Abstract
Context: The current debate regarding whether to decrease the upper limit for the TSH reference range to 2.5 μIU/ml has considerable potential impact on the diagnosis and treatment of subclinical hypothyroidism worldwide.
Objective: We report an analysis of TSH distribution in a population with no evidence of thyroid disease, including a normal thyroid ultrasound.
Design: A subset of the Hanford Thyroid Disease Study cohort was used to examine the TSH distribution in a population having no evidence of thyroid disease, seronegative thyroid autoantibodies, no history of thyroid medications, and a normal thyroid ultrasound. The shape of the TSH distribution was compared with the Gaussian and lognormal distributions.
Setting: This study was performed in the general community.
Participants: Of 1861 Hanford Thyroid Disease Study participants with TSH measured by ELISA who also had thyroid peroxidase antibody measurements, 766 comprised the normal reference group 3 (NRG-3) with no evidence of thyroid disease, including no positive antibodies and normal thyroid ultrasound.
Main Outcome Measure: TSH was measured.
Results: The TSH distribution in the NRG (NRG-3) was right skewed and followed an approximate lognormal distribution. The best estimates of the 97.5th percentile, the percentage above 2.5 μIU/ml, and the percentage above 3.0 μIU/ml for TSH by 3rd generation immunochemiluminometric assay are 4.1 μIU/ml, 20% and 10.2%, respectively.
Conclusion: These results indicate that the TSH reference range should be narrowed and support a value of approximately 4.0 as the upper-reference limit.
This population study suggests that the upper limit of the TSH reference range should be lowered to a value of approximately 4.0.
In the last several years, there has been considerable discussion and disagreement regarding what constitutes the best estimate of the upper-reference limit for TSH, and whether it should be lowered to 2.5 μIU/ml from the 4- to 5.5-upper limit still used by many laboratories (1,2,3). Subclinical hypothyroidism may occur in 5–10% of the general population (4,5,6,7). Since treating euthyroid individuals thought to be hypothyroid or withholding treatment of individuals with subclinical hypothyroidism may adversely impact millions of people worldwide, defining the most appropriate upper reference limit for TSH is an important public health issue.
In practice the TSH distribution has been found to be right skewed. Therefore, reference limits have been determined by log transforming the TSH values, calculating the mean ± 2 sd values, and then exponentiating to obtain the reference limits on the original scale (8). This approach will provide accurate reference limits if normal TSH values follow the lognormal distribution. Recently, it has been asserted that if a reference population is carefully chosen to represent a population without evidence of thyroid disease (excluding those with personal or family histories of thyroid disease, those on medications that affect TSH, and those with abnormal thyroid examinations or positive thyroid autoantibodies), then TSH values drawn in the morning should follow a Gaussian rather than lognormal distribution, with lower and upper-reference limits 0.4 and 2.5 μIU/ml (3,8). It has been stated that “greater than 95% of healthy euthyroid subjects have a serum TSH concentration <2.5 μIU/ml” (8).
We capitalized on an opportunity to evaluate the distribution of TSH values in an unselected general population that was intensively screened for the presence of thyroid disease, including the use of thyroid ultrasonography, and focused on the subset that excluded those with thyroid disease and thyroid ultrasound abnormalities. This is the first such study of thyroid function in an iodine-sufficient population with well-documented evidence of the absence of thyroid disease, including a normal thyroid ultrasound. We evaluated the distribution of TSH values in this population, and the results provide an empirical basis for recommending alternative upper-reference limits for TSH.
Subjects and Methods
The population evaluated in this study was from the Hanford Thyroid Disease Study (HTDS), a retrospective cohort study that investigated whether persons exposed as children to iodine-131 (131I) from the Hanford nuclear facility in eastern Washington state during the 1940s and 1950s were at increased risk for developing thyroid disease. The HTDS has been described extensively elsewhere (9,10,11). The cohort consisted of 5199 persons born in Washington state from January 1, 1940 through December 31, 1946, who were randomly selected from birth certificates. As reported previously, 3440 of 5199 persons were located, agreed to participate in the study, attended a clinic evaluation, and were fully evaluated for the presence of thyroid disease and ultrasound-detected abnormalities (UDAs) (9,10). The median age at examination was 51 yr (range 45–57), and a large majority (97.5%) described themselves as white or Caucasian.
Serum samples were obtained from 3431 (99.7%) individuals, and tested for TSH, free T4 index, and thyroid antibodies. The methods used to measure TSH and thyroid antibodies changed over the course of the HTDS, and this study is restricted to the 1861 with TSH measured by ELISA (Abbott IMX; reference range 0.32–5.01, sensitivity < 0.04; Abbott Laboratories, Abbott Park, IL) who had thyroid antibodies based on thyroid peroxidase antibodies (TPOAbs) (Nichols Institute, San Juan Capistrano, CA). Thyroglobulin antibodies (TgAbs) were subsequently measured on frozen samples after the end of the clinical evaluation phase of the study (Dr. Carole Spencer, University of Southern California, Los Angeles, CA). Positive TPOAbs and TgAbs were defined as more than 2.0 IU/ml and more than 1.0 IU/ml, respectively.
Serum samples were typically drawn between 0755 and 1800 h, with half drawn before 1200 h, and only eight persons drawn between 1800 and 2010 h. Each participant underwent independent thyroid examinations by two thyroidologists and a thyroid ultrasound scan by a certified ultrasonographer (7.5 MHz; Hitachi EUB-310; Tokyo, Japan). Ultrasound prints and videotapes were subsequently reviewed by radiologists for interpretation of thyroid ultrasound abnormalities. The criteria for a normal thyroid ultrasound included the following: 1) a homogeneous echogenic pattern throughout the gland, 2) absence of any focal ultrasound nodules, and 3) absence of diffuse or heterogeneous abnormalities. If the pattern was homogeneous, it was not further classified as hypoechoic, isoechoic, or hyperechoic. Criteria for a normal thyroid ultrasound did not include volume measurements. Color Doppler was not available.
Medical records were requested and reviewed for any participant with suspected preexisting thyroid disease. Individuals with abnormal laboratory function, thyroid examination, or thyroid ultrasound results from the HTDS evaluation were sent letters advising them to seek medical evaluation by their personal physician, and all subsequent medical records related to these recommendations were requested.
All procedures and data collection instruments were approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center, and all study participants provided written informed consent to participate in the study in accordance with institutional and federal requirements.
Three subsets of the cohort were defined as normal reference groups (NRGs), based on the degree to which individuals were free of evidence of thyroid disease. NRG-1 (n = 1448) was composed of those having no evidence of thyroid disease, including no prior history of documented thyroid disease, a normal thyroid physical examination, and no history of taking thyroid hormone. NRG-1 excluded anyone with hyperthyroidism, hypothyroidism, palpable thyroid nodules, or ultrasound abnormalities in the presence of palpable nodules, as well as those taking lithium in the 30 d before the evaluation, Dilantin (Parke-Davis, Pfizer Inc., New York, NY), Tegretol (Novartis, Basel, Switzerland), or glucocorticoids in the 60 d before the evaluation, and amiodarone in the 6 months before the evaluation. It did not exclude those with positive TPOAbs or TgAbs, or UDAs in the absence of any other thyroid disease. NRG-2 (n = 1186) further excluded those with a positive TPOAb and/or positive TgAb, and NRG-3 (n = 766) further excluded those with an abnormal thyroid ultrasound. Of the 766 persons in NRG-3, 97.4% were Caucasian. As reported in detail elsewhere (9,10), no association was found between exposure to Hanford 131I and TSH level, or the cumulative incidence of any form of thyroid disease or ultrasound abnormality. Consequently, TSH levels in individuals from these three subsets of HTDS participants with increasingly strict definitions of the absence of thyroid disease are considered to represent a normal range in an unselected population.
Summary measures of the distribution of TSH were calculated (mean, sd, median, 2.5th and 97.5th percentiles, minimum, maximum), and the distribution was plotted for the entire cohort and by sex, for comparison to previously published studies. These same summary measures were calculated for each of the three NRGs and the distributions plotted. Logistic regression analysis was used to test whether the prevalence of TPOAb seropositivity varied in relation to TSH values after excluding participants with Graves disease or on thyroid hormone. Distributions of TSH levels from blood drawn before vs. after 1200 h were compared using the Kruskal-Wallis test. In further analyses limited to NRG-3, the Shapiro-Wilk statistic (12) was used to test the goodness of fit of Gaussian and lognormal distributions for TSH values.
Because the HTDS TSH results were based on an ELISA that is no longer in use, it was important to determine whether an assay similar to those currently in use would yield comparable results. To investigate this we randomly selected a subset of 50 participants across the range of TSH values and in June 2007 used their previously frozen sera to: 1) determine if the frozen samples had degraded over time, using Abbott Laboratories IMX Ultrasensitive human TSH II assay (analytical sensitivity 0.02 μIU/ml; reference range 0.47–5.01 μIU/ml); and 2) compare the original HTDS ELISA TSH values with those from a currently used third-generation immunochemiluminometric assay (ICMA) ADVIA Centaur TSH-3 assay (Bayer Diagnostics, Bayer AG, Leverkusen, Germany; analytical sensitivity 0.004 μIU/ml; reference range 0.350–5.500 μIU/ml) (for additional details, see the supplemental appendix, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). Comparison of IMX to the original ELISA confirmed that TSH remained stable after storage at −70 C. ICMA values for NRG-3 were predicted from the ELISA values using the regression equation relating the 50 ICMA values to the ELISA values.
Results
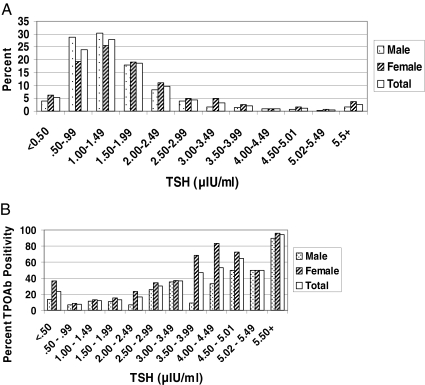
The distribution of TSH by the original HTDS ELISA is shown for the entire cohort (n = 1861) and each sex in Fig. 1A. The mean was 1.81 μIU/ml (males 1.58, females 2.01), and the 97.5th percentile was 5.67 μIU/ml (males 4.40, females 6.50; Table 1). The relationship between TSH and TPOAb seropositivity is shown in Fig. 1B, excluding those with Graves disease or on thyroid hormone. The prevalence of TPOAb seropositivity increased significantly (P < 0.001) with increasing TSH among those with a TSH of at least 0.5 μIU/ml, ranging from 16% for those with a TSH of 2.0–2.49 μIU/ml to 94% for those with a TSH of 5.50 μIU/ml or greater.
Figure 1.
A, TSH distribution for the entire HTDS cohort (n = 1861), by sex (males 895, females 966). B, Percent thyroid peroxidase antibody (TPOAb) positivity by TSH value for the entire HTDS cohort, excluding those with Graves disease and those on thyroid hormone (n = 1707), by sex (males 870, females 837).
Table 1.
TSH distribution using the ELISA for the entire cohort and by NRG
| TSH (μIU/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | sd | Median | Minimuma | Maximum | 2.5th percentileb | 97.5th percentileb | |
| All | 1861 | 1.81 | 2.78 | 1.35 | <0.04 | 88.7 | 0.25 | 5.67 |
| Males | 895 | 1.58 | 1.67 | 1.22 | <0.04 | 26.3 | 0.44 | 4.40 |
| Females | 966 | 2.01 | 3.49 | 1.47 | <0.04 | 88.7 | 0.06 | 6.50 |
| NRG-1c | 1448 | 1.54 | 0.82 | 1.35 | 0.32 | 4.98 | 0.52 | 3.73 |
| Males | 779 | 1.43 | 0.78 | 1.22 | 0.33 | 4.80 | 0.50 | 3.73 |
| Females | 669 | 1.67 | 0.84 | 1.49 | 0.32 | 4.98 | 0.54 | 3.76 |
| NRG-2c | 1186 | 1.45 | 0.73 | 1.30 | 0.32 | 4.79 | 0.53 | 3.37 |
| Males | 673 | 1.39 | 0.73 | 1.21 | 0.36 | 4.79 | 0.52 | 3.38 |
| Females | 513 | 1.53 | 0.72 | 1.40 | 0.32 | 4.05 | 0.53 | 3.37 |
| NRG-3c | 766 | 1.48 | 0.74 | 1.32 | 0.36 | 4.79 | 0.54 | 3.37 |
| Males | 480 | 1.41 | 0.73 | 1.24 | 0.36 | 4.79 | 0.54 | 3.28 |
| Females | 286 | 1.61 | 0.75 | 1.47 | 0.38 | 4.05 | 0.55 | 3.37 |
There were 18 female subjects and one male subject with TSH by ELISA less than 0.04.
Percentiles were estimated nonparametrically, not from fitted Gaussian or lognormal distributions.
NRG-1 excludes those with thyroid disease, on thyroid hormone, and on medications that may affect thyroid function (lithium, amiodarone, glucocorticoids, Dilantin, Tegretol). NRG-2 additionally excludes those with positive antithyroid peroxidase or positive antithyroglobulin, and NRG-3 additionally excludes those with thyroid ultrasound abnormalities.
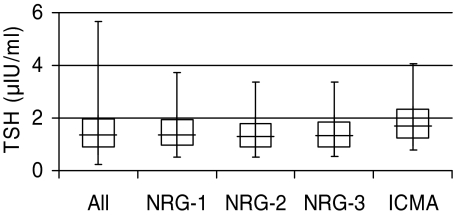
The mean TSH by the original HTDS ELISA in NRG-1 (n = 1448) was 1.54 μIU/ml (males 1.43, females 1.67), and the 97.5th percentile was 3.73 μIU/ml (Table 1). Excluding the 262 with positive TPOAbs and/or positive TgAbs lowered these values slightly, resulting in a mean TSH in NRG-2 (n = 1186) of 1.45 μIU/ml (97.5th percentile 3.37 μIU/ml). Further excluding the 420 with thyroid UDAs did not materially alter the results from that of NRG-2 (Table 1; n = 766; mean = 1.48 μIU/ml; 97.5th percentile = 3.37 μIU/ml). These UDAs included 408 with focal, nonpalpable UDAs and 42 with diffuse, heterogeneous UDAs. The distribution of TSH by the original HTDS ELISA is shown for the entire cohort and for each NRG in Fig. 2.
Figure 2.
Distributions of TSH by ELISA for the HTDS total population (n = 1861) and NRGs (NRG-1: n = 1448; NRG-2: n = 1186; NRG-3: n = 766), and of TSH by ICMA for NRG-3. In these boxplots, the whiskers and box ends represent the 2.5th and 97.5th percentiles, and the 25th and 75th percentiles of the distributions, respectively, and the middle lines represent the medians.
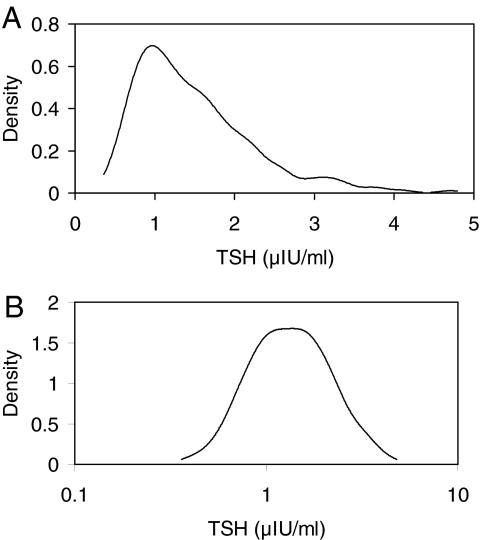
TSH did not differ significantly between those with blood drawn before vs. after 1200 h (P = 0.18), and these groups are, therefore, combined in the following analyses. The distribution of TSH in NRG-3 was right skewed, deviating significantly from a Gaussian distribution (P < 0.0001, Fig. 3A). However, the logarithm of TSH levels in NRG-3 was found to follow an approximate Gaussian distribution (Fig. 3B; P = 0.16). Consequently, the upper-reference limit (mean + 1.96 × sd) was estimated for the log-transformed data, then exponentiated back to the original scale. The resulting value, 3.40 μIU/ml, was very close to the 97.5th percentile of TSH values in NRG-3 (3.37 μIU/ml).
Figure 3.
A, Distribution of TSH by ELISA for the HTDS NRG-3 (n = 766) on a linear scale. B, Distribution of TSH by ELISA for the HTDS NRG-3 (n = 766) on a logarithmic scale.
In the 50 HTDS participants randomly selected for the comparison between assays, the original HTDS ELISA TSH values (range 0.95–6.33 μIU/ml) were highly correlated with those obtained from the Abbott IMX assay (Pearson correlation coefficient ρ = 0.96), and the estimated regression equation relating the IMX and TSH values was not significantly different from IMX = ELISA (supplemental appendix). This was reassuring evidence that the TSH concentration in the stored frozen samples had remained stable over time. Similarly, there was a high degree of correlation between the original HTDS ELISA TSH values and those obtained by the ICMA (ρ = 0.97). On average, the ICMA values were 0.73 μIU/ml higher than the original ELISA values, with fitted regression equation ICMA = 0.16 + 1.16 × ELISA, implying that the 97.5th percentile ELISA value of 3.4 μIU/ml for NRG-3 would correspond to an ICMA value of 0.16 + 1.16 × 3.4 = 4.1 μIU/ml. Similarly, an ICMA value of 2.5 μIU/ml, which would correspond to an ELISA value of (2.5–0.16)/1.16 = 2.0 μIU/ml, was the 80th percentile for NRG-3.
Discussion
We used the HTDS cohort to assess the distribution of TSH levels in a general population, and a subset of individuals in the cohort with documented absence of thyroid disease and normal thyroid ultrasound findings (NRG-3). A key assumption in this approach is that the exposures of these individuals to radioiodine from the Hanford nuclear site in 1944–1957 did not significantly influence their thyroid function, TSH, or thyroid autoantibody levels. The following points provide compelling evidence that NRG-3 represents a normal population without thyroid disease:
We have previously reported that no significant radiation dose-response relationship was found between 131I exposure from Hanford and TSH, any thyroid disease diagnosis, or any ultrasound outcome in this cohort (9,10,13).
The cumulative incidence of all thyroid outcomes studied was consistent with other prevalence studies in unexposed populations (9).
The TSH distribution was right skewed in the entire HTDS cohort, with 15% of individuals having a TSH by ELISA greater than 2.5 μIU/ml. This result suggests that autoimmune thyroiditis is a key factor responsible for the skewed TSH distribution, and is consistent with prior studies (5,14).
The estimated 97.5th percentile for TSH by ICMA in this population is identical to that found in the National Health and Nutrition Examination Survey (NHANES) III normal reference population (5), and similar to that found by Kratzsch et al. (15) in healthy blood donors with normal thyroid glands (Table 2). However, it should be recognized that the HTDS cohort is considered to be iodine sufficient; consequently, the TSH distribution may not be representative of populations with iodine deficiency, such as that reported for the Study of Health in Pomerania in Germany (16).
Table 2.
Median and 97.5th percentiles of the TSH distribution for three population samples
| Study (Ref.) | n | TSH (μIU/ml)
|
|
|---|---|---|---|
| Median | 97.5th percentile | ||
| NHANES III | |||
| Entire cohort (5) | 17,353 | 1.49 | 5.8 |
| Disease free (5)a | 16,533 | 1.49 | 5.52 |
| Disease free risk free (2)b | 14,333 | 1.40 | 4.2 |
| Normal reference population (5)c | 13,344 | 1.39 | 4.12 |
| Kratzsch et al. (15) | |||
| All healthy blood donors | 870 | 1.31 | 3.63 |
| Healthy blood donors with normal thyroid glandd | 453 | 1.36 | 3.77 |
| HTDS | |||
| TSH by ELISA | |||
| Entire cohort | 1861 | 1.35 | 5.67 |
| NRG-1 | 1448 | 1.35 | 3.73 |
| NRG-2 | 1186 | 1.30 | 3.37 |
| NRG-3 | 766 | 1.32 | 3.37 |
| TSH by ICMA for NRG-3 | e | 1.69 | 4.1 |
Excludes those reporting thyroid disease, goiter, or taking thyroid medication.
Disease free group that also excludes those with antithyroid antibodies, those taking lithium, or those discovered biochemically to have overt hyperthyroidism or hypothyroidism.
Disease free risk free group that also excludes those who are pregnant, or are taking estrogen or androgen.
Excludes those with a personal or family history of thyroid disease, an abnormal thyroid ultrasound, or increased antibody.
Based on the regression equation relating the 50 ICMA values to ELISA values.
In determining the TSH reference range, it has been customary to log transform the data because of the skewed distribution. In fact, Guideline 22 of the National Academy of Clinical Biochemistry states “TSH reference intervals should be established from the 95% confidence limits of the log-transformed values of at least 120 rigorously screened normal euthyroid volunteers who have no detectable thyroid autoantibodies, TPOAb or TgAb (measured by sensitive immunoassay), no personal or family history of thyroid dysfunction, no visible or palpable goiter, and no medications (except estrogen)” (8). However, in the recent debate on changing the TSH reference range, it has been asserted that the TSH distribution should follow a Gaussian distribution if the appropriate reference population is used, excluding those with thyroid disease or any family history of thyroid disease, on medication, and using blood samples drawn in the morning (3,8). Furthermore, it has been suggested that until additional data become available, the upper limit of the TSH reference range be 2.5 μIU/ml (3). In the present study, TSH in NRG-3 followed an approximate lognormal rather than Gaussian distribution, in agreement with the National Academy of Clinical Biochemistry guidelines. The upper-reference limit based on “mean + 1.96 sds” of log-transformed TSH was 3.40 μIU/ml, very close to the 97.5th percentile of 3.37 μIU/ml. This result is slightly lower than that recently reported by Kratzsch et al. (15) in a group of 453 healthy blood donors who also had normal thyroid ultrasound examinations in which the 97.5th percentile was 3.77. However, the estimated 97.5th percentile TSH based on our ICMA data for NRG-3 is 4.1 μIU/ml, identical to that seen in NHANES III. It is important to note that these results are derived from a cohort that is nearly entirely Caucasian, who may have higher TSH values compared with non-Caucasians (17).
In assessing whether the upper-reference limit for TSH should be reduced to 2.5 μIU/ml, it is useful to determine what percentage of a population cohort without evidence for thyroid disease has a TSH value above 2.5 μIU/ml. For our NRG-3, the percentage of individuals with TSH (measured by ELISA) over 2.5 μIU/ml was 9.7%. However, from our regression analysis of the relationship between the ICMA and HTDS ELISA values, the proportion with ICMA TSH greater than 2.5 μIU/ml was estimated to be 20%. Thus, the proportion with TSH under 2.5 μIU/ml is estimated to be only 80%; this is significantly different from prior assertions that greater than 95% of euthyroid individuals have TSH levels less than 2.5 μIU/ml (3,8). The proportion of NRG-3 with predicted ICMA values over 3.0 μIU/ml (corresponding to an ELISA value of 2.45 μIU/ml) was also substantial (10.2%), based on our regression analysis. These results are in close agreement with Surks et al. (2), suggesting that analysis of the NHANES III data show that approximately 15% of the reference group (those without known thyroid disease or antithyroid antibodies) have TSH levels above 2.5 μIU/ml. Consequently, lowering the upper-reference limit to 2.5 μIU/ml could result in a significant portion of individuals without evidence for thyroid disease being classified as having an elevated TSH and potentially trigger inappropriate treatment with thyroid hormone. This issue would not be relevant to pregnant women or women planning conception because recent guidelines have recommended that their TSH levels be less than 2.5 μIU/ml (18).
A recent reanalysis of NHANES III data by Spencer et al. (17) has suggested that the upper range skew in TSH may be related to occult thyroid dysfunction caused in part by including antibody negative individuals in cohorts otherwise believed to be free of thyroid disease. The authors further state that it is not possible to establish an accurate TSH upper limit from population studies. One could argue that the TSH upper limit should be somewhat arbitrary because there is not a consensus among endocrinologists whether treating individuals with thyroid hormone for TSH levels between 5 and 10 is warranted. However, endocrinologists make up a small fraction of the practicing physicians who make daily decisions regarding thyroid hormone treatment, and the decision to treat is often based in large part on whether the laboratory reports the TSH to be above or within their reference range. Our primary concern with decreasing the TSH upper limit to 2.5 μIU/mL (or even 3.0, as suggested by the American Association of Clinical Endocrinologists) is that data from NHANES III and our study show that 10–20% of individuals without apparent thyroid disease have TSH levels above 2.5 μIU/mL. Because it is unlikely that all of these individuals have occult thyroid disease, we think a higher upper limit near 4.0 is less likely to result in inappropriate T4 therapy of euthyroid individuals.
Several guidelines regarding the diagnosis and treatment of subclinical hypothyroidism have been published (19,20,21). In addition, there has been much discussion about potential adverse consequences of changing the TSH reference range and the possible impact on individual therapy decisions (22,23,24), the most important of which include treating euthyroid individuals with thyroid hormone and withholding treatment from persons with subclinical hypothyroidism. Clearly, the TSH value alone is insufficient in making a diagnosis of subclinical hypothyroidism and deciding whether treatment with thyroid hormone is appropriate. It is essential that physicians consider many other patient-specific factors, including individual patient symptoms, repeat measurement of TSH to confirm it is elevated and does not represent transient elevation or recovery of subacute thyroiditis, and consideration of whether the person has seronegative autoimmune thyroiditis. An additional clinical factor is recognition of the well-established individual reference range for TSH, which is typically narrower than the population reference range (25). The mean TSH for a given patient may be in either the lower or upper TSH reference range. For example, a patient with a confirmed TSH of 3.6 μIU/ml may have early autoimmune thyroiditis with subclinical hypothyroidism and may benefit from T4 therapy. However, if the history, physical examination, thyroid ultrasound, and thyroid antibody testing are all normal, it is more likely that this TSH value is normal for that individual, and treatment with T4 would be inappropriate.
One potential limitation in this study is the use of an older TSH assay, which although considered acceptable when this study was conducted (1992–1997), has been replaced by third-generation ICMAs. Therefore, we assayed TSH in a randomly selected sample of 50 cryopreserved serum specimens from the original study, and analyzed the feasibility of predicting distributions of TSH measured by ICMA from the existing ELISA values. We demonstrated both that TSH was stable after cryopreservation and that TSH measured by ICMA was consistently higher than the original ELISA values.
There were several other potential limitations of this study. The first is the absence of information on family history of thyroid disease to use as a criterion for defining the reference population. However, all participants in NRG-3 had a normal ultrasound and negative TPOAbs and TgAbs when examined at age 45–57, so it is unlikely that any had a familial predisposition for autoimmune thyroiditis that had not yet been expressed. Second, TSH levels were not uniformly measured during morning hours, however, no significant difference was seen in the distribution when morning TSH values were compared with afternoon values. A third limitation is that we were not able to address ethnicity differences in the TSH distribution because 97.4% of NRG-3 was Caucasian. It is also of interest to note that excluding people with ultrasound abnormalities from our NRG-2 did not further change the TSH distribution in NRG-3. One explanation for this finding is that NRG-2 already excluded persons with indicators of thyroid disease: TPOAbs and TgAbs, palpable thyroid nodules, medications that affect TSH, hyperthyroidism, hypothyroidism, and ultrasound abnormalities associated with these conditions.
The primary strength of this study is that the cohort was randomly selected from birth certificates, and there was no evidence of any substantial bias with regard to selection of the cohort, loss to follow-up, or enrollment and participation (9). In addition, there was nearly complete ascertainment of TSH (99.7%). Furthermore, it is the first study for which a normal thyroid ultrasound is a criterion for excluding thyroid disease in an iodine-sufficient population.
Summary
The results reported here support lowering the upper limit of the TSH reference range below the 4.5–5.0 μIU/ml range that is often reported by many clinical laboratories. However, even after adding normal ultrasonography as a criterion for defining the normal reference population, 20% of the HTDS participants without evidence of thyroid disease have a TSH greater than 2.5 μIU/ml, and 10.2% have a value greater than 3.0. Based on these results, and our concern that an upper limit of 2.5 may result in inappropriate therapy of euthyroid individuals, we recommend an upper limit near 4.0 μIU/ml for the TSH reference range. However, it is essential that patient-specific factors be considered along with a confirmed TSH level before deciding whether thyroid hormone replacement is indicated in an individual patient.
Supplementary Material
Footnotes
This work was supported by the United States Centers for Disease Control and Prevention, contract numbers 03IPA04698, 03IPA04697, 03IPA04699, and 03IPA04700, and Fred Hutchinson Cancer Research Center’s Division of Public Health Sciences.
Disclosure Summary: The authors have nothing to declare.
First Published Online January 29, 2008
Abbreviations: HTDS, Hanford Thyroid Disease Study; 131I, Iodine-131; ICMA, immunochemiluminometric assay; NHANES, National Health and Nutrition Examination Survey; NRG, normal reference group; TgAb, thyroglobulin antibody; TPOAb, thyroid peroxidase antibody; UDA, ultrasound-detected abnormality.
References
- Dickey RA, Wartofsky L, Feld S 2005 Optimal thyrotropin level: normal ranges and reference intervals are not equivalent. Thyroid 15:1035–1039 [DOI] [PubMed] [Google Scholar]
- Surks MI, Goswami G, Daniels GH 2005 The thyrotropin reference range should remain unchanged. J Clin Endocrinol Metab 90:5489–5496 [DOI] [PubMed] [Google Scholar]
- Wartofsky L, Dickey RA 2005 The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab 90:5483–5488 [DOI] [PubMed] [Google Scholar]
- Canaris GJ, Manowitz NR, Mayor G, Ridgway EC 2000 The Colorado thyroid disease prevalence study. Arch Intern Med 160:526–534 [DOI] [PubMed] [Google Scholar]
- Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE 2002 Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, Evans JG, Young E, Bird T, Smith PA 1977 The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol (Oxf) 7:481–493 [DOI] [PubMed] [Google Scholar]
- Cooper DS 2001 Clinical practice. Subclinical hypothyroidism. N Engl J Med 345:260–265 [DOI] [PubMed] [Google Scholar]
- Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, LiVosli VA, Niccoli-Sire P, John R, Ruf J, Smyth PP, Spencer CA, Stockigt JR, Guidelines Committee, National Academy of Clinical Biochemistry 2003 Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 13:3–126 [DOI] [PubMed] [Google Scholar]
- Davis S, Kopecky KJ, Hamilton TE 2002 Hanford Thyroid Disease Study Final Report. http://www.cdc.gov/nceh/radiation/hanford/htdsweb/pdf/htdsreport.pdf. 2–15-2004 [Google Scholar]
- Davis S, Kopecky KJ, Hamilton TE, Onstad L, Griep R, Saporito M, Adams Meyers P, Gross K, Treece G, Brewer G, Tuttle RM, Garbe P 2004 Thyroid neoplasia, autoimmune thyroiditis, and hypothyroidism in persons exposed to iodine 131 from the Hanford Nuclear Site. JAMA 292:2600–2613 [DOI] [PubMed] [Google Scholar]
- Kopecky KJ, Davis S, Hamilton TE, Saporito MS, Onstad LE 2004 Estimation of thyroid radiation doses for the Hanford Thyroid Disease Study: results and implications for statistical power of the epidemiological analyses. Health Phys 87:15–32 [DOI] [PubMed] [Google Scholar]
- Shapiro S, Wilk M 1965 An analysis of variance test for normality. Biometrika 52:591–611 [Google Scholar]
- Kopecky KJ, Onstad L, Hamilton TE, Davis S 2005 Thyroid ultrasound abnormalities in persons exposed during childhood to 131I from the Hanford nuclear site. Thyroid 15:604–613 [DOI] [PubMed] [Google Scholar]
- Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, Grimley EJ, Hasan DM, Rodgers H, Tunbridge F 1995 The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 43:55–68 [DOI] [PubMed] [Google Scholar]
- Kratzsch J, Fiedler GM, Leichtle A, Brugel M, Buchbinder S, Otto L, Sabri O, Matthes G, Thiery J 2005 New reference intervals for thyrotropin and thyroid hormones based on National Academy of Clinical Biochemistry criteria and regular ultrasonography of the thyroid. Clin Chem 51:1480–1486 [DOI] [PubMed] [Google Scholar]
- Volzke H, Alte D, Kohlmann T, Ludemann J, Nauck M, John U, Meng W 2005 Reference intervals of serum thyroid function tests in a previously iodine-deficient area. Thyroid 15:279–285 [DOI] [PubMed] [Google Scholar]
- Spencer CA, Hollowell JG, Kazarosyan M, Braverman LE 2007 National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab 92:4236–4240 [DOI] [PubMed] [Google Scholar]
- Abalovich M, Amino N, Barbour L, Cobin RH, De Groot LJ, Glinoer D, Mandel SJ, Stagnaro-Green A 2007 Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 92(Suppl):s1–s47 [DOI] [PubMed] [Google Scholar]
- Baskin HJ, Cobin RH, Duick DS, Gharib H, Guttler RB, Kaplan MM, Segal RL 2002 American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation of treatment of hyperthyroidism and hypothyroidism. Endocr Pract 8:457–467 [PubMed] [Google Scholar]
- Ladenson PW, Singer PA, Ain KB, Bagchi N, Bigos ST, Levy EG, Smith SA, Daniels GH, Cohen HD 2000 American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med 160:1573–1575 [DOI] [PubMed] [Google Scholar]
- Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, Franklyn JA, Hershman JM, Burman KD, Denke MA, Gorman C, Cooper RS, Weissman NJ 2004 Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA 291:228–238 [DOI] [PubMed] [Google Scholar]
- Chu JW, Crapo LM 2001 The treatment of subclinical hypothyroidism is seldom necessary. J Clin Endocrinol Metab 86:4591–4599 [DOI] [PubMed] [Google Scholar]
- Gharib H, Tuttle RM, Baskin HJ, Fish LH, Singer PA, McDermott MT 2005 Subclinical thyroid dysfunction: a joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and the Endocrine Society. J Clin Endocrinol Metab 90:581–585 [DOI] [PubMed] [Google Scholar]
- McDermott MT, Ridgway EC 2001 Subclinical hypothyroidism is mild thyroid failure and should be treated. J Clin Endocrinol Metab 86:4585–4590 [DOI] [PubMed] [Google Scholar]
- Andersen S, Bruun NH, Pedersen KM, Laurberg P 2003 Biologic variation is important for interpretation of thyroid function tests. Thyroid 13:1069–1078 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.