It has been reported that hyperlipidemia can be alleviated in human beings with an end-to-side portacaval shunt (20, 21). Understanding the mechanism of the effect has important implications, including the possibility of devising other ways of lowering serum lipid levels. In this investigation, the influence of splanchnic venous blood on lipid metabolism has been evaluated in dogs and baboons by altering the portal venous inflow to all, or portions, of the liver and by measuring the effects on different end points, including the serum lipid concentrations and the rate of hepatic lipid synthesis. In other studies, analyses have been made regarding the effect of alloxan-induced insulinopenia and of total pancreatectomy on these processes. The results indicate that the effect of complete portal diversion upon serum lipids is mainly due to diversion of the hormone-rich venous return from the upper splanchnic organs, although the bypass of the nutritionally rich blood returning from the intestines may play a secondary role.
METHODS
Biochemical Studies
All serum and tissue samples were obtained between 9 and 10 a.m. after overnight fasting. The extraction of serum lipids with isopropanol was carried out as described by Kessler and Lederer (9). Tissue lipids were extracted from the liver by the method of Bligh and Dyer (2). For both the serum and liver extracts, cholesterol concentration was determined by the method of Zlatkis and his associates (28). Triglyceride and phospholipid concentrations were quantitated as described by Fletcher (6) and Sunderman and Sunderman (26) respectively.
In vitro hepatic synthesis of cholesterol and triglyceride was measured in dogs. A specimen was excised from the liver and rapidly cooled in ice-cold saline solution. Tissue slices were prepared using 3 to 5 grams of the liver. The slices were incubated for 60 minutes at 37 degrees C. in a final volume of 6 milliliters of Krebs-Hanseleit bicarbonate buffer, pH 7.4, containing 0.01 per cent bovine serum albumin, which was free of fatty acids, 30 micromoles of sodium acetate, and 20 microcuries of [1·14C] acetate. The incubation was terminated by the introduction of 20 milliliters of a 2:1 chloroform and methanol mixture by volume. Tissue lipids were extracted according to the method of Bligh and Dyer (2). An aliquot of the lipid extract was removed for analysis of cholesterol and triglycerides, as described for the serum and tissue analyses. The remainder of the extract was saponified by refluxing it for 90 minutes in the presence of ethanolic potassium hydroxide. The unsaponifiable lipids were extracted from the alkaline hydrolyzate with petroleum ether, and from this extract, cholesterol was isolated by digitonin precipitation. The precipitate was dissolved in scintillation fluid, and the radioactivity was counted in a liquid scintillation counter. The rate of cholesterol synthesis was expressed as counts per minute per milligram of cholesterol per hour. The aqueous phase containing the saponifiable lipids was then acidified with concentrated hydrochloric acid, and the fatty acids were extracted with petroleum ether. The radioactivity in this fraction was taken to represent triglyceride synthesis, with the rate of synthesis being expressed as counts per minute per milligram of triglycerides per hour.
In vivo synthesis of cholesterol and triglycerides was determined on the basis of our recent studies on hepatic deoxyribonucleic acid synthesis (24), during which it was noted that intravenously administrated [CH3-3H] thymidine was metabolized in the dog and that the degraded products were incorporated into various cellular components, such as lipid, protein, and glycogen. Four normal dogs were fasted for 18 to 20 hours and were given 4.5 millicuries of [CH3-3H] thymidine. Two hours later, biopsy specimens were taken, pooled, and fractionated according to the method of Schneider and Greco (17). All the subcellular fractions were labeled with a considerable amount of the isotope when the liver biopsy was taken two hours after the administration of [CH3-3H] thymidine (Table I). In agreement with the earlier findings of Schneider and Greco (17) in rat livers, a considerable amount of radioactivity was incorporated from [CH3-3H] thymidine into tissue lipids. Further fractionation of lipid extract into digitonin precipitable sterols and fatty acids showed that 7 to 16 per cent of the radioactivity was recovered in cholesterol and 80 per cent in triglycerides. In these studies, animals were sacrificed two hours after the administration of [CH3-3H] thymidine. Tissue lipids were extracted, fractionated, and taken for radioactivity measurement in the same way as described for the in vitro studies.
TABLE I.
INCORPORATION OF [CH3-3H) THYMIDINE INTO SUBCELLULAR FRACTIONS OF CANINE LIVER TISSUE WHICH WAS POOLED FROM FOUR NORMAL DOGS
| Distribution of radioactivity | |||||
|---|---|---|---|---|---|
| Particulate fraction | counts per minute per gm. wet tissue | ||||
| Acid-soluble | Lipids | RNA | DNA | Protein | |
| Nuclei......... | 5,491 | 4,789 | 301 | 1,961 | 783 |
| Mitochondria... | 7,741 | 7,354 | 347 | 1,803 | 930 |
| Microsomes..... | 191,772 | 6,992 | 3,594 | 661 | 6,197 |
RNA, Ribonucleic acid.
DNA, Deoxyribonucleic acid.
Additional specimens of the liver were frozen and kept at minus 20 degrees C. until the analyses were completed. For hepatic glycogen, the method of Bloom and his associates (3) was used to separate the trichloroacetic acid soluble glycogen fraction from the insoluble one. Both fractions were quantitated with the anthrone reagent according to the method of Seifter and his colleagues (18).
The protein content of the hepatic tissue was determined on the same sample used for the determination of trichloroacetic acid soluble glycogen. After extraction of the trichloroacetic acid soluble fraction of glycogen, the insoluble precipitate was dissolved in 3 milliliters of 3 per cent alkaline deoxycholate and assayed with the biuret method of Gornall and his associates (7).
Spearman’s rank correlation coefficient (8) was used to test the statistical significance of increasing or decreasing trends over time of the serum lipid components of individual members of and the means for groups 1 and 3. Paired data were analyzed with Student’s t test and with the Wilcoxon and Wilcox (27) matched-pairs signed-rank test, which is the nonparametric equivalent of the paired t test.
Animal Groups
Group 1
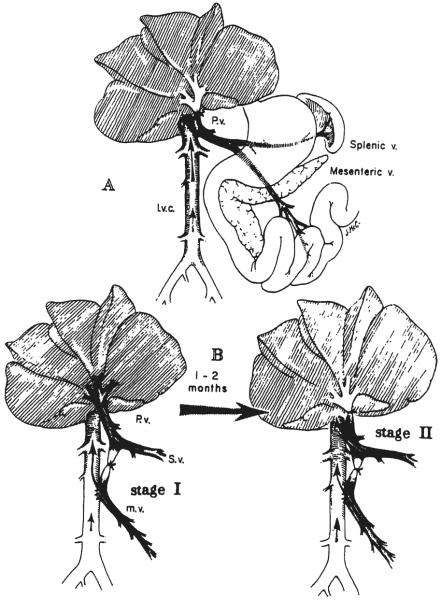
Six dogs had a standard side-to-side portacaval shunt with high ligation of the portal vein, creating a functional complete end-to-side venous bypass, as shown in Figure 1A. They were sacrificed 47 to 62 days later, at a mean of 56±2 (S.E.) days.
Fig. 1.
Experiments shunting splanchnic blood around the liver. A, One stage completely diverting portacaval shunt, as used for group 1 dogs and the test dog of group 2. A large side-to-side shunt was constructed, and then the portal vein was ligated in the hilus of the liver above the last portal tributary. B, Two stage portal diversion carried out in the dogs of group 3. Analogous procedures were performed on the baboons of group 4. At stage 1, the superior mesenteric vein was anastomosed to the inferior vena cava, with proximal ligation just below the entrance of the splenic vein. This achieved bypass of the nutrient rich intestinal venous blood. At stage 2, seven to eight weeks later, venous blood from the pancreas and other upper splanchnic organs was bypassed with a proximal portacaval shunt and hilar ligation of the portal vein.
Group 2
At the age of three and one-half months, the male of a pair of littermate purebred Beagle puppies was given the portacaval shunt depicted in Figure 1A, and the female littermate was kept as a control. The female normally has a lower serum cholesterol level than the male until menopause. The growth curves of the two dogs were similar for the next eight months, with the dog used as the control becoming about 1 kilogram heavier (Fig. 2). From the beginning of the eighth month and under the same dietary conditions of standard kennel rations for both dogs, fasting serum lipids were examined for the next four months. Three hundred and forty-nine days after operation, the test and control dogs were sacrificed.
Fig. 2.
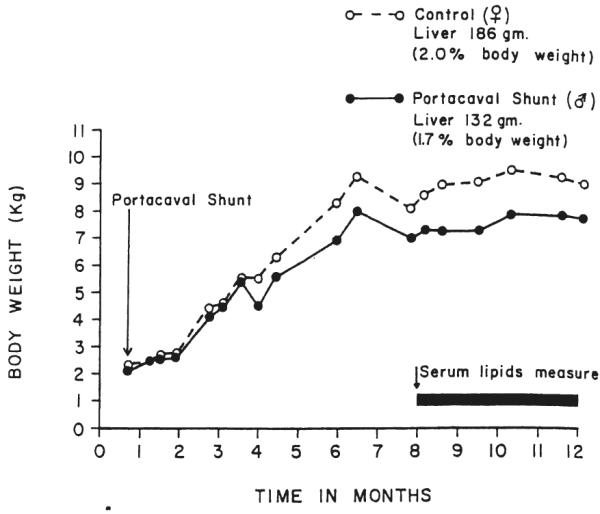
Growth curves of the pair of littermate, certified purebred Beagle puppies of group 2 with and without portal diversion. The test dog, a male, had a portacaval shunt as in Figure 1A at the age of 14 weeks. The female was used as the control, since female dogs normally have lower serum cholesterol concentrations. Sacrifice was one year postoperatively.
Group 3
Nine dogs underwent side-to-side anastomosis of the superior mesenteric vein to the inferior vena cava after excision of an ellipse of both vessels. The mesenteric vein was then ligated above the anastomosis and just below the entrance of the splenic vein (Fig. 1B, left). At the conclusion of the procedure, a selective venous bypass of blood returning from the jejunum, ileum, and proximal part of the colon had been achieved.
After 49±3 (S.E.) days, the dog underwent re-exploration, a side-to-side portacaval shunt was performed at the hilus of the liver, and the portal vein was ligated at its bifurcation into right and left branches. After this second stage, a pancreaticogastroduodenosplenic bypass had been added to the intestinal bypass (Fig. 1B, right), theoretically achieving the same final result as with the one stage shunt of groups 1 and 2. Two of the nine experiments were not satisfactory. One dog died shortly after the second stage procedure, and in the second dog, the portacaval shunt was found, at the time of sacrifice, to be clotted. The remaining seven dogs were sacrificed 65±3 (S.E.) days after the second stage operation; the patency of both the upper and lower shunts was proved at autopsy.
Group 4
Two female baboons weighing 17.2 and 16.1 kilograms were submitted to selective superior mesenteric venous to inferior vena caval end-to-side shunt, with ligation of the proximal mesenteric vein at the pancreas. Before and after this procedure, serum lipid concentrations were studied. Sampling required tranquilization with 1 milligram per kilogram of phencyclidine hydrochloride (Sernylan®) or 5 milligrams per kilogram body weight of ketamine. Three months after the selective intestinal venous bypass, the baboons were again operated upon and a portacaval shunt was performed in the hilus of the liver, diverting the pancreaticogastroduodenosplenic blood. The staged operations in this experiment were comparable with those of the canine studies of group 3 (Fig. 1B).
Group 5
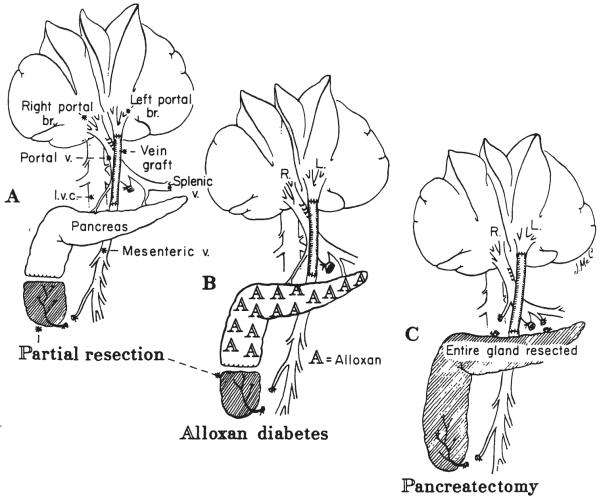
Twelve dogs had division of the splanchnic venous return, so that the intestinal blood was rerouted through a reversed external jugular vein graft to the detached left portal vein. The right portal branch and liver lobes received blood returning from the pancreaticogastroduodenosplenic area (Fig. 3A). Because the end of the inferior lobe of the pancreas often drains into the mesenteric vein and might, therefore, create an experimental artifact, the tip of this lobe was resected. From 41 to 66 days later, mean 58±2 (S.E.) days, the dogs were sacrificed, the patency of the venous inflow on both sides was proved, and tissues were obtained from the right and the left lobes. The main objective in these experiments was to compare the synthesis of cholesterol and triglycerides in the two hepatic sides. In six dogs, the cholesterol synthesis was measured by the in vitro method, and in the other six, the in vivo method was used.
Fig. 3.
Splanchnic division experiments carried out in nondiabetic dogs, A; dogs with alloxan-induced diabetes, B; dogs with total pancreatectomy, C. In all these experiments, blood returning from the upper part of the splanchnic organs went to the right lobes and the intestinal blood went to the left lobes.
Group 6
The same splanchnic division as in group 5 was used in four dogs that had been made alloxan (mesoxalylurea) diabetic (Fig. 3B) from three to eight weeks earlier. Their daily doses of Neutral Protamine Hagedorn insulin or isophane insulin suspension, U.S.P., a modified protamine zinc insulin, were stabilized at from 17.5 to 20.0 units. The dogs were sacrificed 60 to 64 days after the splanchnic division.
Group 7
Five dogs had splanchnic division plus total pancreatectomy at the same operation (Fig. 3C). Fifteen to 20 units of Neutral Protamine Hagedorn insulin per day were required, and the dogs were sacrificed 60 to 64 days postoperatively.
Group 8
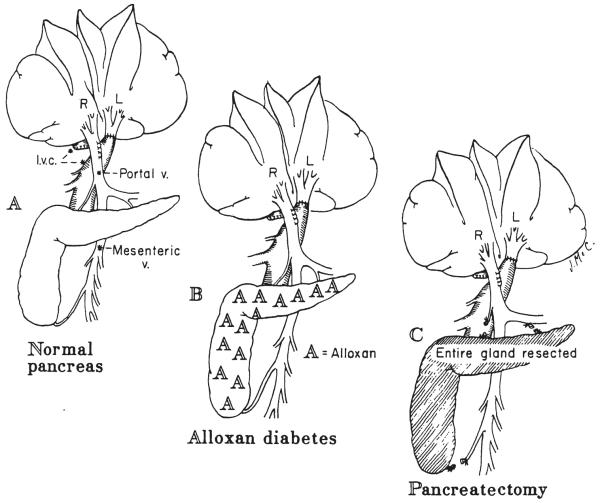
Nine normal dogs were submitted to split transposition, where by the right liver lobes were given all the splanchnic venous return, while the left liver lobes were perfused with vena caval blood (Fig. 4A). They were sacrificed 60 to 63 days later.
Fig. 4.
Partial or split portacaval transposition experiments in which the nonhepatic splanchnic venous return was directed in its entirety to the right lobes, and the inferior vena caval blood was used to perfuse the left liver lobes by anastomosing the supra-adrenal inferior vena cava to the detached left portal branch. A, Nondiabetic dogs; B, dogs with alloxan-induced diabetes; and C, dogs with total pancreatectomy.
Group 9
Three alloxan-induced diabetic dogs, requiring 20 to 25 units of Neutral Protamine Hagedorn insulin per day, were submitted to the split transposition operation (Fig. 4B) one and one-half to seven weeks after the induction of the diabetic state. They were sacrificed 60 to 64 days postoperatively.
Group 10
Five dogs were submitted to split transposition plus total pancreatectomy on the same day (Fig. 4C). Fifty-six to 79 days later, they were sacrificed. Postoperatively, their daily Neutral Protamine Hagedorn insulin doses were 12.5 to 15.0 units.
The dogs in groups 6, 7, 8, 9, and 10 were used for the studies of in vivo synthesis of cholesterol and triglycerides.
Pathologic Studies
The regional effects upon hepatic tissue when a differential blood supply is used, as in groups 5 to 10, have been previously reported (22, 24). Consequently, the histopathologic data in this article will be confined to the effects on the canine liver of one stage complete portal diversion, as was used in groups 1 and 2, and to the effects of the two stage procedures on the canine and baboon livers in groups 3 and 4.
Samples of hepatic tissue were fixed in 10 per cent normal buffered Formalin® (aqueous solution of formaldehyde). Frozen sections were stained with Sudan 4 for fat and, then, the remainder processed and the paraffin sections stained with hematoxylin and eosin, Gordon and Sweet’s silver impregnation method for reticulin fibers, Perls Prussian blue method for iron, trichrome for collagen and fibrin, periodic acid-Schiff method for glycogen, and Pearse’s method for ceroid and lipo-fuscin. Additional small hepatic samples were initially fixed in glutaraldehyde solution, and then postfixed in osmic acid and embedded in Epon® (synthetic embedding medium). Half micron and ultrathin sections were cut. The former were stained with azure II for examination in the light microscope, while the latter were stained with lead citrate and examined in an electronmicroscope. The size of the hepatocytes was determined on hematoxylin and eosin stained sections by a method previously described (22). Midzonal hepatocytes also were used for measuring rough endoplasmic reticulum length per area of cytoplasm by a morphometric method.
RESULTS
Group 1
After complete portal diversion, five of the six dogs seemed healthy. These five dogs maintained their body weights at 95±2 (S.E.) per cent of that recorded preoperatively during a follow-up study of 58±1 (S.E.) days. The sixth dog died 47 days postoperatively with convulsions and other neurologic manifestations of hepatic encephalopathy. The weight of this dog had dropped from 20.9 to 19.5 kilograms.
Biochemical studies
After a transient early postoperative rise, a definite lowering effect of portal diversion on serum cholesterol values was evident within one week. In all six dogs, cholesterol values of more than 2 standard deviations below their respective control means were reached within the first two postoperative weeks (Fig. 5). With the use of Spearman’s rank correlation coefficient, the downward trends after portacaval shunt of serum cholesterol and phospholipid levels (Fig. 5) were shown to be highly significant. Serum triglyceride levels were not significantly altered, however (Fig. 5).
Fig. 5.
Changes in the postabsorptive concentrations of three major lipid components after complete one stage portacaval diversion. These dogs of group 1 were fasted for about 18 hours before blood sampling. In each dog, at least six control samples were obtained over a period of three weeks. The results were averaged for the preoperative value which was the 100 per cent standard against which all postoperative determinations for that dog were compared. Each point with a vertical line represents the mean±one standard error of the six experiments. The actual preoperative means and standard errors for serum triglycerides, cholesterol and phospholipids were 100.2±20.9, 151.3±13.2, and 298.2±20.8 milligrams per 100 milliliters, respectively.
In all the dogs, in vitro synthesis rates of cholesterol and triglycerides were obtained from biopsies at the time of portacaval shunt and at sacrifice 47 to 62 days later. Five of the six dogs showed a striking decrease in both cholesterol and triglyceride synthesis after complete portal diversion (Table II).
TABLE II.
COMPARISON OF IN VITRO SYNTHESIS OF CHOLESTEROL AND TRIGLYCERIDES BEFORE AND AFTER PORTACAVAL SHUNT IN GROUP 1
| Cholesterol synthesis* | Triglyceride synthesis* | |||
|---|---|---|---|---|
| Animal No. | Before | After | Before | After |
| Lipid 15....... | 136 | 146.000 | 0.980 | 4.180 |
| Lipid 16....... | 105 | 0.224 | 0.100 | 0.040 |
| Lipid 17....... | 129 | 6.000 | 609.000 | 3.880 |
| Lipid 18....... | 114 | 5.000 | 41.000 | 1.980 |
| Lipid 19....... | 114 | 0.562 | 5.000 | 1.540 |
| Lipid 20....... | 608 | 74.000 | 101.000 | 33.200 |
| Mean±S.E....... | 201±81 | 39.0±24.0 | 126±98 | 7.00±5.00 |
In counts per minute per milligram cholesterol, or triglycerides, per hour.
S.E., Standard error.
Pathologic studies
In the five dogs that survived the full two month study period, the liver weight was l.5±0.1 (S.E.) per cent of the total body weight. This compared with 2.4±0.2 (S.E.) per cent in six normal dogs, p<0.01.
Histologically, comparisons of the liver at the time of portacaval shunt with specimens from the same dog 56 to 62 days later showed that the liver lobules shrank and that there was a marked diminution in the size of the hepatocytes (Fig. 6). The average hepatocyte size was halved. The amount of stainable glycogen in the liver cell cytoplasm fell. Stainable lipid, which was present initially in small amounts in the hepatocyte cytoplasm of two of the five dogs, increased after portacaval shunt; the remaining dogs at no stage show ed any fat visible by light microscopy. In all the dogs, the Kupffer cells, particularly in the central parts of the lobules, increased in size and number after the operation and came to contain small amounts of hemosiderin. There were more binucleate and tri-nucleate hepatocytes and hepatocyte mitoses. The thin 0.5 micron sections of the livers after portacaval shunt showed variations in the staining properties of the hepatocytes, giving an irregular mixture of light and dark cells; the dark cells were smaller than the light ones and much more numerous.
Fig. 6.

The effect of complete portacaval shunt upon hepatocytesize in five dogs after 57.6±1.1 (S.E.) days. To obtain these quantitative data, a tracing device was attached to a light microscope. In a given sample, 40 hepatocytes were drawn on a standard thickness paper and weighed. This has been shown to be an accurate method for comparing cell sizes by confirmatory planimetry and by studies of unicellular organisms, the size of which could be directly determined. Note that the cell shrinkage averaged about 50 per cent in two months.
Ultrastructurally (Figs. 7, 8, 9, and 10), the cytoplasm of many of the hepatocytes two months after portacaval anastomosis contained lipid vacuoles of variable size, and the number of glycogen granules was greatly reduced. The rough endoplasmic reticulum was reduced in amount and was dilated or fragmented, or both; there were fewer ribosomes on the membranes than normal. The mitochondria sometimes were swollen with disruption of the cristae, and occasionally, they contained crystalline inclusions. The amount of smooth endoplasmic reticulum was not increased. The Golgi apparatus was poorly developed. The nuclei and bile ductules were normal. Small bundies of collagen fibrils were present in the space of Disse, around central veins, and around some of the hepatocytes. The Kupffer cells were enlarged, and there were large masses of lipid and iron-containing material in their cytoplasm.
Fig. 7.
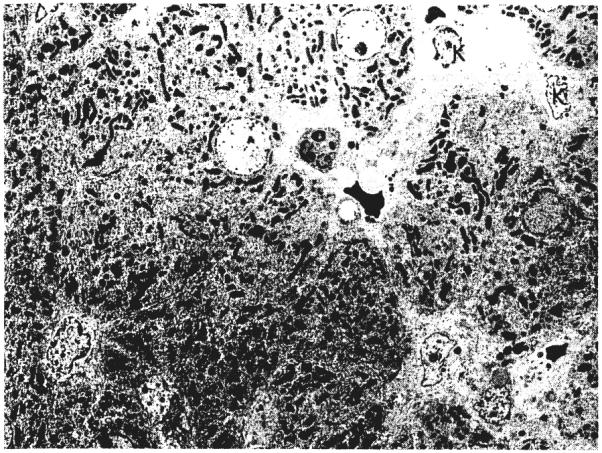
Ultrastructure of the liver of a dog in group 1 before portacaval shunt. The hepatocytes have single nuclei and their cytoplasm lacks lipid. The Kupffer cells, K, are normal in size and do not contain iron. Lead stain, × 1,012.
Fig. 8.
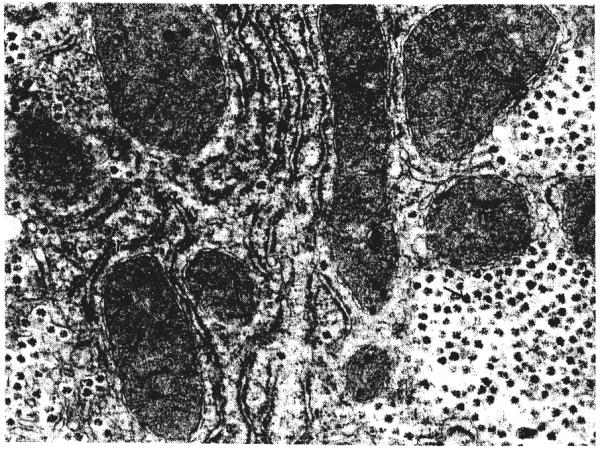
Higher power of liver shown in Figure 7. The cisternae of rough endoplasmic reticulum, r, are not dilated and the membranes are studded with ribosomes. Glycogen granules, arrow, are abundant. m, Mitochondrion. Lead stain, × 19,938.
Fig. 9.
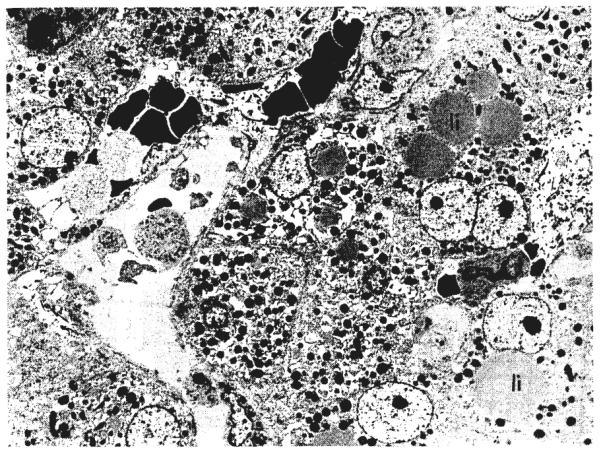
Ultrastructure of liver of same dog in group 1, 62 days after portacaval shunt. The hepatocyte cytoplasm contains dilated cisternae of rough endoplasmic reticulum and lipid vacuoles of various sizes, li. One hepatocyte is binucleate. A Kupffer cell, K, is enlarged and contains lipid and iron pigment. Lead stain, × l,012.
Fig. 10.
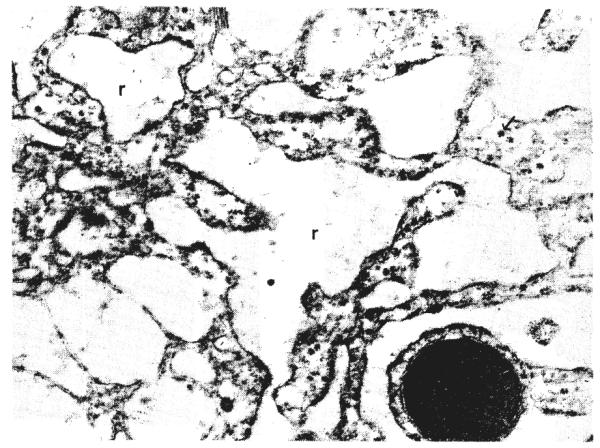
Higher power view of liver after portacaval shunt. The cisternae of rough endoplasmic reticulum, r, are dilated and the membranes lack ribosomes. Glycogen granules, arrow, are scarce. m, Mitochondrion. Lead stain, × 19,938.
Group 2
Biochemical studies
The levels of serum cholesterol and phospholipids in the purebred male Beagle during the period from eight to 12 months after the portacaval shunt were significantly lower than those of the nonoperated upon control Beagle, whereas serum triglyceride levels were higher (Fig. 11).
Fig. 11.
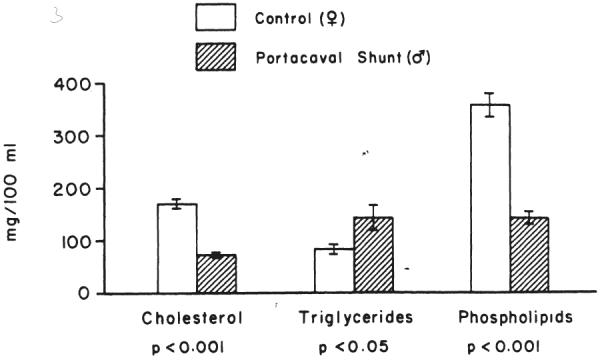
Comparison of the levels of three major serum lipids after 18 hours fasting in a pair of littermate Beagles with or without portacaval shunt. In the test animal, the shunt was performed eight months before beginning sampling; specimens were taken over a period of four months. The mean±one standard error are given for each lipid moiety. The growth curves of the control and test animals are shown in Figure 2.
Comparisons were made of the tissue lipids in the liver biopsies taken at sacrifice of the test and control dogs. The hepatic cholesterol and triglyceride contents were increased by 46 and 114 per cent, respectively, in the dog with the shunt over the values of its littermate control. The hepatic phospholipid content, however, was reduced by 30 per cent in the dog with the shunt (Table III). The rates of in vitro hepatic synthesis of cholesterol and triglycerides in the dog with the shunt were 11 and 14 per cent, respectively, of those in the normal dog (Table III).
TABLE III.
COMPARISON OF LIPID CONTENT AND SYNTHESIS, GLYCOGEN, AND PROTEIN IN THE LIVER BIOPSIES OF A PAIR OF LITTERMATE BEAGLES WITH AND WITHOUT PORTAL DIVERSION
| Control | Portacaval Shunt | |
|---|---|---|
| Hepatic lipids, mgm. per gm. tissue | ||
| Cholesterol........................ | 2.25 | 3.30 |
| Triglycerides...................... | 13.60 | 29.20 |
| Phospholipids...................... | 21.30 | 14.90 |
| Hepatic synthesis of lipids, in vitro, counts per minute per mgm. cholesterol or triglycerides per hour | ||
| Cholesterol...................... | 229.00 | 25.00 |
| Triglycerides...................... | 14.00 | 2.00 |
| Hepatic glycogen, mgrn. per gm. tissue | ||
| Total............................. | 40.90 | 1.79 |
| TCA-soluble...................... | 21.70 | 0.66 |
| Hepatic protein, mgm. per gm. tissue....... | 200.00 | 219.00 |
The test dog, a male, had portacaval shunt and was sacrificed 349 days after operation. The female control littermate was sacrificed the same day.
The content of hepatic glycogen in the test dog was much lower than that in the normal control dog (Table III). The content of hepatic protein in both was almost the same.
Pathologic studies
Both control and test Beagles were healthy, although the female control dog had a final weight about 1 kilogram greater than that of the male dog with the shunt. The liver of the dog with the shunt weighed 1.7 per cent of the final total body weight compared with 2.0 per cent for the unaltered littermate (Fig. 2). The liver in the dog with the shunt had a slightly mottled gross appearance compared with the homogeneous control liver.
Microscopically, the liver lobules and hepatocytes were smaller in the dog with the shunt. The liver cells were 0.083 size units compared with 0.155 in the littermate control. Thus, hepatocyte sizes were approximately halved. The amount of stainable glycogen was much less in the male dog with the shunt than in the female control. Neither dog showed stainable lipid in the liver cells. There were more binucleate hepatocytes and hepatocyte mitoses than normal in the dog with the shunt.
Ultrastructurally, the hepatocytes of the male Beagle showed the same pattern of accumulation of vacuoles containing lipid, loss of glycogen, dilatation and loss of rough endoplasmic reticulum, some distortion of mitochondria, and poor development of the Golgi apparatus that was seen in the dogs of group 1 at two months after portacaval shunt. These changes were not present in the female control dog.
Group 3
The dogs which had mesenteric caval shunts and survived for two months had significant weight loss, averaging 15±4 (S.E.) per cent of their original body weight. After the second stage central portacaval shunt, the seven dogs which lived for another two months sustained an added loss, so that these dogs finished at only 73±3 (S.E.) per cent of the original preoperative weight. In spite of the weight loss, the dogs which completed the study seemed in good condition at its conclusion.
Biochemical studies
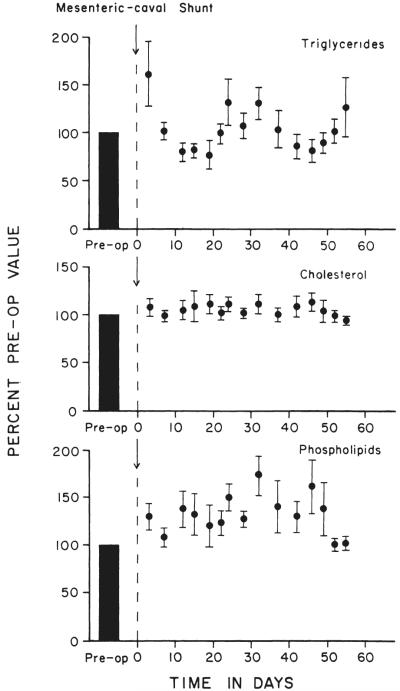
The mesenteric caval shunt in nine dogs had no significant effect upon the serum cholesterol and triglyceride levels (Fig. 12) during the two month postoperative study. Serum phospholipid values were slightly elevated, but this trend was not statistically significant.
Fig. 12.
Changes in the levels of three major lipid components in the serum after mesenteric caval shunt. The samples were drawn after 18 to 20 hours fasting. Note the conversion of the preoperative controls to a 100 per cent standard. The actual preoper ative means and standard err ors for the serum triglycerides, cholesterol, and phospholipids were 68.7±3.9, 141.9±7.3 and 217.8±18.8 milligrams per 100 milliliters, respectively. Each point with a vertical line represents the mean±one standard error of nine experiments.
When the second stage proximal portacaval shunt was added, seven dogs lived for another two months. Six of these surviving dogs were studied, and in each, serum cholesterol values at least 2 standard deviations below the control means were attained after portacaval shunt (Fig. 13). The downward trend in the cholesterol values was highly statistically significant. The serum phospholipid level also fell significantly, with regard to the level after the mesenteric caval shunt, but the final average level was not lower than the preoperative values.
Fig. 13.
Changes in the levels of three major lipid components in the serum of six of the same dogs as in Figure 12 but after proximal portacaval shunt was performed at a second stage. The controls are those obtained several months earlier, before the performance of the stage 1 procedure. Each point with a vertical line represents the mean±one standard error of six experiments.
In vitro synthesis of cholesterol was markedly reduced by mesenteric caval shunt (Fig. 14). The second stage proximal portacaval shunt did not cause further significant change. On the other hand, in vitro synthesis of triglycerides was not decreased by the mesenteric caval shunt but was significantly reduced after proximal portacaval shunt.
Fig. 14.

In vitro synthesis of cholesterol and triglycerides by the liver before and after mesenteric caval and portacaval shunts. The number of animals studied fell from nine to seven as the experiment progressed, since two dogs were lost. The mean±one standard error are represented by the bars and vertical lines.
The tissue lipid values in the livers were determined at the time of sacrifice in six of the dogs of group 1 which had received complete portal diversion at one operation. The same studies were done in the livers of seven dogs of group 3 which also had achieved total portal diversion but at two stages. The results in the two groups were so similar that they were pooled (Table IV). The content of both cholesterol and triglycerides were higher than normal, p<0.05, and that of phospholipids was much lower, p<0.05.
TABLE IV.
COMPARISON OF HEPATIC CONTENT OF LIPIDS IN DOGS WITH OR WITHOUT PORTACAVAL SHUNT
| Tissue lipids, mgm. per gm. wet tissue | No. of experiments | Unoperated upon controls | No. of experiments* | Operated upon animals | P value |
|---|---|---|---|---|---|
| Cholesterol................ | 9 | 2.39±0.10 | 13 | 2.97±0.25 | <0.05 |
| Triglycerides................ | 9 | 9.10±0.50 | 13 | 15.00± 2.50 | <0.05 |
| Phospholipids............... | 9 | 19.10±0.60 | 13 | 16.90±0.80 | <0.05 |
Six of these animals had a one stage portacaval shunt. The other seven also had total portal diversion, but in two stages.
Pathologic studies
The seven dogs of group 3 that completed the study had livers that were 1.8±0.1 (S.E.) per cent of the final body weights. These values compared with 2.4±0.2 (S.E.) per cent in six dogs used as controls, p<0.02.
Histologic comparisons were made from samples obtained at the time of the first mesenteric caval shunt, at the second stage portacaval shunt, and at sacrifice. In the nine dogs that survived to the second stage, these showed that in the 49 to 56 days between the mesenteric caval shunt and biopsy, the liver lobules were reduced in size and the hepatocytes became smaller (Fig. 15). The average reduction in size of the hepatocytes was 41 per cent. The amount of stainable glycogen in the liver cell cytoplasm decreased, and in eight of the nine dogs small amounts of stainable lipid appeared. The Kupffer cells increased in size and number but contained little iron. The number of binucleate hepatocytes and liver cells in mitosis increased. Thin sections showed an irregular arrangement of light and dark cells, the dark predominating.
Fig. 15.

The effect of mesenteric caval and portacaval shunts upon hepatocyte size using the measuring technique described in Figure 6. The average size of the hepatocytes eventually was reduced to a little more than a third of the original dimension. The vertical lines indicate one standard error above and below the mean.
Ultrastructurally, the changes were similar to those seen in the livers of the dogs in group 1 after total portacaval shunt. The cytoplasm of the hepatocytes contained lipid vacuoles, the number of glycogen granules was reduced, and the mitochondria were sometimes swollen and showed some distortion of the cristae. The rough endoplasmic reticulum was dilated, and the number of ribosomes on the membranes was decreased, but estimates by the morphometric technique of Loud (10) showed no reduction in the amount of rough endoplasmic reticulum.
The seven dogs that lived after central portacaval shunt until they were killed two months later showed further atrophy of the hepatocytes (Fig. 15). In the 49 to 62 days after the second operation, the liver cells decreased in size compared with the dimensions two months after stage I by an average of 33 per cent. Glycogen was almost absent from the hepatocyte cytoplasm, lipid vacuoles of variable size were present in large numbers, loss of rough endoplasmic reticulum was marked, mitochondrial swelling and distortion were more common, and collagen fibers were present around hepatocytes and in Disse’s space. The number of binucleate hepatocytes and of hepatocyte mitoses was approximately as before. Over-all, the histologic and ultrastructural changes in the livers of these dogs were remarkably like those encountered in the livers of the dogs in group 1, but on average they tended to be more severe.
Group 4
Both baboons lost weight after each of the two shunt operations. The weight of Baboon 1 fell from 17.2 to 11.4 kilograms. This wasting was partly due to recurrent cellulitis at the base of the tail. In addition, the baboon was reoperated upon four and one-half months after the stage 1 operation and one and one-half months after the stage 2 procedure. A large gallstone and chronic cholecystitis had been noted at both previous operations; the gallbladder, which may have contributed to the anorexia was removed. In addition, an encapsulated sponge that had been left at one of the earlier operations was removed. The second baboon had a weight loss of from 16.1 to 12.0 kilograms, is healthy, and is gaining back her original weight.
The mesenteric caval shunt did not result in reductions in the serum cholesterol level during the three month period of observation. Following the second stage central portacaval shunt, a prompt fall in cholesterol values occurred in both baboons (Fig. 16). In Baboon 1, the downward trend in the serum cholesterol level was significant, p<0.001, but in Baboon 2, the trend did not achieve statistical significance because of the limited number of cholesterol determinations. In both baboons, a small, but significant, decrease in serum phospholipid levels also occurred but not in serum triglyceride values.
Fig. 16.
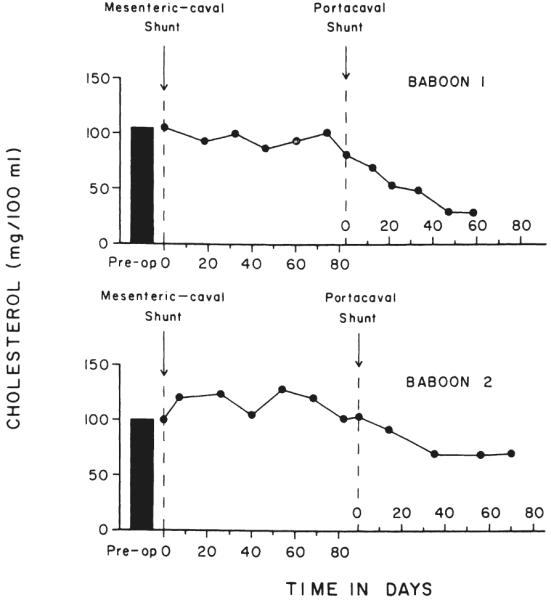
Changes in the postabsorptive serum cholesterol levels in two baboons after sequential mesenteric caval and portacaval shunts. The samples were obtained under tranquilization, after 18 to 20 hours of fasting.
A biopsy of the liver of Baboon 1 was obtained three months after the mesenteric caval shunt at the time proximal portacaval shunt was performed. This was compared with the liver biopsy at the time of reoperation 50 days later. The comparison showed that the hepatocytes, which were already smaller than normal in the first biopsy, were even smaller in the second sample. In the period after the portacaval shunt, the hepatocytes had reduced in size by 33 per cent. The fat content of the hepatocytes was minimal in the first biopsy but marked in the second, and glycogen depletion was greater in the second biopsy. The number of binucleate hepatocytes and hepatocyte mitoses was the same in the two samples.
Ultrastructurally, in the baboon, the changes in hepatocytes were similar to those encountered in the biopsies of the livers of the dogs in group 3 after mesenteric caval shunt and portacaval shunt, respectively. However, loss of rough endoplasmic reticulum predominated; dilatation of the cisternae was much less than in the dogs.
Group 5
Biochemical differences between the right and left lobar complexes after splanchnic division have been described in other publications (22, 24). Here, only the cholesterol and triglyceride synthesis will be considered in animals operated upon 41 to 63 days previously or after an average of 54±3 (S.E.) days. With the in vitro (1-14C] acetate techniques, the right lobes supplied by pancreaticogastroduodenosplenic blood had a higher rate of cholesterol synthesis than the left lobes which were supplied with intestinal venous blood (Fig. 17). In the same three animals plus three more, there was a similar reduction in triglyceride synthesis (Fig. 17).
Fig. 17.

In vitro cholesterol and triglyceride synthesis in the splanchnic division experiments of group 5. The right lobes received pancreaticogastroduodenosplenic blood; the left lobes were supplied by intestinal blood. In each experiment, the liver side with the greater rate of synthesis was arbitrarily assigned a value of 100 per cent, while the other side was accorded a proportionately lesser percentage. The vertical line represents one standard error. The p values are for comparisons of the two sides.
These findings were confirmed using the in vivo tritiated thymidine technique in six dogs. Cholesterol (Fig. 18) and triglyceride (Fig. 19) syntheses 60±1 (S.E.) days after operation were substantially greater in the right lobes.
Fig. 18.
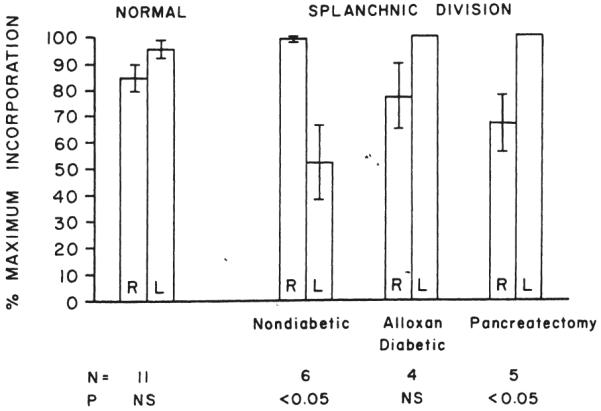
In vivo cholesterol synthesis in the right and left liver lobes in normal dogs and in dogs submitted to splanchnic division. In all of the splanchnic division experiments, the right lobes received pancreaticogastroduodenosplenic blood, while the left lobes were nourished with intestinal venous blood. The animals with splanchnic division were nondiabetic, alloxan-diabetic, or diabetic as the result of total pancreatectomy. The p values compare the synthesis rates for the two sides, the greater rate of synthesis being assigned a value of 100 per cent. For the other side, a proportionately lower percentage was calculated.
Fig. 19.

In vivo triglyceride synthesis in the right and left liver lobes of normal dogs and of nondiabetic and diabetic dogs submitted to splanchnic division. The experiments are the same as those shown in Figure 18.
Groups 6 and 7
In four alloxan-induced diabetic dogs of group 6, studied 62±1 (S.E.) days after splanchnic division, the in vivo cholesterol synthesis was now dominant on the left rather than the right side, though the differences were not significant (Fig. 18).
At 62±1 (S.E.) days after total pancreatectomy and splanchnic division, there was an even more pronounced domination of in vivo cholesterol synthesis by the left lobes (Fig. 18), p<0.05.
A shift in dominance in triglyceride synthesis away from the right lobes was similarly observed with both kinds of diabetes (Fig. 19).
Group 8
After 61±0.1 (S.E.) days, the right liver lobes being perfused with the total splanchnic venous return were shown by the in vivo technique in seven dogs to be more than twice as active in cholesterol synthesis than the left liver lobes, which were receiving systemic venous blood (Fig. 20). The same was true for triglyceride synthesis (Fig. 21).
Fig. 20.
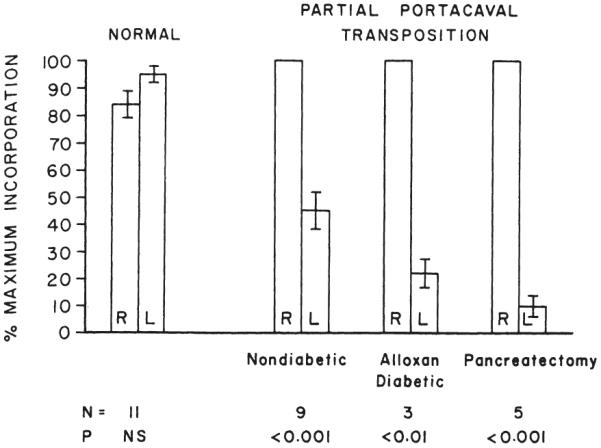
In vivo cholesterol synthesis in the right and left liver lobes in normal dogs and dogs submitted to partial portacaval transposition. In all the latter animals, the right lobes received the splanchnic venous blood, while the left lobes were nourished with blood from the inferior vena cava. The animals with partial portacaval transposition were nondiabetic, alloxan-diabetic, or diabetic as the result of total pancreatectomy. The synthesis rates, standard error, and p values are expressed as in Figure 18.
FIG. 21.

In vivo triglyceride synthesis in the right and left liver lobes of normal dogs and of nondiabetic and diabetic dogs submitted to partial portacaval transposition. The experiments are the same as those shown in Figure 20.
Groups 9 and 10
The imposition of alloxan-induced diabetes in three dogs of group 9 or the diabetes of total pancreatectomy in five dogs of group 10 did not eliminate the predominance of the right lobes in cholesterol or triglyceride synthesis (Figs. 20 and 21) that had been noted in the nondiabetic split transpositions in group 8.
DISCUSSION
Publications from this laboratory beginning ten years ago (12, 13, 23) and recently summarized (19, 22) have shown that venous blood returning from the splanchnic organs has special liver-supporting qualities that are not provided by equal volumes of other kinds of either venous or arterial blood and that can affect hepatic hypertrophy, hyperplasia and basic function. These qualities have been ascribed mainly to the endogenous hormones, especially insulin, that are released by the splanchnic viscera and, thus, arrive at the liver in high physiologic concentrations. The importance of such hormone effects on the liver probably assumes amplified value in hepatic homeostasis, because the richness in nutrients of the same portal blood presumably contributes to significant hormone substrate interrelations.
Collectively, the special portal ingredients have been termed hepatotrophic substances. Their diversion from the liver as with a portacaval shunt produces derangements in either hepatic structure or function which, in the dog, may become severe and life-threatening, but which, fortunately, in the human being with good prior liver function are milder and apparently well tolerated. The species difference has made it possible to apply portal diversion in human beings to two inborn errors of metabolism, glycogen storage disease and homozygous type II hyperlipoproteinemia with considerable metabolic improvement and with no detectable clinical morbidity, such as hepatic encephalopathy during one and one-half to 11 years’ follow-up observation (20, 25). Nevertheless, these clinical applications accept a trade-off of distinctly suboptimal conditions of liver perfusion in return for metabolic improvements that are derivative from the suboptimal conditions.
Our earlier studies on the identification of hepatotrophic factors did not deal with lipid metabolism. However, when the lipid-lowering qualities of portacaval shunt became known (21), it was natural to speculate that the cause for this effect was due more to the bypass of hormone-rich blood from the upper splanchnic organs than to the shunting of nutrient-rich blood returning from the intestines. The results with selective shunting in the dogs and baboons of groups 3 and 4 were consistent with this hypothesis. Mesenteric caval bypass had no lowering effect on any of the serum lipid levels. However, when the venous blood from the upper splanchnic organs was bypassed in the same animals at a second stage, the serum cholesterol and phospholipid values fell just as with the complete one stage portal diversion. Neither the one stage nor two stage operation influenced serum triglyceride levels.
In the animals subjected to complete portal diversion two months previously, in vitro synthesis of cholesterol and triglyceride was strikingly reduced. Similarly, the liver of the Beagle dog subjected to portacaval shunt a year earlier had markedly lower cholesterol and triglyceride synthesis than the liver of its unoperated upon littermate control However, that the cholesterol synthesis may not have been the overriding determinant of serum cholesterol concentrations was shown by the sequential operations on the group 3 dogs. After the selective mesenteric caval shunt of stage 1, in vitro synthesis of cholesterol was also markedly lowered but without effect on serum lipids. A decrease in serum cholesterol concentrations was seen only after the second stage rerouting of blood from the upper splanchnic organs, and this without much of a further reduction of hepatic cholesterol synthesis. Triglyceride synthesis was diminished only after both stages had been completed.
The way in which hepatic cholesterol and triglyceride synthesis could be influenced by the quality of portal perfusion was illustrated by the experiments in which portions of the liver were given different kinds of portal venous inflow. Particularly revealing were the experiments of groups 5, 6, and 7 in which one part of the liver received the hormone rich blood from the upper splanchnic organs, whereas the other part was given intestinal venous effluent. In nondiabetic animals, the hormone-enriched fragment enjoyed a great advantage in cholesterol and triglyceride synthesis. This advantage was lost if the animals were previously or concomitantly made diabetic with alloxan or by total pancreatectomy, respectively.
The experiments of groups 8, 9, and 10 provided further information. The livers of these dogs also were divided into two parts. One side received the total nonhepatic splanchnic venous inflow. The other part had supra-adrenal vena caval inflow, much as in the portacaval transposition of Child and his associates (4). In nondiabetic dogs, the liver tissue fed with splanchnic venous blood had a far higher rate of cholesterol and triglyceride synthesis than the other side receiving systemic venous blood. The advantage of splanchnic blood was hardly affected by the addition of the diabetes caused by alloxan or total pancreatectomy.
Taken together, the results of the staged experiments of group 3 and the double fragment experiments of groups 5 to 10 suggest that the upper splanchnic organs have an important effect on cholesterol and triglyceride synthesis, provided that endogenous insulin is present. Intestinal blood was less supportive of this particular biochemical activity but still greater than systemic blood which provided the lowest potential for hepatic cholesterol and triglyceride synthesis.
In this connection, it is reasonable to point out how little is known in a modern and sophisticated sense about the fine biochemical changes that occur after Eck fistula in animals. Since most of the published studies of this preparation are from another era with the crudest of tools for investigation, the Eck fistula could well become, again, a source of new and important information about a full spectrum of subtle functional changes caused by this procedure.
Such speculation hardly seems idle in view of the pathologic changes in the livers of groups 1 to 4, which underwent total portal diversion in one or two stages. The light microscopic changes were as described by previous authors and consisted of a progressive atrophy of the hepatocytes accompanied by loss of glycogen and accumulation of fat in the cytoplasm of these cells, an increase in the number of binucleate hepatocytes and liver cells in mitosis, and increased numbers of enlarged Kupffer cells containing haemosiderin. The presence of relative hyperplasia in these shrinking livers may have represented some kind of compensatory reaction to the loss of total hepatic mass.
Ultrastructurally, surprisingly little has been written about the effects of portal diversion. Fisher (5), Rubin (16), Oudea (14), and Mallet-Guy (11) and their associates have described changes after this procedure, but only the article by Mallet-Guy and his colleagues (11) was concerned with dogs. In our animals, the dominant change 47 to 62 days after portacaval shunt was in the rough endoplasmic reticulum which decreased in amount, underwent marked dilatation, and became depleted of ribosomes. There was also marked loss of glycogen granules, variable mitochondrial abnormalities, and widespread accumulation in the hepatocyte cytoplasm of lipid vacuoles of various sizes. Essentially the same observations were made by Mallet-Guy and co-workers (11), and our findings fully confirm those of that earlier study. They have much in common also with the three ultrastructural studies made in rats, the main difference being that Oudea and Bismuth (14) also found proliferation of smooth endoplasmic reticulum and lesions in the biliary canaliculi, and Rubin and his colleagues (16) noted an increase in the number of mitochondria.
It was difficult to determine whether the first stage mesenteric caval shunt or the second stage portacaval shunt had the greater effect on group 3 dogs because of great individual variation between animals, but consistent actual loss of rough endoplasmic reticulum, as distinct from dilatation, only occurred after portacaval shunt. The fact that both the mesenteric caval and the central portacaval shunt caused demonstrable changes in hepatic structure as well as function was interpreted as further evidence that portal hepatotrophic factors are manifold and come from different splanchnic organs, even though the single most important substance, as emphasized in other publications (22, 24), is apparently pancreatic in origin and apparently is insulin.
So far, this discussion of the mechanisms of the antilipidemic effect of portal diversion has focused mostly upon altered lipid synthesis in the liver. A second general way by which cholesterol values could be reduced would be by a reduced absorption from the intestine. It is not known whether or not the absorption of cholesterol from the intestine is affected by portal diversion.
The third possibility, and a more likely one, is that the degradation or excretion of cholesterol is accelerated after portacaval shunt. Under normal physiologic conditions, both cholesterol and bile acids have an enterohepatic circulation, cholesterol through the lymph channel and bile salts through the portal vein. The diversion of portal blood to the systemic circulation could create a disturbance in the enterohepatic circulation of bile salts. If the demand for production of bile salts were to become excessive, the conversion of cholesterol to bile acids in the liver would be stimulated, resulting in depletion of the exchangeable cholesterol pool, as suggested by Ahrens (1). Such a possibility has been implicated in a recent report by Paumgartner and his colleagues (15) who showed that bile salt synthesis is augmented in the liver of rats after portacaval shunt.
Finally, it is conceivable that a reduced ability of the liver to release lipoprotein could be a factor. The higher content of tissue cholesterol and triglycerides and the lower content of phospholipids in the livers of animals having complete portacaval shunt would be consistent with this possibility. Thus, the metabolic effect may be at one or more sites within the hepatic cells concerned with the synthesis of lipids and the formation and release of lipoproteins, as would be expected with the changes in organelles that were demonstrated ultrastructurally.
Whatever the mechanism of its antilipidemic effect, it may be well to insert a cautionary note about the clinical use of a portacaval shunt for the treatment of hyperlipidemic states. It is certain that deprivation of the liver cells of first order exposure to hepatotrophic substances, such as insulin, in splanchnic venous blood, can nonspecifically reduce the functional capacity of hepatocytes in many ways of which the ability to perform cholesterol and triglyceride syntheses are only two. The potential risks from altering multiple metabolic functions are serious, including the specter of nervous system deterioration and hepatic encephalopathy. For this reason, we have carried out portal diversion only in two patients with the devastating homozygous type II hyperlipidemia, a disease in which survival into the teen ages is uncommon.
SUMMARY
Complete diversion of portal blood in dogs caused sustained falls in serum cholesterol and phospholipid concentrations and declines in hepatic cholesterol and triglyceride synthesis. The hepatocytes in these canine livers were deglycogenated, and they atrophied to about half of their original size within two months. At the same time, there was evidence of increased mitoses. Ultrastructurally, the dominant change in the hepatocytes was in the rough endoplasmic reticulum which decreased in amount, underwent marked dilatation, and became depleted of ribosomes. There was also marked loss of glycogen granules, variable mitochondrial abnormalities, and widespread accumulation in the hepatocyte cytoplasm of lipid vacuoles.
Bypass of intestinal venous return around the liver through a mesenteric caval shunt did not influence the serum lipid concentrations in dogs and baboons, although cholesterol synthesis was depressed in the canine livers and significant morphologic changes, including atrophy, were produced. In both species, the addition of a second stage central portacaval shunt which diverted venous return from the pancreaticogastroduosplenic area caused declines in serum cholesterol and phospholipid concentrations. After the second operation, hepatic cholesterol synthesis in the dogs was further reduced, and triglyceride synthesis was markedly depressed. The eventual ultrastructural changes were similar to those after one stage portal diversion.
In other experiments on dogs, discrete regions of the liver were provided with portal perfusion from different splanchnic sources during a two month period. When the right lobes received pancreaticogastroduodenosplenic venous blood and the left lobes received intestinal venous effluent, in vivo cholesterol and triglyceride synthesis were higher in the hormone-enriched right lobes. This advantage was eliminated with pre-existing alloxan-induced diabetes or by the concomitant performance of total pancreatectomy in dogs that were treated during the ensuing two months with subcutaneously administered insulin. The nutrient-enriched left lobes had the higher lipid synthesis. In a final series of experiments, the right lobes of dogs were given the total splanchnic flow, and the left lobes were perfused with systemic venous blood by anastomosing the left portal vein to the suprarenal vena cava. The right lobar advantage in lipid synthesis could not be eliminated in this preparation with alloxan-induced diabetes or total pancreatectomy.
These results indicate that a reduction of hepatic lipid synthesis is an important, although not necessarily the sole, factor in the antilipidemic influence of portacaval shunt. The effects upon synthesis and blood lipids apparently are due more to the diversion of endogenous hormones than to the bypass of intestinal nutrients. The substances in portal venous blood that subserve hepatic lipid metabolism are presumably largely the same as the hepatotrophic factors which have been described before as profoundly affecting hepatic structure, function, and the capacity for regeneration. These portal blood factors are multiple and interrelated, but the single most important one seems to be insulin.
Acknowledgments
The work was supported by research grants from the Veterans Administration, by Grant Nos. AI-AM-08898 and AM-07772 from the National Institutes of Health, and by Grant Nos. R-00051 and RR-00069 from the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health.
REFERENCES
- 1.Ahrens EH., Jr. Homozygous hypercholesteraemia and the portacaval shunt. Lancet. 1974;2:449. doi: 10.1016/s0140-6736(74)91828-5. [DOI] [PubMed] [Google Scholar]
- 2.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 3.Bloom WL, Lewis GT, Schumpert MZ, Shen T-M. Glycogen fractions of liver and muscle. J. Biol. Chem. 1951;188:631. [PubMed] [Google Scholar]
- 4.Child CG, Barr D, Holswade GR, Harrison CS. Liver regeneration following portacaval transposition in dogs. Ann. Surg. 1953;138:600. doi: 10.1097/00000658-195310000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher ER, Fisher B. Ultrastructural hepatic changes following partial hepatectomy and portacaval shunt in the rat. Lab. Invest. 1963;12:929. [PubMed] [Google Scholar]
- 6.Fletcher MJ. A colorimetric method for estimating serum triglycerides. Clin. Chim. Acta. 1968;22:393. doi: 10.1016/0009-8981(68)90041-7. [DOI] [PubMed] [Google Scholar]
- 7.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949;177:751. [PubMed] [Google Scholar]
- 8.Kendall MG, Stuart A. The Advanced Theory of Statistics. II. Hafner Publishing Co.; New York: 1961. p. 483. [Google Scholar]
- 9.Kessler G, Lederer H. In: Skeggs ZT Jr., editor. Fluorometric measurements of triglycerides; Automation in Analytical Chemistry, Technicon Symposia; New York: Media. 1965.p. 341. [Google Scholar]
- 10.Loud AV. A quantitative stereological description of the ultrastructure of normal rat liver parenchymal cells. J. Cell. Biol. 1968;37:27. doi: 10.1083/jcb.37.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallet-Guy Y, Hezez G, Feroldi J. Anastomose portocave latéro-latérale experimentale. Documents histologiques et électro-microscopiques. Lyon Chir. 1972;68:436. [PubMed] [Google Scholar]
- 12.Marchioro TL, Porter KA, Brown BI, et al. The effect of partial portacaval transposition on the canine liver. Surgery. 1967;61:723. [PMC free article] [PubMed] [Google Scholar]
- 13.Marchioro TL, Porter KA, Dickinson TC, et al. Physiologic requirements for auxiliary liver homotransplantation. Surg. Gynecol. Obstet. 1965;121:17. [PMC free article] [PubMed] [Google Scholar]
- 14.Oudea P, Bismuth H. L'Anastomose porto-cave expérimentale chez le rat normal. Pathol. Biol., Paris. 1965;13:288. [PubMed] [Google Scholar]
- 15.Paumgartner B, Herz R, Preisig R. In: Saunders SJ, Terblanche J, editors. The effect of liver atrophy on hepatic excretory function; International Liver Conference; New York: The Pitman Publishing Corp.. 1973.p. 288. [Google Scholar]
- 16.Rubin E, Gevirtz R, Cohan P, et al. Liver cell damage produced by portacaval shunt. Proc. Soc. Exp. Biol. Med. 1965;118:235. doi: 10.3181/00379727-118-29807. [DOI] [PubMed] [Google Scholar]
- 17.Schneider WC, Greco AE. Incorporation of pyrimidine deoxyribonucleosides into liver lipids and other components. Biochim. Biophys. Acta. 1971;228:610. doi: 10.1016/0005-2787(71)90725-8. [DOI] [PubMed] [Google Scholar]
- 18.Seifter S, Seymour D, Novic B, Muntwyler E. The estimation of glycogen with the anthrone reagent. Arch. Biochem. 1950;25:191. [PubMed] [Google Scholar]
- 19.Starzl TE. Judd lecture—portal hepatotrophic factors; a century of controversy. In: Najarian JS, Delaney JP, editors. Surgery of the Liver, Biliary Tract and Pancreas. Intercontinental Medical Book Corp.; New York: 1974. pp. 495–524. [Google Scholar]
- 20.Starzl TE, Chase HP, Putnam CW, Nora JJ. Follow-up of patient with portacaval shunt for the treatment of hyperlipidemia. Lancet. 1974;2:714. doi: 10.1016/s0140-6736(74)93285-1. [DOI] [PubMed] [Google Scholar]
- 21.Starzl TE, Chase HP, Putnam CW, Porter KA. Portacaval shunt in hyperlipidemia. Lancet. 1973;2:940. doi: 10.1016/s0140-6736(73)92599-3. [DOI] [PubMed] [Google Scholar]
- 22.Starzl TE, Francavilla A, Halgrimson CG, et al. The origin, hormonal nature and action of hepatotrophic substances in portal venous blood. Surg. Gynecol. Obstet. 1973;137:179. [PMC free article] [PubMed] [Google Scholar]
- 23.Starzl TE, Marchioro TL, Rowlands DT, Jr., et al. Immunosuppression after experimental and clinical homotransplantation of the liver. Ann. Surg. 1964;160:411. doi: 10.1097/00000658-196409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starzi TE, Porter KA, Kashiwagi N, et al. Portal hepatotrophic factors in diabetic and nondiabetic dogs. In preparation. [Google Scholar]
- 25.Starzl TE, Putnam CW, Porter KA, et al. Portal diversion for the treatment of glycogen storage disease in humans. Ann. Surg. 1973;178:525. doi: 10.1097/00000658-197310000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sunderman FW, Sunderman FW., Jr. A method for lipid phosphorus in serum. In: Sunderman FW, Sunderman FW Jr., editors. Lipids and Steroid Hormones in Clinical Medicine. J. B. Lippincott Co.; Philadelphia: 1960. p. 28. [Google Scholar]
- 27.Wilcoxon F, Wilcox RA. Some Rapid Approximate Statistical Procedures. Lederle Laboratories; Pearl River, N. Y.: 1964. p. 7. [Google Scholar]
- 28.Zlatkis A, Zak B, Boyle AJ. A new method for the direct determination of serum cholesterol. J. Lab. Clin. Med. 1953;41:486. [PubMed] [Google Scholar]