During the last decade, the influence of portal venous blood upon the liver has been of increasing interest to hepatologists, as reported by Whelan and Porter (33). Numerous publications from our laboratories have shown how crucial endogenous insulin is in its so-called hepatotrophic benefits but have emphasized that additional humoral and nonhumoral portal substances from splanchnic viscera are, undoubtedly, also of importance (26,27,28,29,30,31).
In the present study, some, almost all or all of the nonhepatic splanchnic viscera were removed, and the effect upon the liver during a two or three day period was determined with or without infusion therapy with insulin, glucagon or epidermal growth factor.
METHODS
Eighty-two normal mongrel dogs, weighing 14 to 25 kilograms, were used. Anesthesia for operation and later sacrifice was with pentobarbital sodium, supplemented with phencyclidine hydrochloride and succinylcholine chloride.
Because of the high mortality during the two or three days of study after evisceration, only 30 of the 82 evisceration experiments could be carried to completion. The completed experimental groups are summarized in Table I.
TABLE I.
DATA FOR EXPERIMENTAL GROUPS
| Group No. | Attempted experiments | No. of completed experiments | Days duration postoperative study | Organs retained | Hormone infused |
|---|---|---|---|---|---|
| COMPARISONS BETWEEN RIGHT AND LEFT LOBES AFTER INSULIN INFUSION INTO LEFT PORTAL VEIN | |||||
| 2 | 16 | 7 | 3 | Ileoco1on, 5 dogs, or colon, 2 dogs | Insulin, 0.54±0.19 S.D., units/kgrn./day |
| 3 | 25 | 6 | 2 | None | Insulin, 0.59±0.07 S.D., units/kgrn./day |
| COMPARISONS OF NORMAL. DOGS VERSUS EXPERIMENTS WITHOUT AND WITH HORMONE INFUSIONS INTO MAIN PORTAL VEIN | |||||
| 4 | 16 | 3 | 3 | Colon | None |
| 5 | 13 | 5 | 2 | None | None |
| 6 | 8 | 5 | 2 | None | Insulin, 0.67 ±0.17 S.D., units/kgrn./day |
| 7 | 2 | 2 | 2 | None | Glucagon,0.53 and 0.25 mgrn./kgrn./day |
| 8 | 2 | 2 | 2 | None | Epidermal growth factor, 5.9 and 5.4 gamma/kgrn./day |
Group 1, not shown, consisted of unoperated upon normal dogs. For different biochemical or morphologic measurements, the numbers of dogs in the control study varied from six to 19.
With one kind of evisceration, all of the nonhepatic splanchnic organs were removed, except the colon or terminal part of the ileum and colon (Fig. 1a and b). By anastomosing the ileum or colon to the distal part of the esophagus, saliva or even fluid could be swallowed and would pass promptly to the anus (Fig. 1a and b). In the other preparation (Fig. 1c), all of the nonhepatic splanchnic viscera were removed including the rectum from above or below by a pull-through procedure. The distal part of the esophagus or the proximal part of the stomach was closed in three layers. The amount of gastric cardia retained never exceeded 1 centimeter.
Fig. 1.
a, Evisceration, retaining a few centimeters of the terminal part of the ileum and all of the colon for anastomosis to the esophagus, part of group 2. In all evisceration procedures, the base of the mesentery was preserved, so that the portal vein still had a small volume of hepatopetal flow. b, Same as Fig. l a, except that the ileum was removed and an esophagocolostomy was performed, part of groups 2 and 4. c, Same as Fig. la, except that the colon and rectum were excised with closure of the esophagus and anus, groups 3, 5, 6, 7 and 8.
Postoperatively, fluids were given intravenously or subcutaneously to maintain hydration and electrolyte balance. This usually required 1.5 to 2.0 liters per day of electrolyte solutions, with or without glucose.
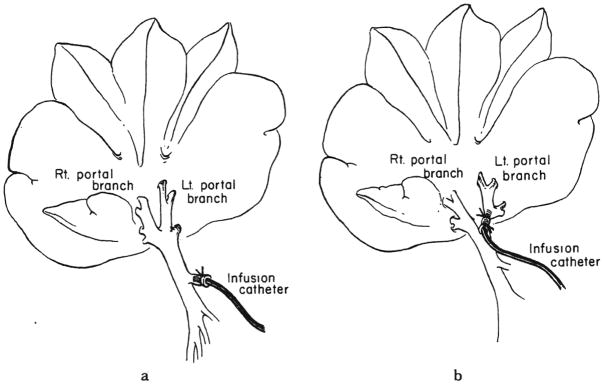
In all the evisceration operations, the base of the mesentery of the small and the large intestine was retained, so that a small volume of hepatopetal portal venous flow was preserved (Fig. 1a, b and c). In groups 2 and 3, insulin was infused into the tied off left portal vein (Fig. 2b); potentially offsetting this hormone advantage for the left lobes was the advantage for the right lobes of flow returning from the mesenteric base. The experimental comparisons in groups 2 and 3 were between the right lobes versus the insulin infused left lobes.
Fig. 2.
Hormone infusion into main portal vein, a, groups 6, 7 and 8 and into left portal vein, b, groups 2 and 3.
With groups 4, 5, 6, 7 and 8, the experimental comparisons were between the livers of dogs two or three days after evisceration and the livers of normal dogs. Hormones in groups 6, 7 and 8 were infused into the main portal vein (Fig. 2a), using a previously described continuous pump system (31).
In group 2 and half of the dogs in group 3, the insulin infused was regular commercial insulin, prepared from beef and pork pancreases. For the other dogs of group 3 and for all the dogs for group 6, research quality crystalline bovine insulin was administered. The glucagon was pure, as was the epidermal growth factor.
About two hours before sacrifice, the dogs were given 0.2 millicurie per kilogram of body weight of (CH3-3H) thymidine by the intravenous route. The specific activity was 47 curies per millimole.
HORMONE MEASUREMENTS
Plasma hormone concentrations were measured. Insulin analysis was by the immunoassay of Herbert and associates (13). Glucagon and glucagon-like immunoreactivity were determined by the radioimmunoassay methods of Faloona and Unger (7). The primary pancreatic glucagon measured with this technique has a molecular weight of 3,500, although other larger moieties have some activity. Glucagon-like immunoreactivity arising from a variety of sources, including the gastrointestinal tract and salivary glands, consists of at least two different molecular weight fractions, as demonstrated by Moody (20). The cross reactivity of glucagon and glucagon-like immunoreactivity is about 2 per cent.
BIOCHEMICAL ANALYSES
At the time of sacrifice, biopsies were made of the liver. Some tissue was used fresh and some was frozen and stored in liquid nitrogen until the analyses were completed. Tissue protein was analyzed by the method of Lowry and colleagues (18).
Deoxyribonucleic acid concentration and synthesis
Extraction and purification of deoxyribonucleic acid were carried out by the method of Schneider and Greco (24). Deoxyribonucleic acid content was measured by Giles and Myers’ (11) modification of the diphenylamine method of Burton (3). The deoxyribonucleic acid content was expressed as micrograms per gram of wet liver. The in vivo incorporation of (CH3-3H) thymidine into deoxyribonucleic acid was expressed as disintegrations per minute per 100 micrograms of the purified deoxyribonucleic acid.
The results in experimental groups 4, 5, 6, 7 and 8 were compared with those of six normal control study dogs. In groups 2 and 3, the right and left lobes were compared against each other postoperatively.
Adenylate cyclase
Fresh dog liver was homogenized and incubated by the method of Makman and Sutherland (19) under basal conditions and conditions of glucagon and sodium fluoride stimulation. To obtain a better dispersal of membrane before analysis, the original procedure of Makman and Sutherland (19) was modified by freezing the homogenate in liquid nitrogen. The cyclic 3′, 5′-adenosine monophosphate formed during incubation was determined by the methods of White and Zenzer (34), Krishna and co-authors (15) and Salomon and co-workers (23). The adenylate cyclase activity was expressed as the nanomoles of cyclic 3′, 5′-adenosine monophosphate formed in 15 minutes by 1 milligram of the protein homogenate.
The results postoperatively in groups 4, 5, 6, 7 and 8 were compared with the values obtained from 19 normal dogs. In groups 2 and 3, the right and left lobes were compared against each other postoperatively.
Cyclic 3′, 5′-adenosine monophosphate
About 50 milligrams of the quick frozen liver was analyzed by the radioimmunoassay method of Harper and Brooker (12) after purification of a trichloroacetic acid extract through a column that had a 200 to 400 mesh.
The results postoperatively in groups 4, 5, 6, 7 and 8 were compared with the values obtained from 12 normal dogs. In groups 2 and 3, the right and left lobes were compared against each other postoperatively.
Pathologic studies
Pieces of liver were fixed in a buffered 10 per cent aqueous solution of formaldehyde. Frozen sections were cut and stained for fat. The tissue was then processed, and paraffin sections were produced. Some of these were stained with a wide range of reagents, while others were prepared for autoradiography by dewaxing and dipping in Ilford K2 nuclear emulsion before drying and exposing for three to six weeks. After passing through a developer, they were stained through the emulsion with methylene blue. Cells were counted as labeled if there were more than 3 grains over the nucleus.
For electron microscopy, specimens were fixed in buffered glutaraldehyde, then postfixed in buffered 2 per cent osmium tetroxide, dehydrated and embedded in epoxy. Sections, 0.5 micromole, were cut, stained and examined by light microscopy and areas selected for electron microscopy. Ultrathin sections were cut, stained with Reynolds lead acetate and with azure II and examined in an electron microscope. The size of hepatocytes in the middle zones of the lobules was determined on stained sections by a method previously described (26), and Loud’s (17) morphometric method was used to determine the length of rough endoplasmic reticulum per area of cytoplasm in these cells.
RESULTS
Hormone Studies
After removal of all the nonhepatic splanchnic viscera (Fig. 1c), detectable circulating glucagon was gone within 24 hours. However, when the colon was retained (Fig. 1b), significant quantities of glucagon were always detectable (Table II). Glucagon-like immunoreactivity was unaffected by evisceration (Table II). Trace quantities of insulin remained one and two days after either type of evisceration, as shown in Table II.
TABLE II.
GLUCAGON, GLUCAGON-LIKE IMMUNOREACTIVITY AND INSULIN ASSAY OF PLASMA
| Time in days after evisceration | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control: | ||||||||||||||||||||||||
| 0 days | 1 day | 2 days | 3 days | 4 days | 5 days | 6 days | 7 days | |||||||||||||||||
| Animal No. |
G | GLI | I | G | GLI | I | G | GLI | I | G | GLI | I | G | GLI | I | G | GLI | I | G | GLI | I | G | GLI | I |
| ALL VISCERA EXCEPT COLON REMOVED | ||||||||||||||||||||||||
| 1 | 110 | 2.7 | 94 | 0 | 2.2 | 5 | 50 | 2.5 | 5 | |||||||||||||||
| 2 | 86 | 1.9 | 27 | 50 | 2.7 | 5 | 0 | 2.9 | 6 | 66 | 2.7 | 5 | 0 | 2.5 | 5 | 40 | 2.5 | 5 | 50 | 2.2 | 5 | 76 | 2.6 | 5 |
| 3 | 76 | 1.4 | 34 | 50 | 2.2 | 6 | 40 | 1.7 | 5 | 50 | 1.5 | 5 | ||||||||||||
| 4 | 50 | 3.4 | 13 | 40 | 5 | 66 | 2.2 | 6 | 145 | 2.9 | 5 | |||||||||||||
| ALL VISCERA REMOVED | ||||||||||||||||||||||||
| 5 | 125 | 3.6 | 120 | 0 | 3.5 | 5 | 0 | 2.8 | 5 | |||||||||||||||
| 6 | 110 | 2.9 | 78 | 0 | 2.4 | 5 | 0 | 2.7 | 5 | |||||||||||||||
| 7 | 76 | 2.1 | 13 | 0 | 1.9 | 5 | 0 | 1.9 | 5 | |||||||||||||||
G, Glucagon, in pgm./ml.
GLI, Glucagon-like immunoreactivity, in ngm./ml.
I, Insulin, in μU./ml.
In the Dallas laboratory of Dr. R. H. Unger, glucagon is considered absent at values <30 pgm., and insulin is considered absent at values <2 μU./ml.
Right Versus Left Lobes After Operation
Biochemical studies
The left lobes of the liver which were receiving commercial or highly purified insulin in groups 2 and 3 had higher adenylate cyclase activity than did the right lobes. Adenyl cyclase activity is separable into receptor and catalytic components. Basal activity is receptor plus catalytic, glucagon stimulated activity is receptor in nature and sodium fluoride stimulated activity is catalytic.
The increases in the insulin perfused tissues were of both the receptor and the catalytic parts of adenyl cyclase, with elevations of the basal, glucagon stimulated and sodium fluoride stimulated components. The higher adenyl cyclase activity in the left lobes was evident in both groups 2 and 3 but reached significance only in group 3 (Table III). In group 3, the results were the same with commercial as with purified insulin. Cyclic 3′, 5′ -adenosine monophosphate concentration was not significantly different between the two sides, in either group 2 or 3, as shown in Table IV.
TABLE III.
ADENYLATE CYCLASE, NANOMOLES PER MILLIGRAM PROTEIN HOMOGENATE PER 15 MINUTES
| Basal, H2O | Glucagon stimulated | NaF stimulated, 10 mM. |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10−9M | 10−8M | 10−7M | 10−6M | 10−5M | |||||||||||
| Group | No. | Rp | Lp | Rp | Lp | Rp | Lp | Rp | Lp | Rp | Lp | Rp | Lp | Rp | Lp |
| 2 | 7 | 27±6 | 50±29 | 64±16 | 96±45 | 77±22 | 110±53 | 91±21 | 129±58 | 107±26 | 145±64 | 117±22 | 147±60 | 172±34 | 233±74 |
| p Value* | NS | NS | NS | NS | NS | NS | NS | ||||||||
| 3 | 6 | 21±4 | 42±27 | 32±7 | 71±43 | 43±7 | 127±101 | 62±11 | 151±99 | 86±19 | 236±122 | 87±28 | 209±128 | 135±38 | 298±128 |
| p Value* | <0.05 | <0.05 | <0.05 | <0.05 | <0.02 | <0.05 | <0.02 | ||||||||
Data are mean ± standard deviation, S.D. All statistical comparisons are between the right, Rp, and left, Lp, lobes at the time of sacrifice using the Students t test.
NaF, Sodium fluoride; NS, not significant.
TABLE IV.
CYCLIC 3′, 5′-ADENOSINE MONOPHOSPHATE, DEOXYRIBONUCLEIC ACID CONCENTRATIONS AND DEOXYRIBONUCLEIC ACID SYNTHESIS
| Cyclic AMP, pM/gm. of wet liver | DNA concentrations, μgm./gm. of wet liver | DNA synthesis, DPM/100 μgm. DNA | |||||
|---|---|---|---|---|---|---|---|
| Group | No. | Rp | Lp | Rp | Lp | Rp | Lp |
| 2 | 7 | 998±308 | 1844±420 | 2513±350 | 2730±256 | 504±117 | 810±273 |
| p Value* | NS | NS | <0.01 | ||||
| 3 | 6 | 1348±392 | 1000±256 | 2873±583 | 2811±806 | 476±194 | 565±220 |
| p Value* | NS | NS | NS | ||||
AMP, Adenosine monophosphate.
DNA, Deoxyribonucleic acid.
Rp, Right lobe.
Lp, Left lobe.
DPM, Disintegrations per minute.
NS, Not significant.
Statistical comparisons are between the right and left lobes by Students t test.
Deoxyribonucleic acid synthesis was heightened in the insulin infused left lobes compared with that in the right lobes. However, this effect was statistically significant only in group 2 (Table IV). Deoxyribonucleic acid concentration was essentially the same on both sides (Table IV).
Pathologic studies
During the two or three days of postoperative observation, the insulin infused left lobes retained their cell size far better than did the acutely atrophying right lobe hepatocytes (Table V). The morphologic characteristics of the hepatocytes of the left lobe were more normal. In the totally eviscerated dogs, the cytoplasm of the hepatocytes in the right lobes became laden with fat droplets. This lipid accumulation was greatly reduced or prevented by insulin infusion. Ultrastructurally, the rough endoplasmic reticulum was nearly normal in amount and appearance in the hepatocytes in the insulin infused left lobes compared with that in the right lobes in which the rough endoplasmic reticulum was reduced in amount, disorganized, depleted of ribosomes and had many dilated cisternae. Both sides of the liver contained fewer glycogen granules than normal, and there were increased numbers of autophagosomes and lipid droplets.
TABLE V.
CELL SIZE AND AUTORADIOGRAPHY
| Mean ± S.D. hepatocyte size, units | Mean ± S.D. labeled hepatocytes per 1,000 hepatocytes | |||||
|---|---|---|---|---|---|---|
| Group | Description | No. | Rp | Lp | Rp | Lp |
| Normal* | Controls | 11 | 0.181±0.04 | 0.175±0.05 | 1.53±0.46 | 1.54±0.37 |
| p Value† | NS | NS | ||||
| 2 | Partial evisceration Insulin into left portal | 7 | 0.115±0.02 | 0.170±0.01 | 0.79±0.06 | 1.63±0.18 |
| p Value† | <0.01 | <0.001 | ||||
| 3 | Total evisceration Insulin into left portal | 7 | 0.09±0.01 | 0.166±0.02 | 0.67±0.04 | 1.32±0.4 |
| p Value† | <0.01 | <0.01 | ||||
NS, Not significant.
The data comparing the right and left lobes in these 11 normal dogs were obtained from earlier experiments, Surg. Gynecol. Obstet., 1975, 141: 843. Students t test compares the right and left lobes.
Statistical comparison between right. Rp, and left, Lp, lobes at time of sacrifice using Students t test.
The rate of spontaneous cell renewal, as reflected by the mitotic index and by thymidine labeling, was twice as great in the insulin infused left lobes in both groups 2 and 3 (Table V). In group 3, the results in half the dogs that received commercial insulin were the same as in the other half that received highly purified insulin.
Preoperative Versus Postoperative
Biochemical studies
Compared with normal controls, the adenylate cyclase activity under basal and stimulatory conditions was elevated in the dogs of group 4 which had all of the nonhepatic splanchnic viscera removed, except for the colon (Table VI). In some instances, these increases were statistically significant (Table VI).
TABLE VI.
ADENYLATE CYCLASE, NANOMOLES PER MILLIGRAM PROTEIN HOMOGENATE PER 15 MINUTES
| Glucagon stimulated | NaF stimulated, 10 mM. | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | No. | Basal, H2O | 10−9M | 10−8M | 10−7M | 10−6M | 10−5M | |
| Normal | 18 | 18.6±4.8 | 40.3±12.6 | 59.2±13.2 | 77.2±17.2 | 93±21.8 | 97±24.2 | 126±39.3 |
| 4 | 3 | 33.3±14.9 | 75.9±18.1 | 105±45.6 | 179.4±96.3 | 287±128 | 238.6±104.5 | 352±128.6 |
| pValue | NS | NS | NS | NS | NS | <0.05 | <0.05 | |
| 5 | 5 | 8.8±4.6 | 14.1±6.9 | 24.9±16.7 | 35.3±19.9 | 53.2±36.3 | 57.2±48.9 | 81.6±54.7 |
| p Value | <0.01 | <0.001 | <0.001 | <0.001 | <0.01 | <0.02 | NS | |
| 6 | 5 | 24±6.6 | 37.5±8.7 | 54.4±12.5 | 76.8±24 | 111.4±25.5 | 127.9±31.2 | 222±71.5 |
| p Versus normal | NS | NS | NS | NS | NS | <0.05 | <0.05 | |
| p Versus group 5 | <0.01 | <0.01 | <0.02 | <0.02 | <0.02 | <0.05 | <0.01 | |
| 7 | 2 | 15.5±10.6 | 16.8±10.8 | 31.4±29.1 | 40.6±37.4 | 61±48 | 66.6±49 | 120.2±113 |
| p Versus normal | NS | <0.05 | <0.02 | <0.02 | NS | NS | NS | |
| p Versus Group 5 | NS | NS | NS | NS | NS | NS | NS | |
| 8 | 2 | 11.5±6.3 | 13.5±9.1 | 20.3±4.3 | 24.6±7.4 | 44.4±17.5 | 42.5±14.5 | 82.8±32.2 |
| p Versus normal | NS | <0.01 | <0.001 | <0.001 | <0.01 | <0.01 | NS | |
| p Versus group 5 | NS | NS | NS | NS | NS | NS | NS | |
In each group, the test group is compared by the Students t test with the normal dogs. In groups 6, 7 and 8, a second companion, lower p value, is made with the eviscerated dogs of group 5 which did not receive hormones.
NaF, Sodium fluoride; NS, not significant.
In contrast, the adenylate cyclase activity was reduced to a highly significant degree under basal and stimulatory conditions in the dogs of group 5 which had the colon as well as the other non-hepatic splanchnic viscera excised. The adenylate cyclase activity was not restored by infusion of glucagon, group 7, or by epidermal growth factor, group 8, but it was returned essentially to, or even above, normal when research quality insulin was infused into the main portal vein, group 6, Table VI.
Hepatic cyclic 3′, 5′-adenosine monophosphate was increased above the normal level in dogs which had evisceration, except for the colon, group 4, and in completely eviscerated dogs which were given glucagon intraportally, group 7, Table VII.
TABLE VII.
CYCLIC 3′, 5′-ADENOSINE MONOPHOSPHATE
| Group | No. | Cyclic AMP, pM./gm. of wet liver | p Value* |
|---|---|---|---|
| Normal | 12 | 1076±213 | |
| 4 | 3 | 1581±787 | <0.1>0.05 |
| 5 | 5 | Not done | Not done |
| 6 | 5 | 1360±413 | NS |
| 7 | 2 | 1516±426 | <0.1>0.05 |
| 8 | 2 | 780±244.6 | NS |
Comparison by the Students t test with the normals.
AMP, 3′, 5′·Adenosine monophosphate.
Hepatic deoxyribonucleic acid synthesis was depressed when evisceration of the nonhepatic splanchnic organs was complete, group 5, or when glucagon, group 7, or epidermal growth factor, group 8, were infused intraportally after complete evisceration (Table VIII). In contrast, deoxyribonucleic acid synthesis was restored to normal when research quality insulin was infused into the portal vein, group 6, Table VIII. Incongruously, in view of the results with autoradiography, the dogs of group 4 with retained colons had spontaneous deoxyribonucleic acid synthesis more than twice that of normal.
TABLE VIII.
DEOXYRIBONUCLEIC ACID CONCENTRATION AND SYNTHESIS
| Group | No. | DNA concentration, μgm./gm. of wet liver | p Value* | DNA synthesis, DPM/1,000 μgm. of DNA | p Value* | Agreement with ARG† |
|---|---|---|---|---|---|---|
| Normal | 6 | 2691 ± 381 | 461 ± 107 | Yes | ||
| 4 | 3 | 5553 ± 748 | <0.001 | 1039 ± 47 | <0.001 | No |
| 5 | 5 | 2963 ± 507 | NS | 188 ± 33 | <0.001 | Yes |
| 6 | 5 | 2930 ± 632 | NS | 564 ± 380 | NS | Yes |
| 7 | 2 | 3066 ± 11 81 | NS | 277 ± 139 | <0.05 | Yes |
| 8 | 2 | 4392 ± 830 | <0.01 | 248 ± 8 | <0.05 | Yes |
All statistical comparisons are with the normal dogs using the Students t test.
See actual ARG, autoradiography, in Table IX.
NS, Not siginificant.
DPM, Disintegrations per minute.
Pathologic studies
At the end of two days of postoperative observation, the liver cells in dogs infused intraportally with purified insulin had less atrophy, less fat and fewer ultrastructural changes than did the untreated dogs or the dogs infused with glucagon or epidermal growth factor (Table IX). The insulin treated dogs of group 6 also had a normal mitotic index and a normal rate of thymidine incorporation, as measured by autoradiography compared with that of all the other groups of test dogs in which the rate of spontaneous cell renewal was about half normal.
TABLE IX.
HEPATOCYTE SIZE AND AUTORADIOGRAPHIC LABELING
| Group | Type experiment | No. | Mean ± S.D., hepatocyte Size | p Value* | Mean ± S.D. labeled hepatocytes per 1,000 hepatocytes | p Value* |
|---|---|---|---|---|---|---|
| Normal | Control | 17† | 0.173±0.038 | — | 1.55±0.33 | — |
| 4 | Partial evisceration; no infusion | 3 | 0.107±0.017 | <0.001 | 0.69±0.02 | <0.001 |
| 5 | Total evisceration; no infusion | 5 | 0.086±0.010 | <0.001 | 0.62±0.035 | <0.001 |
| 6 | Total evisceration plus insulin | 5 | 0.160±0.010 | NS | 1.48±0.42 | NS |
| 7 | Total evisceration plus glucagon | 2 | 0.097±0.008 | <0.001 | 0.69±0.015 | <0.001 |
| 8 | Total evisceration plus epidermal growth factor | 2 | 0.091±0.005 | <0.001 | 0.65±0.035 | <0.001 |
Experimental groups compared with normal dogs using Students t test.
Eleven of the normals were the left lobar samples of previously studied dogs, the other six were new controls.
The subnormal rate of hepatocyte mitosis in the dogs of group 4 (Table IX) was inconsistent with the elevated deoxyribonucleic acid synthesis shown for those same dogs in Table VIII. These disparate findings in the dogs with a retained distal part of the colon in group 4 could be partly explained by the infiltration of the livers by mono-nuclear cells, many of them in division, and by the active proliferation of the endothelial cells lining the vessels of the liver. Something odd seemed to have happened to these dogs to account for the vascular damage and to have caused the cellular invasion. The hepatocytes also contained much more fat than was present in the right lobes of the comparable dogs in group 2.
DISCUSSION
Many past studies of portal hepatotrophic factors have lasted for weeks or months. The shortest observation times used by us in the past have been four days (29, 30, 31). It was well established from these studies that the anatomic changes in the liver caused by deprivation of portal venous blood are well advanced within four days.
In the present experiments, it was necessary to reduce the duration of the experiments even further. The reason was that survival for more than two or three days was extremely difficult to achieve after complete, or nearly complete, evisceration. This situation was not improved by the infusion of replacement hormones. Fortunately, the evolution of the end points under study was fast enough to permit conclusions, even after two or three days.
An unequivocal conclusion from the pathologic studies in the eviscerated dogs was that insulin, as a single factor, had a profound effect upon the structure and spontaneous cell renewal of hepatocytes. In past studies in dogs with a portacaval shunt, it has been demonstrated that insulin administered into the tied off central portal vein of livers deprived of portal blood augmented the rate of mitoses, prevented atrophy and preserved the ultrastructural qualities of hepatocytes (30, 31). The same effect of insulin was seen in the eviscerated dogs of the present study and approximately to the same degree. Glucagon and epidermal growth factor had no demonstrable effect on the autoradiographic or structural measurements.
The effects of insulin upon the biochemical composition of the liver are well known, as we (26, 27, 28) and others have reported. In the present investigation, attention was directed to deoxyribonucleic acid, adenyl cyclase and cyclic 3′, 5′-adenosine monophosphate. Deoxyribonucleic acid synthesis was heightened by insulin infusion, which was consistent with the autoradiographic studies. This was particularly evident in experiments in which insulin was provided for one portal branch only, allowing a comparison of the lobes on that side with the contralateral lobes.
Somewhat unexpected was the seeming maintenance of adenyl cyclase activity under the influence of insulin. After total nonhepatic splanchnic evisceration, including the pancreas, hepatic adenyl cyclase levels were generally depressed under both basal and stimulatory conditions. The prevention of this effect by intraportal administration of either commercial or purified insulin was paradoxic, since insulin in contrast with glucagon has been thought by Sutherland and Robison (32) to be an adenyl cyclase and cyclic 3′, 5′-adenosine monophosphate inhibitor. The effect of insulin in the evisceration experiments was presumably due to nonspecific membrane stabilization. It is well known that insulin can stabilize cell membranes and, as reviewed by Kono and Barham (14), Freychet and associates (10) and Cuatrecasas (4), that it can have wide ranging beneficial effects upon the organelles of hepatocytes and other kinds of cells. In the absence of exogenous insulin in the eviscerated dogs, the intraportal infusion of glucagon to two dogs in many times the physiologic dosages to the damaged liver cells did not increase adenyl cyclase. At the same time, the cyclic 3′, 5′-adenosine monophosphate concentrations underwent the expected increases.
The only inconsistency internally within these experiments or with past work done in our laboratories was the biochemical profile observed in the dogs of group 4 which retained their colon after evisceration. The deoxyribonucleic acid concentration and deoxyribonucleic acid synthesis were incongruously high in the livers of these dogs, as was the level of adenyl cyclase activity relative to all the other evisceration groups. The probability that these biochemical findings were due to some kind of experimental artifact rather than to an hepatotrophic effect of the retained colon was evident from the morphologic and autoradiographic studies which revealed no evidence of a beneficial colonic influence upon the liver.
Results of these studies further underscore the primacy of insulin as a hepatotrophic substance in experimental systems that do not involve hepatectomy. The probable relevance of this kind of information to hepatic regeneration has been the subject of a number of recent reports from our own laboratories (29) and by Broelsch (1), Bucher and Swaffield (2), Duguay and Orloff (5, 6), Farivar and colleagues (8), Fisher and co-authors (9), Richman and associates (22), Skivolocki and co-workers (25), Whittemore and colleagues (35), as well as by Price (21) and Leffert (16). In spite of the extensiveness of these studies, the role of portal factors in regeneration of the liver has not been clarified. In future studies, it will be necessary to repeat work in which hepatectomy is carried out at the same time as the evisceration procedures to test directly the influence of such extirpation with or without hormone replacement upon the expected hepatic regeneration process. Investigations of this kind have been pioneered by Price (21), Bucher and Swaffield (2) and Whittemore and associates (35), but the results have not been consistent.
SUMMARY
All, or nearly all, of the nonhepatic splanchnic viscera were removed in dogs. In most untreated dogs, the liver cells underwent changes similar to those caused by portacaval shunt, including structural deterioration of organelles and fatty metamorphosis. The rate of division of the hepatocytes, as measured by the mitotic index and by autoradiography, was depressed as were deoxyribonucleic acid synthesis and adenylate cyclase activity. These changes were restored to, or toward, normal with the intraportal administration of commercial or purified insulin but not with glucagon or epidermal growth factor. The results of both the pathologic and biochemical studies were consistent, except for an incongruity in some of the dogs in which the colon was retained.
Acknowledgments
The work was supported by research grant Nos. MRIS 8118-01 and 7227-01 from the Veterans Administration; by Grant Nos. AM-17260 and AM-07772 from the National Institutes of Health, and by Grant No s. RR-00051 and RR-00069 from the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health.
Contributor Information
Thomas E. Starzl, Denver, Colorado
Antonio Francavilla, Bari, Italy
Kendrick A. Porter, London, England
Joseph Benichou, Denver, Colorado
References
- 1.Broelsch CE, Lee S, Charter AC, III, et al. Regeneration of liver isografts transplanted in continuity with splanchnic organs. Surg Forum. 1974;25:394. [PubMed] [Google Scholar]
- 2.Bucher NLR, Swaffield M. Regulation of hepatic regeneration in rats by synergistic action of insulin and glucagon. Proc Natl Acad Sci U S A. 1975;72:1157. doi: 10.1073/pnas.72.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton K. A study of the conditions and mechanisms of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956;62:315. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuatrecasas P. Insulin Action. New York and London: Academic Press; 1972. The nature of insulin-receptor interaction; p. 137. [Google Scholar]
- 5.Duguay LR, Orloff MJ. Regulation of liver regeneration by the pancreas in dog. Surg Forum. 1976;27:355. [PubMed] [Google Scholar]
- 6.Idem. Role of the pancreas in regulation of liver regeneration in dogs. Surg Forum. 1977;28:387. [PubMed] [Google Scholar]
- 7.Faloona GR, Unger RH, Glucagon . In: Methods of Hormone Radioimmunoassay. Jaffe BM, Berman HR, editors. New York: Academic Press; 1974. pp. 317–330. [Google Scholar]
- 8.Farivar M, Wands JR, Isselbacher KJ, Bucher NLR. Effect of insulin and glucagon on fulminant murine hepatitis. N Eng J Med. 1976;295:1517. doi: 10.1056/NEJM197612302952706. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Szuch P, Fisher ER. Evaluation of a humoral factor in liver regeneration utilizing liver transplants. Cancer Res. 1971;31:322. [PubMed] [Google Scholar]
- 10.Freychet P, Roth S, Mavlle MD. Monoidoinsulin; demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971;43:400. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- 11.Giles KW, Myers A. An improved diphenylamine method for the estimation of deoxyribonucleic acid. Nature. 1965;206:93. [Google Scholar]
- 12.Harper JF, Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2’0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1:207. [PubMed] [Google Scholar]
- 13.Herbert V, Kam-Seng L, Gottlieb CW, Bleicher SJ. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965;25:1375. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- 14.Kono T, Barham FW. The relationship between the insulin-limiting capacity of fat cells and cellular response to insulin. J BioI Chern. 1971;246:6210. [PubMed] [Google Scholar]
- 15.Krishna G, Weiss B, Brodie BB. A simple sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968;163:379. [PubMed] [Google Scholar]
- 16.Leffert HL. Growth control of differentiated fetal rat hepatocytes in primary monolayer culture—VII. J Cell BioI. 1974;62:792. doi: 10.1083/jcb.62.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loud AV. A quantitative stereological description of the ultrastructure of normal rat liver parenchymal cells. J Cell BioI. 1968;37:27. doi: 10.1083/jcb.37.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J BioI Chem. 1951;193:265. [PubMed] [Google Scholar]
- 19.Makman MH, Sutherland EW. Use of liver adenyl cyclase for assay of glucagon in human gastrointestinal tract and pancreas. Endocrinology. 1964;75:127. doi: 10.1210/endo-75-1-127. [DOI] [PubMed] [Google Scholar]
- 20.Moody AJ. Gastrointestinal glucagon-like immunoreactivity. In: Lefebure PJ, Unger RH, editors. Glucagon: Molecular Physiology, Clinical and Therapeutic Implications. New York: Pergamon Press; 1972. pp. 319–341. [Google Scholar]
- 21.Price JB., Jr Insulin and glucagon as modifiers of DNA synthesis in the regenerating rat liver. Metabolism. 1976;25:1427. doi: 10.1016/s0026-0495(76)80157-6. [DOI] [PubMed] [Google Scholar]
- 22.Richman RA, Claus TH, Pilkis SJ, Friedman DL. Hormonal stimulation of DNA synthesis in primary cultures of adult rat hepatocytes. Proc Natl Acad Sci U S A. 1976;73:3588. doi: 10.1073/pnas.73.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salomon Y, Londos C, Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974;58:541. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- 24.Schneider WC, Greco AE. Incorporation of pyrimidine deoxyribonucleotides into liver lipids and other components. Biochem Biophys Acta. 1971;228:610. doi: 10.1016/0005-2787(71)90725-8. [DOI] [PubMed] [Google Scholar]
- 25.Skivolocki WP, Duguay LR, Orloff MJ. Effect of pancreatic hormones on liver regeneration in a double-liver rat bioassay. Surg Forum. 1977;28:385. [PubMed] [Google Scholar]
- 26.Starzl TE, Francavilla A, Halgrimson CG, et al. The origin, hormonal nature and action of portal venous hepatotrophic substances. Surg Gynecol Obstet. 1973;137:179. [PMC free article] [PubMed] [Google Scholar]
- 27.Starzl TE, Lee IY, Porter KA, Putnam CW. The influence of portal blood upon lipid metabolism in normal and diabetic dogs and baboons. Surg Gynecol Obstet. 1975;140:381. [PMC free article] [PubMed] [Google Scholar]
- 28.Starzl TE, Porter KA, Kashiwagi N, et al. The effect of diabetes mellitus on portal blood hepatotrophic factors in dogs. Surg Gynecol Obstet. 1975;140:549. [PMC free article] [PubMed] [Google Scholar]
- 29.Starzl TE, Porter KA, Kashiwagi N, Putnam CW. Portal hepatotrophic factors, diabetes mellitus and acute liver atrophy, hypertrophy and regeneration. Surg Gynecol Obset. 1975;141:843. [PMC free article] [PubMed] [Google Scholar]
- 30.Starzl TE, Porter KA, Putnam CW. Intraportal insulin protects from the liver injury of portacaval shunt in dogs. Lancet. 1975;2:1241. doi: 10.1016/s0140-6736(75)92076-0. [DOI] [PubMed] [Google Scholar]
- 31.Starzl TE, Porter KA, Watanabe K, Putnam CW. Effects of insulin, glucagon, and insulin/glucagon infusions on liver morphology and cell division after complete portacaval shunt in dogs. Lancet. 1976;1:821. doi: 10.1016/s0140-6736(76)90477-3. [DOI] [PubMed] [Google Scholar]
- 32.Sutherland EW, Robison GA. The role of cyclic AMP in the control of carbohydrate metabolism. Diabetes. 1969;18:797. doi: 10.2337/diab.18.12.797. [DOI] [PubMed] [Google Scholar]
- 33.Whelan J, Porter R. Hepatotrophic Factors, Ciba Foundation Study Group No 55. London: J. & A. Churchill, Ltd.; 1977. [Google Scholar]
- 34.White AA, Zenser TE. Separation of cyclic 3′, 5′-adenosine monophosphate (cyclic AMP) from other nucleotide in aluminum oxide columns. Anal Biochem. 1971;41:372. doi: 10.1016/0003-2697(71)90156-4. [DOI] [PubMed] [Google Scholar]
- 35.Whittemore AD, Kasuya M, Voorhees AB, Jr, Price JB., Jr Hepatic regeneration in the absence of portal viscera. Surgery. 1975;77:419. [PubMed] [Google Scholar]