Abstract
Context: The kindred described is the only known instance of a germ line loss of function mutation of estrogen receptor (ER)-α.
Objective: Our objective was to assess the impact of a loss of function mutation in the ER-α gene on histomorphometry, bone volumetric density, bone geometry and skeletal growth, and ER-α heterozygosity on spine density and adult height in an extended pedigree.
Design and Participants: A longitudinal follow-up of the propositus with homozygous loss of function mutation of ER-α and single contact evaluation of the kindred were performed.
Main Outcome Measures: Iliac crest bone biopsy and peripheral quantitative computed tomography of propositus with serial measures of areal spine bone mineral density (aBMD) by dual-energy x-ray absorptiometry and bone age were performed. Members of pedigree were evaluated for ER-α mutation carrier status and spine aBMD.
Results: Bone biopsy revealed marked osteopenia (cortex: 641 μm), low trabecular volume (10.6%), decreased thickness (76.2 μm), normal trabecular number, and low activation frequency (0.099/yr). Radial periosteal circumference was similar, endosteal circumference larger, and trabecular and cortical volumetric bone mineral density markedly lower (158 and 1092 mg/cm3, respectively) than controls. Spine aBMD at age 28.5 yr (0.745 g/cm2) decreased to 0.684 g/cm2 (Z score −3.85) in 3.5 yr. Bone age advanced from 15–17.5 yr. Kindred analysis revealed that gene carriers had spine aBMD Z scores less than zero (P = 0.003), but carriers and nonmutant members were similar (−0.84 ± 0.26 vs. −0.64 ± 0.16).
Conclusion: Homozygous ER-α disruption markedly affects bone growth, mineral content, and structure but not periosteal circumference. ER-α heterozygosity appears to not impair spine aBMD.
A longitudinal study on bone parameters of a large population with a loss of function mutation in estrogen receptor alpha reveals that this disruption markedly affects bone growth, mineral content, and structure, but not periosteal circumference and areal spine bone mineral density.
Estrogen is well known to be important in females for reproductive function and bone growth and metabolism. However, evidence has emerged from rodent models (1) and rare disorders of estrogen production or response in humans (2,3) that the physiological role for estrogen is far more complicated, involving actions in males as well as females and responses in a surprising array of target tissues in both genders, distinct from bone and reproductive systems (4). Estrogen actions are further complicated by the presence of two distinct but related receptors, estrogen receptor (ER)-α (4) and β (5), both with different splice variants. In addition, complementary investigations focused on androgen synthesis and response have provoked a reexamination of the relative roles of androgen and estrogen in males and females (4,6). In this study the investigation of a large kindred with a loss of function mutation in ER-α afforded a unique opportunity to examine the effect of ER-α disruption on bone parameters.
Perhaps the most striking effects on the human skeleton of impaired estrogen action or synthesis in males are tall stature secondary to delayed epiphyseal fusion and osteopenia (2,3). Estrogen therapy in aromatase-deficient patients results in rapid epiphyseal fusion and marked improvement in bone mineral content (7,8,9,10,11,12). Although peripheral quantitative computed tomography (pQCT) assessment was performed in two of the aromatase deficiency case studies (11,12), there have been no histomorphometric analyses to this point. As a result, detailed structural knowledge of the consequences of profound estrogen deficiency in males is lacking. Clarification of estrogen action on the skeleton has been attempted by studies relating various markers of bone status to polymorphisms in ER-α (13). An association between ER-α and bone mineral density (BMD) has been observed, but, in general, studies of ER-α alleles in relation to areal spine bone mineral density (aBMD) have yielded inconsistent results. This study involving follow-up evaluation of the propositus with a unique loss of function mutation in ER-α, combined with an assessment of the impact on bone of carrier status in members of his extended kindred, attempts to provide further insight into the relative roles of androgen and estrogen on the skeleton.
Patients and Methods
Follow-up of propositus
As previously reported, the propositus at age 28.5 yr presented with tall stature, eunuchoid body proportions (upper segment to lower segment ratio, 0.88), severe genu valgum, acanthosis nigricans, Tanner V sexual maturity, and unfused epiphyses (3). Initial clinical evaluation revealed elevated insulin, glucose intolerance, increased estradiol, hypergonadotropism, and marked osteopenia. Sequencing of ER-α revealed a mutation in exon 2, cytosine-to-thymine transition at codon 157 of both alleles, resulting in a premature stop codon. Both parents were heterozygous carriers. At age 29.7 yr, an iliac crest biopsy was performed. After a 3-month period of healing, a correction of the left leg was performed, and a sample of distal femur bone was used to isolate bone marrow stromal cells (14). Subsequently, the propositus was periodically evaluated in our Clinical Research Center.
Patient recruitment
Family members were contacted and, after informed consent, were enrolled in the study. Vital signs, height, and weight measures were obtained as was a general medical history, which, in particular, detailed relevant reproductive, nutritional, and skeletal system histories. Subsequently, subjects had spine aBMD measurements by dual-energy x-ray absorptiometry (DXA), and blood was obtained for ER-α carrier status determination on lymphocyte DNA.
Iliac crest biopsy and histomorphometric analysis
At age 29.7 yr, the propositus received oral loading with tetracycline (500 mg orally twice daily on d −16 and −15) and Declomycin (Wyeth, Madison, NJ; 500 mg orally twice daily on d −4 and −3), followed by a left iliac crest bone biopsy (d 0). The biopsy specimen was treated with the Villanueva bone stain solution for 3 d, dehydrated in graded concentrations of alcohol, and embedded in methyl methacrylate. Three consecutive sections were cut at 10-μm thickness. Sections were then either counterstained with Goldner’s trichrome, von Kossa, or remained unstained. Quantitative static and dynamic histomorphometry was performed using the Osteomeasure Histomorphometry digitizing image analysis system (OsteoMetrics, Inc., Decatur, GA). Cortical thickness, tissue area, trabecular bone area and perimeter, osteoid length and thickness, eroded surface (ES), wall thickness, double-label and single-label lengths, and interlabeling distance were measured based on methods of Parfitt et al. (15).
DXA (Hologic QDR-2000; Hologic Inc., Waltham, MA) was used to determine aBMD of the spine, and pQCT (XCT-2000; Stratec, Pforzheim, Germany) was used to measure volumetric BMD (vBMD) and bone size parameters at the 4 and 20% distal radius. Slices were obtained at the 4 and 20% of the measured arm length from the distal radius, using a voxel size of 0.4 mm and scan speed of 30 mm/sec with a one-block rotation. Slices were analyzed using ContMode2, Peel Mode 2 and a threshold of 400 mg/cm3 to obtain trabecular density (4% site only). Cortical bone was identified at the 4% site, using CortMode 3 with an automatic threshold, and at the 20% site, using CortMode 1 and a density threshold of 710 mg/cm3. Cortical density at the 4% site was not determined due to partial volume effects with small cortical thicknesses. Cortical thicknesses and periosteal and endosteal circumferences were calculated using the circular ring procedure. Normative data for pQCT on men, aged 20–40 yr from an ongoing study, were obtained and analyzed in a similar fashion (16). Radiographs for assessment of bone maturation were performed at Cincinnati Children’s Hospital Medical Center. Bone age (BA) determinations were performed by comparison with standards of Greulich et al. (17). All studies not considered part of standard medical care were approved by the Cincinnati Children’s Hospital Institutional Review Board.
Cell culture and ER analysis
Establishment of the immortalized propositus cell line and subsequent culture were performed as previously described (14). Immortalized normal marrow stromal cell fibroblasts were obtained from a 28-yr-old female donor. For RT-PCR, mRNA was extracted from cultured cells and PCR amplified using the primers ER-β 3′ p8 CAG CAT CTC CAG CAG CAG GTC ATA C and ER-β 5′ P9 AAA TCT TTG ACA TGC TCC TGG C (95 C, 4 min; 62 C, 1 min; 72 C, 1 min; 94 C, 1 min; 29 cycles followed by a final extension for 72 C for 10 min). ER-β PCR product (362 bp) DNA was sequenced using a PerkinElmer Applied Biosystems 377 automated sequencer (Applied Biosystems, Foster City, CA).
Nuclear extracts of cells were resolved by 4–20% SDS-PAGE gradient gels, transferred to nitrocellulose in 25 mm Tris-base, 192 mm glycine 20% methanol, and probed with ER21 antibody directed toward the amino terminus of ER-α (gift of Geoffrey Greene, University of Chicago, Chicago, IL) or PAI-310 antibody directed against ER-β (Affinity BioReagents, Inc., Golden, CO). First antibody dilution was 1:2,000, and second antibody (goat antirabbit horseradish peroxidase; Pierce, Chicago, IL) dilution was 1:20,000. Pierce’s enhanced chemiluminescence reagents were used for detection.
Single-stranded conformational polymorphism analysis
All family members who provided consent were screened for ER-α mutation carrier status by using isotopic single-stranded conformational polymorphism analysis on DNA isolated from whole blood lymphocytes using a phenol-chloroform extraction method as previously described (3,18). The forward primer sequence for exon 2 was 5′-CCCAGGCCAAATTCAGATAA-3′, and the reverse primer sequence was 5′-CGTTTTCAACACACTATTAC-3′.
Statistical analysis
A Student’s t test was used to compare spine aBMD between patients who were heterozygotes or wild type for ER-α mutations.
Laboratory tests
Initial hormonal assays and urine bone metabolic markers were performed by Nichols Institute (San Juan Capistrano, CA). Subsequent reproductive hormone assays were performed by Endocrine Sciences (Burlington, NC) unless otherwise indicated.
Results
Longitudinal data on bone growth, aBMD, and serum hormonal concentrations
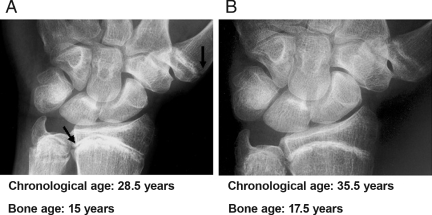
Initial BA, performed at age 28.5 yr, demonstrated a delayed bone maturation of 15 yr (Fig. 1, Panel A). As previously reported (3), serum hormone profile revealed total testosterone 445 ng/dl [normal range (nr) 265–800], estradiol 119 pg/ml (nr 10–50), FSH 33 mIU/ml (nr 2–15), LH 37 mIU/ml (nr 2–20), and IGF-I 528 ng/ml (nr 123–465). At the chronological age of 31.5 yr, BA was 15.5 yr (data not shown). At age 33.2 yr, repeat hormonal profile revealed estradiol 3.7 ng/dl (nr 0.8–3.5), total testosterone 364 ng/dl (nr 350-1030), FSH 28 mIU/ml (nr 2–9.2), and LH 9.4 mIU/ml (nr 1.5–9). At the time of last follow-up at age 35.5 yr, BA was 17.5 yr (Fig. 1, Panel B). Reproductive hormone levels were not obtained. During the period of follow-up, measured stature increased slightly (204 cm at age 28.5 yr to 206.2 cm at age 33.5 yr), although the presence of genu valgum and the subsequent orthopedic procedures on the both distal femurs limited the validity of this parameter. Arm span increased 3 cm (213 cm at age 28.5 yr to 216 cm at age 38.5 yr), but length of the hands and feet was unchanged. The breadth of the wrist at presentation was 7.2 cm [normal adult male (mean ± sd) 6.0 ± 0.5, adult female 5.3 ± 0.3], and cord of the arc of the radiocarpal joint was 4.6 cm (normal adult male 3.1 ± 0.2, normal adult female 2.7 ± 0.2). The width of the distal femurs was both approximately 130 mm by radiography and, based on an estimate of 15% magnification, was estimated to be 113 mm (normal adult male 90 ± 7, normal adult female 80 ± 7).
Figure 1.
Representative radiographs of the propositus. A, Radiograph of left wrist and hand performed at presentation, age 28.5 yr; BA is 15 yr, and open epiphyses are apparent (arrows). B, Radiograph of left wrist and hand performed at age 35.5 yr; BA is 17.5 yr, and the epiphyses are near complete closure.
Spine aBMD at age 28.5 yr was 0.745 g/cm2 and remained stable through age 33.2 yr at 0.759 g/cm2 (+1.8%) but decreased to 0.684 g/cm2 (Z score −3.85) at 35.5 yr (−9.8%). After initial diagnosis, a nutritional supplement of vitamin D (400 IU/d), calcium carbonate (400 mg elemental/d), and magnesium sulfate (240 mg elemental/d) was instituted, otherwise no specific bone-focused drug therapy was initiated. At age 34.5 yr, after a fall on icy pavement, a fracture of the right humerus was sustained that healed well without disability.
Markers of bone turnover, vBMD, and bone geometry
Between the ages of 28.5 and 30 yr, multiple blood samples were collected for monitoring bone turnover. Mean serum osteocalcin concentrations were 3.9 ng/ml [range 1.2–7.5, n = 10 (nr 1.8–6.6 ng/ml); Incstar, Stillwater, MN], serum alkaline phosphatase concentrations were 161 IU/liter [n = 11, range 121–196 (nr 32–92 IU/liter); VP Autoanalzyer; Abbott Laboratories, Abbott Park, IL], and carboxy terminal telopeptide region of type I collagen concentrations were 92 μg/ml [n = 11, range 8.5–10.8 (normal adult range 1.8–5.0 μg/ml); Incstar].
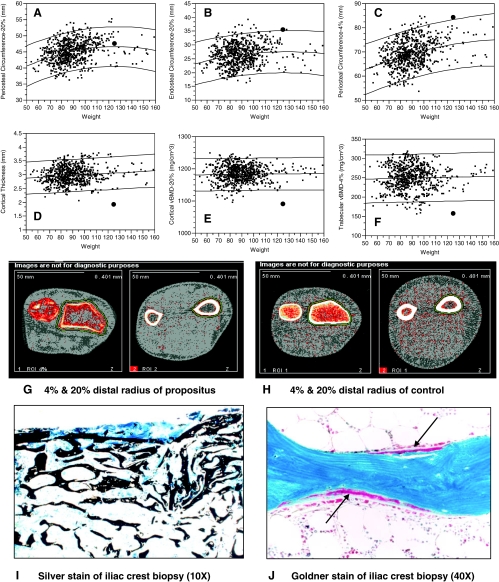
At age 35.5 yr, a pQCT measurement of the left wrist at the 4 and 20% distal sites revealed markedly impaired cortical (1092 mg/cm3) and trabecular (158 mg/cm3) vBMD (Fig. 2, Panels E and F). Figure 2 also shows pQCT scan images of the 4 and 20% radius site for propositus (Panel G) and one normal control man (Panel H) with similar arm length. The larger bone area, decreased trabecular vBMD at the 4% distal radius, and decreased cortical thicknesses are apparent. The lower cortical thickness, combined with normal periosteal circumference, resulted in markedly lower cortical bone area (80 cm2; normal of men with similar weight: 90–135 cm2).
Figure 2.
pQCT and histomorphometry: A–F, pQCT results of left radius by weight of the propositus [identified by the bold circle (•)]. Normative pQCT bone data are from men aged 20–40 yr (16); mean and 95% confidence intervals are shown. A, Periosteal circumference at the 20% distal radius was normal compared with men of similar weight. B, Endosteal circumference was increased. C, Periosteal circumference at the 4% distal radius was greater compared with men of similar weight. D, Cortical thickness was decreased. E, Cortical vBMD was low. F, Trabecular vBMD was low. G and H, pQCT images. G, pQCT images at the 4% (left side of panel) and 20% (right side of panel) distal radius for the propositus. Normal periosteal circumference, with thinner cortex and decreased cortical area, is apparent at the 20% distal radius, whereas decreased trabecular vBMD (as evidenced by the predominance of red colored voxels) and greater periosteal circumference are evident at the 4% distal radius. H, pQCT images at the 4% (left side of panel) and 20% (right side of panel) distal radius for a normal man with similar arm length to the propositus. H, Histomorphometry. I, Silver stain of left iliac crest biopsy (10×); mineralized cortex and trabeculae (black stained areas) are thin. Trabecular number and separation are normal. Note the distorted trabeculae in the right upper region of the image near the cortex. This was created by the mechanical forces generated during the performance of the biopsy and is consistent with increased bone fragility. J, Goldner stain of the iliac crest biopsy (40×); two bone remodeling units at different stages of completion of the bone formation process are present (arrows). Trabeculum is blue, osteoid is red, and the osteoblasts are the monolayer of cells immediately overlying the newly deposited osteoid.
Histomorphometric analysis
At 29.7 yr, a left iliac crest biopsy was performed as described in the Patients and Methods. The static histomorphometric data are listed in Table 1. Most striking is thinner than normal cortical bone, similar to osteoporotic females, and attenuated trabecular thickness, despite normal trabecular number and spacing. This can be visually appreciated in the von Kossa stained section of the biopsy (Fig. 2, Panel I) showing thin trabeculae and an attenuated cortex rendered distorted by the biopsy. Also apparent is endocortical trabecularization such that the endosteal portion of the cortical bone is mostly converted to trabecular bone. The osteoid surface (OS), as reflected in the OS/bone surface (BS) parameter, and osteoid thickness are markedly reduced, suggesting little bone formation. Finally, the bone resorption parameter, ES/BS, was markedly low, indicating little active bone resorption.
Table 1.
Structural and static histomorphometric analysis of iliac crest biopsy
| Units | Propositus | Normal male age range: 21–30 yr (mean ± sd, Ref. 34) | Normal children age range: 14–17 yr (mean ± sd, Ref. 35) | Hypogonadal osteoporotic male mean age: 61 ± 6 yr (mean ± sd, Ref. 36) | Eugonadal osteoporotic male mean age: 58 ± 10 yr (mean ± sd, Ref. 36) | Aging male mean age: 67 ± 6 yr (mean ± se, Ref. 37) | Normal female mean age: 32 yr (Ref. 34) | Osteoporotic female mean age: 67 ± 7 yr (mean ± sd, Ref. 38) | |
|---|---|---|---|---|---|---|---|---|---|
| Structural parameters | |||||||||
| Ct Wi | μm | 641 | 1151 ± 110 mean ± se (Ref. 39) | 1180 ± 350 | 962 ± 154 | 786 ± 193 | 930 ± 67 mean ± se (Ref. 40) | 940 ± 170 (Ref. 38) | 586 ± 264 |
| BV/TV | % | 10.6 | 18.2 ± 7.0 | 25.7 ± 5.3 | 14.2 ± 1.2 | 13.7 ± 2.9 | 19.7 ± 6.4 | 20.2 ± 7.1 | 13.9 ± 4.5 |
| Tb Th | μm | 76 | 135 ± 29 | 157 ± 22 | 126 ± 30 | 132 ± 23 | 133 ± 28 | 140 ± 31 | 124 ± 27 |
| Tb N | No./mm | 1.4 | 1.3 ± 0.3 | 1.6 ± 0.2 | 1.2 ± 0.2 | 1.0 ± 0.2 | 1.5 ± 0.3 | 1.4 ± 0.3 | 1.1 ± 0.2 |
| Tb Sp | μm | 640 | 661 ± 189 | 461 ± 70 | 581 ± 182 | 594 ± 168 | 928 ± 218 | ||
| Bone formation parameters | |||||||||
| OS/BS | % | 2.6 | 14.8 ± 10.4 | 25.7 ± 8.0 | 24.1 ± 13.5 | 14.0 ± 7.2 | 15.0 ± 7.9 | 6.6 ± 5.2 | 16.0 ± 7.1 |
| O Th | μm | 3.6 | 10.6 ± 5.8 | 6.3 ± 1.0 | 11.1 ± 1.4 | 9.2 ± 1.3 | 11.7 ± 5.5 | 7.6 ± 2.2 | 8.5 ± 2.1 |
| W Th | μm | 61.3 | 41.1 ± 3.2 (Ref. 35) | 44.4 ± 3.2 | 54.3 ± 3.6 | 56.7 ± 4.8 | 44.8 ± 5.6 | 32.1 ± 4.1 | 28.0 ± 4.4 |
| Bone resorption parameters | |||||||||
| ES/BS | % | 0.6 | 3.9 ± 1.7 | 18.0 ± 15.7 | 3.9 ± 1.4 | 4.0 ± 2.0 (Ref. 38) | 4.8 ± 2.7 |
sd and se were obtained from different sources as indicated in the cited references. BV/TV, Trabecular area; Ct Wi, cortical thickness; O Th, osteoid width; Tb N, trabecular number; Tb Sp, trabecular separation; Tb Th, trabecular thickness; W Th, wall thickness.
The dynamic histomorphometric data are listed in Table 2. Most notable are dramatically lower mineral apposition rate (MAR), adjusted MAR, and bone formation rate (BFR) and a markedly reduced activation frequency (FAQ). However, the formation period for individual bone remodeling units appears to be normal. A Goldner stained section (Fig. 2, Panel J) of the biopsy demonstrates two normal appearing bone remodeling units at different stages of completion of the bone remodeling process.
Table 2.
Kinetic histomorphometric analysis of iliac crest
| Units | Propositus | Normal male age range: 21–31 yr (mean ± se, Ref. 22) | Normal female mean age: 24 yr (mean ± se, Ref. 22) | Normal children (male and female) age range: 14–17 yr (mean ± sd, Ref. 35) | Osteoporotic female mean age: 67 ± 7 yr (mean ± sd, Ref. 39) | |
|---|---|---|---|---|---|---|
| MAR | μm/d | 0.5 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.5 ± 0.1 |
| Aj MAR | μm/d | 0.2 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | |
| FP | d | 98 | 112 ± 12 | 145 ± 25 | 114 ± 32 | 72 ± 54 |
| MLT | d | 24.1 | 10.4 ± 1.9 | 14.9 ± 2.3 | 15.3 ± 3.6 | 74.0 ± 47.1 |
| BFR/BS | μm3/μm2·d | 0.22 | 0.10 ± 0.03 | |||
| BFR/BV | %/yr | 4.7 | 48.2 ± 18.5 | 14.3 ± 10.1 | ||
| FAQ | yr−1 | 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.8 ± 0.3 |
sd and se were obtained from different sources as indicated in the cited references. Aj MAR, Adjusted apposition rate; BV, bone volume; FP, formation period; MLT, mineralization lag time.
Expression of ER-α and β in cultured stromal cells
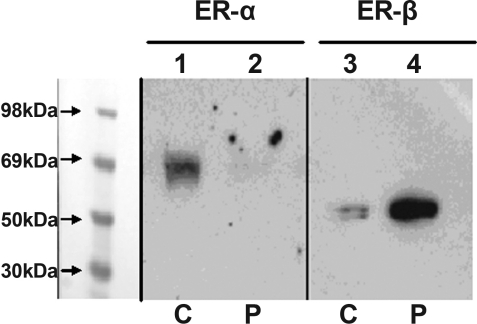
mRNA was extracted from immortalized stromal cells derived from the left distal femur of propositus (3,14). ER-β mRNA amplicon was detectable in the stromal cells, and the identity was confirmed by sequencing RT-PCR product and comparing with ER-β mRNA sequence, GenBank accession no. X99101 (data not shown). Figure 3 shows Western blot analysis of ER-α and ER-β expression in immortalized propositus and control cells. ER-β is readily detectable in the stromal cells of the propositus with a suggestion of higher levels than control; in contrast, ER-α is evident only in the control.
Figure 3.
ER-α/-β protein expression in immortalized bone marrow stromal cells. Nuclear extracts were isolated from immortalized stromal cells from the propositus (P) (lanes 2 and 4) and an age-matched 28-yr-old female donor (C) (lanes 1 and 3) (14). Nuclear extract protein preparations, equalized for protein content, were analyzed by SDS-PAGE and Western blot. The samples were probed with antisera against amino terminal sequence of ER-α using ER21 (lanes 1 and 2) or to ER-β using PAI-310 (lane 3 and 4) as described in Patients and Methods.
Effect of mutation status on adult height and aBMD
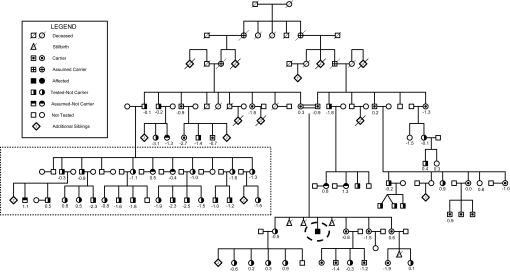
A total of 67 members of the family representing three generations participated; both carrier status and spine aBMD measurements were available for 60 individuals, 17 of whom were carriers (28%). There was no significant difference in adult height of carriers compared with noncarriers (height of males: noncarriers 178.2 ± 4.2 cm, carrier 179.9 ± 7.7; females: noncarriers 164.4 ± 5.9 cm, carrier; 165.6 ± 6.2). Body mass index measurements were similar in adult males [body mass index (kg/m2) of carriers 28 ± 1, wild type 28 ± 2] and adult females (carriers 33 ± 2, noncarriers 33 ± 3). BMD Z scores for this pedigree are shown in Fig. 4. Clinical histories did not reveal medical conditions that could account for decreased aBMD; notably, there were no histories of spontaneous or pathological fractures. The mean spine aBMD Z score among the carriers was significantly less than zero (P = 0.003); however, spine aBMD Z scores were similar among carriers compared with wild type (−0.84 ± 0.26 vs. −0.64 ± 0.16). Excluding one large nuclear family, due to a high prevalence of low aBMD (Fig. 4, dashed box), did not significantly change these results, with aBMD Z scores among carriers and wild types of −0.84 ± 0.26 vs. −0.38 ± 0.26 (P = 0.2).
Figure 4.
Pedigree. Carrier status and spine aBMD Z scores are shown. Propositus is highlighted with a dashed oval. Spine aBMD Z scores did not differ between carriers and wild types, either with or without the inclusion of the large nuclear family (noncarriers) with an increased prevalence of low aBMD (outlined within rectangular box in lower left of pedigree).
Discussion
The action mediated through the ER-α receptor that is most distinct for estrogen in the human skeleton is the stimulation of the final phases of epiphyseal maturation. In the propositus, unlike normal males in which there is a self-limited surge of estrogen coinciding with a growth spurt (19), there appears to have been a prolonged phase of elevated estrogens associated with sustained linear growth in his third decade but with minimal BA progression despite apparent normal testosterone and IGF-I levels. However, without detailed hormonal data beyond the age of 33 yr, conclusions regarding the relative contribution of the GH-IGF-I axis and sex steroids are limited at best.
Although tall stature was ultimately achieved, marked eunuchoid body proportions ensued with debilitating genu valgum. The natural progression, observed in the propositus, is strikingly similar to reports in five aromatase-deficient men (7,8,9,10,12,20), all of whom presented either in their third or fourth decade with BAs ranging from 14.5–16.5 yr, tall stature, genu valgum, and eunuchoid body proportions. By contrast, a sixth case presented at age 17.1 yr with a BA of 12 5/12 yr, normal stature, and apparently normal body proportions (11). Importantly, in the cases in which estrogen treatment has been initiated, there has been rapid epiphyseal maturation (7,8,9,10), but only in the adolescent-aged individual was a significant growth acceleration observed (11). A tentative conclusion is that normal to increased stature can ultimately ensue over time without estrogen and that BA maturation tends to arrest at about 15 yr. However, final epiphyseal fusion, coupled with a growth spurt, is observed only if estrogen exposure occurs at younger BAs.
There are a number of other implications. The first is that manipulation of estrogen production and/or signaling has the potential for augmenting final height. Recent studies using aromatase inhibitors show potential for increasing stature in a variety of clinical conditions (21). The second is that modulation of estrogen exposure, while simultaneously maintaining normal circulating androgens, has intriguing potential benefits. Age-appropriate masculinization can be achieved while suppressing BA maturation. More speculatively, maintenance of androgen allows for greater increments in height per unit of BA advancement, and this is a markedly different outcome from the tapered height velocity observed in long-standing hypogonadism (19). The third, and perhaps as important implication, is that there are senescence factors, intrinsic to the epiphysis, that serve to place an overall limit on final stature. The near final epiphyseal fusion in the propositus supports this notion (Fig. 1, Panel B). Not only is linear growth without estrogen largely completed by the middle to end of the third decade in the propositus, and in the aromatase-deficient cases, but also the increased stature is accompanied by abnormalities of the body proportion, marked genu valgum and osteopenia. Stature increments, substantially beyond the normal genetic potential, are not likely to be achieved safely by manipulating estrogen action for more than a few years.
Another striking set of findings in the skeleton of the propositus is marked thinning of the cortex with increased trabecularization and low radial cortical vBMD. The cortical abnormalities are present in the face of normal to increased periosteal circumference (size), but increased endosteal circumference, and, therefore, decreased cortical bone area. Cortical width is greater in men and is considered to be secondary to increased periosteal apposition rate induced by androgens (6). In contrast, postmenopausal women are susceptible to increased endosteal bone loss relative to men, display greater cortical porosity (22), and exhibit less periosteal apposition of new bone. Although estrogen is considered by some to exert primarily inhibitory actions on periosteal expansion, there is recent evidence that many of the actions of estrogen on periosteum may be to stimulate expansion through ER-α, but in a dosage sensitive manner, and potentially, in collaboration with androgens (23). In addition, low doses of estrogen may increase the mechanical sensitivity of the periosteum indirectly, through stimulation of IGF-I, whereas higher estrogen concentrations may reduce the mechanical sensitivity of bone through ER-β and lower IGF-I concentrations (11,24). Although complete longitudinal data are lacking, the IGF-I concentrations in the propositus were slightly elevated for an adult male, and testosterone levels were persistently within normal limits (3), reinforcing the possibility that the GH/IGF-I axis, along with androgens, may have contributed to the increase in periosteal circumference in the face of ER-α disruption.
The decreased trabecular volume, coupled with decreased bone formation, is a more predictable outcome of the severe disruption in estrogen action (25,26). However, the preservation of trabecular number in the propositus is distinctly uncharacteristic of both male and female hypogonadism, and is more typical of the aging skeleton with normal androgen concentrations (27). In addition, the increased markers of bone turnover are surprisingly high, in light of the low FAQ, and decreased bone formation by histomorphometric analysis. It is possible that the bone turnover markers in the immediate years after presentation were predominantly reflecting the increased bone turnover of a “growing” skeleton as evidenced by open epiphyses and, therefore, were not revealing markers of trabecular function in this setting. The preserved trabecular number in the propositus may be, in part, explained by residual actions of estrogen through ER-β (28) (Fig. 3) and the normal androgen levels. Investigations of the androgen insensitivity syndrome clearly suggest an important contribution of androgens to bone (29). Similarly, studies involving supplementation of anabolic steroids such as tibolone (30) or testosterone (31) to standard estrogen/progesterone replacement in postmenopausal women also point to important actions of androgens on trabecular bone. Regardless, a potential clinical implication is that interventions that augment ER-α pathways preserve the anabolic actions of androgen, and account for any compensatory actions of estrogen through ER-β, may be particularly effective in maintaining and/or augmenting trabecular bone.
In our study, osteopenia was not a consequence of haplo-insufficiency of the ER-α receptor compared with noncarriers. The ER-α gene has eight exons, seven introns, and extends over 200 kb (32). An association between ER-α and BMD has been observed in some studies, mostly focusing on two polymorphisms within intron 1 recognized by the XbaI (C351G) (X and x = absence or presence of restriction sites) and PvuII (T397C) (P and p = absence or presence of restriction sites) restriction enzymes, and on a variable TA repeat in the ER-α gene promoter (13). A variety of metaanalyses of ER-α have, in fact, shown evidence of an association between the X allele and both BMD and fractures, with higher BMD values and reduced fracture risk in XX homozygotes (33). An intriguing mechanistic possibility has occurred from studies showing that the p allele overlaps the consensus recognition sites for transcription factors. Because promoter-reporter assays suggest that the presence of the P allele might amplify Myb-induced ER-α transcription (13), it is possible that polymorphisms that eliminate the P allele could affect levels ER-α protein. However, in our study the number of carriers was relatively low, and there may have been insufficient power to detect bone differences at the spine. The means differed by approximately one half of a sd, which would have been statistically significant with a sample size of approximately 80 carriers. It is likely that other studies, in which less profound effects on allelic function are being assessed, will require large subject numbers.
In summary, detailed analysis of homozygous-affected propositus suggests a complex contribution of both estrogen and androgen in bone growth, mineral content, and skeletal structural integrity. ER-α disruption results in delayed epiphyseal maturation, tall stature, trabecular thinning, marked cortical thinning, significantly reduced cortical vBMD, and normal to increased periosteal expansion. This supports an important role for ER-α in mediating the actions of estrogen on the male skeleton. However, effects of estrogen, through ER-β, are possible, and androgen, particularly by preserving trabecular number and augmenting both periosteal and epiphyseal growth, has important actions on bone. In contrast, haplo-insufficiency for the ER-α receptor was not associated with osteopenia or differences in adult stature, suggesting that major disruption in ER-α expression is required before a clear impact on the skeleton can be appreciated.
Acknowledgments
We thank Patrick G. Kirk, M.D., JoAnn Horn, R.N., Caitlin Berry, and the staff of the Clinical Research Center, Cincinnati Children’s Hospital Medical Center for their assistance. E.P.S. thanks James A. Fagin and the late Judson J. Van Wyk for their advice and encouragement.
Footnotes
This research was supported by the Clinical Research Center, Cincinnati Children’s Hospital Medical Center, and the Division of Internal Research at the National Institute of Environmental Health Sciences.
Disclosure Information: The authors have nothing to disclose.
First Published Online May 27, 2008
Abbreviations: aBMD, Areal spine bone mineral density; BA, bone age; BFR, bone formation rate; BMD, bone mineral density; BS, bone surface; DXA, dual-energy x-ray absorptiometry; ER, estrogen receptor; ES, eroded surface; FAQ, activation frequency; MAR, mineral apposition rate; nr, normal range; OS, osteoid surface; pQCT, peripheral quantitative computed tomography; vBMD, volumetric bone mineral density.
References
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90:11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K 1995 Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 80:3689–3698 [DOI] [PubMed] [Google Scholar]
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS 1994 Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331:1056–1061 [DOI] [PubMed] [Google Scholar]
- Deroo BJ, Korach KS 2006 Estrogen receptors and human disease. J Clin Invest 116:561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA 1996 Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E, Bianchi G, Khosla S, Kanis JA, Orwoll E 2006 Bone fragility in men–where are we? Osteoporos Int 17:1577–1583 [DOI] [PubMed] [Google Scholar]
- Herrmann BL, Janssen OE, Hahn S, Broecker-Preuss M, Mann K 2005 Effects of estrogen replacement therapy on bone and glucose metabolism in a male with congenital aromatase deficiency. Horm Metab Res 37:178–183 [DOI] [PubMed] [Google Scholar]
- Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER 1997 Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med 337:91–95 [DOI] [PubMed] [Google Scholar]
- Bilezikian JP, Morishima A, Bell J, Grumbach MM 1998 Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. N Engl J Med 339:599–603 [DOI] [PubMed] [Google Scholar]
- Maffei L, Murata Y, Rochira V, Tubert G, Aranda C, Vazquez M, Clyne CD, Davis S, Simpson ER, Carani C 2004 Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab 89:61–70 [DOI] [PubMed] [Google Scholar]
- Bouillon R, Bex M, Vanderschueren D, Boonen S 2004 Estrogens are essential for male pubertal periosteal bone expansion. J Clin Endocrinol Metab 89:6025–6029 [DOI] [PubMed] [Google Scholar]
- Rochira V, Zirilli L, Madeo B, Aranda C, Caffagni G, Fabre B, Montangero VE, Roldan EJ, Maffei L, Carani C 2007 Skeletal effects of long-term estrogen and testosterone replacement treatment in a man with congenital aromatase deficiency: evidences of a priming effect of estrogen for sex steroids action on bone. Bone 40:1662–1668 [DOI] [PubMed] [Google Scholar]
- Gennari L, De Paola, V, Merlotti D, Martini G, Nuti R 2007 Steroid hormone receptor gene polymorphisms and osteoporosis: a pharmacogenomic review. Expert Opin Pharmacother 8:537–553 [DOI] [PubMed] [Google Scholar]
- Dieudonne SC, Xu T, Chou JY, Kuznetsov SA, Satomura K, Mankani M, Fedarko NS, Smith EP, Robey PG, Young MF 1998 Immortalization and characterization of bone marrow stromal fibroblasts from a patient with a loss of function mutation in the estrogen receptor-α gene. J Bone Miner Res 13:598–608 [DOI] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR 1987 Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610 [DOI] [PubMed] [Google Scholar]
- Specker B, Binkley L, Fahrenwald N 2004 Rural vs. non-rural differences in BMC, volumetric BMD, and bone size: a population-based cross-sectional study. Bone 35:1389–1398 [DOI] [PubMed] [Google Scholar]
- Greulich WW, Pyle SI, Waterhouse AM 1971 A radiographic standard of reference for the growing hand and wrist. Chicago: Press of Case Western Reserve University [Google Scholar]
- Blin N, Stafford DW 1976 A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res 3:2303–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O, Marino R, De LF, Phillip M, Baron J 2005 Endocrine regulation of the growth plate. Horm Res 64:157–165 [DOI] [PubMed] [Google Scholar]
- Maffei L, Rochira V, Zirilli L, Antunez P, Aranda C, Fabre B, Simone ML, Pignatti E, Simpson ER, Houssami S, Clyne CD, Carani C 2007 A novel compound heterozygous mutation of the aromatase gene in an adult man: reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clin Endocrinol (Oxf) 67:218–224 [DOI] [PubMed] [Google Scholar]
- Dunkel L 2006 Use of aromatase inhibitors to increase final height. Mol Cell Endocrinol 254–255:207–216 [DOI] [PubMed] [Google Scholar]
- Brockstedt H, Kassem M, Eriksen EF, Mosekilde L, Melsen F 1993 Age- and sex-related changes in iliac cortical bone mass and remodeling. Bone 14:681–691 [DOI] [PubMed] [Google Scholar]
- Venken K, De Gendt K, Boonen S, Ophoff J, Bouillon R, Swinnen JV, Verhoeven G, Vanderschueren D 2006 Relative impact of androgen and estrogen receptor activation in the effects of androgens on trabecular and cortical bone in growing male mice: a study in the androgen receptor knockout mouse model. J Bone Miner Res 21:576–585 [DOI] [PubMed] [Google Scholar]
- Vanderschueren D, Venken K, Ophoff J, Bouillon R, Boonen S 2006 Clinical review: sex steroids and the periosteum–reconsidering the roles of androgens and estrogens in periosteal expansion. J Clin Endocrinol Metab 91:378–382 [DOI] [PubMed] [Google Scholar]
- Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S 2000 Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 106:1553–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specker BL 2006 Influence of rapid growth on skeletal adaptation to exercise. J Musculoskelet Neuronal Interact 6:147–153 [PubMed] [Google Scholar]
- Mosekilde L 1989 Sex differences in age-related loss of vertebral trabecular bone mass and structure–biomechanical consequences. Bone 10:425–432 [DOI] [PubMed] [Google Scholar]
- Sims NA, Dupont S, Krust A, Clement-Lacroix P, Minet D, Resche-Rigon M, Gaillard-Kelly M, Baron R 2002 Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-β in bone remodeling in females but not in males. Bone 30:18–25 [DOI] [PubMed] [Google Scholar]
- Marcus R, Leary D, Schneider DL, Shane E, Favus M, Quigley CA 2000 The contribution of testosterone to skeletal development and maintenance: lessons from the androgen insensitivity syndrome. J Clin Endocrinol Metab 85: 1032–1037 [DOI] [PubMed] [Google Scholar]
- Castelo-Branco C, Vicente JJ, Figueras F, Sanjuan A, Martinez de Osaba MJ, Casals E, Pons F, Balasch J, Vanrell JA 2000 Comparative effects of estrogens plus androgens and tibolone on bone, lipid pattern and sexuality in postmenopausal women. Maturitas 34:161–168 [DOI] [PubMed] [Google Scholar]
- Raisz LG, Wiita B, Artis A, Bowen A, Schwartz S, Trahiotis M, Shoukri K, Smith J 1996 Comparison of the effects of estrogen alone and estrogen plus androgen on biochemical markers of bone formation and resorption in postmenopausal women. J Clin Endocrinol Metab 81:37–43 [DOI] [PubMed] [Google Scholar]
- Swope DL, Castranio T, Harrell JC, Mishina Y, Korach KS 2002 AF-2 knock-in mutation of estrogen receptor α: Cre-loxP excision of a PGK-neo cassette from the 3′ UTR. Genesis 32:99–101 [DOI] [PubMed] [Google Scholar]
- Ioannidis JP, Ng MY, Sham PC, Zintzaras E, Lewis CM, Deng HW, Econs MJ, Karasik D, Devoto M, Kammerer CM, Spector T, Andrew T, Cupples LA, Duncan EL, Foroud T, Kiel DP, Koller D, Langdahl B, Mitchell BD, Peacock M, Recker R, Shen H, Sol-Church K, Spotila LD, Uitterlinden AG, Wilson SG, Kung AW, Ralston SH 2007 Meta-analysis of genome-wide scans provides evidence for sex- and site-specific regulation of bone mass. J Bone Miner Res 22:173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler CM, Pettifor JM, Mesquita JM, Bird MD, Schnaid E, Smyth AE 1990 Histomorphometry of iliac crest bone in 346 normal black and white South African adults. Bone Miner 10:183–199 [DOI] [PubMed] [Google Scholar]
- Glorieux FH, Travers R, Taylor A, Bowen JR, Rauch F, Norman M, Parfitt AM 2000 Normative data for iliac bone histomorphometry in growing children. Bone 26:103–109 [DOI] [PubMed] [Google Scholar]
- Jackson JA, Kleerekoper M, Parfitt AM, Rao DS, Villanueva AR, Frame B 1987 Bone histomorphometry in hypogonadal and eugonadal men with spinal osteoporosis. J Clin Endocrinol Metab 65:53–58 [DOI] [PubMed] [Google Scholar]
- Mullender MG, Tan SD, Vico L, Alexandre C, Klein-Nulend J 2005 Differences in osteocyte density and bone histomorphometry between men and women and between healthy and osteoporotic subjects. Calcif Tissue Int 77:291–296 [DOI] [PubMed] [Google Scholar]
- Kimmel DB, Recker RR, Gallagher JC, Vaswani AS, Aloia JF 1990 A comparison of iliac bone histomorphometric data in post-menopausal osteoporotic and normal subjects. Bone Miner 11:217–235 [DOI] [PubMed] [Google Scholar]
- Steiniche T, Vesterby A, Eriksen EF, Mosekilde L, Melsen F 1986 A histomorphometric determination of iliac bone structure and remodeling in obese subjects. Bone 7:77–82 [DOI] [PubMed] [Google Scholar]
- Clarke BL, Ebeling PR, Jones JD, Wahner HW, O'Fallon WM, Riggs BL, Fitzpatrick LA 1996 Changes in quantitative bone histomorphometry in aging healthy men. J Clin Endocrinol Metab 81:2264–2270 [DOI] [PubMed] [Google Scholar]