Abstract
Context: Cocaine- and amphetamine-regulated transcript (CART) codes for a peptide widely distributed in nervous and endocrine tissues. CART immunoreactivity (CART-LI) has been detected in human insulinomas.
Objective: The objective of the study was to investigate the measurement of plasma CART-LI as a tumor marker of neuroendocrine malignancy.
Design and Subjects: Plasma CART-LI levels were measured in 401 patients with a range of diagnoses: neuroendocrine malignancy (n = 131), after removal of neuroendocrine malignancy (n = 27), without any form of tumor or renal impairment (n = 192), with renal impairment (n = 17) and with nonneuroendocrine tumors (n = 34). Chromatography methods were used to investigate CART-LI circulating in human plasma.
Results: The upper limit of normal calculated for CART-LI was 150 pmol/liter. Mean circulating plasma CART-LI among neuroendocrine tumor patients was 440 pmol/liter, 56% of subjects having levels greater than 150 pmol/liter. Measuring CART-LI in addition to chromogranin (Cg)-A improved the sensitivity for neuroendocrine malignancy from 85 to 91%, whereas combined use of CgA and CgB had a joint sensitivity of 89%. Of 38 patients with pancreatic neuroendocrine tumors, 71% had plasma CART-LI levels greater than 150 pmol/liter, increasing to 95% in those classified with progressive disease (n = 20, mean CART-LI 625 pmol/liter), compared with 80% for CgA. Chromatographic analysis suggests that circulating CART-LI is present as one major form, which may correspond to CART (62–102) or another unknown form.
Conclusions: We demonstrate CART-LI as a specific tumor marker in patients with a range of neuroendocrine tumors. Used in combination with CgA, CART-LI measurement has the potential to improve sensitivity in diagnosis and follow-up of neuroendocrine tumors, in particular progressive pancreatic neuroendocrine tumors.
Cocaine- and amphetamine-regulated transcript immunoreactivity may be a novel, specific marker for diagnosing neuroendocrine tumors. Although this marker alone is not as sensitive as chromogranin A, when the two are combined, they are more sensitive than chromagranins A and B, especially for pancreatic tumors.
Neuroendocrine cells are generally defined by the production of a neurotransmitter, neuromodulator, or neuropeptide hormone; the presence of dense core secretory granules from which hormones are released by exocytosis in response to external stimuli; and the absence of axons and synapses (1). Outside the brain, the main source of regulatory peptides is from diffuse neuroendocrine components of the gastroenteropancreatic system (2).
Neuroendocrine tumors are a heterogeneous group, secreting a variety of peptides and biogenic amines. Functioning neuroendocrine tumors are characterized by various endocrine profiles, which may give rise to recognizable clinical syndromes. In particular, subtypes of pancreatic neuroendocrine tumors have been defined by their specific production of hormones such as insulin, glucagon, vasoactive intestinal polypeptide, and gastrin (3,4). More than 50% of neuroendocrine tumors are carcinoid tumors. Most commonly originating in the midgut, these are defined by characteristic morphology and staining patterns but also by their ability to synthesize serotonin (5,6).
Originally discovered as a transcript up-regulated in the rat nucleus accumbens in response to psychostimulant administration, cocaine- and amphetamine-regulated transcript (CART) and the peptide it codes for have since been detected in the central, peripheral, and enteric nervous systems and a number of endocrine tissues, including the pancreas (7). CART is highly conserved between species (8). CART (55–102) is the putative active peptide; however, variable processing by prohormone convertase enzymes gives rise to multiple CART fragments (9,10). The CART system has been suggested to play a role in a number of physiological and pathophysiological functions, including regulation of feeding, drug abuse, stress, depression, and insulin secretion (11,12,13).
CART is also produced by a variety of islet-derived transplantable tumors (14), and CART immunoreactivity (LI) has been demonstrated within human insulinomas (12). We therefore investigated the possible use of plasma CART-LI concentrations as a tumor marker of neuroendocrine malignancy.
Patients and Methods
Reference range data
To determine whether patients needed to be fasted before plasma samples were taken for CART measurement, the effect of food intake on plasma CART levels was examined. A group of 12 volunteers (five males, seven females, age ranging from 22 to 35 yr) who had fasted overnight had levels measured 30 min before and 30, 60, 80, 100, 120, 150, and 180 min after a test breakfast. The meal comprised of two eggs, toast, marmalade, and orange juice and contained approximately 560 kcal. After the results of this study, the normal range of plasma CART-LI level was determined using samples collected from 29 healthy volunteers (19 females and 10 males, mean age 32 yr). Circulating CART-LI levels have reported to show a circadian rhythm in rats (15). CART-LI was therefore measured in a group of ad libitum-fed volunteers (n = 5) at 0900, 1200, 1500, and 1800 h.
Patients and blood samples
CART-LI levels were measured in plasma samples from 131 patients with neuroendocrine tumors derived from a range of sites; midgut (n = 51), pancreatic (n = 38), unknown primary (n = 24), hindgut (n = 5), pulmonary (n = 5), thymic (n = 2), paraganglionic (n = 2), gastric (n = 2), ovarian (n = 1), and renal (n = 1). Patients were divided into those with stable disease (n = 68) or progressive disease (n = 63). Patients were categorized into progressive disease if they had increase in size of tumor and/or experienced worsening symptoms, requiring increased management. Patients with stable disease were described as having stable symptoms and/or no growth in tumor size. Treatments that patients received included somatostatin analogs, I131-metaiodobenzylguanidine therapy, capecitabine, interferon, cisplatin, and yttrium 90-labeled octreotide treatment. Results were compared with those using chromogranin (Cg)-A and CgB assays.
To see whether CART-LI measurement could aid in the detection of nonfunctioning neuroendocrine tumors, levels were also measured in patients who had undergone complete removal of neuroendocrine tumors (n = 27) and in other cases including newly diagnosed pituitary tumors (n = 6), various nonneuroendocrine tumors (n = 34), prostate cancer (n = 153), and patients with renal impairment (n = 17). Patients with a range of other conditions or complaints having blood tests at the hospital phlebotomy department were also accepted and tested for plasma CART-LI levels (n = 192). Possible effects of age or sex on circulating levels of CART-LI were analyzed using data, respectively, from 132 of these patients whose age could be confirmed and a subset of these patients whose sex could be confirmed.
Sample collection and storage
All blood samples were collected into heparinized tubes containing 400 kallikrein inhibitory units aprotinin (Trasylol; Bayer, UK) per milliliter of blood and immediately centrifuged. Plasma was separated and stored at −20 C until assay. To assess the stability of CART-LI in human plasma, six samples were taken from two subjects, half into lithium heparin tubes containing Trasylol and half into lithium heparin tubes without Trasylol. Before centrifugation and separation of the plasma, the samples were left at room temperature for different lengths of time up to 8 h and then stored at −20 C.
RIA
An established RIA directed to CART 55–102 was used as previously described (16). Briefly, CART 55–102 was purchased from the Peptide Institute (Osaka, Japan). The antiserum was raised in a rabbit immunized with human CART (55–102) peptide conjugated to BSA by glutaraldehyde (17). The 125I-labeled synthetic CART (55–102) was prepared by the direct iodogen method and purified by reverse-phase HPLC.
The assay had a sensitivity of 1.9 ± 0.5 fmol/tube (means ± sem), n = 5, with 95% confidence interval. The specific activity of freshly prepared CART (55–102) peptide label, as estimated by self displacement in the assay, was 58 Bq/fmol. A standard curve of 10–50 fmol/tube of CART (55–102) was set up in conjunction with dilutions of four samples from patients with high levels of CART-LI. The resulting binding curves showed good parallelism (data not shown). The interassay variation at 157 pmol/liter (15.7 fmol/tube, n = 14) was 8% and 426 pmol/liter (42.6 fmol/tube, n = 14) was 7%. The intraassay variation at 157 pmol/liter varied from 4 to 9% (n = 5), and at 426 pmol/liter, the range was from 8 to 12% (n = 5).
CgA and CgB assays were carried out using antibodies raised to detect pancreastatin for CgA and glycine-alanine-tryptophan-lysine (CgB 420–493) fragment for CgB, as previously described (18,19). Normal ranges for CgA and CgB are less than 60 pmol/liter and less than 150 pmol/liter, respectively.
Peptide extraction procedure
Peptide was extracted from plasma using Sep-Pak C18 cartridges (Waters, Milford, MA) as previously described (20).
Chromatography
CART-LI was characterized as previously described by gel chromatography and reverse-phase fast protein liquid chromatography (FPLC) eluting with an 18–24% gradient of acetonitrile/water 0.05% (vol/vol) trifluoroacetic acid over 50 min (21). Elution coefficient (Kav) for all gel chromatography elution profiles was calculated also as previously described (21).
Statistics
One-way ANOVA was used to assess the variation in plasma CART levels among healthy volunteers throughout the day. Independent Student t tests were used to compare mean CART-LI levels between groups of patients with stable and progressive disease and between patients with midgut or pancreatic neuroendocrine tumors.
Ethics
All subjects with neuroendocrine tumors were anonymized. All other subjects in the study gave written informed consent for the measurement of CART-LI in plasma samples, and ethical approval was obtained from the Hammersmith and Queen Charlotte’s and Chelsea Hospitals Research Ethics Committee (no. 04/Q0406/80). Studies were performed in accordance with the Declaration of Helsinki. Blood was taken from all subjects between 0900 and 1200 h.
Results
Reference range
The mean plasma CART-LI level among the 29 healthy volunteers from our laboratory was 80 pmol/liter (sd 30). The upper limit of normal was calculated as 150 pmol/liter, based on mean + 2 sd, and rounded to the nearest 50 pmol/liter. Henceforth, where normal or raised CART-LI levels are referred to, it is based on this reference range. There was no significant change in the mean plasma CART of 12 volunteers after consumption of a 560-kcal meal, suggesting that circulating CART levels are not acutely affected by food intake (Fig. 1). Also, there was no significant variation in plasma CART level measured in five healthy volunteers throughout the day [0900 (fasting), 1200, 1500, and 1800 h]. Plasma samples from two subjects showed no decrease in CART-LI when left at room temperature for up to 8 h. The addition of Trasylol to lithium heparin tubes made no difference in CART-LI when compared with lithium heparin tubes without Trasylol.
Figure 1.
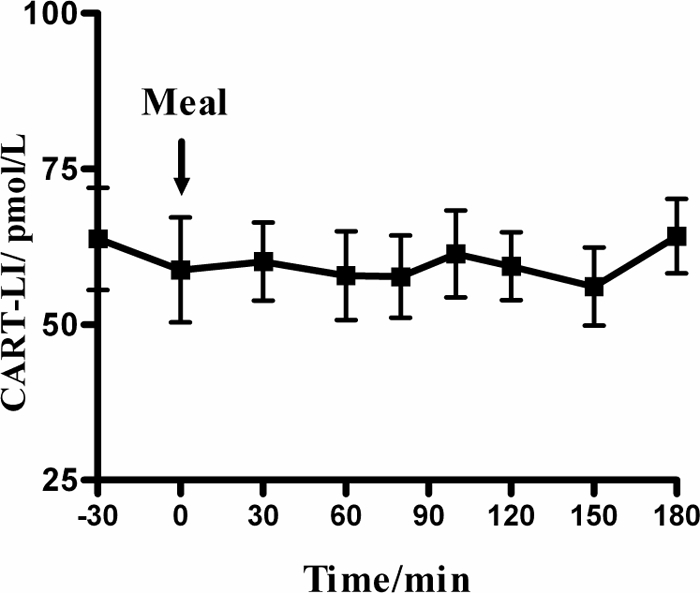
Mean plasma CART-LI level of 12 subjects before and after consumption of a test meal.
Chromatography
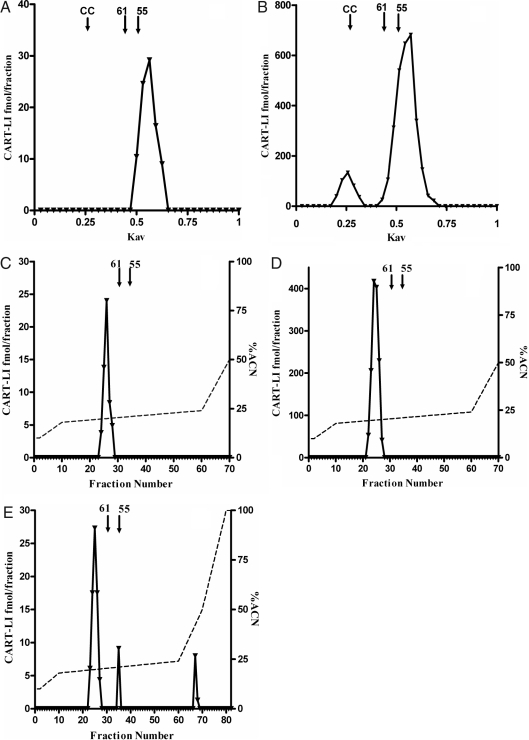
Samples from subjects with high- and low-plasma CART were fractionated using G-50 Sephadex chromatography. All columns had a recovery of more than 70%. CART 55–102 and CART 61–102 standards elute with a Kav of 0.50 and 0.44, respectively. Three different plasma samples from normal subjects were fractionated by gel chromatography (n = 3); Fig. 2A shows a representative elution profile of plasma CART-LI of this. The single CART-LI peak detected elutes with a Kav of 0.56. Figure 2B shows a representative (n = 4) elution profile of plasma CART-LI from a subject with high levels of plasma CART-LI. Two peaks were detected. The first contains 14% of total CART-LI detected and elutes with a Kav of 0.26, giving it a molecular weight of approximately 12,500. The second larger peak contains 86% of total CART-LI detected and elutes with a Kav of 0.56, corresponding to the single peak detected in Fig. 2A.
Figure 2.
Sephadex G-50 column chromatography showing representative elution profiles of plasma CART-LI from a healthy subject, 75 pmol/liter (A) and plasma from a patient with a high level of CART-LI, approximately 20,000 pmol/liter (B). CC, Elution position of horse heart cytochrome C; 61, elution position of CART (61–102); 55, elution position of CART (55–102). Representative FPLC profiles of plasma CART-LI taken from a healthy subject (C), plasma from a patient with a high level of CART-LI (D), profile of fractions containing first peak (E) from B. Solid line, CART-LI concentration; broken line, percent ACN.
Reverse-phase FPLC
Reverse-phase C-18 FPLC chromatography was used to further analyze CART-LI in human plasma. All columns had a recovery greater than 72%. CART 55–102 and CART 61–102 standards eluted at 20.8 and 20.4% acetonitrile, respectively. Figure 2C shows a representative (n = 4) elution profile of extracted plasma CART-LI from a subject with normal circulating CART-LI levels. The single CART-LI peak detected elutes with 20% ACN. Figure 2D shows a representative (n = 5) elution profile of extracted plasma CART-LI from a subject with high circulating CART-LI levels. The single CART-LI peak detected also elutes with 20% ACN and thus corresponds to the CART-LI peak detected in plasma from normal subjects. To determine whether the first peak in Fig. 2B could be resolved further, the fractions containing this peak were dried down in a Savant vacuum centrifuge, reconstituted in distilled water and 0.05% (vol/vol) trifluoroacetic acid and fractionated by reverse-phase C-18 FPLC. Figure 2E shows a representative (n = 3) elution profile of the CART-LI peaks detected. The first contains 80% of the total CART-LI detected and elutes at 19.6% ACN. The second two peaks both contain 10% of the total CART-LI detected and elute at 21.0 and 41.6% ACN.
CART-LI levels in neuroendocrine tumor patients
Overall, CART was positive in 73 of the 131 neuroendocrine tumor cases tested (56%). Of the main tumor types, CART was raised in 23 midgut tumor cases (45%), 27 pancreatic tumors (71%), and 15 tumors of unknown origin (63%). Ninety-five percent of patients with progressive pancreatic neuroendocrine tumors had raised plasma CART-LI levels (n = 20). Mean plasma CART-LI levels were significantly higher among patients with progressive disease (n = 63), compared with patients with stable neuroendocrine tumors of all types (n = 68) (P = 0.035) (Table 1). A comparison of gastrointestinal neuroendocrine tumors (NETs) (n = 58), pancreatic neuroendocrine tumors (n = 38), and nongastrointestinal NETs (n = 11) revealed that pancreatic neuroendocrine tumors have a significantly (P = 0.0402) higher mean CART-LI level (540 ± 66 pmol/liter) than gastrointestinal NETs (337 ± 51 pmol/liter); however, there is no significant difference between pancreatic neuroendocrine and nongastrointestinal NETs (510 ± 136 pmol/liter) (mean ± sem). All 27 cases under observation after the removal of a neuroendocrine tumor had plasma CART-LI within the normal range.
Table 1.
Mean plasma CART-LI concentration (picomoles per liter), sd, number of patients with positive test, and sensitivity of CART, CgA, and CgB assays against disease status (stable or progressive) of patients with known neuroendocrine tumors
| Site of NET | Disease status | n | CART
|
CgA
|
CgB
|
|||
|---|---|---|---|---|---|---|---|---|
| Mean (sd) | +ve (%) | Mean (sd) | +ve (%) | Mean (sd) | +ve (%) | |||
| Midgut | Stable | 24 | 250 (348) | 7 (29) | 490 (342) | 24 (100) | 120 (43) | 6 (25) |
| Progressive | 27 | 447 (429) | 16 (60) | 697 (396) | 25 (93) | 147 (57) | 12 (44) | |
| Pancreatic | Stable | 18 | 426 (443) | 8 (44) | 352 (409) | 12 (67) | 168 (114) | 11 (61) |
| Progressive | 20 | 625 (348) | 19 (95) | 379 (362) | 16 (80) | 166 (57) | 12 (60) | |
| Unknown primary | Stable | 12 | 520 (455) | 7 (58) | 622 (362) | 12 (100) | 202 (114) | 8 (67) |
| Progressive | 12 | 606 (434) | 8 (67) | 794 (338) | 11 (92) | 209 (76) | 9 (75) | |
| Hindgut | Stable | 4 | 279 (326) | 2 (50) | 361 (438) | 3 (75) | 142 (27) | 1 (25) |
| Progressive | 1 | 70 | 0 (0) | 141 | 1 (100) | 89 | 0 (0) | |
| Lung | Stable | 4 | 532 (540) | 2 (50) | 299 (468) | 2 (50) | 127 (85) | 2 (50) |
| Progressive | 1 | 270 | 1 (100) | 60 | 0 (0) | 49 | 0 (0) | |
| Thymic | Stable | 2 | 58 (27) | 0 (0) | 37 (5) | 0 (0) | 61 (9) | 0 (0) |
| Progressive | 0 | |||||||
| Para-ganglioma | Stable | 1 | 1000 | 1 (100) | 153 | 1 (100) | 196 | 1 (100) |
| Progressive | 1 | 1000 | 1 (100) | 184 | 1 (100) | 161 | 1 (100) | |
| Gastric | Stable | 2 | 133 (103) | 1 (50) | 124 (77) | 2 (100) | 133 (29) | 1 (50) |
| Progressive | 0 | |||||||
| Ovary | Stable | 1 | 77 | 0 (0) | 1000 | 1 (100) | 157 | 1 (100) |
| Progressive | 0 | |||||||
| Renal | Stable | 0 | ||||||
| Progressive | 1 | 74 | 0 (0) | 50 | 0 (0) | 180 | 1 (100) | |
| All sites | Stable | 66 | 372 (411) | 28 (42) | 448 (383) | 57 (86) | 150 (88) | 30 (46) |
| Progressive | 54 | 535 (419) | 36 (67) | 592 (420) | 46 (85) | 167 (65) | 32 (60) | |
CART +ve, Plasma CART-LI greater than 150 pmol/liter; CgA +ve, plasma CgA greater than 60 pmol/liter; CgB +ve, plasma CgB greater than 150 pmol/liter.
Comparing and combining CgA and CART assays
Eight neuroendocrine tumor cases were identified with CART-LI greater than 150 pmol/liter but normal CgA: five tumors of pancreatic origin and one each of unknown, lung, and hindgut origin. Forty patients had CART levels greater than 1000 pmol/liter; 13 (34%) with midgut neuroendocrine tumors, and 12 (30%) with neuroendocrine tumors of pancreatic origin. In comparison, such high levels of CgA (>1000 pmol/liter) were more commonly from midgut tumors (46%) and less so from pancreatic neuroendocrine tumors (13%). CART-LI has 95% sensitivity for progressive pancreatic neuroendocrine tumors, compared with only 80% sensitivity for CgA. Combined use of CgA and CART assays gave a positive result in 91% of cases, compared with 85% using CgA assay alone. The combined measurement of CgA and CgB had a lower sensitivity of 89%. Measuring CgA, CgB, and CART-LI did not improve the sensitivity over that of CgA in conjunction with CART.
CART-LI levels in patients without neuroendocrine tumors
Of 192 patients without tumors or renal impairment [93 females and 100 males, average age 53 yr (sd 18 yr)], only one had raised CART-LI levels (Fig. 3). Overall, the mean CART-LI level for the group was 76 pmol/liter (sd 30 pmol/liter). Renal impairment was associated with an increase in plasma CART-LI. The mean level among a group of 17 such patients was 200 pmol/liter (sd 33 pmol/liter), with a maximum value of 282 pmol/liter (with glomerular filtration rate 16 ml/min).
Figure 3.
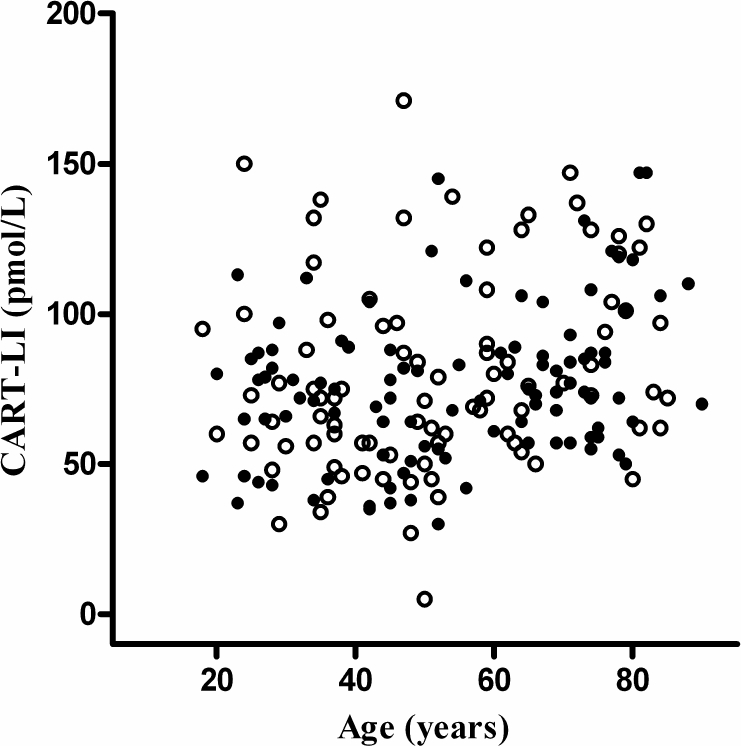
CART-LI level against age for 192 patients without known or suspected neuroendocrine tumors, in the absence of known renal impairment or other form of cancer. •, Male; ○, female.
Of 34 patients with nonneuroendocrine tumors and normal renal function, three were found to have elevated levels of CART-LI. These were adenocarcinomas of the bowel (159 pmol/liter), pancreas (457 pmol/liter), and prostate (393 pmol/liter) (Table 2). The mean CART-LI level among 153 samples from patients with prostate cancer was 84 pmol/liter (sd 38 pmol/liter). Seven had raised plasma CART-LI levels, up to a maximum of 226 pmol/liter, although renal function was unknown.
Table 2.
Mean plasma CART-LI concentration (picomoles per liter), sd, range, and number of patients testing positive by the assay (>150 pmol/liter) in patients with a range of non-NETs
| Tumor site | No. of patients | Mean plasma CART-LI | sd | Range | CART +ve |
|---|---|---|---|---|---|
| Throat | 4 | 84.0 | 5.9 | 79.0–92.0 | 0 |
| Testicle | 2 | 61.7 | 7.8 | 56.2–67.2 | 0 |
| Bowel | 5 | 93.2 | 39.9 | 57.9–159.0 | 1 |
| Prostate | 8 | 105.3 | 117.0 | 50.0–392.5 | 1 |
| Brain | 4 | 88.2 | 38.2 | 53.3–138.8 | 0 |
| Bladder | 2 | 99.7 | 37.2 | 73.3–126.0 | 0 |
| Breast | 4 | 83.1 | 31.5 | 70.0–138.1 | 0 |
| Pancreas | 5 | 150.2 | 171.8 | 56.0–456.6 | 1 |
CART-LI levels measured in 132 subjects aged 18–89 yr did not correlate with age (r2 = 0.0904). There was also no significant difference in CART-LI levels between 49 male (72 ± 2.8 pmol/liter) and 49 female (74 ± 3.8 pmol/liter) patients (mean ± sem).
Discussion
A substantial proportion of neuroendocrine tumors not associated with specific hormonal markers or clinical syndromes, previously defined as nonfunctioning, may still be secretory. A major advancement in the diagnosis of neuroendocrine tumors, and the screening of individuals predisposed to neuroendocrine malignancy, has been the discovery of nonspecific markers such as CgA and CgB (18,19,22,23,24). CgA, cosecreted with amines and peptides, is generally accepted as the best available neuroendocrine tumor marker (25,26). However, CgA is not 100% sensitive for neuroendocrine tumors, and consequently, there is a need for additional nonspecific tumor markers (27).
We report for the first time increased CART-LI in patients with neuroendocrine malignancy. Raised in many forms of neuroendocrine tumor, high levels were associated particularly with those of pancreatic origin, with up to 95% of patients with in progressive pancreatic neuroendocrine tumors having raised circulating CART-LI. This is in accord with previous reports describing the presence of CART in insulinomas and gastrinomas as well as healthy islet cells (12). In the current study, CgA was particularly sensitive for midgut tumors and tumors of unknown origin, which often have a similar natural history to those derived from the midgut (28). This pattern has been shown previously, with highest levels of CgA found in patients with carcinoid tumors (22). In accord with this, positive immunostaining for CgA is found in virtually all enterochromaffin cells but is not universal among neuroendocrine tumors (29,30). Such evidence supports the use of additional tumor markers as adjuncts to CgA to maximize the sensitivity of immunohistochemical diagnosis. In our center CgB is measured routinely as a complement to CgA, and other centers have reported similar use of CgB assays (31). In this study, combined use of CgA and CART assays also gave a positive result for a greater number of neuroendocrine tumor patients than combining CgA and CgB, suggesting a greater crossover of tumors producing both CgA and CgB than CgA and CART.
Circulating neuroendocrine tumor markers are useful in not only the diagnosis of tumors but also patient follow-up and the classification of disease status (32). The results of this study showed significant difference in plasma CART-LI levels between patients with stable and progressive disease. This suggests that CART-LI, like CgA, might be used to help monitor treatment response, whether the aim is curative or simply to slow disease progression. To further define the potential use of CART-LI measurement as a marker in neuroendocrine tumors, it will be necessary to conduct longitudinal studies and examine the effect of tumor resection on circulating levels of CART-LI. It would also be useful to investigate whether treatments such as somatostatin analogs influence CART-LI levels.
Our results demonstrated that CART-LI was not only sensitive for neuroendocrine disease but also specific. Only one of 192 patients without tumor or renal impairment had a raised plasma CART-LI level of 171 pmol/liter. This patient had a history of cluster headaches and was being investigated for suspected inflammatory bowel disease. We found that, as might be expected, renal impairment was associated with an elevation of plasma CART-LI. However, levels were not as high as those found in neuroendocrine tumor patients. Inflammatory bowel disease and renal impairment are also associated with raised circulating CgA (27). Three of 34 patients with nonneurendocrine tumors had CART-LI levels above the normal range, with primary tumors of the bowel, prostate, and pancreas. The highest levels were seen in the patients with the tumors of the prostate and pancreas. Neuroendocrine differentiation of prostatic carcinomas is a recognized factor affecting prognosis, and elevated CgA values correlate with a worse outcome (33,34). Of a further 153 blood samples from prostate tumor patients, 5% had elevated plasma CART-LI. As renal function was unknown, these results must be treated with caution. The mean CART-LI level of five patients with nonneuroendocrine pancreatic tumors was 150.2 pmol/liter. This was the highest level of all the nonneuroendocrine tumors. Neuroendocrine differentiation is a recognized feature of pancreatic tumors and is also associated with a rise in CgA in certain patients (26).
In rats, circulating CART peptide exhibits a diurnal variation, with peak levels in the evening. In contrast, CART levels in the rhesus monkey are higher in the morning. In both species, the diurnal rhythm corresponds with changes in circulating glucocorticoids (15,35). Strong evidence suggests that hypothalamic CART is associated with the regulation of the hypothalamic-pituitary-adrenal axis in animal models (11,16,35). Plasma CART-LI levels in the five healthy volunteers in this study did not significantly differ throughout the day, and comparisons between individuals showed no standard pattern of diurnal variation. There was no correlation with plasma cortisol levels. In addition, there was no effect of fasting or feeding on circulating CART-LI levels. Plasma CART-LI may therefore play a different role in humans, compared with other mammals.
Size exclusion chromatography and FPLC analysis suggests that CART-LI circulates in human plasma in one major form. Using size exclusion chromatography, this form elutes with a Kav of 0.56. There may also be small quantities of other CART-LI fragments in plasma because we detected a smaller additional peak eluting with a Kav of 0.26 in patients with very high levels of CART-LI. It is possible that this peak is also present in normal subjects, but there is insufficient present in the sample to be detected. This fraction elutes slightly earlier than cytochrome C, which has a molecular weight of 12,384, suggesting this peak may represent a protein with a similar molecular weight. It is possible that this peak represents preproCART. Kuhar and Yoho (36) previously detected a fragment of CART in rat adrenal gland with a molecular mass of approximately 13.5 kDa by Western blotting, which they proposed could be preproCART. Neither of the elution positions of the CART-LI peaks in our studies correspond with CART (55–102) or CART (61–102). CART (62–102) is not commercially available, and it is therefore possible that the peak represents this form of CART peptide, although the large difference in Kav between plasma CART-LI and CART (61–102) would suggest otherwise. FPLC analysis also suggested that there is one major CART fragment in human plasma. The CART-LI peak detected in plasma from normal subjects corresponded to the peak detected in plasma from patients with high levels of CART-LI but again did not correspond to CART (55–102) or CART (61–102).
Circulating forms of CART peptide have previously been analyzed using surface-enhanced laser desorption ionization-time-of-flight mass spectrometry in a study by Yanik et al. (37), who detected small amounts of a peptide with a similar molecular weight to CART (55–102) and higher levels of a peptide with a similar molecular weight to CART (62–102). In view of these results, it is possible that the single major peak detected in our study does correspond to CART (62–102). Yanik et al. also detected two smaller additional fragments with larger molecular weights that they proposed might represent intermediate forms of CART peptide. It is possible that the techniques used in our study are insufficiently sensitive to detect the low levels of other forms of CART peptides reported in the previous study. It is also possible human CART may be posttranslationally modified. Further studies are necessary to characterize this possible modification in more detail. CART (1–89) and CART (10–89) extracted from rat adrenal glands are believed to have an unknown posttranslational modification, adding approximately 80 mass units to their molecular weight (38). Further fractionation by FPLC of the first peak obtained by size exclusion chromatography separated three peaks, none of which corresponded to the CART peptide standards.
Studies conducted by Koylu et al. (39) found CART peptide levels were higher in males than females and that CART levels increased with age. In our studies we found no significant difference in circulating CART levels with age or sex. It is possible subtle differences in the patient groups account for the discrepancies between these results.
Plasma CART-LI may represent a new tumor marker of neuroendocrine malignancy. Although less sensitive than CgA as a general marker of neuroendocrine tumors, the combined sensitivity of CART-LI and CgA is better than that of CgA and CgB. There is a particular association of CART-LI with neuroendocrine tumors of pancreatic origin. Further studies are now required to fully assess the value of measuring plasma CART-LI as an adjunct to CgA in the diagnosis of neuroendocrine disease.
Footnotes
This work was supported by program grants from the Medical Research Council (G7811974) and Wellcome Trust (072643/Z03/Z) and European Union FP6 Integrated Project Grant LSHM-CT-2003-503041. We are also grateful for support from the National Institute for Health Research Biomedical Research Centre funding scheme.
Disclosure Statement: The authors have nothing to disclose.
First Published Online January 22, 2008
Abbreviations: CART, Cocaine- and amphetamine-regulated transcript; Cg, chromogranin; FPLC, fast protein liquid chromatography; Kav, elution coefficient; LI, immunoreactivity; NET, neuroendocrine tumor.
References
- Langley K 1994 The neuroendocrine concept today. Ann NY Acad Sci 733:1–17 [DOI] [PubMed] [Google Scholar]
- Polak JM, Bloom SR 1986 Regulatory peptides of the gastrointestinal and respiratory tracts. Arch Int Pharmacodyn Ther 280(Suppl 2):16–49 [PubMed] [Google Scholar]
- Taheri S, Meeran K 2000 Islet cell tumors: diagnosis and medical management. Hosp Med 61:824–829 [DOI] [PubMed] [Google Scholar]
- Barakat MT, Meeran K, Bloom SR 2004 Neuroendocrine tumours. Endocr Relat Cancer 11:1–18 [DOI] [PubMed] [Google Scholar]
- Modlin IM, Lye KD, Kidd M 2003 A 5-decade analysis of 13,715 carcinoid tumors. Cancer 97:934–959 [DOI] [PubMed] [Google Scholar]
- Caplin ME, Buscombe JR, Hilson AJ, Jones AL, Watkinson AF, Burroughs AK 1998 Carcinoid tumour. Lancet 352:799–805 [DOI] [PubMed] [Google Scholar]
- Murphy KG 2005 Dissecting the role of cocaine- and amphetamine-regulated transcript (CART) in the control of appetite. Brief Funct Genomic Proteomic 4:95–111 [DOI] [PubMed] [Google Scholar]
- Douglass J, Daoud S 1996 Characterization of the human cDNA and genomic DNA encoding CART: a cocaine- and amphetamine-regulated transcript. Gene 169:241–245 [DOI] [PubMed] [Google Scholar]
- Dylag T, Kotlinska J, Rafalski P, Pachuta A, Silberring J 2006 The activity of CART peptide fragments. Peptides 27:1926–1933 [DOI] [PubMed] [Google Scholar]
- Stein J, Steiner DF, Dey A 2006 Processing of cocaine- and amphetamine-regulated transcript (CART) precursor proteins by prohormone convertases (PCs) and its implications. Peptides 27:1919–1925 [DOI] [PubMed] [Google Scholar]
- Koylu EO, Balkan B, Kuhar MJ, Pogun S 2006 Cocaine and amphetamine regulated transcript (CART) and the stress response. Peptides 27:1956–1969 [DOI] [PubMed] [Google Scholar]
- Wierup N, Sundler F 2006 CART is a novel islet regulatory peptide. Peptides 27:2031–2036 [DOI] [PubMed] [Google Scholar]
- Hunter RG, Philpot K, Vicentic A, Dominguez G, Hubert GW, Kuhar MJ 2004 CART in feeding and obesity. Trends Endocrinol Metab 15:454–459 [DOI] [PubMed] [Google Scholar]
- Jensen PB, Kristensen P, Clausen JT, Judge ME, Hastrup S, Thim L, Wulff BS, Foged C, Jensen J, Holst JJ, Madsen OD 1999 The hypothalamic satiety peptide CART is expressed in anorectic and non-anorectic pancreatic islet tumors and in the normal islet of Langerhans. FEBS Lett 447:139–143 [DOI] [PubMed] [Google Scholar]
- Vicentic A 2006 CART peptide diurnal variations in blood and brain. Peptides 27:1942–1948 [DOI] [PubMed] [Google Scholar]
- Stanley SA, Murphy KG, Bewick GA, Kong WM, Opacka-Juffry J, Gardiner JV, Ghatei M, Small CJ, Bloom SR 2004 Regulation of rat pituitary cocaine- and amphetamine-regulated transcript (CART) by CRH and glucocorticoids. Am J Physiol Endocrinol Metab 287:E583–E590 [DOI] [PubMed] [Google Scholar]
- Reichlin M 1980 Use of glutaraldehyde as a coupling agent for proteins and peptides. Methods Enzymol 70:159–165 [DOI] [PubMed] [Google Scholar]
- Sekiya K, Ghatei MA, Salahuddin MJ, Bishop AE, Hamid QA, Ibayashi H, Polak JM, Bloom SR 1989 Production of GAWK (chromogranin-B 420–493)-like immunoreactivity by endocrine tumors and its possible diagnostic value. J Clin Invest 83:1834–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AE, Bretherton-Watt D, Hamid QA, Fahey M, Shepherd N, Valentino K, Tatemoto K, Ghatei MA, Bloom SR, Polak JM 1988 The occurrence of pancreastatin in tumours of the diffuse neuroendocrine system. Mol Cell Probes 2:225–235 [DOI] [PubMed] [Google Scholar]
- Patterson M, Murphy KG, le Roux CW, Ghatei MA, Bloom SR 2005 Characterization of ghrelin-like immunoreactivity in human plasma. J Clin Endocrinol Metab 90:2205–2211 [DOI] [PubMed] [Google Scholar]
- Murphy KG, Abbott CR, Mahmoudi M, Hunter R, Gardiner JV, Rossi M, Stanley SA, Ghatei MA, Kuhar MJ, Bloom SR 2000 Quantification and synthesis of cocaine- and amphetamine-regulated transcript peptide (79–102)-like immunoreactivity and mRNA in rat tissues. J Endocrinol 166:659–668 [DOI] [PubMed] [Google Scholar]
- O’Connor DT, Deftos LJ 1986 Secretion of chromogranin A by peptide-producing endocrine neoplasms. N Engl J Med 314:1145–1151 [DOI] [PubMed] [Google Scholar]
- Taupenot L, Harper KL, O’Connor DT 2003 The chromogranin-secretogranin family. N Engl J Med 348:1134–1149 [DOI] [PubMed] [Google Scholar]
- Sobol RE, Memoli V, Deftos LJ 1989 Hormone-negative, chromogranin A-positive endocrine tumors. N Engl J Med 320:444–447 [DOI] [PubMed] [Google Scholar]
- Bajetta E, Ferrari L, Martinetti A, Celio L, Procopio G, Artale S, Zilembo N, Di BM, Seregni E, Bombardieri E 1999 Chromogranin A, neuron specific enolase, carcinoembryonic antigen, and hydroxyindole acetic acid evaluation in patients with neuroendocrine tumors. Cancer 86:858–865 [DOI] [PubMed] [Google Scholar]
- Nobels FR, Kwekkeboom DJ, Coopmans W, Schoenmakers CH, Lindemans J, De Herder WW, Krenning EP, Bouillon R, Lamberts SW 1997 Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuron-specific enolase and the α-subunit of glycoprotein hormones. J Clin Endocrinol Metab 82:2622–2628 [DOI] [PubMed] [Google Scholar]
- Stridsberg M, Eriksson B, Oberg K, Janson ET 2003 A comparison between three commercial kits for chromogranin A measurements. J Endocrinol 177:337–341 [DOI] [PubMed] [Google Scholar]
- Kirshbom PM, Kherani AR, Onaitis MW, Feldman JM, Tyler DS 1998 Carcinoids of unknown origin: comparative analysis with foregut, midgut, and hindgut carcinoids. Surgery 124:1063–1070 [DOI] [PubMed] [Google Scholar]
- Portela-Gomes GM, Stridsberg M, Johansson H, Grimelius L 1997 Complex co-localization of chromogranins and neurohormones in the human gastrointestinal tract. J Histochem Cytochem 45:815–822 [DOI] [PubMed] [Google Scholar]
- Wiedenmann B, Waldherr R, Buhr H, Hille A, Rosa P, Huttner WB 1988 Identification of gastroenteropancreatic neuroendocrine cells in normal and neoplastic human tissue with antibodies against synaptophysin, chromogranin A, secretogranin I (chromogranin B), and secretogranin II. Gastroenterology 95:1364–1374 [DOI] [PubMed] [Google Scholar]
- Stridsberg M, Eriksson B, Fellstrom B, Kristiansson G, Tiensuu JE 2007 Measurements of chromogranin B can serve as a complement to chromogranin A. Regul Pept 139:80–83 [DOI] [PubMed] [Google Scholar]
- Nehar D, Lombard-Bohas C, Olivieri S, Claustrat B, Chayvialle JA, Penes MC, Sassolas G, Borson-Chazot F 2004 Interest of chromogranin A for diagnosis and follow-up of endocrine tumours. Clin Endocrinol (Oxf) 60:644–652 [DOI] [PubMed] [Google Scholar]
- Angelsen A, Syversen U, Haugen OA, Stridsberg M, Mjolnerod OK, Waldum HL 1997 Neuroendocrine differentiation in carcinomas of the prostate: do neuroendocrine serum markers reflect immunohistochemical findings? Prostate 30:1–6 [DOI] [PubMed] [Google Scholar]
- Berruti A, Dogliotti L, Mosca A, Bellina M, Mari M, Torta M, Tarabuzzi R, Bollito E, Fontana D, Angeli A 2000 Circulating neuroendocrine markers in patients with prostate carcinoma. Cancer 88:2590–2597 [DOI] [PubMed] [Google Scholar]
- Vicentic A, Dominguez G, Hunter RG, Philpot K, Wilson M, Kuhar MJ 2004 Cocaine- and amphetamine-regulated transcript peptide levels in blood exhibit a diurnal rhythm: regulation by glucocorticoids. Endocrinology 145:4119–4124 [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Yoho LL 1999 CART peptide analysis by Western blotting. Synapse 33:163–171 [DOI] [PubMed] [Google Scholar]
- Yanik T, Dominguez G, Kuhar MJ, Del Giudice EM, Loh YP 2006 The Leu34Phe ProCART mutation leads to cocaine- and amphetamine-regulated transcript (CART) deficiency: a possible cause for obesity in humans. Endocrinology 147:39–43 [DOI] [PubMed] [Google Scholar]
- Thim L, Kristensen P, Nielsen PF, Wulff BS, Clausen JT 1999 Tissue-specific processing of cocaine- and amphetamine-regulated transcript peptides in the rat. Proc Natl Acad Sci USA 96:2722–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koylu E, Erdogan M, Erdogan M, Karadeniz M, Saygili F, Balkan B, Pogun S, Kuhar M, Plasma CART peptide levels in thyroid patients: body mass Index, gender, and age-related changes. 36th Annual Meeting of the Society of Neuroscience, Atlanta, GA 2006 (Poster 003) [Google Scholar]