Abstract
Controlled trials to assess the therapeutic benefit of orthotopic hepatic transplantation (OHTx) for primary sclerosing cholangitis (PSC) cannot be justified in view of improvement of patient survival after this operation since 1981. However, the actual patient survival with OHTx can be compared with the Mayo model estimated survival probabilities without OHTx. This model, which encompasses physical, biochemical and histopathologic parameters of PSC, was constructed from a study of 392 conservatively treated PSC patients at five international centers in England and North America.
We compared the actual survival of 216 adult patients with the diagnosis of advanced PSC who underwent hepatic replacement with the expected survival estimated by the Mayo PSC natural history model, “the simulated control technique.” OHTx was performed at the University of Pittsburgh and Mayo Medical Center between 5 December 1981 and 26 December 1990. The mean (plus or minus standard deviation) post-OHTx follow-up period was 34±25 months (range of zero to 104 months). Before transplantation, biliary or portal hypertensive operation, or both, was performed upon 104 patients. At operation, the mean age of recipients was 42.1±11.3 years and the mean value of total serum bilirubin was 13.3±13.0 milligrams per deciliter. Extensive septal fibrosis and cirrhosis were histologically documented in 97 percent of the patients, with splenomegaly in 63 percent. Immunosuppressive therapy was based primarily on cyclosporin in 184 recipients and FK-506 in 32.
Within six months, the Kaplan-Meier survival probability after OHTx (0.89) already was higher than predicted by the Mayo model (0.83). At five years, the Kaplan-Meier actual survival with OHTx was 0.73 compared with 0.28 expected Mayo model survival. The overall increased survival rate with transplantation was statistically significant (chi-square equals 126.6; p<0.001). At all risk stratifications, OHTx significantly improved survival with a p value of 0.031 (low risk), 0.001 (moderate risk) and <0.001 (high risk). Thus, OHTx is effective therapy for PSC. Disease gravity and unsuspected cholangiocarcinoma in the excised native liver adversely influenced short and long term survival rates after transplantation, respectively.
Hepatic transplantation has evolved during the last decade to be the most effective and definitive therapeutic modality for patients with end stage hepatic diseases, including primary sclerosing cholangitis (PSC) (1-3). Obviously, random assignment of these patients to a conservative treatment “control group” is considered to be ethically and clinically inappropriate.
At the Mayo Medical Center, a simulated control technique “natural history model” for predicting the probability of survival in conservatively treated patients with PSC has been made (4) and recently refined (5). A similar model, but with different risk variables, was designed for primary biliary cirrhosis (6), which was used in a recent study (7) to assess the efficacy of orthotopic hepatic transplantation (OHTx) in these patients.
To provide control data for evaluating the efficacy of OHTx in improving survival for patients with advanced PSC, the Mayo PSC natural history model was mathematically applied to the combined population of patients with PSC who underwent hepatic replacement at the University of Pittsburgh and Mayo Medical Center. Actual survival after transplantation was compared with the survival estimated with the use of the Mayo model. These data were carefully analyzed to estimate the survival advantage of OHTx in this unique population.
MATERIALS AND METHODS
Mayo PSC natural history model
The control group used in the current evaluation of the effect of OHTx on patient survival was formed according to data generated by the Mayo model for assessing survival in relation with the natural history of PSC (5). This is a Cox proportional hazards regression model (8, 9) that uses a small number of variables to predict survival in patients who are conservatively treated. The model was made from a combined data set consisted of 426 patients with PSC who had been referred to five international medical centers (three in the United States and two in the United Kingdom), between July 1973 and September 1989. The final model, however, was fit only to 392 patients who had complete data for all of the variables included in the model (5). All of the patients met the standard clinical, biochemical, radiologic and histologic criteria for the diagnosis of PSC (10-12). Patients were excluded from the study if they were less than 16 years of age or had histologically proved concomitant cholangiocarcinoma at the time of referral. Patients with attempts of surgical or roentgenologic decompression, or both, of the biliary tract were not excluded because these interventions are not thought to alter survival meaningfully. Of the 392 patients, 49 received a hepatic transplant after the initial referral and the follow-up evaluation of these patients was censored at transplantation. It is important to note that 24 patients of this group were found to have unsuspected cholangiocarcinoma by examining the hepatectomy specimen after transplantation or at autopsy.
The Mayo PSC model uses the age of the patient and three other different variables that measure disease severity—total serum bilirubin, splenomegaly and histologic stage on hepatic biopsy. These variables were measured for all patients at time of referral. The model combines these four prognostic variables to obtain a risk score (R) for each patient using the equation: R=0.041 (age in years) + 0.535 loge (bilirubin in milligrams per deciliter) + 0.705 (splenomegaly score) + 0.486 (histologic score). Splenomegaly was defined based upon clinical or roentgenologic, or both, data and the score was given zero for absence and one for presence of splenic enlargement. Histologic stage was determined according to the pathologic changes seen on hepatic biopsies. All hepatic specimens taken at biopsy were restaged according to the Ludwig criteria (13). The results of statistical analysis suggest that a particularly useful score of histologic stage consists of using the value 1 for stage 1 and 2, the valve 2 for stage 3 and the value 4 for stage 4 (4, 5). The value of the individual prognostic variables for patients that were used to build and validate the model, as well as the calculated risk score, are given in Table I.
TABLE I.
VALUES FOR MAYO MODEL VARIABLES IN PATIENTS WITH PRIMARY SCLEROSING CHOLANGITIS TREATED WITH AND WITHOUT HEPATIC TRANSPLANTATION
| Variable | Natural history group, n=392 |
Transplanted group, n=216 |
|
|---|---|---|---|
| Age, yrs. | |||
| Mean±S.D. . . . . . . . . . . . . | 41.3±13.9 | 42.1±11.3 | |
| Range . . . . . . . . . . . . . . . | 17 to 80 | 18 to 69 | |
| Bilirubin, mgm./dl. | |||
| Mean ±S.D. . . . . . . . . . . . . | 4.3±6.3 | 13.3±13.0 | |
| Range . . . . . . . . . . . . . . . | 0.2 to 36 | 0.2 to 83 | |
| Splenomegaly . . . . . . . . . . . . | 120 (30.6) | 137 (63.4) | |
| Histologic stage* . . . . . . . . . . | 2.7±1.1 | 3.8±0.5 | |
| 1 and 2 . . . . . . . . . . . . . . | 160 (40.8) | 7 (3.2) | |
| 3 . . . . . . . . . . . . . . . . . . | 116 (29.6) | 33 (15.3) | |
| 4 . . . . . . . . . . . . . . . . . . | 116 (29.6) | 176 (81.5) | |
| Risk score† | |||
| Mean ±S.D. . . . . . . . . . . . . | 3.3±1.3 | 5.0±0.9 | |
| Range . . . . . . . . . . . . . . . | 0.6 to 6.8 | 1.7 to 6.8 | |
Mean ±S.D.
According to the Mayo model.
Numbers in parentheses are percentages.
The R is then used to generate a predicted survival probability curve for each patient based upon the risk level. This curve shows the probability that the individual will live a given number of years after the risk variables have been measured. Technical details are available elsewhere (5). The model was internally cross validated by using data splitting (14) and intramural techniques. The 95 percent confidence intervals for the estimated survival probability measured by the jackknife procedure (15, 16) showed acceptable accuracy for most of the patients who were individually studied among the total population, as well as the risk groups (5).
Patient population
Between December 1981 and December 1990, 1,785 patients underwent primary OHTx at the University of Pittsburgh Medical Center. At Mayo Medical Center, another 236 patients received OHTx during the period from March 1985 to December 1990. Cyclosporin and steroids were the baseline immunosuppressive treatment for all patients until the introduction of the new immunosuppressive agent FK-506 in March of 1989 (17). Beginning in November 1984, OKT3 was used to treat some episodes of allograft rejection.
Before transplantation, the diagnosis of the underlying hepatic disease was established in each patient on the basis of thorough clinical, biochemical, roentgenologic and histologic assessments in addition to other metabolic and immunologic tests. The diagnosis of PSC was given to 173 of the patients at the Pittsburgh facility and 58 of the patients at the Mayo Clinic. The diagnosis was confirmed in all patients by serial histologic examination of the hepatectomy specimen. Based upon the selection criteria adopted to build the Mayo PSC natural history model, 15 of these combined 231 patients were excluded. Seven patients had the pretransplant diagnosis of concomitant cholangiocarcinoma and four were less than 16 years of age. The remaining four patients had concomitant pathologic lesions that could influence patient survival, namely Hodgkin's lymphoma, eosinophilic granuloma, A1 antitrypsin deficiency and multiorgan polycystic disease.
Of the 216 patients included in the study, 141 were male and 75 were female. A history of inflammatory intestinal disease was documented in 174 patients and total colectomy was required in 29 because of either active intestinal disease or complicating carcinoma. Biliary or portal hypertensive, or both, surgical treatment was performed before transplantation in 104 patients. Attempts of surgical or roentgenologic, or both, biliary drainage were reported in 73 patients. The primary indications for OHTx in most patients were poor hepatic function or large esophageal varices, with repeated massive upper gastrointestinal hemorrhage, or both. Unsuspected cholangiocarcinoma was detected in the hepatectomy specimen in 11 patients. All patients received transplantation between 5 December 1981 and 26 December 1990 (median transplant date was mid-January 1988). The primary immunosuppressive agent was cyclosporin in 184 patients and FK-506 in 32. During the study period, 30 of the patients receiving cyclosporin were changed to FK-506 and one of the FK-506 group was changed to cyclosporin.
The descriptive statistics for the 216 hepatic recipients are given in Table I. The four prognostic variables used were measured at the time of transplantation and obtained from the medical records of the patients. The criteria used to measure these variables were essentially the same ones used for the natural history “Mayo model” group. Almost all the histologic slides obtained from the hepatectomy specimen were reviewed and restaged by the same pathologic team, adopting the same histologic criteria (13). Values for the individual risk factors among the patients at the Pittsburgh facility and Mayo Clinic are listed in Table II. Follow-up evaluation was complete for all patients as of 30 June 1991.
TABLE II.
VALUES FOR MAYO MODEL VARIABLES IN 216 PRIMARY SCLEROSING CHOLANGITIS LIVER RECIPIENTS STRATIFIED ACCORDING TO THE CENTER OF TRANSPLANTATION
| Variable | University of Pittsburgh, n=158 |
Mayo Medical Center, n=58 |
p Value | |
|---|---|---|---|---|
| Age, yrs.* . . . . . . | 41.6±11.7 | 43.5±10.0 | 0.2 | |
| Bilirubin, mgm./dl.* | 14.1±13.4 | 11.2±11.5 | 0.06 | |
| Splenomegaly . . . . | 116 (73.4) | 21 (36.2) | <0.001 | |
| Histologic stage* . . | 3.7±0.6 | 3.9 ±0.2 | 0.002 | |
| 1 and 2 . . . . . . | 7 (4.4) | 0 (0) | ||
| 3 . . . . . . . . . . | 30 (19) | 3 (5.2) | ||
| 4 . . . . . . . . . . | 121 (76.6) | 55 (94.8) | ||
| Risk score* . . . . . | 5.1±0.9 | 4.9±0.9 | 0.2 | |
Mean±S.D.
Numbers in parentheses are percentages.
Statistical analysis
The comparison between the Pittsburgh group and patients receiving transplants at Mayo was done using the rank sum tests for continuous and the chi-square test for categorical prognostic variables. Survival time was defined as the time that elapsed from the initial OHTx until death or the date of last follow-up evaluation. All patients were included in the analysis, including those who required multiple transplants. Thirty-seven patients had undergone transplantation twice, seven received three grafts, two received four grafts and one patient received six grafts. When analyzing the effect of the immunosuppressive regimen on patient survival, we censored patients at the time of their change from one agent to the other.
The actual post-transplantation survival rate was estimated by the Kaplan-Meier product limit estimator (18). The predicted survival rate without transplantation was computed for each patient using the Mayo natural history model, along with the values of the four prognostic variables at the time of transplantation. The values for actual and model predicted survival rates were compared graphically and by means of a statistical test.
For the graphic method, the individual probabilities for predicted survival rate were averaged to produce a mean Mayo model survival curve to be compared with the Kaplan-Meier survival curve. For purposes of presentation, the averaged Mayo model survival probabilities were computed at only a few times after transplantation (three months, six months and at yearly intervals) and then interpolated linearly.
The difference between the survival predicted with the Mayo model and the actual survival was tested using the one sample log rank test (19). This test uses the Mayo model predicted survival curve for each patient as a mathematical control for that patient.
To assess the efficacy of OHTx for patients with different levels of disease severity, the 216 patients receiving OHTx were classified into three risk groups based upon the Mayo model risk score. The risk score cutoff values dividing the groups (4.8 and 5.4) were selected so that the three groups had roughly equal numbers of total deaths after transplantation. The low-risk group had 78 patients, with 15 deaths; the moderate risk group had 53 patients, with 15 deaths and the high-risk group had 85 patients, with 17 deaths. Within each risk group, the actual and predicted survival were compared using the one sample log rank test. The relation between the Mayo model risk score and the actual post-transplantation survival rate was assessed using the Cox proportional hazards regression procedure. As in the study of efficacy of OHTx for primary biliary cirrhosis (7), two analyses were used. One analysis examined the entire follow-up period and the other was restricted to the first three months after transplantation.
Because of the coexistence of other potential risk factors that were not included in the Mayo model, patients were subclassified according to the presence of inflammatory intestinal disease, history of pretransplant biliary or portal hypertensive, surgical treatment and detection of cholangiocarcinoma in the hepatectomy specimen. The effect of each of these risk factors on the actual survival rate was tested using log rank test (20). Cox regression, with the risk score as a covariate, was used to balance statistically the disease severity in these subgroups (8).
The influence of the available immunosuppressive agents on post-transplantation outcome was assessed by subclassifying the total population into three subgroups—patients who underwent transplantation before OKT3 was available (November 1984) and treated with cyclosporin, patients who underwent transplantation after OKT3 was used to treat severe rejection and received cyclosporin as the primary immunosuppressive agent and patients who were primarily treated from the outset with FK-506. The log rank test and Cox regression procedure were used to compare survival rates in these three groups. The differences in disease severity at baseline were controlled for by including the risk score from the Mayo model as a covariate.
Survival calculations and log rank tests were done by using the SAS procedure SURVDIFF and Cox regressions were calculated by using the SAS procedure PHGLM (21). For all of the statistical analyses used, two-tailed p values were reported.
RESULTS
Patients with PSC receiving OHTx at the Pittsburgh facility and Mayo Clinic were combined based upon a comparable risk score (p=0.159) and a similar actual (Kaplan-Meier) survival (p=0.365). The entire study population had more advanced disease than the patients used to build the natural history model (Table I). The mean value of serum bilirubin, incidence of splenomegaly and histologic stage of hepatic disease were substantially higher in the patients receiving transplantation at the time of operation compared with the natural history group at the time of referral. Consequently, the patients receiving transplantation had higher risk scores, with a mean value of 5.0±0.9 compared with 3.3±1.3 for the Mayo model data set group. However, because the range of the risk scores is similar in both groups, we are not extrapolating beyond the range of the model when we predict the survival of the patients receiving transplantation.
With a mean follow-up period of 34±25 months (range of zero to 104 months), 47 patients died after OHTx. Nineteen of these deaths occurred during the first three months postoperatively with fulminant sepsis or graft failure, or both, being the main causes of death. The actual post-transplantation Kaplan-Meier survival rate for the 216 patients with PSC and the averaged Mayo model expected survival rate are depicted in Figure 1. The two curves were very similar during the first three months after transplantation, a period during which the observed post-transplantation survival probability decreased sharply. The curves diverged later during the postoperative course. At six months after transplantation, the mean actual survival probability was 0.89±0.02 and the average Mayo model survival probability was 0.83. At one year, these estimates were 0.86±0.02 and 0.70, respectively. At five years, the difference substantially increased with a value of 0.73±0.04 for actual survival and 0.28 for expected survival. The one sample log rank test showed the difference between the overall actual survival after transplantation and the expected survival estimated by the Mayo model to have a high level of significance (chi-square equals 126.6; p<0.001).
Fig. 1.
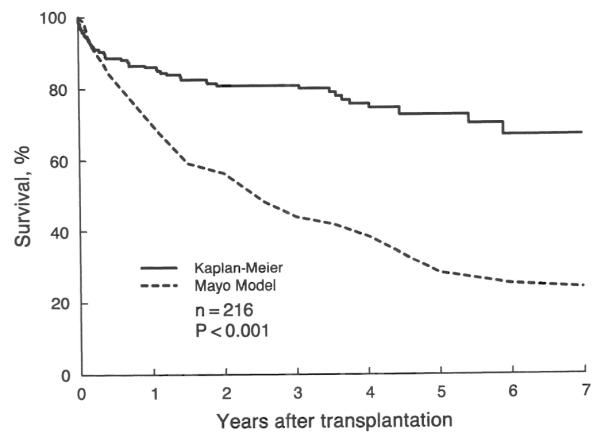
Actual (Kaplan-Meier) survival after transplantation in 216 patients with primary sclerosing cholangitis and their estimated survival without transplantation as predicted by the Mayo model “simulated control.”
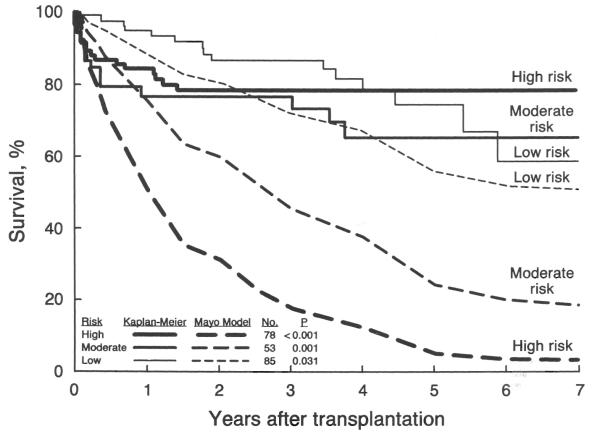
The values of the individual risk factors for each of the three risk groups are listed in Table III. The actual (Kaplan-Meier) survival rate and the average survival rate based on the Mayo model for each of the risk groups are illustrated in Figure 2. At each risk level, the actual survival rate after transplantation was significantly better than the survival rate predicted by the Mayo model, with a p value of 0.031 for the low, 0.001 for the moderate and less than 0.001 for the high-risk group.
TABLE III.
VALUES FOR MAYO MODEL VARIABLES IN 216 PATIENTS WITH PRIMARY SCLEROSING CHOLANGITIS CLASSIFIED IN RISK SUBGROUPS
| Variable | Low, n=78 | Moderate, n=53 | High, n=85 | |
|---|---|---|---|---|
| Age, yrs.* . . . . . . . | 36.3±9.8 | 43.0±12 | 46.8±9.7 | |
| Bilirubin, mgm./dl.* . | 6.2±5.4 | 12.7±13.9 | 20.2±13.8 | |
| Splenomegaly . . . . . | 29 (37.2) | 35 (66) | 73 (85.9) | |
| Histologic stage* . . . | 3.6±0.7 | 3.7±0.6 | 4.0±0.2 | |
| 1 and 2 . . . . . . . | 5 (6.4) | 2 (3.8) | 0 (0) | |
| 3 . . . . . . . . . . . | 21 (26.9) | 10 (18.9) | 2 (2.4) | |
| 4 . . . . . . . . . . . | 52 (66.7) | 41 (77.3) | 83 (97.6) | |
| Risk score* . . . . . . | 4.1±0.6 | 5.1±0.2 | 5.9±0.4 | |
Mean ±S.D.
Numbers in parentheses are percentages.
Fig. 2.
Actual (Kaplan-Meier) survival after transplantation in three risk groups of patients with primary sclerosing cholangitis and the estimated survival without transplantation as predicted by the Mayo natural history model. These risk groups were formed on the basis of pretransplantation Mayo-model risk scores with a cutoff value of 4.8 for the low risk and 5.4 for the moderate risk group.
Even patients at low risk, with better chance of survival with no treatment according to Mayo model, still had better post-transplantation survival (Fig. 2). The actual survival rate after transplantation, however, was not significantly different among the three risk groups (chi-square equals 2.7; p=0.259). A Cox analysis assessing the association of the risk score with the overall actual survival rate for each patient indicated that the Mayo model risk score was not a significant predictor of survival after transplantation (chi-square equals 1.6; p=0.202). However, when the analysis was restricted to the first 90 days after transplantation, the Mayo model risk score became a significant predictor of actual survival (chi-square equals 6.0; p=0.014).
The possible effect of the potential risk factors that are not included in the Mayo model on patient survival was also examined in this study. Patients with concomitant inflammatory intestinal disease or who underwent biliary or portal hypertensive, or both, operation, tended to have a less favorable survival after OHTx (Figs. 3 and 4, respectively). However, these reductions in the actual survival rate were not statistically significant (for inflammatory intestinal disease, p=0.117; for biliary and portal hypertensive operation, p=0.148). These findings remained nonsignificant even after adjustment for the risk scores.
Fig. 3.
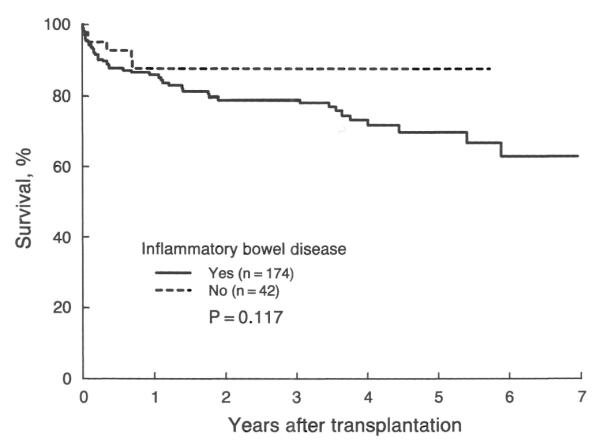
Kaplan-Meier actual survival after transplantation in patients with primary sclerosing cholangitis with and without inflammatory intestinal disease. The actual survival rate at five year is 0.70±0.09 and 0.88±0.05 for each subgroup, respectively.
Fig. 4.
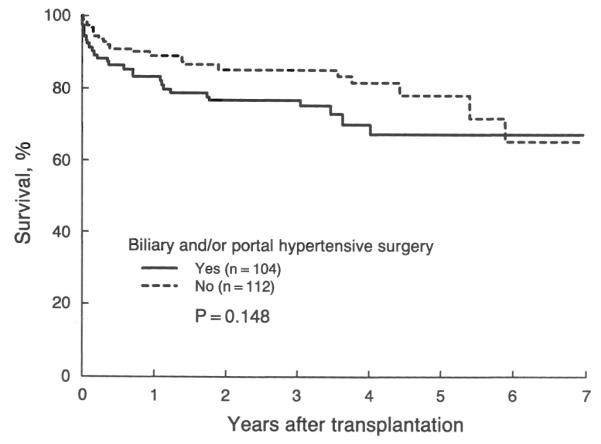
Actual (Kaplan-Meier) survival after transplantation in patients with primary sclerosing cholangitis with and without a history of biliary and portal hypertensive operation before transplantation. The actual survival rate at five year is 0.67±0.06 and 0.78±0.05 for each subgroup, respectively.
The difference in the long term actual survival rate after transplantation between patients who had cholangiocarcinoma in the excised liver that was unsuspected preoperatively and those who had not had such complication is illustrated in Figure 5. Recurrence of the tumor was the cause of death in four of the five deaths that occurred among the patients with cholangiocarcinoma. Statistically, unsuspected cholangiocarcinoma was associated with a significant (chi-square equals 5.0; p=0.026) reduction in the actual survival rate after transplantation, even after adjustment for the risk scores (chi-square equals 3.7; p=0.054). Meanwhile, the cumulative survival rate measured by the Kaplan-Meier method was similar (chi-square equals 2.7; p=0.103) to that predicted by the Mayo model (Fig. 6).
Fig. 5.
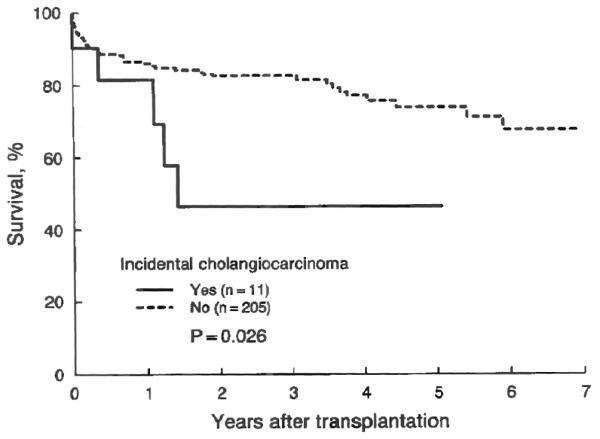
Kaplan-Meier survival plot after transplantation in patients with primary sclerosing cholangitis with and without detection of cholangiocarcinoma in the excised liver. The difference was statistically significant, with a five-year survival rate of 0.47±0.17 and 0.75±0.04, respectively.
Fig. 6.
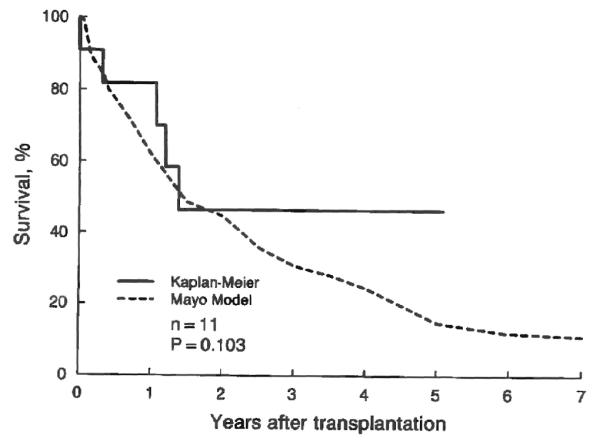
Actual (Kaplan-Meier) survival after transplantation in patients with primary sclerosing cholangitis who had incidental cholangiocarcinoma and the estimated (Mayo Model) survival rate without transplantation. The three year survival rate is 0.47±0.17 and 0.31, respectively.
There was evidence of an improvement in outcome over time. The 32 recipients of OHTx who received FK-506 after March 1989 had better one year actual survival rates (0.97±0.03) compared with the 184 patients who were treated with cyclosporin (0.84±0.03) since December 1981. In the group receiving cyclosporin, the 159 patients who underwent transplantation after the introduction of OKT3 (November 1984) also had better one year Kaplan-Meier survival rates (0.86±0.03) compared with the 25 patients who received OHTx before that time (0.72±0.09). The difference in the overall survival rate between the three groups was not significant (chi-square equals 4.9; p=0.086) (Fig. 7). Because there was no evidence of a change in the level of the risk score for the patients receiving transplantation since 1981 (Fig. 8), this improvement most likely reflects the use of more effective immunosuppressive agents in addition to the improved medical technologic factors and increased surgical, as well as clinical, experience.
Fig. 7.
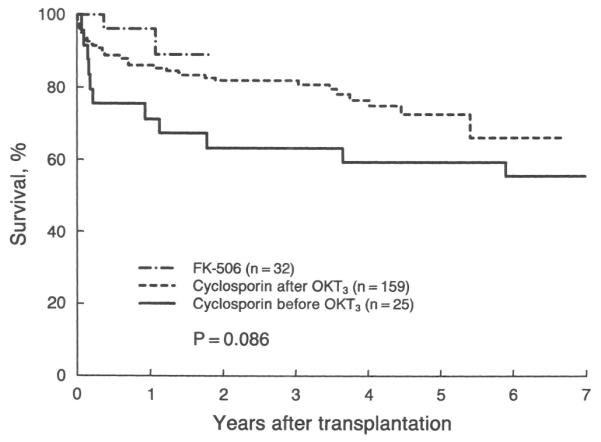
Actual (Kaplan-Meier) survival after transplantation in patients with primary sclerosing cholangitis subclassified according to the used immunosuppressive regimen.
Fig. 8.
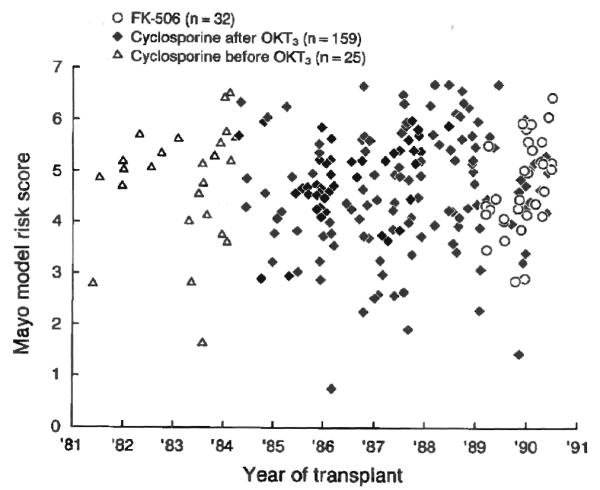
Scatter plots for the Mayo model risk score values of the patients with primary sclerosing cholangitis who received transplantation each year among the three immunosuppressive groups.
At the last follow-up evaluation, the functional status of each patient was assessed according to the classification system of the United Network for Organ Sharing. Information on functional status was available for all of the 169 patients with PSC receiving OHTx who were alive at that date. Of these, 145 were working full time, 20 were working part time, three were homebound and unable to work and one patient was hospitalized.
DISCUSSION
Different mathematical models have been made and proven useful in predicting survival of conservatively treated patients with different types of hepatic diseases (22-27). In patients with PSC, these models have used univariate (28-33) and multivariate (4, 34, 35) approaches. The Cox regression analysis has proved to be a useful statistical method for identifying variables that have independent prognostic significance.
The multivariate technique to predict survival among patients with PSC was first used in 1987 (34). Such an attempt was followed by two other independent trials conducted at Mayo Clinic and King's College using a similar statistical approach (4, 35). In these two models, a risk score could be calculated and translated into a survival curve to estimate survival for the individual patient. Although the results of both studies provide some objective evidence with regard to disease severity and estimation of survival, neither one was cross-validated and the general application to the PSC patients remained unknown.
Recently, others (5) were able to refine and cross-validate the Mayo model by applying the Cox regression procedure to a geographically heterogenous population, which included an expanded pool of patients with PSC that were referred to five international medical centers, including King's College. It is a four variable model that includes age, total serum bilirubin level, presence or absence of splenomegaly and hepatic histologic stage. In addition to age, the histologic stage seems to be a common survival predictor in most studies (4, 5, 35). However, the invasive nature of the percutaneous needle biopsy of the liver with the possibility of sampling errors seems to be a limiting factor to the beneficial use of histologic stage for repeated and accurate estimation of predicted patient survival. The levels of serum bilirubin in these patients reflect not only the degree of parenchymal damage to the liver, but also the extent and type of biliary sclerosis characteristic of PSC. The development of biliary sludge and the occurrence of intermittent bacterial cholangitis can have a significant transient influence on serum bilirubin levels, which should be considered in each patient to avoid underestimation of the predicted survival. The enlargement of the spleen indicates the occurrence of significant portal hypertension.
Using the simulated control technique as estimated by the PSC Mayo model, the results of this study showed a statistically significant improvement in overall survival rate of patients with PSC after transplantation, despite the initial mortality related to operation and events in the perioperative period. The improvement survival rate at five years was greatest for the high and moderate risk groups, with a difference between actual and average predicted survival rate of 74 and 42 percent, respectively. Even in the lowest risk group, that is, those who had the best prognosis according to the model for survival with no treatment, there was a significant improvement in survival rate at five years of 18 percent. Such beneficial effect of OHTx has been previously documented in other noncontrolled studies (1-3).
The similarity between actual survival and Mayo model estimates of survival during the first three months after transplantation suggests that the perioperative mortality was related to the pretransplantation status of each patient, which was assessed by the Mayo model. This is statistically confirmed by the significant relationship between the Mayo model risk score and the three months' actual survival rate after transplantation. The crossing of the actual “Kaplan-Meier” survival curves for the three risk groups during the late postoperative course (Fig. 2) suggests that the relation between pretransplantation risk and post-transplantation survival is not long term.
There are some important limitations to applying the Mayo model to transplant recipients with PSC. First, the patients who received OHTx at Pittsburgh and Mayo Medical centers were at a substantially higher risk than the 392 patients of whom the Mayo model was constructed. Such high values of the individual risk factors and calculated risk score probably reflect the later stage of the illness of the patient receiving transplantation. Because the precision of the model decreases for patients at higher risk of dying (5), the model may be less reliable in such high-risk patients, making it difficult to assess the improvement in the survival after transplantation.
A second limitation concerns the risk of cholangiocarcinoma among patients with PSC (36-40). The actual survival of the patients who had undergone transplantation and were found to have cholangiocarcinoma in the excised liver was similar to the estimated survival predicted by the Mayo model. The failure to achieve significant improvement in survival among these patients may be the result of the limited benefit of hepatic replacement in these patients or the overestimation of their predicted survival, or both, because the calculated risk score for the Mayo model did not and could not include a component for such undiagnosed “incidental” cholangiocarcinoma. As the diagnostic ability to detect early cholangiocarcinoma is improved, this refinement may influence decisions for OHTx in such patients.
A third limitation is the existence of other risk factors that are not reflected in the Mayo model risk score and could influence actual and predicted survival rates. Although not statistically significant, a history of inflammatory intestinal disease and pretransplant biliary or portal hypertensive, or both, surgical treatment was associated with a less favorable actual survival rate after transplantation. Accordingly, the current Mayo model could overestimate the predicted survival rate of these patients. However, the Mayo model predicted survival rates in patients who had previous biliary drainage could be underestimated because of the expected reduction in serum bilirubin level after such palliative treatment. However, the current group of patients who had a history of surgical, endoscopic or roentgenologic, or both, drainage of the biliary tract showed higher (p=0.001) serum bilirubin levels at the time of transplantation (16.6±13.7 milligrams per deciliter) compared with those who did not receive such therapy (11.6±12.4 milligrams per deciliter).
A final limitation is that bias would have been introduced into this evaluation of the effect of transplantation if the patients receiving transplantation uniformly had had a worse or a better prognosis than the patients in the Mayo model, even after adjustment for differences in severity of disease as measured by the risk score. Because the disease was at a more advanced stage in the patients receiving transplantation, the prognosis could have remained worse after adjustment for risk, leading to an underestimate of the benefit of transplantation. However, they could have been in better condition than the patients in the Mayo model after adjustment for risk, because of the careful screening performed when the patients were selected for an extensive surgical procedure. This would have led to an overestimation of the transplantation benefit. In our judgment, if bias did exist, the net tendency was toward underestimating the true benefit of transplantation.
SUMMARY
OHTx substantially improves the long term survival rate in patients with PSC who are at a wide variety of risk levels as measured by the Mayo model. The results reported herein may underestimate the improvement in survival to be expected in the future because post-transplantation survival rates have improved with advanced medical technologic factors, greater surgical experience, better postoperative care and new more effective immunosuppressive agents, such as OKT3 and FK-506. Of the patients who survive transplantation, 98 percent return to work at least part time.
Acknowledgments
Aided by Research Grants from the Veterans Administration and from the National Institute of Health, Bethesda, Maryland.
REFERENCES
- 1.Gordon RD, Iwatsuki S, Tzakis AG, et al. The Denver-Pittsburgh liver transplant series. In: Terasaki PL, editor. Clinical Transplants. University of California Los Angeles Tissue Typing Laboratory; Los Angeles: 1987. pp. 43–50. [PMC free article] [PubMed] [Google Scholar]
- 2.Marsh JW, Iwatsuki S, Makowka L, et al. Orthotopic liver transplantation for primary sclerosing cholangitis. Ann. Surg. 1988;207:21–25. doi: 10.1097/00000658-198801000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langnas AN, Grazi GL, Stratta RJ, et al. Primary sclerosing cholangitis: the emerging role for liver transplantation. Am. J. Gastroenterol. 1990;85:1136–1141. [PubMed] [Google Scholar]
- 4.Wiesner RH, Grambsch PM, Dickson ER, et al. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology. 1989;10:430–436. doi: 10.1002/hep.1840100406. [DOI] [PubMed] [Google Scholar]
- 5.Dickson ER, Murtaugh PA, Grambsch PM, et al. Primary sclerosing cholangitis: refinement and validation of survival model. Gastroenterology. 1992;103:1893–1901. doi: 10.1016/0016-5085(92)91449-e. [DOI] [PubMed] [Google Scholar]
- 6.Dickson ER, Grambsch PM, Fleming TR, et al. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology. 1989;10:1–7. doi: 10.1002/hep.1840100102. [DOI] [PubMed] [Google Scholar]
- 7.Markus BH, Dickson ER, Grambsch PM. Efficacy of liver transplantation in patients with primary biliary cirrhosis. N. Engl. J. Med. 1989;320:1709–1713. doi: 10.1056/NEJM198906293202602. [DOI] [PubMed] [Google Scholar]
- 8.Cox DR. Regression model and life-table. J. R. Stat. Soc. 1972;34:187–202. [Google Scholar]
- 9.Christensen E. Multivariate survival analyses using Cox's regression model. Hepatology. 1987;7:1346–1358. doi: 10.1002/hep.1840070628. [DOI] [PubMed] [Google Scholar]
- 10.MacCarty RL, LaRusso NF, Wiesner RH, et al. Primary sclerosing cholangitis: findings on cholangiography and pancreatography. Radiology. 1983;149:39–44. doi: 10.1148/radiology.149.1.6412283. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig J, MacCarty RL, LaRusso NF, et al. Intrahepatic cholangiectases and large-duct obliteration in primary sclerosing cholangitis. Hepatology. 1986;6:560–568. doi: 10.1002/hep.1840060403. [DOI] [PubMed] [Google Scholar]
- 12.Dickson ER, LaRusso NF, Wiesner RH. Primary sclerosing cholangitis. Hepatology. 1984;4:33–35. doi: 10.1002/hep.1840040711. [DOI] [PubMed] [Google Scholar]
- 13.Ludwig J, Barham SS, LaRusso NF, et al. Morphologic features of chronic hepatitis associated with primary sclerosing cholangitis and chronic ulcerative colitis. Hepatology. 1981;1:632–640. doi: 10.1002/hep.1840010612. [DOI] [PubMed] [Google Scholar]
- 14.Picard RR, Berk KN. Data splitting. J. Am. Stat. Assoc. 1990;44:140–147. [Google Scholar]
- 15.Efron B. The jackknife, the bootstrap, and other resampling plans. Society for Industrial and Applied Mathematics; Philadelphia: 1982. [Google Scholar]
- 16.Miller RG. The jackknife—a review. Biometrika. 1974;61:1–15. [Google Scholar]
- 17.Starzl TE, Todo S, Fung J, et al. FK-506 for human liver, kidney, and pancreas transplantation. Lancet. 1989;2:1000–1004. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958;53:457–481. [Google Scholar]
- 19.Harrington DP, Fleming TR. A class of rank test procedures for censored survival-data. Biometrika. 1982;69:553–566. [Google Scholar]
- 20.Mantel N, Haenszel W. Statistical aspect of the analysis of data from retrospective studies of disease. J. Natl. Cancer Instit. 1959;22:719–748. [PubMed] [Google Scholar]
- 21.Cary, editor. SAS Institute SAS User's Guide: Statistics, Version 5. SAS Institute; North Carolina: 1985. [Google Scholar]
- 22.Wiesner RH, Dickson ER, Krom RAF, et al. Estimating survival in patients with primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune chronic active hepatitis: applications to liver transplantation. Hepatology. 1985;5:1020. [Google Scholar]
- 23.Roll J, Boyer JL, Barry D, et al. The prognostic importance of clinical and histologic features in asymptomatic and symptomatic primary biliary cirrhosis. N. Engl. J. Med. 1983;308:1–7. doi: 10.1056/NEJM198301063080101. [DOI] [PubMed] [Google Scholar]
- 24.Newberger J, Altman DG, Christensen E, et al. Use of a prognostic index in evaluation of liver transplantation for primary biliary cirrhosis. Transplantation. 1986;41:713–716. doi: 10.1097/00007890-198606000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Christensen E, Newberger J, Crowl J, et al. Beneficial effects of azathioprine and prediction of prognosis in primary biliary cirrhosis. Gastroenterology. 1985;89:1084–1091. doi: 10.1016/0016-5085(85)90213-6. [DOI] [PubMed] [Google Scholar]
- 26.Grambsch PM, Dickson ER, Wiesner RH, et al. Application of the Mayo primary biliary cirrhosis survival model to Mayo liver transplant patients. Mayo Clin. Proc. 1989;64:699–704. doi: 10.1016/s0025-6196(12)65350-6. [DOI] [PubMed] [Google Scholar]
- 27.Bonsel GJ, Klopmaker IJ, VantVeer F, et al. Use of prognostic models for assessment of value of liver transplantation in primary biliary cirrhosis. Lancet. 1990;335:493–497. doi: 10.1016/0140-6736(90)90734-m. [DOI] [PubMed] [Google Scholar]
- 28.Gross JB, Ludwig J, Wiesner RH, et al. Abnormalities in tests of copper metabolism in primary sclerosing cholangitis. Gastroenterology. 1985;89:272–278. doi: 10.1016/0016-5085(85)90326-9. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson ML, Donaldson PT, Portmann BC, et al. Natural history of primary sclerosing cholangitis (PSC); HLA antigens as predictors of prognosis. Gut. 1987;28:1345–1346. [Google Scholar]
- 30.Lebovics E, Palmer M, Woo J, et al. Outcome of primary sclerosing cholangitis. Arch. Intern. Med. 1987;147:729–731. [PubMed] [Google Scholar]
- 31.Herrmann R, Dooley JS, Sherlock S, et al. Natural history and mortality in primary sclerosing cholangitis. Gut. 1988;29:1430. [Google Scholar]
- 32.Porayko MK, Wiesner RH, LaRusso NF, et al. Patients with a symptomatic primary sclerosing cholangitis frequently have progressive disease. Gastroenterology. 1990;98:1594–1602. doi: 10.1016/0016-5085(90)91096-o. [DOI] [PubMed] [Google Scholar]
- 33.Martin FM, Rossi RL, Nugent FW, et al. Surgical aspects of sclerosing cholangitis—results in 178 patients. Ann. Surg. 1990;212:551–558. doi: 10.1097/00000658-199010000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helzberg JH, Petersen JM, Boyer JL. Improved survival with primary sclerosing cholangitis: a review of clinicopathologic features and comparison of symptomatic and asymptomatic patients. Gastroenterology. 1987;92:1869–1875. doi: 10.1016/0016-5085(87)90618-4. [DOI] [PubMed] [Google Scholar]
- 35.Farrant JM, Hayllor KM, Wilkinson M, et al. Natural history and prognostic variables in primary sclerosing cholangitis. Gastroenterology. 1991;100:1710–1717. doi: 10.1016/0016-5085(91)90673-9. [DOI] [PubMed] [Google Scholar]
- 36.Thompson HH, Pitt HA, Tompkins RK, et al. Primary sclerosing cholangitis: a heterogenous disease. Ann. Surg. 1982;196:127–136. doi: 10.1097/00000658-198208000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wee A, Ludwig J, Coffey RJ, et al. Hepatobiliary carcinoma associated with primary sclerosing cholangitis and chronic ulcerative colitis. Humn. Pathol. 1985;16:719–726. doi: 10.1016/s0046-8177(85)80158-1. [DOI] [PubMed] [Google Scholar]
- 38.Mir-Madjlessi SH, Farmer RG, Sivak MV. Bile duct carcinoma in patients with ulcerative colitis. Dig. Dis. Sci. 1987;32:145–154. doi: 10.1007/BF01297102. [DOI] [PubMed] [Google Scholar]
- 39.Rosen CB, Nagorney DM, Wiesner RH, et al. Cholangiocarcinoma complicating primary sclerosing cholangitis. Ann. Surg. 1991;213:21–25. doi: 10.1097/00000658-199101000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abu-Elmagd KM, Selby R, Iwatsuki S, et al. Cholangiocarcinoma and primary sclerosing cholangitis: clinical characteristics and effect on survival after transplantation. Transplant. Proc. 1993;25:1124–1125. [PMC free article] [PubMed] [Google Scholar]