Although considerable experimental data have accumulated concerning many organs, there have been few reports of homotransplantation of the liver. Until 1959, the only work had been done by Welch and his associates (9, 34), who were able to obtain function from liver homografts transplanted into the pelvis. Recently, Moore and his associates presented the first accounts of successful homotransplantation of the canine liver to animals with total hepatectomy (21,22). Maximum survival after operation was 12 days.
The techniques to be described for homotransplantation of the liver have previously been briefly outlined (12, 28). The influence of portal flow upon the homografted liver has been analyzed in detail not only be-cause this factor proved to be an important determinant of success or failure but also because the resultant information may have application in a variety of other experimental situations, including those involving hemorrhagic shock. Maximum survival after liver homotransplantation has been 20½ days.
GENERAL METHODS
Seventy-nine liver transplants were performed, using healthy adult mongrel dogs of 10 to 25 kilograms. In most cases, the weights of the donor and recipient animals were closely matched. Both dogs were anesthetized with 25 to 30 milligrams of sodium pentobarbital per kilogram and, after tracheal intubation, placed on respirators. The arterial pressure of the recipient dog was monitored during and immediately after operation with an arterial catheter connected to an aneroid manometer. Venous pressures were read directly from a water manometer attached to an indwelling catheter. The operation was performed under sterile conditions by two, or occasionally three surgeons. In analysis of results, the first 27 hepatic transplants were excluded from consideration because a variety of methods of liver preparation were used with a resultant inconstant quality of grafts.
PRELIMINARY STEPS IN THE RECIPIENT DOG
The abdomen was opened with an upper midline incision. A segment of aorta, 1½ to 2 centimeters in length, was mobilized just above the inferior mesenteric artery for later anastomosis. Next, the mesentery of the caudate lobe of the liver was incised, and the inferior vena cava encircled with a tape, above the entrance of the adrenal veins. The gastrohepatic ligament to the left of the portal triad was then doubly ligated and divided. Finally, common duct and gastroduodenal artery were doubly ligated and divided as close to the duodenum as possible. The portal vein was cleaned off toward the liver until its bifurcation was encountered, taking care to ligate all lymphatics on its surface. When these steps were completed, the only uninterrupted structures remaining in the gastrohepatic ligament were the hepatic artery and the portal vein.
A portacaval shunt was then constructed in as inferior a position as was convenient (Fig. 1b). The exact size and technique of this shunt varied with the different techniques of venous reconstruction during implantation, but in every case the presence of the anastomosis was necessary for decompression of the splanchnic system during implantation of the liver. When the anastomosis was completed, the abdominal wound was closed with towel clips, and attention directed to the donor dog.
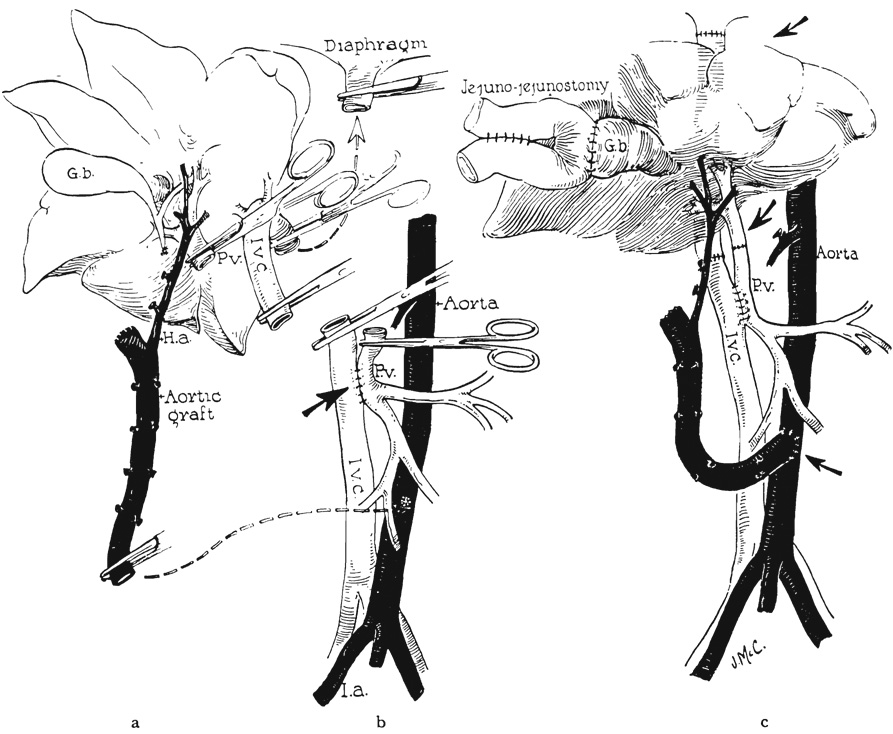
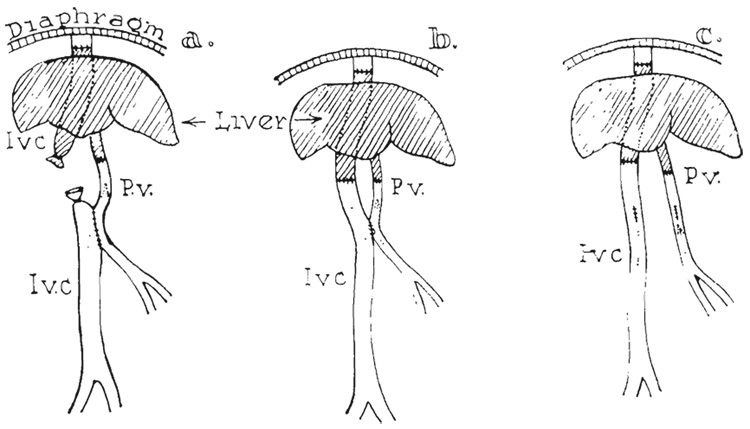
FIG. 1.
Basic technique of homotransplantation. a, Donor liver ready for transplant. Note aortic graft removed in continuity with hepatic artery and liver graft. b, Recipient with portacaval shunt and liver removed. c, Donor liver in place.
PREPARATION OF THE DONOR LIVER
During this operation on the recipient, the donor dog was immersed in an ice bath, and the body temperature reduced to 25 to 30 degrees C. The abdomen of the donor dog was opened through a long midline incision. The abdominal aorta was mobilized proximally to the level of the superior mesenteric artery, ligating and dividing all branches (Fig. 2). The superior mesenteric artery was encircled with a ligature but left intact for the time being.
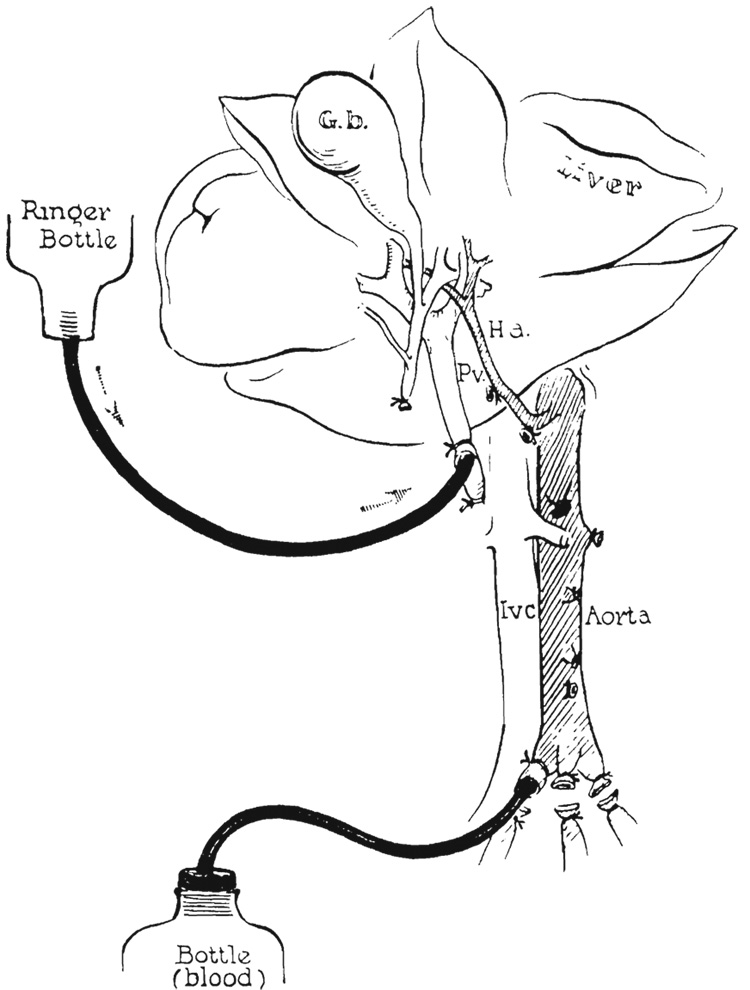
FIG. 2.
Preparation of donor liver. Aortic graft prepared. Perfusion of cooled Ringer’s solution by gravity through the portal vein and collection of blood from the aorta.
Next, the stomach and spleen were retracted sharply to the right, and the celiac axis was dissected free from its origin to the trifurcation, where splenic and left gastric arteries were ligated and divided (Fig. 2). Attention was then directed to the portal triad. The gastrohepatic ligament to the left of the triad was first ligated and divided, and the common duct and gastroduodenal artery doubly ligated and divided near the duodenum. The portal vein was freed from its surrounding adventitia in which there were many lymph channels which required ligation. In order to obtain an adequate length of portal vein for subsequent anastomosis, the pancreatic vein was doubly ligated and divided. Several large lymph nodes usually still loosely connected the portal vein and hepatic artery inferiorly, but these were stripped out easily with blunt dissection, leaving the hepatic artery and portal vein skeletonized (Fig. 2). The donor liver was now ready for perfusion in situ.
The previously placed ligature around the superior mesenteric artery was tied. The portal vein was ligated as far inferiorly as possible, and the blunt end of a standard intravenous infusion set directed up the portal vein to the liver. Through this, 1,000 cubic centimeters of cooled (5 to 10 degrees C.) lactated Ringer’s solution were used for gravity perfusion of the liver with a pressure head of 60 to 80 centimeters of water (Fig. 2). As soon as perfusion was begun, the animal was bled to death through a catheter inserted into the aorta (Fig. 2). The collected blood was subsequently used for transfusion of the recipient. During the perfusion, the interior of the liver cooled to 10 to 20 degrees C.
The liver was then removed after incision of the diaphragmatic ligaments by transection of the portal vein, the upper abdominal aorta, and the vena cava above and below the liver (Fig. 1a), The open upper end of the aortic graft was ligated. The vena caval cuff above the liver was carefully scrutinized for small holes which, if unrecognized, will lead to air embolus or hermorrhage after the liver is revascularized. A small incision in the gallbladder was made at the site of the proposed cholecystenterostomy to prevent autolysis. The liver was then brought to the table of the recipient animal.
Before evolving this method for preparation of the donor liver, a number of unsatisfactory techniques were employed. These failed generally either because of the use of heparin in the donor with consequent bleeding after transplantation or because the liver was not cooled enough to withstand the effects of ischemia.
HEPATECTOMY IN THE RECIPIENT DOG AND TRANSPLANTATION OF THE DONOR LIVER
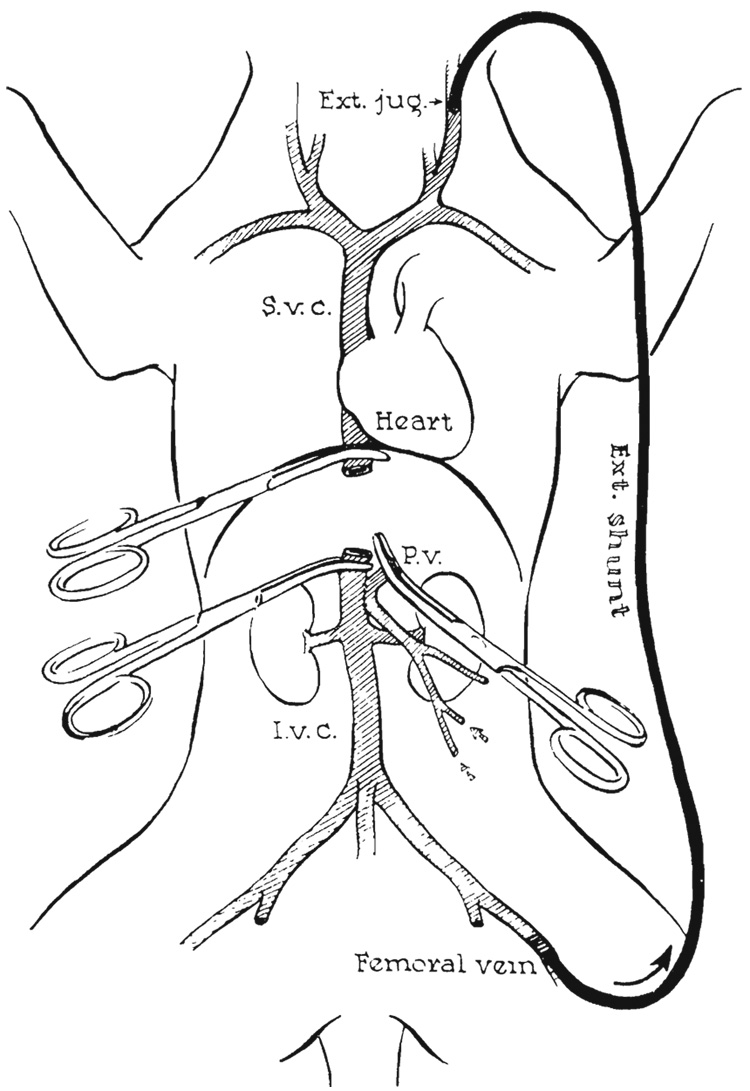
After removal of the donor liver, the operating team returned immediately to the recipient dog and inserted an external polyethylene bypass from the femoral to jugular veins (Fig. 3), as described by Kaupp and Starzl (13). After ligation and division of the hepatic artery, the vena cava, above and below the liver, and the portal vein were grasped with Potts’ clamps and the liver was removed (Fig. 3). A brisk flow through the external shunt caused decompression of the blocked splanchnic as well as of the caval beds.
FIG. 3.
Decompression of portal and caval venous systems during complete occlusion of vena cava and portal vein by external shunt from femoral vein to external jugular vein.
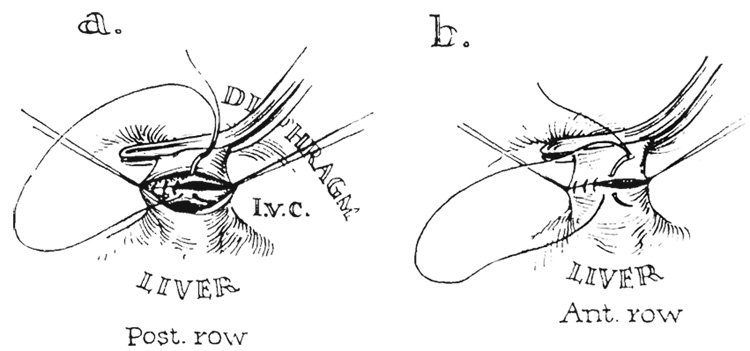
The cooled donor liver was positioned in the upper part of the abdomen of the recipient animal (Fig. 1 a and b). Anastomosis was performed with continuous No. 5-0 arterial silk between the cuff of recipient vena cava at the diaphragm, which was sharply pulled down to facilitate exposure (Fig. 4), and the short cuff of vena cava on the donor liver. Because of the limited space and short segments of vessels, it was necessary to sew the posterior row from within the vessels (Fig. 4).
FIG. 4.
Technique of caval-caval anastomosis with posterior row being sutured from within.
Re-establishment of the portal and vena caval return was done in three different ways (Fig. 5). The choice of procedure influenced the type of portacaval shunt previously performed (during preliminary steps the recipient dog). The first method of venous reconstruction employed the principle of the reverse Eck fistula (Fig. 5a), in which the preliminary portacaval shunt was made at least 1 centimeter in length. After completion of the caval-caval anastomosis above the liver, an end-to-end portal-portal anastomosis was performed, and the open stumps of donor and recipient vena cava below the liver were ligated (Fig. 5a ). With release of the clamps, the venous return of both the caval and splanchnic systems were directed through the liver (Fig. 5a).
FIG. 5.
Methods of venous reconstruction. a, Reverse Eck fistula. b, Anatomic reconstruction with small porta-caval shunt. c, Complete anatomic reconstruction with closure of preliminary portacaval shunt.
The second method resulted in anatomic venous reconstruction with the addition of a small portacaval shunt (Fig. 5b). In this case, the preliminary portacaval anastomosis was made small, 4 to 7 millimeters long, without the excision of an ellipse from either vessel. After completing the upper caval-caval anastomosis, the lower caval-to-caval and portal-to-portal anastomoses were performed in anatomic position (Fig. 1c and 5b).
The third method of venous reconstruction resulted in normal venous pathways. The preliminary portacaval anastomosis was 8 to 10 millimeters in length and was used only to decompress the portal system (via the external polyethylene bypass) during the period of acute portal-caval occlusion as the liver was implanted. The portal-portal and lower caval-caval anastomoses were performed anatomically with No. 6-0 and No. 5-0 silk respectively (Fig.1c). Before the abdomen was closed the portacaval shunt was taken down and the venotomies were closed with No. 6-0 arterial silk (Fig. 5c).
After completing the venous anastomoses, the aortic graft was placed in the right para-vertebral gutter and attached with an end-to-side anastomosis to the recipient dog’s aorta, at a variable distance below the renal arteries (Fig. 1c). No. 6-0 arterial silk was used and a button of recipient aorta removed.
Internal biliary drainage was performed with a cholecystojejunostomy, below which was established a jejunojejunostomy (Fig. 1c), Both gastrointestinal anastomoses were performed with a two-layer catgut and silk technique. Splenectomy was carried out, and the abdomen was closed with multiple layers of fine silk.
POSTOPERATIVE CARE
One million units of aqueous penicillin were given on the morning of operation and twice daily during the entire postoperative period. As soon as the operation was completed, a large gastric tube was passed and kept in position until no longer tolerated. Frequent endotracheal aspirations or bron-choscopies were performed until the animal was fully awake.
During operation, 500 cubic centimeters of blood were generally given. During the first 8 to 10 postoperative hours, it was almost always necessary to give another 500 cubic centimeters, even to those dogs in which no bleeding could ever be demonstrated. Intravenous fluids were usually administered for the first 2 or 3 days, after which many dogs tolerated a low protein diet. If the animals stopped eating, intravenous or clysis therapy was reinstituted.
RESULTS
Tolerance of the devascularized liver to ischemia
The time between removal of the cooled donor liver and its revascularization in the recipient dog was 45 minutes, at the least, and in many instances 2 hours. After more than 2 hours, ischemic liver injury occurred which almost always precluded success by any of the described means of reconstruction. When blood flow was restored after ischemia of longer than 2 hours, the liver became tense and dark in color, with histologic evidence of intense congestion. Death followed in a few hours either from hemorrhagic gastroenteritis, as a result of hemorrhage from small capsular tears in the distended liver, or from acute hepatic failure.
In preparation of the donor liver, it was important that the liver be cool before any ischemia occurred. For this reason, total body cooling (25 to 30 degrees C.) was carried out in the donor dog before the abdomen was opened. In the event of accidental ischemia during dissection of the portal triad or aortic pedicle, before the liver was further cooled by perfusion, some protection was afforded.
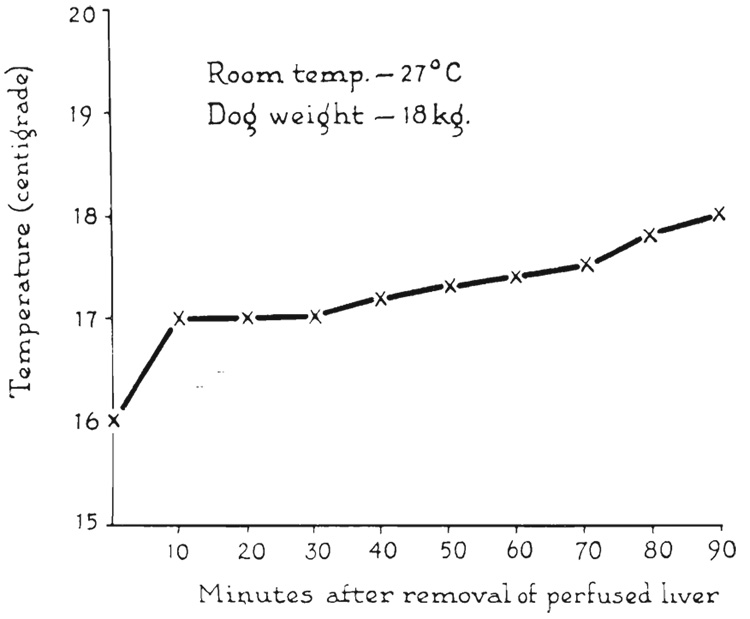
After the liver had been perfused with cold Ringer’s solution, its temperature was well maintained at approximately 10 to 16 degrees C. after removal. Shown in Figure 6 is a typical warming curve of the interior of a donor liver, in which the temperature rise was less than 2 degrees C. in 1½ hours.
FIG. 6.
Rewarming curve of interior of donor liver exposed to room temperature for 1½ hours.
The value of the external bypass during liver transplantation
In performing the first 20 liver transplants, an external bypass was not used during the period of portacaval occlusion. since in previous work it had been determined that short term occlusion in the presence of a portacaval shunt was well tolerated (29). Survivals of as long as 6 days were obtained with this technique, but in subsequent experiments the external bypass was routinely used for several reasons. The portacaval occlusion was often for as long as 2 hours, during the latter part of which profound hypotension usually developed. At autopsy, many of the dogs had cardiac lesions similar to the infarcts described by Tanturi after temporary portal occlusion. Finally, hemorrhagic gastroenteritis proved to be a common postoperative complication, and it was not possible to know if this was due to the acute portal hypertension during implantation or to splanchnic venous stasis which developed postoperatively.
With the external bypass, severe hypotension did not develop. Some fall in blood pressure occurred, but the mean pressure almost always remained above 100 millimeters of mercury. The bowel remained pink. Clotting of the siliconized tube did not generally occur during the bypass.
Acute effect of venous reconstruction with the reverse Eck fistula
Twenty-six hepatic transplants were performed by the method of the reverse Eck fistula (Fig. 5a). Only 4 of the 26 dogs survived for more than 4 days. The other 22 animals died at varying times up to 4 days, in 11 experiments as a result of the inability of the transplanted liver to transmit the required venous flow, with consequent hemorrhagic gastroenteritis. The other early deaths were caused by hemorrhage in 3 dogs, portal thrombosis in 3 dogs, atelectasis in 2 dogs, air embolus in 1 dog, and unknown etiology in 2 dogs.
Most of the unsuccessful experiments followed a highly characteristic pattern. After revascularization, it became obvious that sequestration of blood was occurring in the liver which became swollen, tense, and extremely dark in color. Shortly after, large amounts of lymphatic fluid escaped from the surface of the liver capsule. The portal vein and inferior vena cava distended with pressures as high as 250 millimeters of saline. Superficial lacerations in the transplanted liver began to hemorrhage, and frequently the previously dry portal-portal or portacaval anastomosis began to ooze, sometimes uncontrollably. Within a few minutes, the bowel and stomach lost their normal pink color and assumed a faintly cyanotic hue. Within a few hours, large quantities of fluid accumulated in the gut—at first clear and later blood-stained.
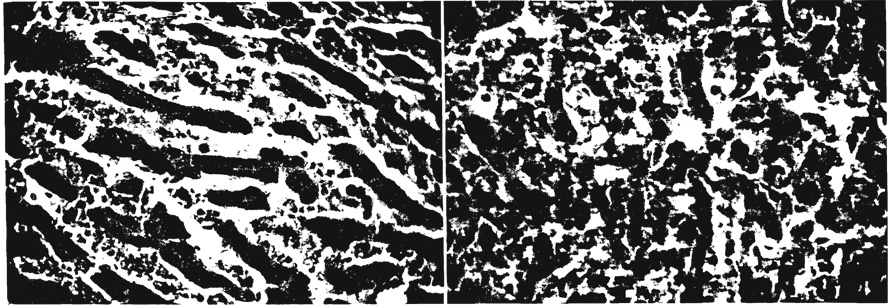
In such cases, a progressively increasing rate of blood transfusion was necessary to maintain the blood pressure. Bloody diarrhea developed, and the animals died in 5 to 15 hours, despite aggressive therapy. Histologic sections of the liver showed acute congestion and early disruption of hepatic cord architecture (Fig. 7a). Sections of the large and small intestine revealed marked congestion and interstitial edema of the entire wall (Fig. 8a and b).
FIG. 7.
Histologic changes in hepatic outflow block. a. Liver after 10 hours, showing congestion and early loss of architecture with dilated sinusoids. b. Another liver at 36 hours showing persistent congestion and marked disorganization of hepatic carchitecture. ×214.
FIG. 8.
Intcstinal changes resulting from outflow hepatic block in dogs with reverse Eck fistula. a, Small intestine after 10 hours showing mucosal and submucosal congestion and submucosal edema. Note intact epithelium. ×11. b, Small intestine in another dog after 18 hours. There is congestion and loss of epithelium of distal portions of villi. ×44. c, Small intestine in a dog dying after 36 hours. Base of villi have partially intact epithelium, but distal villi are edematous and congested with marked slough of epithelial cove ring. ×44.
In other experiments, the animals survived the immediate postoperative period, only to die 1 to 4 days postoperatively, usually from gastrointestinal hemorrhage. In these animals, there was histologic evidence of persistent hepatic congestion with distention and coalescence of sinusoids (Fig. 7b). Hepatic cell loss was apparent, especially in central areas. Associated slough of intestinal mucosa and inflammatory infiltration of intestinal villi occurred (Fig. 8c).
Four of the 26 liver transplants revascularized with the reverse Eck fistula survived longer than 4 days, or long enough for homograft rejection to be expected to occur. Maximum survival was 9 days.
Numerous complications characterized this group of animals. Formation of ascites was excessive, and dissection of the fluid through the incision with wound breakdown occurred in all long term survivals. Pneumonitis and thrombosis of the vascular anastomosis were also late causes of death. Only 1 of the long term survivors was able to eat.
Acute effect of anatomic venous reconstruction with addition of a portacaval shunt
Fifteen transplants were performed by means of anatomic reconstruction of the venous system with the addition of a small portacaval shunt (Fig. 5b). The portacaval anastomosis was deliberately constructed small— 4 to 7 millimeters—so that a definitely elevated portal pressure would be necessary in order for large flows to cross the shunt. In this wav, it was hoped to decompress the liver and splanchnic circulation in the event of high portal pressures, without unnecessarily diverting portal blood from the liver. Fifteen livers were transplanted in this manner and 6 animals survived for more than 4 days. Nine dogs died in the early pastoperative period: 2 of hemorrhage, 4 of atelectasis. 2 of hemorrhagic gastroenteritis, 1 as a result of portal thrombosis. An important contributory cause of death in many of these dogs was a distinctive syndrome consisting of intractable vomiting and retching which began immediately after operation and continued until the death of the dog. This was thought, but not proved, to be due to acute liver failure.
With this technique, the acute congestion of the liver and bowel described for the reverse Eck fistula was seen in only 2 experiments. Generally, the liver became soft and light in color after revascularization, and the bowel remained pink except in those cases in which the shunt was inadvertently made too small to be functional.
Despite the obvious disadvantage of reducing the blood flow to the liver, this technique of reconstruction proved to be the most versatile in one respect. It was possible to perform transplantation without closely matching the size of the donor and recipient animals. Donors were used which weighed as much as 8 to 10 kilograms less than the recipient animals, a disparity in size which precluded success in the other two methods because of the development of splanchnic and hepatic congestion.
Six of the 15 dogs with this type of venous reconstruction lived for more than 4 days. Only 2 animals were able to eat consistently. Maximum survival was 9 days. Although it is thought that homograft rejection occurred, a variety of other complications were found at autopsy which could have been independent causes of death.
Acute effect of anatomic venous reconstruction
Eleven dogs had reconstitution of the liver in an anatomic manner, and 8 survived for more than 4 days. The causes of early death were hemorrhagic gastroenteritis in 2 cases, and atelectasis in the third. When this technique was used, the acute bowel congestion described with reverse Eck fistula reconstruction was seldom encountered provided the weights of the donor and recipient dogs were closely matched. However, efforts to use significantly smaller donor than recipient animals resulted in acute bowel congestion and early death.
The clinical behavior of this group of dogs was the most satisfactory of all techniques tried. All 8 long term dogs resumed oral intake promptly. usually after 2 days, and continued to eat until shortly before death, which was presumably due to liver rejection. The average long term survival exceeded that obtained with either of the other methods, as did maximum survival which was 20½ days.
DISCUSSION
The unusual susceptibility of the liver to anoxia has undoubtedly deterred progress in hepatic transplantaion. Welch and associates (9), who transplanted the whole organ to the pelvis of recipient dogs, pointed out that the transferred tissue could not survive if devascularized for more than 30minutes under normothermic conditions. Disclosures of the protective effect of hypothermia on the ischemic liver by Raffuci, Bernhard, and Huggins and their associates have made transplantation studies more practical.
Despite the protection of hypothermia, undue prolongation of ischemia results in a characteristic liver injury in which the revascularized organ entraps large quantities of blood. The inability of blood to escape is probably due to constriction of small intraparenchymal hepatic veins as shown by Deysach and Thomas and Essex. These vessels have been demonstrated by Arey to have extraordinarily well developed muscular coats in the dog. Similar hepatic congestion, aptly termed “blue liver syndrome” or “outflow block,” has been described in the dog in a variety of physiologic circumstances including: anaphylaxis and peptone shock by Mautner and Pick, Simonds, and Weil; temporary devascularization of the liver by Hines and Roncoroni and State and Lichtenstein; histamine injection by Mautner and Pick and Bauer and his associates; perfusion of the isolated liver by Makravarti and Tripod, Maegaith, and Kestens and his associates; endotoxin shock by MacLean and Weil: and hemorrhagic shock by Frank (6) and Wiggers and their associates.
With transplantation of the liver, the degree of ischemic injury is only one of the influences which contribute to the grave complication of outflow block. The most important secondary factor is the volume of venous blood which is transported to the liver. In the normal dog, it has been shown by Meyer and others that both the splanchnic and inferior caval venous return can be directed through the liver without harmful effects. But the anoxic transplanted liver apparently reacts to such augmented venous flow with outflow block. In contrast, the blue liver developed infrequently when venous inflow was normal or when a decompressing portacaval anastomosis was retained as a permanent feature of the reconstruction. The importance of regulating portal vein inflow in avoiding outflow block during isolated liver perfusion has also been commented up on by McDermott and associates (14), as well as numerous earlier workers.
Another factor contributing to the susceptibility to outflow block of the transplanted or the isolated perfused liver may be the state of denervation in both instances. The confusing literature on vasomotor control of the liver has been summarized by Child. The dominant autonomic influence appears to be sympathetic, with reduction of the size of the liver and increased outflow, upon stimulation. Parasympathetic stimulation has resulted in less clear findings. Similarly, epinephrine solutions cause shrinkage of the liver with increased hepatic vein outflow, as shown by Bauer and his associates and by Maegaith, while acetylcholine has a less pronounced but reverse effect. From this information, it would seem quite possible that the denervated liver undergoes vasomotor alterations which promote stasis. Such a mechanism, in addition to contributing to outflow block, could explain the necessity for the large transfusions which are necessary postoperatively, even in the dogs which survive for long term study.
The sequence of events in the bowel following outflow block in the transplanted liver is of interest because of the resemblance of the pathologic changes to those seen in irreversible hemorrhagic shock. For many years, the liver has been suspected of playing an important role in the development of irreversibility. Numerous observers, including Selkurt (25), Wiggers, and Friedman and their associates, have reported increased hepatic vascular resistance after experimental hemorrhage, and it has been reasonable to believe that consequent splanchnic pooling and reduction of circulating blood volume would result. This concept was apparently strengthened by the demonstration of Fine and his associates (7) that viviperfusion of the liver during hemorrhage prevented the hemorrhagic gastro-enteritis of irreversible shock. However, Fine’s group (6) also demonstrated that decompression of the portal system with portacaval shunt to prevent impounding of blood in the splanchnic system did not prevent the intestinal lesions. Subsequently, attention has been focused on other than hepatic factors in irreversibility, and recently the intestine itself has been suggested as an important primary target organ in shock by Schweinburg and his associates, Selkurt, and Lillehei.
The assessment of the relative importance of the liver and that of bowel in determining irreversibility of shock is still not settled, although there is evidence that both play an important role. In the present study, an experimental situation was present in which only the liver was subjected to anoxia. The blood pressure generally was never below 100 before, during, or immediately after liver was revascularized. Yet, in the dogs developing outflow hepatic block, shock soon ensued which ultimately was intractable to further transfusion and which eventuated in fatal hemorrhagic gastroenteritis. In this situation, the damage inflicted upon the liver by the transplantation would seem to be solely responsible for the inception and development of irreversible shock.
SUMMARY
The homologous canine liver has been transplanted to recipient animals in which total hepatectomy and splenectomy have been performed. The longest survival after placement of the liver homograft was 20½ days.
Protection from hepatic ischemia for as long as 2 hours was obtained by cooling the donor liver to 10 to 20 degrees C. The arterial supply was restored through a hepatic artery-aortic pedicle which was removed in continuity with the liver and anastomosed to the descending aorta of the recipient. Internal biliary drainage was established.
The volume of venous flow transmitted to the transplanted liver has been shown to be an important determinant of success. When this was excessive, as when both the portal and inferior caval flows were directed through the liver, hepatic outflow block usually developed with consequent fatal congestion of the hepatic and splanchnic beds. When the portal flow was normal or reduced, outflow block rarely occurred.
An attempt has been made to relate the development of outflow block as it occurred in the transplanted liver to other circumstances, including hemorrhagic shock, in which similar phenomena have been observed.
Acknowledgments
Aided by Grant A-3176 from the U. S. Public Health Service, Nalional Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Arey LB. Throttling veins in the livers of certain mammals. Anat. Rec. 1941;81:21. [Google Scholar]
- 2.Bauer W, Dale HH, Poulsson LT, Richards DW. The control of circulation through the liver. J. Physiol., Lond. 1932;74:343. doi: 10.1113/jphysiol.1932.sp002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernhard WF, McMurrey JD, Curtis GW. Feasibility of partial hepatic resection under hypothermia. N. England J. M. 1955;253:159. doi: 10.1056/NEJM195508042530501. [DOI] [PubMed] [Google Scholar]
- 4.Child CG. The Hepatic Circulation and Portal Hypertension. Philadelphia: W. B Saunders Co; 1954. pp. 90–106. [Google Scholar]
- 5.Deysach LJ. The nature and location of the “sphincter mechanism” in the liver as determined by drug actions and vascular injections. Am. J. Physiol. 1941;132:713. [Google Scholar]
- 6.Frank HA, Glotzen P, Jacob SW, Fine J. Traumatic shock – XIX, hemorrhagic shock in Eck fistula dogs. Am. J. Physiol. 1951;167:508. doi: 10.1152/ajplegacy.1951.167.2.508. [DOI] [PubMed] [Google Scholar]
- 7.Frank HA, Seligman AM, Fine J. Traumatic shock – XIII, the prevention of irreversibility in hemorrhagic shock by viviperfusion of the liver. J. Clin. Invest. 1946;25:22. doi: 10.1172/JCI101685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman EW, Frank HA, Fine J. Portal circulation in experimental hemorrhagic shock; in vivo roentgen ray studies. Ann. Surg. 1951;134:70. doi: 10.1097/00000658-195107000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodrich EO, Welch HF, Nelson JA, Beecher TS, Welch CS. Homotransplantation of the canine liver. Surgery. 1956;39:244. [PubMed] [Google Scholar]
- 10.Hines JR, Roncoroni M. Acute hepatic ischemia in dogs. Surg. Gyn. Obst. 1956;102:689. [PubMed] [Google Scholar]
- 11.Huggins C, Carter E, McDermott W. Differential hypothermia in experimental hepatic surgery. Arch. Surg. 1957;74:327. doi: 10.1001/archsurg.1957.01280090025005. [DOI] [PubMed] [Google Scholar]
- 12.Kaupp HA, Lazarus RE, Starzl TE. Complete homotransplantation of the liver after total hepatectomy in dogs. Presented at the American Association for Study of Liver Disease; Chicago. Nov. 5, 1959; Gastroenterology; 1960. p. 794. (Abstract) [Google Scholar]
- 13.Kaupp HA, Starzl TE. The use of an external by-pass during experimental total hepatictomy. Surgery. in press. [PMC free article] [PubMed] [Google Scholar]
- 14.Kestens PJ, Austen WG, McDermott WV., Jr . Surgical Forum: Clinical Congress 1959. Vol. X. Chicago: American College of Surgeons; 1960. Biochemical and physiologic studies on the viable extracorporeal liver; p. 225. [PubMed] [Google Scholar]
- 15.Lillehei RC. The intestinal factor in irreversible hemorrhagic shock. Surgery. 1957;42:1043. [PubMed] [Google Scholar]
- 16.Maclean LD, Weil MH. Hypotension (shock) in dogs produced by Escherichia coli endotoxin. Circ. Res. 1956;4:546. doi: 10.1161/01.res.4.5.546. [DOI] [PubMed] [Google Scholar]
- 17.Maegaith BG. Microanatomy of the hepatic vascular system. Trans. Tenth Conf.; May 21–22, 1951; Josiah Macv Jr. Foundation; 1951. pp. 181–204. [Google Scholar]
- 18.Makravarti M, Tripod J. The action in the perfused liver of acetylcholine, sympatheticomimetic substances and local anesthetics. J Physiol., Lond. 1940;97:316. doi: 10.1113/jphysiol.1940.sp003810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mautner H, Pick EP. Ueber die durch Schockgitte erzeugten Zirkulationsveränderungen: der Einfluss der Leber auf Blutdruck und Schlag-volumen. Arch. exp Path., Lpz. 1929;142:271. [Google Scholar]
- 20.Meyer WH, Starzl TE. The reverse porta-caval shunt. Surgery. 1959;45:531. [Google Scholar]
- 21.Moore FD, Smith LL, Gruber UH, Demissianos HV, Burnap TK, Dammin GJ. One stage homotransplantation of the canine liver after total hepatectomy. Presented at the American College of Surgeons meeting; Sept. 28, 1959; Atlantic City: [Google Scholar]
- 22.Moore FD, Wheeler HB, Demissianos HV, Smith LL, Belankura O, Abel K, Greenberg JB, Dammin GJ. Experimental whole organ transplantation of the liver and of the spleen. Ann. Surg. in press. [PMC free article] [PubMed] [Google Scholar]
- 23.Raffucci RL, Lewis FJ, Wangensteen OH. Hypothermia in experimental hepatic surgery. Proc. Soc. Exp. Biol., N. Y. 1953;83:639. doi: 10.3181/00379727-83-20443. [DOI] [PubMed] [Google Scholar]