot be attributed to unwise selection of the diagnostic thresholds for either IFG or IGT, because the correlation between FPG and 2hG levels in the whole data set is not existent (r = 0.08).
Isolated IFG vs. Isolated IGT in youth: commonalities and dissimilarities
Our study design allowed us to provide a detailed metabolic portrait of isolated IFG, IGT, and the combined form of IFG/IGT. Although the IFG and IGT groups show alterations in glucose levels under different physiological conditions, they share the same defect in β-cell function, i.e. a reduction in glucose sensitivity of first-phase secretion, and the same increase in the basal hepatic insulin resistance index. The isolated IFG group displays normal peripheral insulin sensitivity, whereas the isolated IGT group is affected by a more severe degree of insulin resistance.
Our data suggest that in the obese adolescent, the defects in glucose sensitivity of first-phase secretion and in the insulin sensitivity of basal EGP set the stage for the emergence of a prediabetic fasting glucose level, but the severity of peripheral insulin resistance is the factor that determines whether the prediabetic phenotype will be manifested in the glucose challenge state.
Multiple regression analysis confirmed this general pattern, showing that glucose sensitivity of first-phase insulin secretion is a primary determinant of FPG, whereas insulin resistance for both HGP and peripheral glucose utilization is strictly related to glucose tolerance, as assessed by 2hG.
How do obese children with isolated IGT maintain normal fasting glucose levels? Basal insulin secretion rates, basal glucagon levels, and basal HGP were similar in isolated IGT and isolated IFG children, thereby showing that islet α- or β-cells and regulation of hepatic glucose output could not justify lower fasting glucose levels in the IGT children. However, basal glucose clearance rate was higher in adolescents with isolated IGT than in adolescents with isolated IFG or, most importantly, with NGT, thereby ensuring normal fasting levels. This pattern strongly suggests that the increased basal glucose clearance reflects a compensatory mechanism, which is at work in the isolated IGT but not in the isolated IFG children.
Glucose utilization in the basal state is due to organs with insulin-independent utilization (primarily central nervous system and red blood cells) and to another component, which is primarily attributed to basal glucose effectiveness and is spread among different organs (i.e. muscle and liver) (23,3). Because the rate of the first component is approximately fixed, the increase in basal glucose clearance observed in the adolescents with isolated IGT is due to the metabolic routes accounting for basal glucose effectiveness. It is remarkable that in the dog model of fat feeding-induced insulin resistance and glucose intolerance (24), but with normal fasting glucose, a progressive increase in glucose effectiveness, as assessed by the iv glucose tolerance test, was documented, a finding that closely parallels our observation in the isolated IGT adolescent. Thus, still unidentified compensatory mechanisms, reflected in increased basal glucose effectiveness, are activated by fat-induced insulin resistance/glucose intolerance and help to preserve normal fasting glucose levels.
The combined IFG/IGT phenotype is more complex. The metabolic phenotype of these adolescents is not the simple sum of the isolated IFG and the isolated IGT phenotypes. Our data demonstrate that the emergence of the combined phenotype requires an additional defect in β-cell function, which impairs glucose sensitivity of second-phase secretion. This defect was previously described by us to be present only in childhood T2DM (12). However, in that study, our comparator for the prediabetic state was formed by children with isolated IGT, not by children with IFG/IGT. Hence, one wonders whether IFG/IGT should not be considered just as diabetes, but the answer to this question can be found only in longitudinal studies aimed to establish whether specific diabetic complications arise in individuals with IFG/IGT before they reach the diabetic status.
One can argue that the IFG/IGT subjects were slightly older and at a later Tanner stage of development compared with the other groups, which together may have contributed to their more severe metabolic phenotype. Insulin resistance increases significantly with the onset of puberty and returns almost to prepubertal levels by the end of the puberty (25,26). However, in the presence of moderate to severe obesity, both the effects of gender and pubertal stage of development on insulin sensitivity are not so well differentiated as in lean adolescents, which may be due to the overriding effects of obesity on insulin sensitivity and the rather narrow range of insulin resistance.
One strength of our paper is the multiethnic nature of the cohort, but given the limited number of subjects from each ethnic group in each one of the prediabetic groups, we are unable to address the question of how ethnic variation in insulin sensitivity and secretion might contribute to the onset of the different prediabetic phenotypes.
In summary, our data elucidate that in the obese adolescent, IFG is a prediabetic state linked primarily to alterations in glucose sensitivity of first-phase insulin secretion and in insulin sensitivity of endogenous (liver) glucose output. As such, IFG may be considered a disorder of glucose-sensing organs (β-cells and liver).
The IGT state is far more complex, in that it adds a defect in peripheral insulin sensitivity and a (compensatory?) increase in basal glucose clearance, presumably due to a stimulation of basal glucose effectiveness.
The combined phenotype IFG/IGT is hallmarked by profound insulin resistance and by a new additional defect in β-cell function.
Supplementary Material
Footnotes
This work was supported by grants from the National Institutes of Health (NIH) (R01-HD40787, R01-HD28016, and K24-HD01464 to S.C. and M01-RR00125 to the Yale Clinical Center Research), by a Ministero dell'Universitá e della Ricerca Scientifica e Tecnologica 60% Grant from the University of Verona (to R.C.B.), and by the Stephen I. Morse Pediatric Diabetes Research Fund (to R.W.).
Disclosure Summary: The authors have nothing to declare.
First Published Online February 26, 2008
Abbreviations: σ1, glucose sensitivity of first-phase insulin secretion; σ2, glucose sensitivity of second-phase insulin secretion; BMI, body mass index; BSR, basal secretion rate; CV, coefficient of variation; EGP, endogenous glucose production; FPG, fasting plasma glucose; FPI, fasting plasma insulin; 2hG, 2-h glucose; HGP, hepatic glucose production; HOMA-IR, homeostasis model assessment of insulin resistance; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; LBM, lean body mass; M/LBM, glucose disposal rate; NGT, normal glucose tolerance; OGTT, oral glucose tolerance test; T2DM, type 2 diabetes mellitus.
References
- Unwin N, Shaw J, Zimmet P, Alberti KG 2002 Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med 19:708–723 [DOI] [PubMed] [Google Scholar]
- Abdul-Ghani MA, Tripathy D, DeFronzo RA 2006 Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 29:1130–1139 [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA 2005 β-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 90:493–500 [DOI] [PubMed] [Google Scholar]
- Tripathy D, Carlsson M, Almgren P, Isomaa B, Taskinen MR, Tuomi T, Groop LC 2000 Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the Botnia Study. Diabetes 49:975–980 [DOI] [PubMed] [Google Scholar]
- Weyer C, Bogardus C, Pratley RE 1999 Metabolic factors contributing to increased resting metabolic rate and decreased insulin-induced thermogenesis during the development of type 2 diabetes. Diabetes 48:1607–1614 [DOI] [PubMed] [Google Scholar]
- Sato Y, Komatsu M, Katakura M, Ohfusa H, Yamada S, Yamauchi K, Hiramatsu K, Ichikawa K, Aizawa T, Hashizume K 2002 Diminution of early insulin response to glucose in subjects with normal but minimally elevated fasting plasma glucose. Evidence for early β-cell dysfunction. Diabet Med 19:566–571 [DOI] [PubMed] [Google Scholar]
- Meyer C, Pimenta W, Woerle HJ, Van Haeften T, Szoke E, Mitrakou A, Gerich J 2006 Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care 29:1909–1914 [DOI] [PubMed] [Google Scholar]
- Williams DE, Cadwell BL, Cheng YJ, Cowie CC, Gregg EW, Geiss LS, Engelgau MM, Narayan KM, Imperatore G 2005 Prevalence of impaired fasting glucose and its relationship with cardiovascular disease risk factors in US adolescents, 1999–2000. Pediatrics 116:1122–1126 [DOI] [PubMed] [Google Scholar]
- Duncan GE 2006 Prevalence of diabetes and impaired fasting glucose levels among US adolescents: National Health and Nutrition Examination Survey, 1999–2002. Arch Pediatr Adolesc Med 160:523–528 [DOI] [PubMed] [Google Scholar]
- Baranowski T, Cooper DM, Harrell J, Hirst K, Kaufman FR, Goran M, Resnicow K 2006 The STOPP-T2D Prevention Study Group. Presence of diabetes risk factors in a large U.S. eighth-grade cohort. Diabetes Care 29:212–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R, Dufour S, Taksali SE, Tamborlane WV, Petersen KF, Bonadonna RC, Boselli L, Barbetta G, Allen K, Rife F, Savoye M, Dziura J, Sherwin R, Shulman GI, Caprio S 2003 Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 362:951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R, Caprio S, Trombetta M, Taksali SE, Tamborlane WV, Bonadonna R 2005 β-Cell function across the spectrum of glucose tolerance in obese youth. Diabetes 54:1735–1743 [DOI] [PubMed] [Google Scholar]
- Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, Savoye M, Rieger V, Taksali S, Barbetta G, Sherwin RS, Caprio S 2002 Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med [Erratum (2002) 346:1756] 346:802–810 [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL 2002 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11:1–190 [PubMed] [Google Scholar]
- Weiss R, Taksali SE, Dufour S, Yeckel CW, Papademetris X, Cline G, Tamborlane WV, Dziura J, Shulman GI, Caprio S 2005 The “obese insulin-sensitive” adolescent: importance of adiponectin and lipid partitioning. J Clin Endocrinol Metab 90:3731–3737 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD, Andres R 1979 Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223 [DOI] [PubMed] [Google Scholar]
- Mari A, Camastra S, Toschi E, Giancaterini A, Gastaldelli A, Mingrone G, Ferrannini E 2001 A model for glucose control of insulin secretion during 24 h of free living. Diabetes 50(Suppl 1):S164–S168 [DOI] [PubMed] [Google Scholar]
- Mari A, Tura A, Gastaldelli A, Ferrannini E 2002 Assessing insulin secretion by modeling in multiple-meal tests: role of potentiation. Diabetes 51(Suppl 1):S221–S226 [DOI] [PubMed] [Google Scholar]
- Steil GM, Rebrin K, Janowski R, Darwin C, Saad MF 2003 Modeling β-cell insulin secretion: implications for closed-loop glucose homeostasis. Diabetes Technol Ther 5:953–964 [DOI] [PubMed] [Google Scholar]
- Akaike H 1974 A new look at the statistical model identification. IEEE Trans Automat Control 19:716–723 [Google Scholar]
- Mari A, Tura A, Gastaldelli A, Ferrannini E 2002 Assessing insulin secretion by modeling in multiple-meal tests: role of potentiation. Diabetes 51(Suppl 1):S221–S226 [DOI] [PubMed] [Google Scholar]
- Dalla Man C, Caumo A, Cobelli C 2002 The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng 49:419–429 [DOI] [PubMed] [Google Scholar]
- Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA 2006 Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 55:1430–1435 [DOI] [PubMed] [Google Scholar]
- Mittelman SD, Van Citters GW, Kim SP, Davis DA, Dea MK, Hamilton-Wessler M, Bergman RN 2000 Longitudinal compensation for fat-induced insulin resistance includes reduced insulin clearance and enhanced beta-cell response. Diabetes 49:2116–2125 [DOI] [PubMed] [Google Scholar]
- Moran A, Jacobs Jr DR, Steinberger J, Hong CP, Prineas R, Luepker R, SinaikoAR 1999 Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes 48:2039–2044 [DOI] [PubMed] [Google Scholar]
- Goran MI, Gower BA 2001 Longitudinal study on pubertal insulin resistance. Diabetes 50:2444–2450 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.