Abstract
This study evaluates the bond strength and related properties of photo-polymerizable, remineralizing amorphous calcium phosphate (ACP) polymeric composite-adhesive systems to dentin after various periods of aqueous aging at 37 °C. An experimental ACP base and lining composite was made from a photo-activated resin comprising 2,2-bis[p-(2’-hydroxy-3’-methacryloxypropoxy)phenyl]propane (Bis-GMA), triethylene glycol dimethacrylate (TEGDMA), 2-hydroxyethyl methacrylate (HEMA) and zirconyl dimethacrylate (ZrDMA); designated BTHZ. An experimental orthodontic composite was formulated from a photo-activated resin comprising ethoxylated bisphenol A dimethacrylate (EBPADMA), TEGDMA, HEMA and methacryloxyethyl phthalate (MEP); designated ETHM. In both composite series three fillers were compared: 1) freshly precipitated zirconium-modified ACP freshly precipitated (as-prepared Zr-ACP), 2) milled Zr-ACP and 3) an ion-leachable fluoride glass. In addition to the shear bond strength (SBS), work to fracture and failure modes of the orthodontic composites were determined. The SBS of the base and lining ACP composites appeared unaffected by filler type or immersion time. In the orthodontic ACP composite series, milled ACP composites showed initial mechanical advantages over as-prepared ACP composites, and produced higher incidence of a failure mode consistent with stronger adhesion. After six months of aqueous exposure, 80 % of specimens failed at the dentin-primer interface, with a 42 % overall reduction in bond strength. BTHZ and ETHM based ACP composites are potentially effective anti-demineralizing-remineralizing agents with possible clinical utility as protective base-liners and orthodontic cements, respectively. The analysis of the bond strength and failure modalities suggests that milled ACP composites may offer greater potential in clinical applications.
Keywords: amorphous calcium phosphate, photo-polymerizable composites, shear bond strength, dentin
1. Introduction
Calcium phosphate-based restoratives are generally appealing due to their biocompatibility. Specifically, amorphous calcium phosphate (ACP)-containing dental materials may provide an extended supply of Ca and PO4 ions needed to re-form damaged mineral structures, thus counteracting the recurrent decay that is known to develop near the surfaces of teeth in contact with conventional fillings (almost 50 % of all dental fillings require replacement because of recurrent caries). Such cariostatic ACP composites may be particularly useful for patients who are susceptible to caries as a result of radiation therapy and diseases or medications that cause dry mouth. When embedded in polymerized methacrylate matrices and exposed to an aqueous environment, ACP releases sufficient remineralizing Ca and PO4 ions in a sustained manner to promote redeposition of thermodynamically stable, apatitic tooth mineral [1, 2].
A problem with dental composites of all types is their inability to resist cracking under masticatory stress due to their relatively low strength and toughness. In the case of ACP composites the uncontrolled aggregation of ACP particles was identified as one of the main reasons for a poor interfacial interaction with dental resins that contributes to the mechanical instability of these materials [3] and their inferiority when compared with silanized glass-reinforced composites. To overcome this shortcoming we have focused on developing strategies for improving the ACP filler/polymer matrix interfacial properties (and, in turn, composite properties) by better controlling the particle size distribution and surface properties of ACP fillers or by fine-tuning the resin [4–8]. Formulating ACP base and lining material and developing ACP adhesive cements capable of minimizing the demineralization that frequently occurs under orthodontic brackets are major goals of this ongoing research. Towards this end, extensive physicochemical studies have been and continue to be performed in our group on various ACP polymeric composites.
In this study, we report on the adhesive properties of experimental, photo-polymerizable ACP composites formulated for base and lining and orthodontic applications. The first working hypothesis was that bioactive, hybridized ACP filler, when used in composites, will not adversely affect the strength of the composite-adhesive-dentin bond in comparison with similar glass-filled composites based on the same resins after various periods of aqueous immersion. Both as-prepared and milled ACP composites were used in this study to assess the possible correlation between the ACP particle size and state of agglomeration and the composite adhesive properties. The second working hypothesis was that the improvements offered by milling ACP will increase the uniformity at the adhesive-composite interface, reduce the incorporation of flaws and aid maintenance of the bond strength over longer periods of aqueous immersion. The work to fracture and failure modality of de-bonded composites also were utilized to differentiate the more subtle variations in mechanical and fracture behavior of these composites.
2. Materials and Methods
2.1. Resin formulations
Two matrix resins were formulated from commercially available monomers (Esstech, Essington, PA, USA). The chemical nomenclature and the corresponding acronyms used here of the resin and photo-initiator system components are listed in Table 1. The compositions of the resins are provided in Table 2. Both BTHZ and ETHM resin were photo-activated by the addition of CQ and 4EDMAB (Aldrich Chemical Company, Milwaukee, WI, USA) as photo-activator and photo-reductant components, respectively, of the photo-initiator system.
Table 1.
Monomers and components of photo-initiator system employed in the study.
| Chemical name | Acronym | Type or function |
|---|---|---|
| 2,2-Bis[p-(2’-hydroxy-3’- methacryloxypropoxy)phenyl]propane Ethoxylated bisphenol A dimethacrylate |
Bis-GMA EBPADMA |
Base monomer |
| Triethylene glycol dimethacrylate | TEGDMA | Diluent monomer |
| 2-Hydroxyethyl methacrylate Methacryloxyethyl phthalate Zirconyl dimethacrylate |
HEMA MEP ZrDMA |
Surface active monomer |
| Camphorquinone | CQ | Photo-activator |
| Ethyl-4-N,N-dimethylaminobenzoate | 4EDMAB | Photo-reductant |
Table 2.
Composition (mass fraction, %) of the resins evaluated in the study.
| Component | BTHZ* resin | ETHM** resin |
|---|---|---|
| Bis-GMA | 35.5 | - |
| EBPADMA | - | 62.8 |
| TEGDMA | 35.5 | 23.2 |
| HEMA | 27.0 | 10.4 |
| ZrDMA | 1.0 | - |
| MEP | - | 2.6 |
| CQ | 0.2 | 0.2 |
| 4EDMAB | 0.8 | 0.8 |
Resin acronyms are obtained by combining the initial letter of the acronym for each monomeric constituent of the matrix.
2.2. Synthesis and physicochemical characterization of ACP fillers
ACP fillers were synthesized following a modified preparation protocol proposed by Eanes et al.[9].The hybridizing agent, ZrOCl2 was used to introduce zirconia into precipitating ACP at a mole fraction of 10 % based on Ca content. The resulting zirconia-hybridized filler is designated Zr-ACP. To avoid exposure to moisture that may lead to the premature conversion of ACP into crystalline apatite, the filler was kept over anhydrous calcium sulfate under vacuum (2.7 kPa) until used in composite formulation (as-prepared Zr-ACP) or after being milled (milled Zr-ACP). The amorphous state of the as-prepared Zr-ACP was verified by powder X-ray diffraction (XRD; Rigaku X-ray diffractometer; Rigaku-USA Inc., Danvers, MA, USA) and Fourier-transform spectroscopy (FTIR; Nicolet Magna-IR FTIR System 550, Nicolet Instrument Corporation, Madison, WI, USA). Wet ball-milling of Zr-ACP powder was performed as follows: 25 g Zr-ACP, 500 g high density ZrO2 grinding balls (diameter = 2 mm; Glen Mills Inc., Clifton, NJ, USA) and 150 mL analytical grade isopropanol (J.T. Baker, Phillipsburg, NJ, USA) were sealed in the grinding jar and milled for 2 h at 400 rpm with rotation direction being reversed every 15 min (programmable planetary mill PM100; Retch Inc., Newton, PA, USA). Milled Zr-ACP was separated from the grinding balls by sieving and the remaining isopropanol was evaporated in a vacuum oven (24 h at 70 °C). The dry milled powder was then screened by XRD and FTIR to verify that no detectable conversion to apatite had occurred during the milling process, examined by scanning electron microscopy (SEM), and its particle size distribution (PSD) was determined. Fluoride-releasing, strontium glass (Sr-glass) was used as received from the manufacturer (Caulk Dentsply, Milford, DE, USA). The particle size distribution (PSD) of the fillers was determined using laser obscuration concurrently with a computerized inspection system (CIS-100 Particle Size Analyzer; Ankersmid Ltd., Yokneam, Israel). Approximately 3 mg of filler was dispersed in 3 g of ETHM resin and the suspension briefly ground with a mortar and pestle to simulate the effect of hand spatulation. Five replicate measurements were performed for each filler type.
2.3. Preparation of Composites
The photo-polymerizable ACP composite pastes were made by hand-blending the BTHZ or ETHM resin (mass fraction 60 %) with as-prepared Zr-ACP and milled Zr-ACP (mass fraction 40 %). Almost double the amount of Sr-glass (mass fraction 75 %) was combined with the resins (mass fraction 25%) to achieve the same consistency of the glass-filled composite pastes. The homogenized pastes were kept under a moderate vacuum (2.7 kPa) overnight to reduce the air entrained during mixing.
2.4. Bonding Protocol
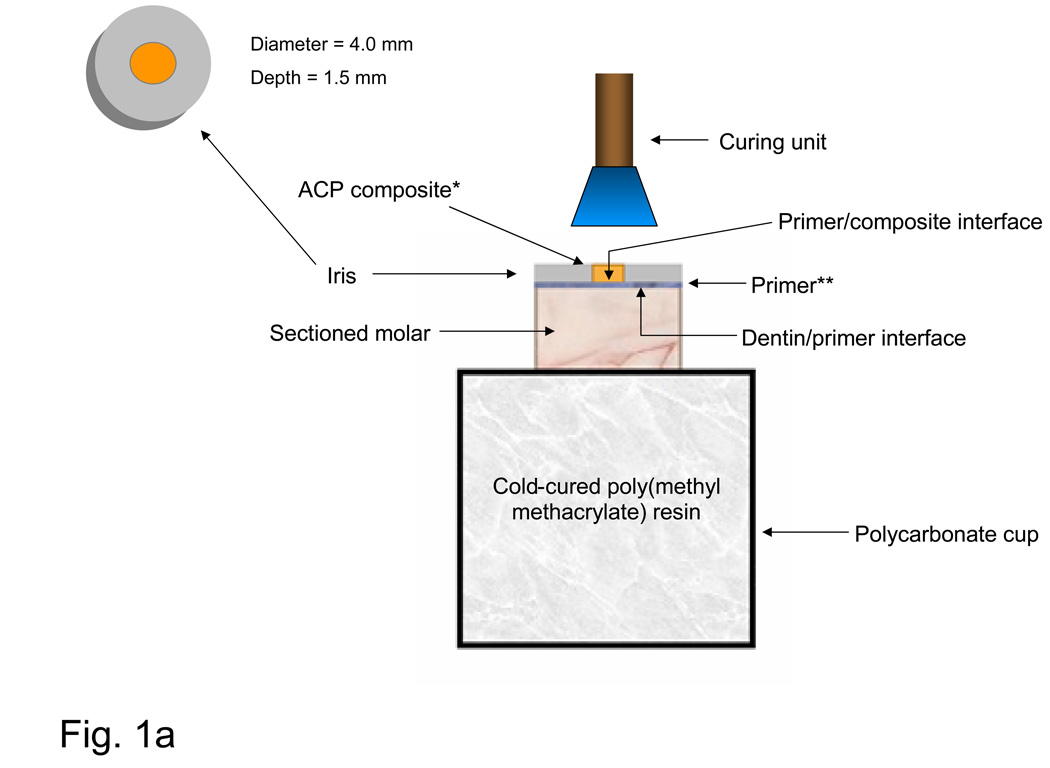
To test the shear bond strength (SBS) of the experimental composites to dentin through an adhesive system, the occlusal surfaces of extracted, caries-free, human molars were removed and their roots embedded in polycarbonate holders with chemical curing poly(methyl methacrylate) tray resin (Bosworth Fastray Powder and Liquid, Bosworth Company, Skokie, IL, USA). The exposed dentin surfaces were ground flat perpendicular to the longitudinal axis of the teeth with 320 grit silicon carbide paper (Fig. 1a). The bonding protocol included the following steps. Ground dentin surfaces were first dried, then etched for 15 s with phosphoric acid gel (mass fraction H3PO4 38 %; Etch-Rite®, Pulpdent Corporation, Watertown, MA, USA). The acid was rinsed away with distilled water for 10 s, and a moistened paper towel (Kimwipes®; Kimberly-Clark Global Sales, Inc., Roswell, GA, USA) was used to blot the surface to a near-dry condition. Two protocols were used to prime the moist dentin surfaces. In the ACP base-lining composite series, dentin surfaces were sequentially primed first with N-phenylglycine (NPG; mass fraction 5 %) solution in acetone for 30 s, and then with five consecutive coats of pyromellitic glycerol dimethacrylate (PMGDMA; mass fraction 20 %) in acetone solution and camphorquinone (CQ; mass fraction 0.028 %) as photo-activator. In the ACP orthodontic composite series, only one coating of PMGDMA-acetone primer (DenTASTIC UNO, Pulpdent Corporation, Watertown, MA, USA) was applied. Following the application of NPG and PMGDMA, or PMGDMA alone, the surfaces were air-dried for 10 s to remove acetone and visible-light cured for 10 s (Spectrum Curing Light, Dentsply Caulk Limited, Milford, DE USA). A poly(tetrafluoroethylene) (PTFE)-coated iris (4 mm in diameter and 1.5 mm thick) that defined the bonding area was positioned on the tooth surface, filled by the experimental composite and light-cured for 20 s for the experimental base-liner composites and 60 s for the orthodontic composites. ACP base-liner specimens were completed by applying a commercial resin-based composite (TPH, Dentsply Caulk, Milford, DE, USA), which was cured for an additional 60 s. The assemblies were then exposed to air for 5 min to allow further dry-curing at room temperature, after which they were immersed at 37 °C in either distilled water (ACP base-liner series) or a saliva-like solution [10] (orthodontic ACP series) for up to 6 months.
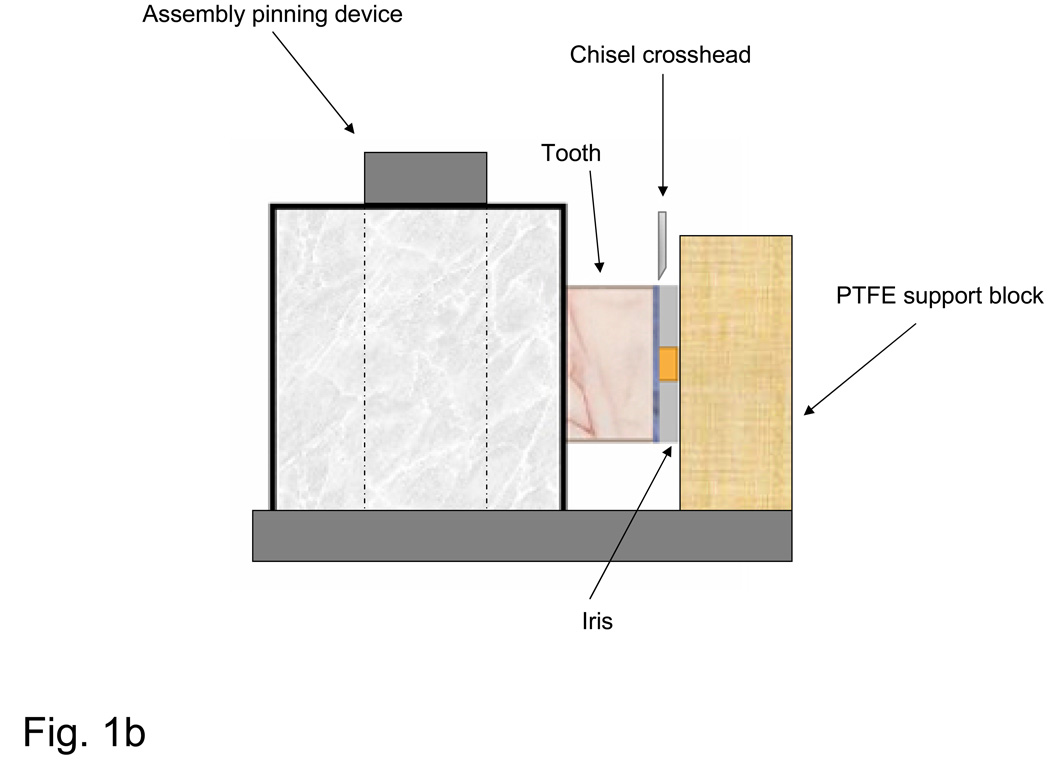
Fig. 1.
Schematic of bonding protocol (a) and the shear bond strength measurement assembly (b). *ACP base-lining composite plus TPH or orthodontic ACP composite alone: **NPG+PMDMA (base-lining ACP composite specimens) or PMDMA only (orthdodontic ACP composite specimens).
2.5. Shear Bond Strength (SBS) Measurements
De-bonding was performed by using the shear test method [11] with a computer-controlled universal testing machine (Instron 5500R, Instron Corp., Canton, MA, USA). Specimens were placed in the test apparatus and fixed such that their iris surface was flush against the PTFE support block (Fig. 1b). The SBS was determined normal to the longitudinal axis of the tooth, at a crosshead speed of 0.5 mm/min. It was calculated according to the following equation:
| (1) |
where P = applied load at failure and D = diameter of the cured composite as defined by the inner diameter of the iris.
2.6. Fracture Surface Evaluation
Initial qualitative assessments of specimen fracture surfaces (tooth and iris) were made immediately after testing using a stereomicroscope at various magnifications (Leica StereoZoom 6, Leica Microsystems, Wetzlar, Germany), and any observations regarding the surface condition (i.e. irregularities, general mode of failure) were recorded. Tooth and iris surfaces were then digitally photographed (Leica MZ16 Optical Stereomicroscope, Wetzlar, Germany; QCapture Pro, QImaging Corporation, Surrey, British Columbia, Canada) and imaging software was used to measure the surface areas of different failure modes (ImageJ, National Institutes of Health, Bethesda, MD, USA).
2.7. Statistical Analysis
Experimental data were analyzed by ANOVA (α= 0.05). Significant differences between specific groups were determined by all pair-wise multiple comparisons (two-tail t-test; unequal variances). One standard deviation (SD) is given in this paper for comparative purposes as the estimated standard uncertainty of the measurements.
3. Results
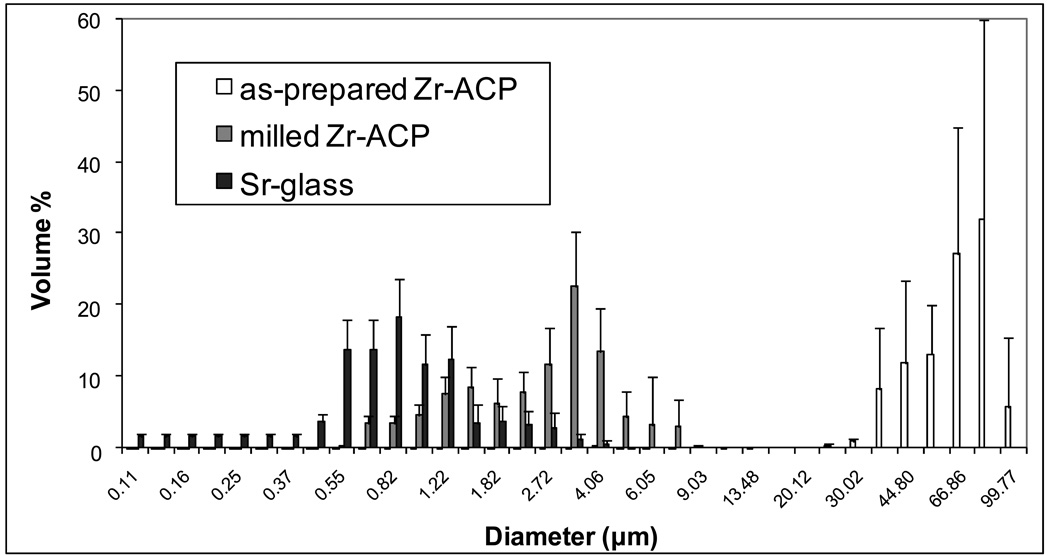
The amorphous character of the as-prepared and unmilled ACP fillers utilized in the study was verified by XRD and FTIR analysis (spectra not shown). Their XRD patterns consisted of two diffuse broad bands in 2θ = (4 to 60)° region, while the FTIR spectra showed wide absorbance bands typical for non-crystalline phosphates in the (1200 to 900) cm−1 and (630 to 500) cm−1 regions. PSD data (Fig. 2) indicate that the milling process reduced the median diameter (dm) of ACP by almost 95 %, i.e. from (58.23 ± 14.44) µm for as-prepared Zr-ACP to (3.10 ± 0.88) µm for milled Zr-ACP. The Sr-glass filler had a dm of (2.64 ± 0.86) µm and its volume distribution was close to that of the milled Zr-ACP.
Fig. 2.
Differential volume particle size distributions of as-prepared Zr-ACP, milled Zr-ACP and Sr-glass used in the study. Indicated values represent the means of five replicate runs in each group. Standard deviation (SD) is indicated by bars
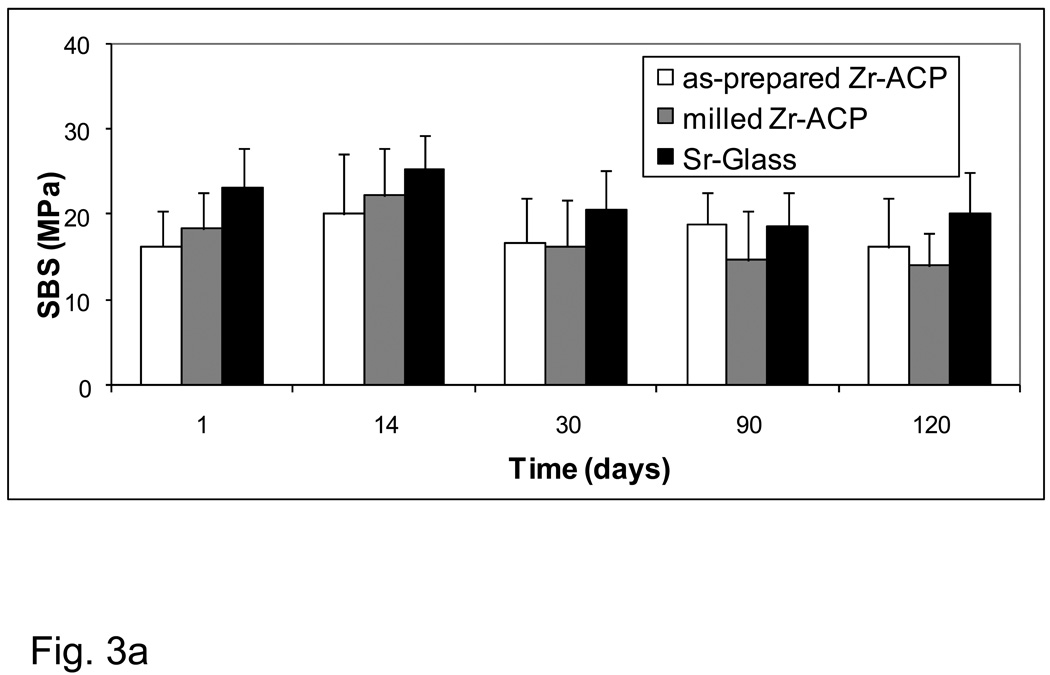
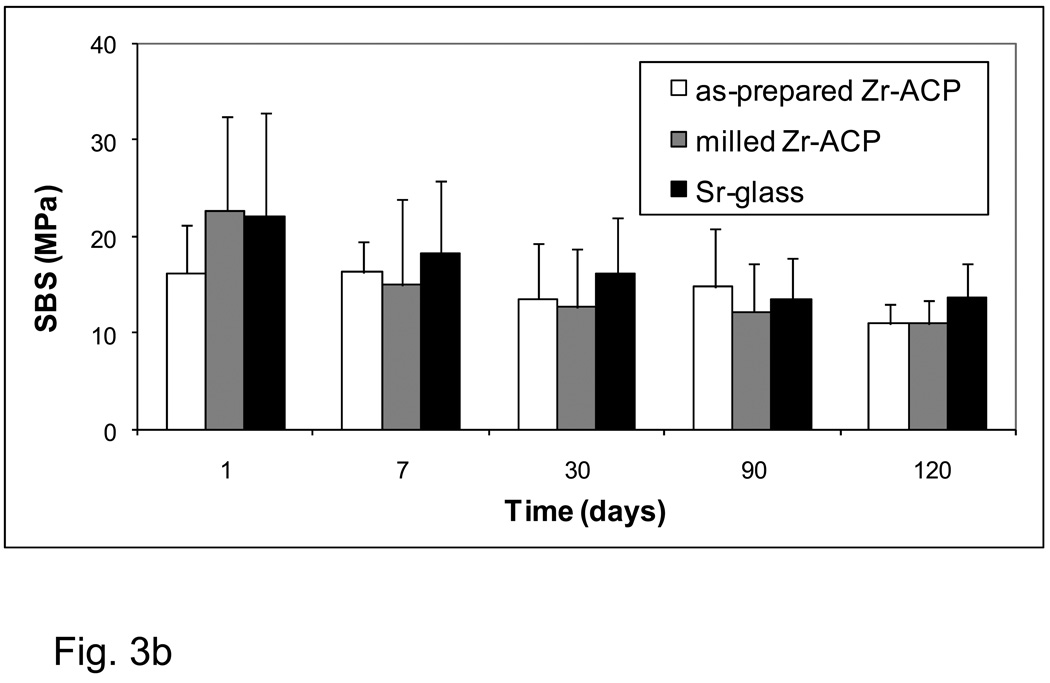
The aging-related changes in the SBS values of the experimental base-lining composites (BTHZ resin matrix; Fig. 3a) and of the experimental orthodontic composites (ETHM resin matrix; Fig. 3b) showed identical patterns. All-pair comparisons revealed stronger bonds for Sr-glass composite groups compared with both types of ACP composite only for the 24 h immersion data. At longer time intervals, the differences in mean values between the filler groups were not statistically significant.
Fig. 3.
Shear bond strength (SBS) of the base-lining experimental composites (a) and the orthodontic experimental composites (b) as a function of aging time. Indicated are the mean values + one standard deviation (SD) for the number of specimens n ≥ 7 (a) and n ≥ 5 (b).
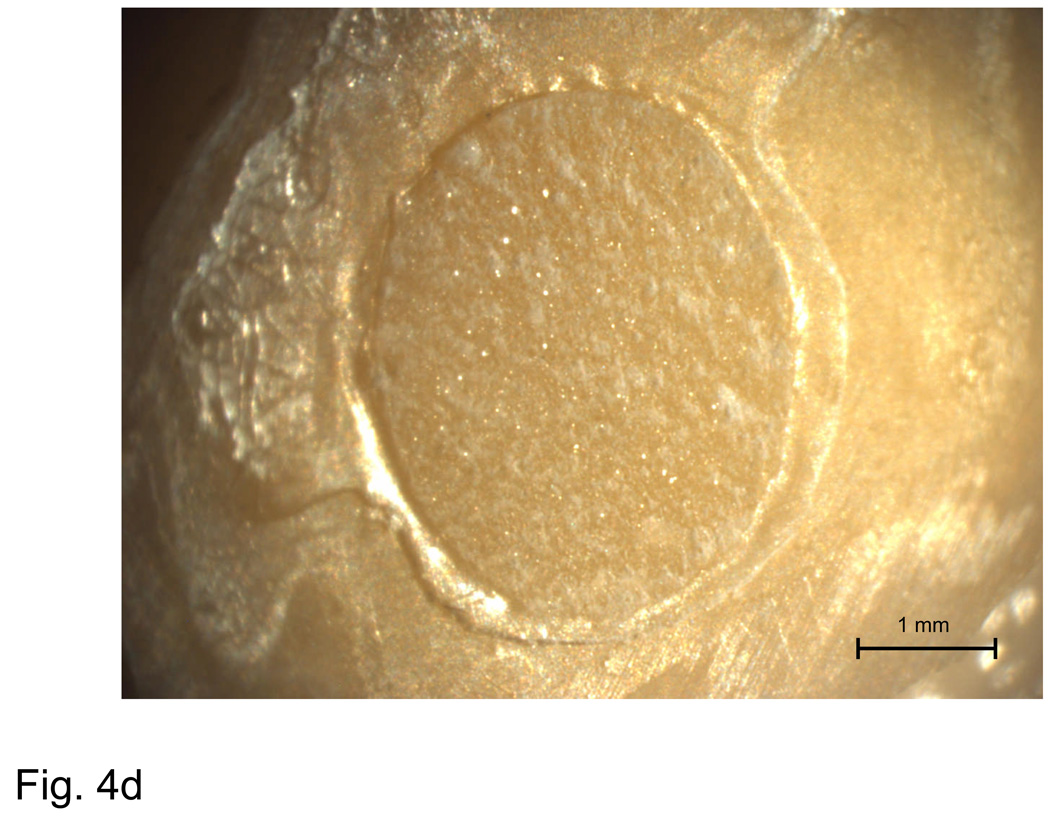
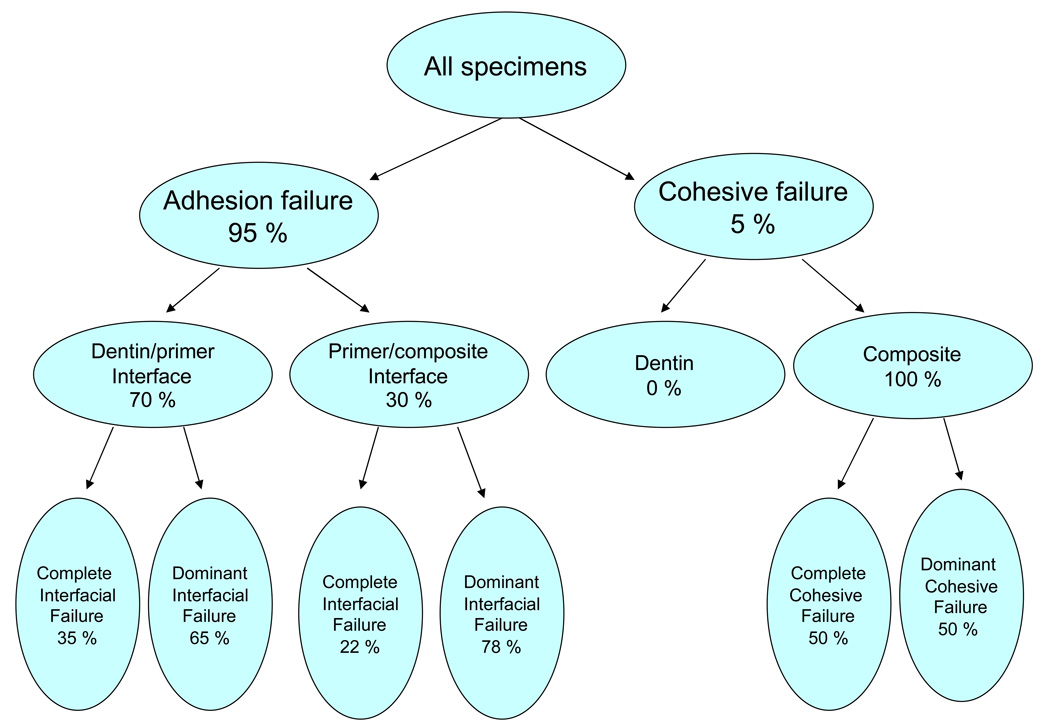
The failure analysis by optical microscopy of de-bonded specimens in base/lining composite group was performed on a limited number of specimens (8/group). After 2 weeks of water storage failures occurred predominantly at the dentin-adhesive resin interface regardless of the type of the filler. Further study of the fracture surfaces obtained upon de-bonding of the experimental orthodontic composites pointed to three distinct modes of failure: cohesive failure within the composite and adhesion failures at either the dentin-primer or primer-composite interface. Typical examples of different fracture surfaces are shown in Fig. 4a–d. The failure modes were broken down into two subcategories: complete, in which the fracture surface showed only composite or adhesive interface, and dominant, in which the fracture surface showed mixed adhesive and composite interfaces, with the majority occupied by composite or an adhesive interface. The distinction between complete and dominant was made in order to shed light on differences in mechanical behavior brought about by the transition from complete to mixed interfacial failures. A breakdown of the observed failure modes for the orthodontic composites is provided in Fig. 5.
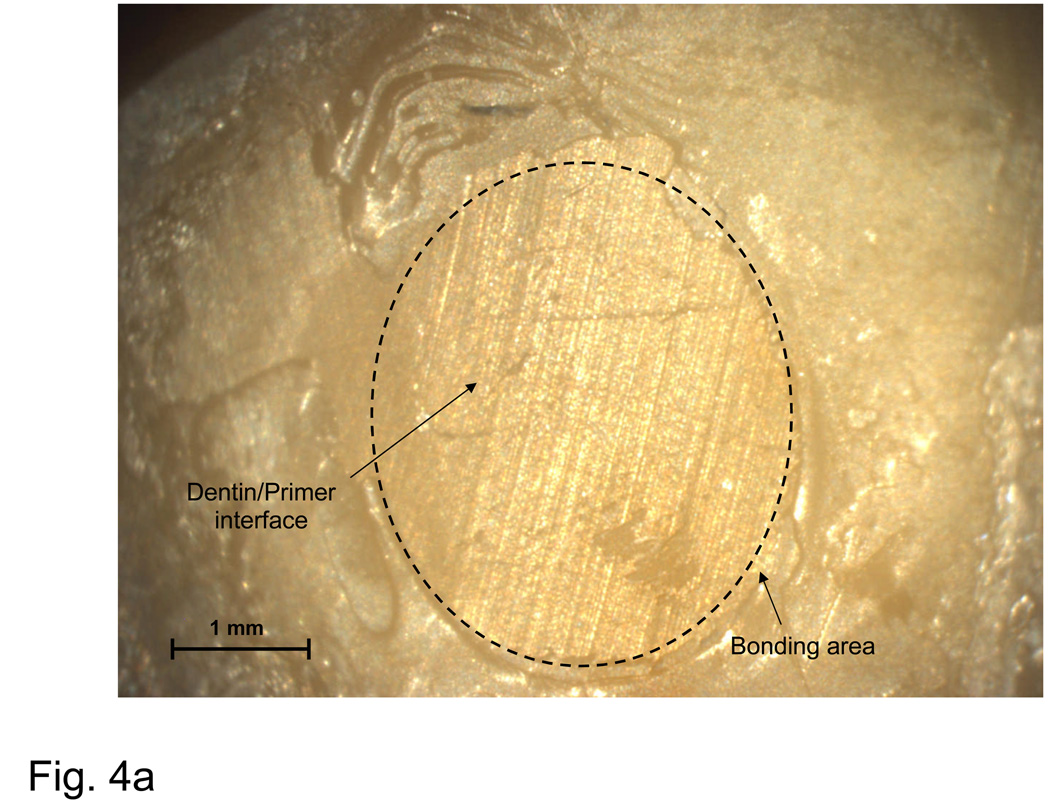
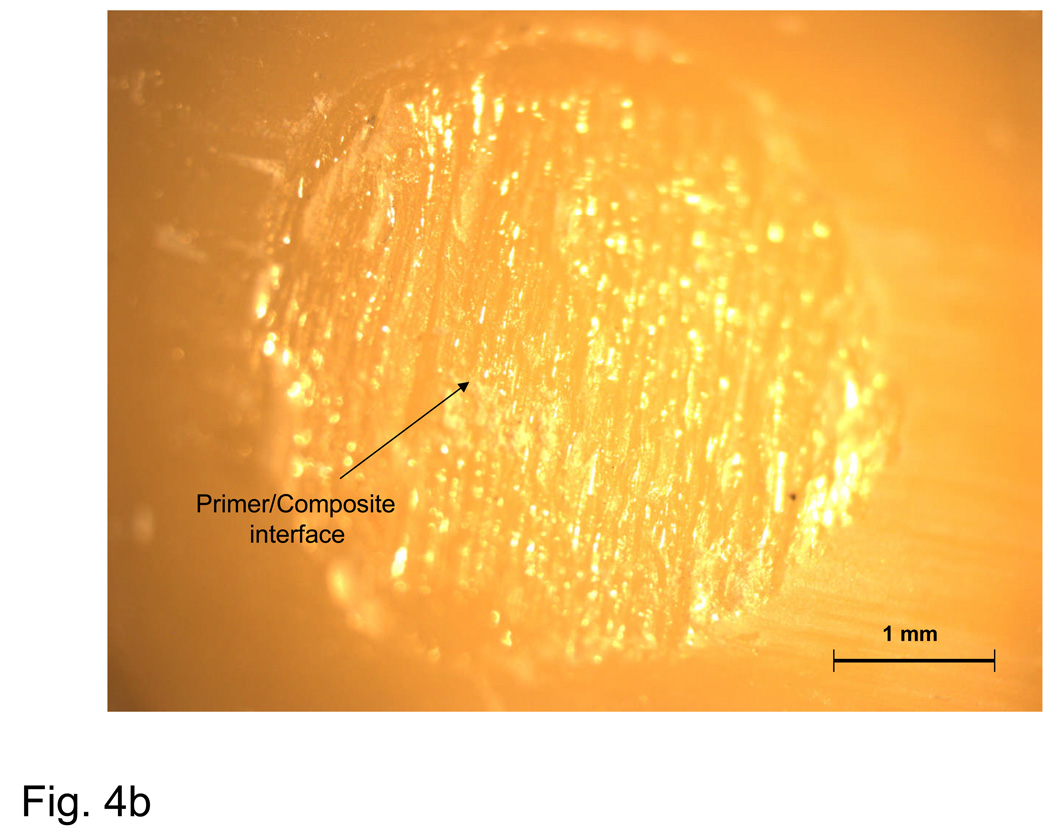
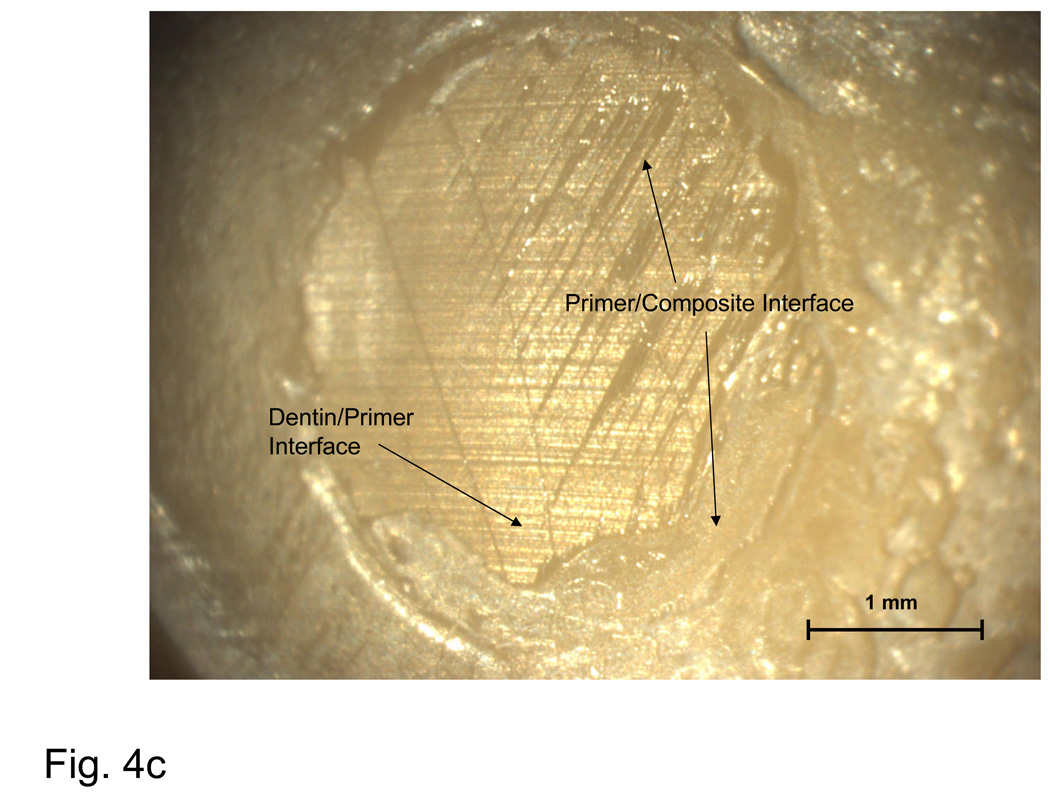
Fig. 4.
Typical examples of an adhesion failure at the dentin-primer interface (a), failure at the primer-composite interface (b), adhesion failure at both interfaces, as well as cohesive failure within the composite (c), and a cohesive failure within the composite (d).
Fig. 5.
A breakdown of the observed failure modes for the orthodontic composites (as-prepared Zr-ACP, milled ACP and Sr-glass formulations combined) indicating the percentage of specimens that fall under each category.
A fracture surface common to both complete and most dominant dentin-primer failures is illustrated in Fig. 4a. Regions of visible dentin are denoted as ‘dentin-primer interface’. The parallel grinding lines that continue outside of the dotted circle that delineates the bond area are referred to as a ‘peel layer’. Fig. 4b is typical of the primer-composite failure mode. In this type of failure, primer appears on the surface in the form of parallel or fanned striations, possibly indicating that the primer itself had failed cohesively. Striations are distinguishable from the grinding lines by their visibly raised surfaces (grinding lines are shallow enough that areas on which they occur appear flat). Aside from containing all three distinct failure modes, the bond area shown in Fig. 4c shows a region of flat, uniform primer surface suggestive of de-lamination at the interface and indicates poor adherence of the composite. Fig. 4d is representative of a complete cohesive failure, marked by the raised, irregular surface of the composite.
Dominant failure at the dentin-primer interface occurred in 43 % of all specimens. The corresponding complete interfacial failures accounted for 22 % of the observed fracture surfaces. Complete failure at the primer/composite interface accounted for less than 7 % of failures, and did not occur at every immersion time. The same is true of complete and dominant cohesive failures within the composite, which accounted for 5 % of the observed failures. However, cohesive failures occurred only for Sr-glass composites, with the SBS of complete cohesive failures consistently in the top 15 % of all treatments at corresponding immersion times.
4. Discussion
To obtain a dental resin with a tractable viscosity for composite formulation we have included into BTHZ formulation considerable amounts of the more flexible TEGDMA and HEMA (an adhesion-to-dentin-promoting co-monomer). The additive ZrDMA was included to improve inorganic-organic interface coupling. The fluid nature of BTHZ plus the intensified hydrogen bonding that occurs in matrices with relatively high HEMA contents (BTHZ resin contained mass fraction of 27 % HEMA) favor formation of highly-converted, densified polymeric matrices [12] that also may have an important role in determining the bonding properties of their composites.
We have shown [3, 5, 13] that as-prepared Zr-ACP, which is highly agglomerated, disperses unevenly throughout matrices regardless of the compositional makeup of the resin. The existence of large agglomerates in the resin leads to inadequate wetting of the filler and results in composite surface irregularities. Additionally, the ACP filler-matrix interface is predisposed to random spatial and other changes during water sorption as a result of continuous mineral ion release from composites. As a result, composites formulated with the as-prepared Zr-ACP generally exhibit lower strength and are more affected by water exposure than the corresponding unfilled copolymer resins [1]. In recent studies it was shown [14] that employing resin matrices based on the use of the more flexible, low viscosity, EBPADMA instead of the rigid, high viscosity, and somewhat more hydrophilic Bis-GMA along with milled Zr-ACP instead of as-prepared Zr-ACP, resulted in composites of improved mechanical stability, even under aqueous challenge. Contrary to that study, the SBS results reported here showed practically no improvement with the use of milled Zr-ACP filler.
It is generally accepted that, in addition to the chemical and physical bonding to the substrate, gradients in fracture toughness and elastic modulus across the bonded interface are most likely the governing factors in the joint strength and durability of the composite-dentin bond. Another factor may be the adhesion test methodology. The chisel-on-iris shear method used here may not be sensitive enough to detect subtle differences in the adhesiveness of the tested materials and, perhaps, the wire-loop-on-composite shear bond test, which would involve much higher stress concentrations, might be a more appropriate test to discriminate the adhesive properties of the composites. Uniaxial tensile test methods such as the micro-tensile method also should have greater sensitivity than shear methods in elucidating dentin adhesion. Although other factors, such as tooth-intrinsic flaws and the method of dentin pre-treatment, may have a decisive function in determining the strength of the composite-adhesive-dentin bond, the possible role of the filler type certainly cannot be ignored. However, the results of this study have shown that as-prepared and milled Zr-ACP, despite the flaws that as-prepared ACP filler may introduce into the microstructure of composites, did not adversely affect their short and mid-term bonding behavior to adhesive resins. Composites formulated with either type of Zr-ACP performed at least as well as Sr-glass-reinforced composites, while providing an additional bioactive component that may arrest demineralization and possibly promote remineralization of enamel and dentin. This study suggests that the experimental Zr-ACP-BTHZ resin composite may be a suitable lining or base material with the viscosity of a flowable composite and sufficient strength to serve as a base material [2]. The Zr-ACP-ETHM formulation may be well suited as a cement for orthodontic application, aiding in minimizing the demineralization of enamel often seen adjacent to and under the brackets.
Testing the SBS of strong adhesives commonly produces cohesive failures in dentin [15, 16], a failure mode not usually observed in clinical settings. In this study, the majority of failure modes were observed to occur at the interface between the primer and dentin substrate, and very rarely within the composite itself (indeed, never for milled ACP composites). This suggests strongly that some critical discrepancies exist between the test methods and the clinical situations they are intended to mimic. It follows, then, that an entirely new adhesion test needs to be developed or that existing methods need to be altered to improve their clinical relevance. Additionally, the high degree of scatter inherent in shear bond test methods is a problem that continues to require attention. The biological variability of the human species and anisotropic nature of the tooth itself both present significant obstacles to accurate, reliable measurements, and though widespread test standardization (loading mechanism, crosshead speed, etc.) may achieve some progress in this direction, altering the test method in the interest of clinical relevance might prove more beneficial in the long run. Therefore, modifying the SBS test method to include a fatigue component [17–20] or combined fatigue and modified-bond- geometry (e.g., micro-shear and micro-tensile) approach may be indicated for future studies.
5. Conclusions
The type of bioactive ACP filler and its particle size distribution did not significantly affect the strength of the composite-adhesive-dentin bond for these experimental, anti-demineralizing-remineralizing resin composites. Composites formulated with milled ACP produced a higher incidence of the failure mode consistent with stronger adhesion, thus offering a potential advantage in clinical bonding applications.
Acknowledgements
Reported work was supported by the National Institute of Dental and Craniofacial Research (NIDCR: grant DE 13169 to the American Dental Association Foundation (ADAF) and the National Institute of Standards and Technology (NIST)/NIDCR Interagency Agreement YI-DE-7005-01). It is a part of the dental material research program conducted by NIST in cooperation with ADAF and was also supported by both NIST and ADAF. Generous contribution of the monomers from Esstech, Essington, PA, USA, is gratefully acknowledged.
Footnotes
“Official contribution of the National Institute of Standards and Technology; not subject to copyright in the United States”
Publisher's Disclaimer: Disclaimer. Certain commercial materials and equipment are identified in this article to specify the experimental procedure. In no instance does such identification imply recommendation or endorsement by NIST or ADAF or that the material and equipment identified is necessarily the best available for the purpose.
References
- 1.Skrtic D, Antonucci JM, Eanes ED. J. Res. (Natl. Inst. Stands. Technol.) 2003;108:167. doi: 10.6028/jres.108.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonucci JM, Skrtic D. In: Polymers for Dental and Orthopedic Applications. Shalaby SW, Salz U, editors. Boca Raton, FL: CRC Press; 2007. pp. 217–242. [Google Scholar]
- 3.Skrtic D, Antonucci JM, Eanes ED, Eidelman N. Biomaterials. 2004;25:1141. doi: 10.1016/j.biomaterials.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Antonucci JM, Skrtic D, Hailer AW, Eanes ED. In: Polymeric Drugs & Drug Delivery Systems. Ottenbrite RM, Kim SW, editors. Lancaster, PA: Technomics Publ. Co.; 2000. pp. 301–310. [Google Scholar]
- 5.Antonucci JM, Skrtic D. J. Bioact. Compatible Polym. 2005;20:29. doi: 10.1177/0883911506064476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonucci JM, Liu DW, Skrtic D. J. Dispersion Sci. Technol. 2007;28:819. doi: 10.1080/01932690701346255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skrtic D, Antonucci JM, Eanes ED, Brunworth RT. J. Biomed. Mater. Res. 2002;59:597. doi: 10.1002/jbm.10017. [DOI] [PubMed] [Google Scholar]
- 8.Skrtic D, Antonucci JM, Liu DW. Acta Biomaterialia. 2006;2:85. doi: 10.1016/j.actbio.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eanes ED, Gillessen IH, Posner AS. Nature. 1965;208:365. doi: 10.1038/208365a0. [DOI] [PubMed] [Google Scholar]
- 10.Ten Cate JM, Duijsters PPE. J. Caries Res. 1982;16:201. doi: 10.1159/000260599. [DOI] [PubMed] [Google Scholar]
- 11.Venz S, Dickens B. J. Dental Res. 1993;72:582. doi: 10.1177/00220345930720030501. [DOI] [PubMed] [Google Scholar]
- 12.Skrtic D, Stansbury JW, Antonucci JM. Biomaterials. 2003;24:2443. doi: 10.1016/s0142-9612(02)00574-4. [DOI] [PubMed] [Google Scholar]
- 13.Lee SY, Regnault WF, Antonucci JM, Skrtic D. J. Biomed. Mater. Res. 2007;80B:11. doi: 10.1002/jbm.b.30561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skrtic D, Antonucci JM. J. Biomater. Appl. 2007;21:375. doi: 10.1177/0885328206064823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Versluis A, Tantbirojn D, Douglas WH. J. Dental Res. 1997;76:1298. doi: 10.1177/00220345970760061001. [DOI] [PubMed] [Google Scholar]
- 16.Pashley DH, Sano H, Ciucchi B, Yoshiyama M, Carvalho RM. J. Dental Mater. 1995;11:117. doi: 10.1016/0109-5641(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 17.Frankenberger R, Krämer N, Petschelt A. Clin. Oral Invest. 1999;3:11. doi: 10.1007/s007840050072. [DOI] [PubMed] [Google Scholar]
- 18.Frankenberger R, Strobel WO, Krämer N, Lohbauer U, Winterscheidt J, Winterscheidt B, Petschelt A. J. Biomed. Mater. Res. 2003;67B:712. doi: 10.1002/jbm.b.10052. [DOI] [PubMed] [Google Scholar]
- 19.Frankenberger R, Pashley DH, Reich SM, Lohbauer U, Petschelt A, Tay FR. Biomaterials. 2005;26:2043. doi: 10.1016/j.biomaterials.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Soappman MJ, Nazari A, Porter JA, Arola D. Dental Mater. 2007;23:608. doi: 10.1016/j.dental.2006.05.003. [DOI] [PubMed] [Google Scholar]