Abstract
Background: Hypovitaminosis D is prevalent in youth worldwide, but the safety of vitamin D at doses exceeding 200 IU/d is unknown in this age group. We assessed the safety of high doses of vitamin D3 administered to apparently healthy schoolchildren.
Methods: To assess short-term safety, 25 subjects randomly received placebo or vitamin D3 at doses of 14,000 IU/wk for 8 wk. To assess long-term safety, 340 subjects randomly received placebo, vitamin D3 as 1,400 IU/wk or 14,000 IU/wk for 1 yr. Biochemical variables were monitored at 0, 2, 4, 6, and 8 wk and 8 wk off therapy in the short-term study and at 0, 6, and 12 months in the long-term study.
Results: In both the short- and long-term studies, mean serum calcium and 1,25-hydroxyvitamin levels did not change in any group. In the short-term study, mean 25-hydroxyvitamin concentrations increased from 44 (± 11) to 54 (± 19) ng/ml in the treated groups (P = 0.033). In the long-term study, mean 25-hydroxyvitamin D levels increased from 15 ± 8 to 19 ± 7 ng/ml (P < 0.0001) in subjects receiving 1,400 IU/wk and from 15 ± 7 to 36 ± 22 ng/ml (P < 0.0001) in the group receiving 14,000 IU/wk. No subject developed vitamin D intoxication.
Conclusion: Vitamin D3 at doses equivalent to 2000 IU/d for 1 yr is safe in adolescents and results in desirable vitamin D levels.
Although hypovitaminosis D is prevalent in youth worldwide, the recommended adequate intake remains at only 200 IU/day. The lack of evidence-based vitamin D safety studies is a major limitation to current recommendations. Two randomized controlled trials demonstrate the short and long term safety of vitamin D3 at doses of 2000 IU/day in children and adolescents.
Vitamin D is an essential hormone for bone growth and development in children and skeletal health in adults (1). During adolescence bone mass increases substantially, a process in large part determined by the genetic background of the individual, but that is also modulated by environmental factors including exercise, calcium, and vitamin D intake. Nutrition guidelines targeted to optimize bone health in children and adolescents have focused on calcium and exercise but have omitted vitamin D (2,3). Recommendations from the Institute of Medicine in 1999 for adequate daily intake of vitamin D are 200 IU for children (4). Similarly, a 2003 report of the American Academy of Pediatrics recommends 200 IU/d to be continued through childhood and adolescence (5). Higher doses were not considered due to lack of evidence for a beneficial effect at the time of the above recommendations.
Hypovitaminosis D is prevalent worldwide in children and adolescents (6,7). Direct and indirect evidence support a role for vitamin D on bone accrual and musculoskeletal health in the youth (6,8,9,10). However, in contrast to adults, in whom extensive short- and long-term studies have documented the safety of vitamin D supplementation in doses of up to 10,000 IU/d (11,12), the data in the pediatric age group are lacking (6). This is a major obstacle to modification of the daily allowance in this age group to doses that would enhance musculoskeletal health and possibly other outcomes (1,6,13).
We present detailed short- and long-term safety data using relatively high doses of vitamin D3 in schoolchildren, aged 10–17 yr. The positive impact of vitamin D on bone mass, bone area, and lean mass in this trial was previously reported in detail (9).
Subjects and Methods
Intervention
Short-term study
A 16-wk, single-blinded pilot trial to determine the safety of high-dose vitamin D3, a dose used subsequently in the long-term study (see below). Students were randomized to a treatment for 8 wk (August to October) followed by a period of 8 wk off therapy (December). Treatment consisted of placebo oil (n = 9) or vitamin D3 at doses of 350 μg (14,000 IU)/ml of vitamin D3 given once weekly, either as Vigantol (n = 8 subjects, Vigantol oil; Merck KGaA, Darmstadt, Germany) or cholecalciferol dissolved at the same concentrations in pharmaceutical grade ethanol (n = 9 subjects, crystalline cholecalciferol USP; Sigma, St. Louis, MO; Sigma group). The Sigma crystalline vitamin D3 in ethanol was used because its safety in adults had been characterized in previous studies (12,14). The vitamin D solutions were prepared in the Clinical Pathology Laboratory at Mt. Sinai Hospital (Toronto Canada) as detailed in the Appendix. The vitamin D3 was administered by the investigators at the study site to ensure 100% compliance. The study took place in the summer to early fall, a period when the highest levels of vitamin D are reached (7). The high dose, i.e. the equivalent of 2000 IU/d, was chosen as half the dose labeled as the lowest observed adverse effect level in adults and that would result in desirable serum 25-hydroxyvitamin D (25-OHD) levels (12). Vigantol oil vitamin D3 was provided as a solution by its manufacturer, and all doses were confirmed to be within 10% of the expected value by HPLC analysis after dissolution of dose to mobile phase (see Appendix).
Long-term study
This was a 1-yr, double-blind, randomized, placebo-controlled trial evaluating the safety and efficacy of vitamin D supplementation on musculoskeletal health (9). Students took placebo oil, low-dose vitamin D (1400 IU/wk or 35 μg/wk), or high-dose vitamin D (14,000 IU/wk or 350 μg/wk), both as vigantol oil (Vigantol oil; Merck KGaA).
The low dose corresponds to the current adequate intake for vitamin D in this age group (4). The high dose was chosen because it represented half the dose that is safe in adults and results in desirable 25-OHD levels (12). It also had been confirmed to be safe in the short-term pilot study described above and conformed with the upper level for vitamin D for this age group (15). Students were called by study personnel every 2 wk to improve compliance. Every 3 months, students returned the bottles and received new bottles. The mean percent intake of the assigned dose of vitamin D given was 98 ± 2.6% for placebo, 97.5 ± 3% for the low dose arm, and 97 ± 3% for the high-dose arm. There was no difference in compliance between the two genders (data not shown).
Subjects
Short-term study
Twenty-five students (15 boys and 10 girls), aged 10–17 yr were recruited from the pool of subjects who had completed a previous study evaluating the prevalence of hypovitaminosis D in children (7).
Long-term study
Three hundred forty (172 boys and 168 girls) apparently healthy subjects were recruited from four schools from the Greater Beirut area to ensure balanced representation geographically and socioeconomically; 340 completed the 1-yr study (9). The age group studied was 10–17 yr, a critical age for bone mass accretion (16).
Subjects were excluded from either study if they reported disorders or medications known to affect bone metabolism. Both studies were approved by the institutional review board, and informed consent was obtained from all study subjects and their parents.
Data collection
Students had a physical examination at baseline in both studies and at 1 yr in the long-term study. In the short-term study, serum 25-OHD was measured at baseline, 2, 4, 6, and 8 wk of therapy and after 8 wk off therapy. Serum calcium and 1,25-hydroxyvitamin (1,25-OHD) were measured at baseline, 8 wk, and 8 wk off. Blood collection took place fasting, right before the due weekly dose of vitamin D. In the long-term study, serum calcium was measured at study entry, 6 months, and 12 months and vitamin D metabolites at baseline and 12 months. Blood collection did not take place with any specific timing in relation to the last dose of vitamin D received.
Assays and measurements
Total serum calcium (uncorrected) was measured in the institutional chemistry laboratory by a colorimetric method using the Hitachi 192 analyzer (Roche Molecular Biochemicals, Mannheim, Germany).
In the short-term study, serum 25-OHD and 1,25-OHD were assayed in the Clinical Pathology Laboratory at Mt. Sinai Hospital (Reinhold Vieth). In the long-term study, serum 25-OHD and 1,25-OHD were measured in the core endocrinology laboratory at the American University of Beirut. The core laboratory is a participant in the international vitamin D quality assurance program DEQAS (www.deqas.org). In both studies serum 25-OHD was measured by a competitive protein binding assay using the DiaSorin RIA (Diasorin, Incstar, Sallugia, Italy), with intra- and interassay coefficients of variation (CVs) less than 13% at a serum concentration of 47 ng/ml. This assay measures both 25-OHD2 and 25-OHD3. The intraassay CV percent for measuring 25-OHD in our laboratory is 5 ± 9%, based on 124 specimens run as separate samples in the assay, with a mean 25-OHD level of 19 ± 5 ng/ml. Serum 1,25-OHD was measured by RIA using the IDS kit with intra- and interassay CVs less than 10% at serum concentrations between 10 and 100 pg/ml (IDS Immuno-Diagnostic Systems, Boldon, UK). Blood for hormonal studies was stored as serum at −70 C. For the short-term study, all samples were run in the same assay, and for the long-term study, samples from each subject were assayed in the same assay at study completion.
Statistical analysis
Descriptive statistics and parametric (paired and independent t tests) and nonparametric tests (paired t test Wilcoxon signed rank and Wilcoxon rank sum test comparisons) were used to evaluate the effect of supplementation on biochemical measures in the long- and short-term studies, respectively. Repeated-measures analyses were used to test whether time trends differ between the three treatment groups and whether there is a time effect within each treatment. To assess the serial profile of serum biochemistries within each treatment group or values at baseline or 1 yr among the three treatment groups, Kruskal Wallis and ANOVA models were used. Analyses were carried out using the SPSS software version 14.0 (SPSS, Chicago, IL). Results were expressed as means (± sd). P < 0.05 was considered statistically significant.
Results
Baseline characteristics of the study subjects
Short-term study
The mean age of the study subjects was 13.7 ± 2.1 yr, height 158 ± 12 cm, and weight 57 ± 17 kg. At study entry, the mean serum calcium was 10.4 ± 0.5 mg/dl, mean serum 25-OHD level was 42 ± 11 ng/ml, and mean serum 1,25-OHD was 60 ± 14 pg/ml.
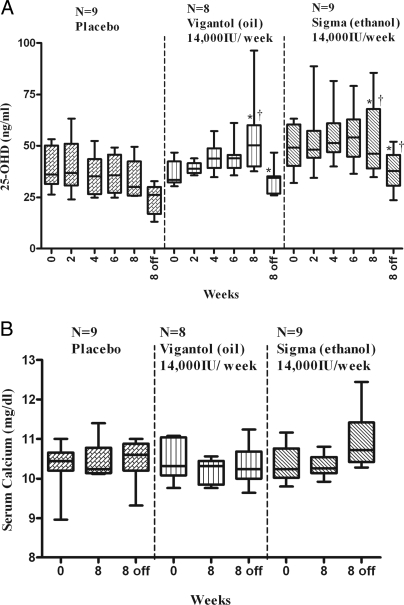
The baseline characteristics of the subjects including height and weight were similar among the treatment groups (data not shown). Baseline serum calcium levels and 1,25-OHD were similar in the three groups, but mean serum 25-OHD was higher at baseline in the Sigma group, compared with Vigantol oil group (P = 0.028, Fig. 1A).
Figure 1.
Serum 25-OHD (A) and calcium (B) at 0, 2, 4, 6, and 8 wk (August to October) and 8 wk off therapy (December) in the short-term study, by treatment group. The box plot shows the median (50th percentile) as a line and the first (25th percentile) and third quartile (75th percentile) of the distribution as the lower and upper parts of the box. The whiskers shown above and below the boxes represent the largest and smallest observed values that are less than 1.5 box lengths from the end of the box. Analyses conducted using repeated-measures ANOVA. In the Sigma group, mean 25-OHD was 49 ± 12 ng/ml at baseline, 53 ± 19 ng/ml (P = 0.440) after 8 wk of supplementation and decreased to 38 ± 10 ng/ml at 2 wk off therapy (P = 0.01 vs. the 8 wk value). In the Vigantol group, mean 25-OHD concentration group increased from 37 ± 6 ng/ml at baseline to 54 ± 20 ng/ml (P = 0.043) after 8 wk of supplementation and then decreased to 33 ± 7 ng/ml at 2 wk off therapy (P = 0.018 compared with the 8 wk value). *, No significant difference in 25-OHD levels between D3 groups at similar time points; †, 25-OHD levels were significantly higher in D3 group(s), compared with the placebo group, at 8 wk and 8 wk off therapy (P < 0.05). There were no significant differences in serum calcium levels achieved at 8 wk and 8 wk off therapy among the three groups (ANOVA). Biochemical assays are reported in metric units. To convert from metric to SI units, multiply calcium by 0.25 (millimoles per liter); 25-OHD by 2.496 (nanomoles per liter).
Long-term study
The mean age of the study subjects was 13.1 ± 2 yr, height 154 ± 12 cm, and weight 50 ± 15 kg. At study entry, the mean serum calcium was 10 ± 0.4 mg/dl, mean serum 25-OHD level 15 ± 8 ng/ml, and mean serum 1,25-OHD 81 ± 27 pg/ml.
The baseline characteristics of the subjects lifestyle variables, age, height, weight, serum calcium, 25-OHD, and 1,25-OHD were similar among the treatment groups in the overall group and within each gender (data not shown).
Effects of 14,000 IU/week of vitamin D3 on serum levels of calcium and vitamin D metabolites: short-term study
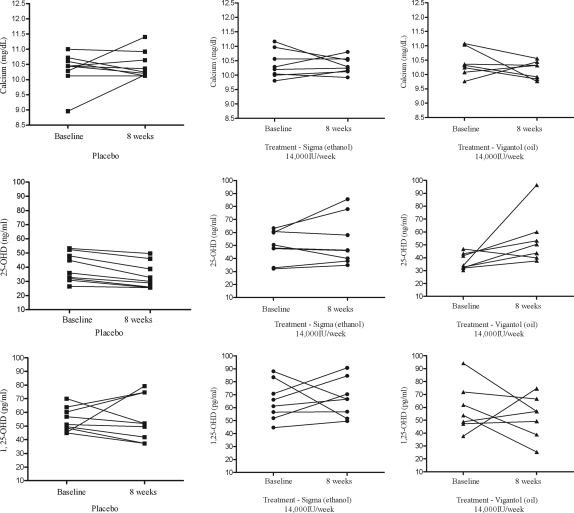
A repeated-measures model was fit with treatment and time as fixed effects and including an interaction term. The interaction was significant (P = 0.029). indicating different time trends between the treatments groups. Hence, for each treatment a repeated-measures model containing only time as a fixed effect was fitted. All three repeated-measures models indicated that there was a time effect, P <.001 for placebo, P = 0.002 for Vigantol, and P = 0.019 for Sigma. In the placebo group, there was a steady decrease in mean 25-OHD levels; mean values at baseline, 8 wk, and at 8 wk off were significantly different from each other (P < 0.01, Fig. 1A). Conversely, mean 25-OHD rose in the treatment groups during treatment and declined to or below baseline values, by 8 wk after stopping therapy (Fig. 1A). Individual values for serum calcium, 25-OHD, and 1,25-OHD at baseline and 8 wk by treatment group are shown in Fig. 2.
Figure 2.
Effect of vitamin D3 supplementation on serum calcium, 25-OHD, and 1,25-OHD in the short-term study, by treatment group. Individual values for serum calcium, 25-OHD, and 1,25-OHD at baseline and 8 wk for the 25 subjects are shown. Biochemical assays are reported in metric units. To convert from metric to SI units, multiply calcium by 0.25 (millimoles per liter); 25-OHD by 2.496 (nanomoles per liter); 1,25-OHD by 2.6 (picomoles per liter).
There were no significant differences in serum calcium levels achieved at 8 wk, or 8 wk off therapy between the three groups.
Effects of vitamin D3 at doses of 14,000 IU/wk, 1,400 IU/wk, and placebo over 1 yr on serum levels of calcium and vitamin D metabolites: long term study
There were no significant differences at baseline in mean serum levels of calcium, phosphate, alkaline phosphatase, 25-OHD, or 1,25-OHD among the three treatment groups in the overall group and by gender (Tables 1 and 2). However, because there were gender differences in the mean levels of calcitriol reached at 1 yr, results for all biochemical variables are reported separately for each gender.
Table 1.
Mean (± sd) serum levels of calcium, phosphorus, alkaline phosphatase, 25-OHD, and 1,25-OHD at 0, 6, and 12 months in girls by treatment group: placebo (PBO; n = 55), low-dose vitamin D3 (1,400 IU/wk, n = 58), and high-dose vitamin D3 (14,000 IU/wk, n = 55)
| Calcium (mg/dl)
|
|||
|---|---|---|---|
| 0 month | 6 months | 12 months | |
| PBO | 10 (0.4) | 10 (0.4) | 10 (0.4) |
| Low dose (1,400 IU/wk) | 9.9 (0.3) | 10 (0.4) | 10 (0.3) |
| High dose (14,000 IU/wk) | 9.9 (0.4) | 10 (0.4) | 10 (0.3) |
| Phosphorus (mg/dl)
|
|||
|---|---|---|---|
| 0 month | 6 months | 12 months | |
| PBO | 4 (0.5) | 4.6 (0.5) | 4.5 (0.6) |
| Low dose (1,400 IU/wk) | 4.3 (0.5) | 4.4 (0.8) | 4.5 (0.6)a |
| High dose (14,000 IU/wk) | 4.3 (0.7) | 4.4 (0.6) | 4.5 (0.6)a |
| Alkaline phosphatase (IU/liter)
|
|||
|---|---|---|---|
| 0 month | 6 months | 12 months | |
| PBO | 199 (116) | 160 (83) | 145 (76)a |
| Low dose (1,400 IU/wk) | 208 (112) | 192 (103) | 172 (93)a |
| High dose (14,000 IU/wk) | 232 (139) | 198 (115) | 176 (102)a |
| 25-OHD (ng/ml)
|
|||
|---|---|---|---|
| 0 month | 6 months | 12 monthsb | |
| PBO | 14 (7) | 16 (8)a | |
| Low dose (1,400 IU/wk) | 14 (9) | 17 (6)a | |
| High dose (14,000 IU/wk) | 13 (8) | 38 (31)a | |
| 1,25-OHD (pg/ml)
|
|||
|---|---|---|---|
| 0 month | 6 months | 12 monthsb | |
| PBO | 86 (30) | 76 (30)a | |
| Low dose (1,400 IU/wk) | 78 (29) | 78 (29) | |
| High dose (14,000 IU/wk) | 83 (27) | 104 (33)a | |
Biochemical assays are reported in metric units. To convert from metric to SI units, multiply calcium by 0.25 (millimoles per liter); phosphorous by 0.32 (millimoles per liter); 25-OHD by 2.496 (nanomoles per liter); and 1,25-OHD by 2.6 (picomoles per liter).
P < 0.001 between 0 and 12 months by paired t test.
P < 0.05 by one-way ANOVA between treatment groups: P < 0.0001 between PBO and high; P < 0.0001 between high and low.
Table 2.
Mean (± sd) serum levels of calcium, phosphorus, alkaline phosphatase, 25-OHD, and 1,25-OHD at 0, 6, and 12 months in boys by treatment group: placebo (PBO; n = 56), low-dose vitamin D3 (1400 IU/wk, n = 56), and high-dose vitamin D3 (14,000 IU/wk, n = 60)
| Calcium (mg/dl)
|
|||
|---|---|---|---|
| 0 month | 6 months | 12 months | |
| PBO | 10 (0.4) | 10 (0.3) | 10 (0.3) |
| Low dose (1,400 IU/wk) | 10 (0.4) | 10 (0.3) | 10 (0.3) |
| High dose (14,000 IU/wk) | 10 (0.3) | 10 (0.4) | 10 (0.3) |
| Phosphorus (mg/dl)
|
|||
|---|---|---|---|
| 0 month | 6 months | 12 months | |
| PBO | 4.6 (0.5) | 4.8 (0.7) | 4.9 (0.7)a |
| Low dose (1,400 IU/wk) | 4.6 (0.6) | 4.8 (0.6) | 5 (0.8)a |
| High dose (14,000 IU/wk) | 4.6 (0.6) | 4.7 (0.6) | 5 (0.6)a |
| Alkaline phosphatase (IU/liter)
|
|||
|---|---|---|---|
| 0 month | 6 months | 12 months | |
| PBO | 280 (89) | 260 (83) | 250 (93)a |
| Low dose (1,400 IU/wk) | 306 (98) | 291 (103) | 274 (98)a |
| High dose (14,000 IU/wk) | 281 (106) | 260 (101) | 248 (104)a |
| 25-OHD (ng/ml)
|
|||
|---|---|---|---|
| 0 month | 6 months | 12 monthsb | |
| PBO | 16 (6) | 17 (6) | |
| Low dose (1,400 IU/wk) | 16 (7) | 20 (7)a | |
| High dose (14,000 IU/wk) | 16 (7) | 35 (9)a | |
| 1,25-OHD (pg/ml)
|
|||
|---|---|---|---|
| 0 month | 6 months | 12 months | |
| PBO | 79 (26) | 78 (31) | |
| Low dose (1,400 IU/wk) | 85 (21) | 88 (27) | |
| High dose (14,000 IU/wk) | 80 (28) | 90 (31) | |
Biochemical assays are reported in metric units. To convert from metric to SI units, multiply calcium by 0.25 (millimoles per liter); phosphorous by 0.32 (millimoles per liter); 25-OHD by 2.496 (nanomoles per liter); 1,25-OHD by 2.6 (picomoles per liter).
P < 0.05 between 0 and 12 months by paired t test.
P < 0.05 by one-way ANOVA between treatment groups: P < 0.0001 between PBO and high; P < 0.0001 between high and low.
Girls
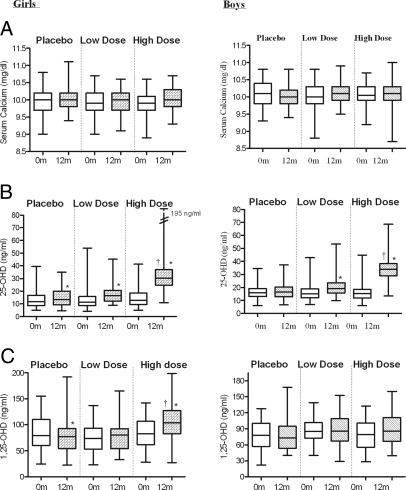
In the placebo group, mean 25-OHD concentrations were 14 ± 7 ng/ml at baseline and 16 ± 8 ng/ml at 1 yr (P = 0.041). In those who received 1400 IU of Vigantol oil per week, mean 25-OHD increased from 14 ± 9 ng/ml at baseline to 17 ± 6 ng/ml at 1 yr (P = 0.011). In those who received 14,000 IU of Vigantol oil per week, mean 25-OHD increased from 13 ± 8 ng/ml at baseline to 38 ± 31 ng/ml at 1 yr (P < 0.0001, Table 1 and Fig. 3). The mean serum 25-OHD level achieved at 1 yr was significantly higher in the high dose, compared with both the placebo and low-dose groups (P < 0.0001 by ANOVA, Table 1).
Figure 3.
Effect of Vitamin D3 supplementation on serum calcium (A), 25-OHD (B), and 1,25-OHD (C) in girls (A) and boys (B), in the long-term study, by treatment group. Low-dose group received 1,400 IU D3 per week and high-dose group 14,000 IU D3 per week. The box plot shows the median (50th percentile) as a line and the first (25th percentile) and third quartile (75th percentile) of the distribution as the lower and upper parts of the box. The whiskers shown above and below the boxes represent the largest and smallest observed values that are less than 1.5 box lengths from the end of the box. *, P < 0.05 in mean value of corresponding variable between 0 and 12 months within a treatment group; †, P < 0.0001 in mean value of corresponding variable at 12 months in the high-dose group, compared with both the low-dose and placebo groups. Biochemical assays are reported in metric units. To convert from metric to SI units, multiply calcium by 0.25 (millimoles per liter); 25-OHD by 2.496 (nanomoles per liter); 1,25-OHD by 2.6 (picomoles per liter).
Mean 1,25-OHD concentrations were comparable at study entry in the three groups. The level went down slightly in the placebo group and remained unchanged, compared with baseline in the two D3-treated groups (Table 1 and Fig. 3). The mean serum calcitriol level reached at 1 yr in the high-dose group was higher than that achieved in the placebo and low-dose groups (P < 0.0001 by ANOVA, Table 1).
Mean serum calcium did not change between baseline and 12 months within any of the treatment groups, and there was no difference in the mean serum calcium level among the three groups at 12 months (Table 1 and Fig. 3). Mean serum phosphate increased significantly, compared with baseline in both the low-dose and high-dose vitamin D arms, but no differences were noted between the two groups at 12 months (Table 1).
Boys
Mean 25-OHD concentrations in the placebo group were 16 ± 6 ng/ml at baseline and 17 ± 6 ng/ml at 1 yr (P = NS). In the group who received 1400 IU of vigantol oil per week, mean levels increased from 16 ± 7 ng/ml at baseline to 20 ± 7 ng/ml at 1 yr (P = 0.0034). In the group who received 14,000 IU of vigantol oil per week mean levels increased from 16 ± 7 ng/ml at baseline to 35 ± 9 ng/ml at 1 yr (P < 0.0001, Table 2 and Figure 3). The mean serum 25-OHD level at 1 yr was higher in the high-dose group, compared with the placebo and low-dose groups (P < 0.001 by ANOVA, Table 2).
Mean 1,25-OHD concentrations were comparable at study entry in the three groups and did not change over 1 yr (Table 2 and Fig. 3). There was no significant difference in the 1,25-OHD levels achieved at 1 yr among the three treatment groups.
Mean serum calcium did not change between baseline and 12 months within any of the treatment groups, and there was no difference in the mean serum calcium level among the three groups at 12 months (Table 2 and Figure 3). Mean serum phosphate increased significantly compared with baseline in all three groups, but there were no differences among the groups at 12 months (Table 2).
Safety of the treatment
Short-term study
At 8 wk of therapy, three subjects had a serum calcium at or above the upper limit of normal for their age (10.7 mg/dl) (17): two had received placebo and one Sigma D3 (Fig. 2 and Table 3). Three subjects reached a serum 25-OHD above 60 ng/ml at 8 wk of therapy: one received Vigantol oil and two received Sigma vitamin D3. Table 3 details all relevant biochemical parameters in these subjects. None had evidence of vitamin D intoxication, defined as frank elevations in both serum calcium and 25-OHD levels within the same individual (Table 3).
Table 3.
Biochemical profile of individuals with high serum calcium (>10.7 mg/liter) or high 25-OHD (>60 ng/ml) at 8 wk in the short-term study
| Variable unit | Subject | Treatment | Calcium (mg/dl) | 25-OHD (ng/ml) | 1,25-OHD (pg/ml) |
|---|---|---|---|---|---|
| High calcium | Male | Placebo | 11.4 | 50 | 75 |
| Male | Placebo | 10.9 | 26 | 50 | |
| Femalea | Sigma | 10.8 | 78 | 91 | |
| High 25-OHD | Female | Vigantol | 10.3 | 96 | 57 |
| Male | Sigma | 9.9 | 86 | 70 | |
| Femalea | Sigma | 10.8 | 78 | 91 |
Biochemical assays are reported in metric units. To convert from metric to SI units, multiply calcium by 0.25 (millimoles per liter); 25-OHD by 2.496 (nanomoles per liter); and 1,25-OHD by 2.6 (picomoles per liter).
Subject with concomitant high calcium and high 25-OHD.
Long-term study
Seven of 340 subjects (2%) had serum calcium levels above the upper limit of normal for children (10.7 mg/dl) at 1 yr. The serum calcium values ranged from 10.8 to 11.1 mg/dl in these subjects, of whom five were in the placebo group and one each in the low-dose and high-dose vitamin D groups. Similarly, five subjects (1.5%) had elevated 25-OHD levels at the end of the study ranging from 63 to 195 ng/ml; all were in the high-dose group, but none had concomitant hypercalcemia. The individual profile including serum Ca, 25-OHD, and 1,25-OHD of subjects who either had an elevated serum calcium or 25-OHD are detailed in Table 4 (Table 4).
Table 4.
Biochemical profile of individuals with high serum calcium (>10.7 mg/liter) or high 25-OHD (>60 ng/ml) at 1 yr, in the long-term study
| Study unit | Subject | Tanner stage | Treatment | Calcium (mg/dl) | 25-OHD (ng/ml) | Alk P (IU/liter) | 1,25-OHD (pg/ml) | PO4(mg/dl) |
|---|---|---|---|---|---|---|---|---|
| High calcium | Female | IV | Placebo | 11.1 | 9 | 118 | 5.3 | |
| Male | II | High dose | 11 | 32 | 289 | 103 | 5.3 | |
| Male | V | Low dose | 10.9 | 23 | 218 | 90 | 3.7 | |
| Male | II | Placebo | 10.8 | 21 | 306 | 131 | 4.5 | |
| Male | II | Placebo | 10.8 | 13 | 431 | 77 | 6.3 | |
| Male | IV | Placebo | 10.8 | 24 | 280 | 95 | 4.0 | |
| Female | V | Placebo | 10.8 | 16 | 88 | 50 | 4.5 | |
| High 25-OHD | Female | IV | High dose | 10.4 | 195 | 154 | 35 | 4.0 |
| Female | V | High dose | 10.5 | 161 | 75 | 70 | 4.0 | |
| Female | IV | High dose | 9.7 | 103 | 86 | 27 | 4.1 | |
| Male | V | High dose | 10.5 | 69 | 89 | 98 | 4.7 | |
| Male | V | High dose | 9.9 | 63 | 155 | 145 | 5.0 |
Biochemical assays are reported in metric units. To convert from metric to SI units, multiply calcium by 0.25 (millimoles per liter); phosphorous by 0.32 (millimoles per liter); 25-OHD by 2.496 (nanomoles per liter); 1,25-OHD by 2.6 (picomoles per liter). PO4, Phosphorus.
Adverse events
Twelve boys (6.5%) and 11 girls (9.8%) dropped out of the 1-yr trial. There were no differences in dropout rates by treatment group in either sex. The reasons for dropout included being afraid of needle pricks, unable to make appointments, not liking the taste of the medication, and changing their mind about the study. One girl dropped out at 7 months because of the development of glomerulonephritis, documented by biopsy, and treated as poststreptococcal glomerulonephritis. She was in the low-dose vitamin D treatment group.
Discussion
This report provides a substantial database establishing the long-term safety of weekly high doses of vitamin D at a critical time for bone mass accretion. At doses equivalent to 50 μg/d (2000 IU/d) Vitamin D3 was well tolerated and led to desirable 25-OHD levels in children and adolescents. There was no evidence of vitamin D intoxication, either in the short-term or long term study.
Although hypovitaminosis D is prevalent in children and adolescent worldwide (6), the recommended daily allowance of 200 IU/d is not sufficient to remedy this universal problem (4,5). In children, the effects of increasing levels of 25-OHD on calcitriol may be different from in adults because of differences in growth hormone, sex steroids, and their effects on the 1α-hydroxylase enzyme (18,19,20). But few are the studies that have used relatively high doses of vitamin D in the pediatric age group. One week of 40 μg (1600 IU)/d of vitamin D3 was not sufficient to prevent vitamin D insufficiency (21). A single oral dose of 150,000 IU vitamin D2 given to 79 children, aged 8.6 yr, maintained serum 25-OHD levels in midteens 5 months later, without inducing hypercalcemia or hypercalciuria (22). Similarly, administration of three oral doses of 100,000 IU of D3 every 2 months to male adolescents maintained serum 25-OHD levels in the low 20s (nanograms per milliliter) 2 months after the last dose (23). Finally, 50,000 IU vitamin D per day administered to seven Iranian girls for 20 d raised 25-OHD from 7.5 ± 2 to 23 ± 4 ng/ml (24). In this report, subjects given the equivalent of 2000 IU/d of vitamin D3 increased 25 OHD levels from the midteens to the mid-30s (nanograms per milliliter), and no subject had evidence for vitamin D intoxication. Toxicity is harder to achieve during rapid skeletal growth, a period characterized by mild secondary hyperparathyroidism and tendency for hypocalcemia (25). The safety of 2000 IU/d vitamin D3 in this report is consistent with studies using high doses of vitamin D in adults (1,11,12).
Although most, but not all, experts agree that a 25-OHD level of 30 ng/ml is desirable in adults (13,26,27,28), what constitutes an optimal D level for the younger subjects is more debatable. A desirable level may be defined based on homeostatic pathways involved in normal vitamin D physiology (6,28). Studies on the relationship between 25-OHD and PTH (6,7,29,30,31), seasonal variations in calciotropic hormones (7,29,30,32), and supplementation studies (22,23) suggest that at 25-OHD concentrations exceeding 30 ng/ml, PTH and calcitriol values are stable (22,23), similarly to data in adults (33). Adolescent girls who received 14,000 IU/wk vitamin D3 raised their 25-OHD from the mid teens (nanograms per milliliter) to the mid-30s, with a rise in calcitriol levels (long term study). Conversely, the use of the same dose in subjects with a baseline 25-OHD level of 40 ng/ml did not increase calcitriol further (short term study). The above suggests that 25-OHD levels in the teens in nanograms per deciliter are indeed suboptimal for children and adolescents, similarly to adults (6). Finally, the substantial increments in lean mass, bone area, and bone mass, specially in girls who received high-dose vitamin D group, confirm that mean 25-OHD levels in the mid-30s may indeed be desirable in adolescents (9). The mean baseline 25-OHD levels in the short-term study were higher than in the long term study because subjects were recruited in the summer and belonged to a high socioeconomic group (7). The fall-off in 25-OHD at the 8-wk-off time point indicates the combined effects of dose discontinuation and the time of year (December).
The dose of vitamin D required to produce the above desirable level is difficult to define due to differential exposure, variability in baseline 25-OHD levels, and differences in potencies between vitamin D2 and D3 (34,35,36). In children, similarly to adults, the mean 25-OHD response to each 100 IU of additional oral vitamin D3 is approximately 1 ng/ml (2.5 nmol/liter) (8,9). Therefore, in children with a serum 25-OHD concentration less than 20 ng/ml (<50 nmol/liter), a vitamin D dose equivalent to 2000 IU/d, preferably as vitamin D3 as opposed to vitamin D2 (35,36), would be an advisable replacement dose. There is no evidence to suggest that weekly administration of vitamin D3 is inferior to daily administration; furthermore, weekly administration may improve compliance. Indeed, at doses equivalent to 600 IU D3/d, there were no differences between the 25-OHD level reached with daily and weekly vitamin D administration, whereas the mean 25-OHD level reached with monthly administration was significantly lower (37).
The study has several limitations. Urinary calcium excretion and the potential development of asymptomatic kidney stones through routine ultrasonography were not assessed. Furthermore, urinary calcium would have been an earlier safety marker, increasing to maintain serum calcium normal. The observations reported do not apply to individuals in different periods of bone modeling or to study groups with adequate calcium intake or vitamin D status. The 25-OHD levels achieved herein may not be reproducible due to the well-described interassay variations between the various kits (38,39,40). However, the endocrine core laboratory measuring vitamin D levels in this report is a participant in an international quality assurance program for vitamin D assays (www.deqas.org). Furthermore, the increments in 25-OHD observed herein are in line with anticipated increments when taking the doses of vitamin D used into consideration (8).
Low vitamin D levels are associated with an increased risk of certain cancers (breast and prostate), autoimmune disorders, and cardiovascular diseases (1,41,42). Sunshine exposure or vitamin D intake during the teenage years are predictors of breast cancer (43) and prostate cancer (44). There are no trials unequivocally establishing the beneficial impact of vitamin D on the above diseases; however, post hoc analyses from a calcium-vitamin D trial revealed a decreased risk of insulin resistance in high-risk individuals (45). The high prevalence of hypovitaminosis D worldwide across all age groups, the fact that many diseases of adulthood are rooted in the pediatric age group, and the safety data available to date render it quite compelling to modify the current recommendations regarding adequate vitamin D intake for not only adults but also children (6,46).
Supplementation of children and adolescents with 2000 IU/d vitamin D3 is well tolerated and safe. This is particularly relevant in view of the increasingly recognized musculoskeletal benefits of vitamin D in not only the adult but also the pediatric age group and the pleotropic effects of vitamin D on multiple physiologic and pathologic processes.
Supplementary Material
Acknowledgments
The authors thank the administrators, school nurses, parents, and students from the American Community School, the International College, Amlieh School, and Ashbal Al Sahel School for their support in making the study possible. The authors also thank Mrs. U. Usta for her assistance in preparing the vitamin D solutions and implementing the randomization protocol, Mrs. S. Mroueh for her expert technical assistance in the acquisition and analyses of the bone mineral density scans, and Mrs. C. Hajj Shahine for her tireless efforts in running the hormonal assays.
Footnotes
This work was largely supported by an educational grant from the Nestle Foundation and a grant from Merck KGaA.
Disclosure Information: J.M., M.N., S.K., R.E.-R., and Z.M, have nothing to disclose. R.V. receives lecture fees from Merck KGaA. G.E.-H.F. serves on the SERM Advisory Board of Eli Lilly and received lecture fees from Eli Lilly and grant support from Eli Lilly, Sanofi-Aventis, and Novartis.
First Published Online April 29, 2008
Abbreviations: CV, Coefficient of variation; 25-OHD, 25-hydroxyvitamin D; 1,25-OHD, 1,25-hydroxyvitamin.
References
- Holick MF 2007 Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- Weaver CM, Peacock M, Johnston Jr CC 1999 Adolescent nutrition in the prevention of postmenopausal osteoporosis. J Clin Endocrinol Metab 84:1839–1843 [DOI] [PubMed] [Google Scholar]
- Baker SS, Cochran WJ, Flores CA, Georgieff MK, Jacobson MS, Jaksic T, Krebs NF 1999 American Academy of Pediatrics. Committee on Nutrition. Calcium requirements of infants, children, and adolescents. Pediatrics 104(5 Pt 1):1152–1157 [PubMed] [Google Scholar]
- 1999 Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. http://www.nap.edu/html/dri_calcium/tables.html (accessed on June 11, 2008) [Google Scholar]
- Gartner LM, Greer FR 2003 Section on Breastfeeding and Committee on Nutrition. American Academy of Pediatrics. Prevention of rickets and vitamin D deficiency: new guidelines for vitamin D intake. Pediatrics 111(4 Pt 1):908–910 [DOI] [PubMed] [Google Scholar]
- El-Hajj Fuleihan G, Vieth R 2007 Vitamin D insufficiency and musculoskeletal health in children and adolescents. In Burckhardt P, Heaney P, Dawson-Hughes B, eds. Nutritional aspects of osteoporosis, 2006. International Congress Series 1297. San Diego: Elsevier B.V.; 91–108 [Google Scholar]
- El-Hajj Fuleihan G, Nabulsi M, Choucair M, Salamoun M, Hajj Shahine C, Kizirian A, Tannous R 2001 Hypovitaminosis D in healthy schoolchildren. Pediatrics 107:1–7 [DOI] [PubMed] [Google Scholar]
- Viljakainen HT, Natri AM, Kärkkäinen M, Huttunen MM, Palssa A, Jakobsen J, Cashman KD, Mølgaard C, Lamberg-Allardt C 2006 A positive dose-response effect of vitamin D supplementation on site-specific bone mineral augmentation in adolescent girls: a double-blinded randomized placebo-controlled 1-year intervention. J Bone Miner Res 21:836–844 [DOI] [PubMed] [Google Scholar]
- El-Hajj Fuleihan G, Nabulsi M, Tamim H, Maalouf J, Salamoun M, Khalifeh H, Shoucair M, Arabi A, Vieth R 2006 Effect of vitamin D replacement on musculoskeletal parameters in healthy school children: a randomized controlled trial. J Clin Endocrinol Metab 91:405–412 [DOI] [PubMed] [Google Scholar]
- Maalouf J, Beck T, Nabulsi M, Arabi A, El-Hajj Fuleihan G 2006 Impact of vitamin D supplementation on hip structural analysis parameters in children and adolescents. J Bone Miner Res 21(Suppl 1):S29 (Abstract) [Google Scholar]
- Vieth R 1999 Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 69:842–856 [DOI] [PubMed] [Google Scholar]
- Vieth R, Chan P, MacFarlane GD 2001 Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr 73:288–294 [DOI] [PubMed] [Google Scholar]
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R 2005 Estimates of optimal vitamin D status. Osteoporos Int 16:713–716 [DOI] [PubMed] [Google Scholar]
- Vieth R, Kimball S, Hu A, Walfish PG 2004 Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 μg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutr J 3:8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1997 Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. http://books.nap.edu/openbook.php?record_id=5776&page=250 (accessed on June 11, 2008) [Google Scholar]
- Glastre C, Braillon P, David L, Cochat P, Meunier PJ, Delmas PD 1990 Measurement of bone mineral content of the lumbar spine by dual energy x-ray absorptiometry in normal children: correlations with growth parameters. J Clin Endocrinol Metab 70:1330–1333 [DOI] [PubMed] [Google Scholar]
- Lockitch G, Halstead AC, Albersheim S, MacCallum C, Quigley G 1988 Age- and sex-specific pediatric reference intervals for biochemistry analytes as measured with the Ektachem-700 analyzer. Clin Chem 34:1622–1625 [PubMed] [Google Scholar]
- Aksnes L, Aarskog D 1982 Plasma concentrations of vitamin D metabolites in puberty: effect of sexual maturation and implications for growth. J Clin Endocrinol Metab 55:94–101 [DOI] [PubMed] [Google Scholar]
- Ilich JZ, Badenhop NE, Jelic T, Clairmont AC, Nagode LA, Matkovic V 1997 Calcitriol and bone mass accumulation in females during puberty. Calcif Tissue Int 61104–61109 [DOI] [PubMed] [Google Scholar]
- Maalouf J, Mahfoud Z, Arabi A, Nabulsi M, El-Hajj Fuleihan, G 2005 Calciotropic hormones, mineral and bone metabolism across puberty. Bone 36(Suppl 1):Abstract S50–S60 [Google Scholar]
- Docio S, Riancho JA, Perez A, Olmos JM, Amado JA, Gonzallez-Macias J 1998 Seasonal deficiency of vitamin D in children: a potential target for osteoporosis-preventing strategies? J Bone Miner Res 13:544–548 [DOI] [PubMed] [Google Scholar]
- Oliveri B, Cassinelli H, Mautalen C, Ayala M 1996 Vitamin D prophylaxis in children with a single dose of 150,000 IU of vitamin D. Eur J Clin Nutr 50:807–810 [PubMed] [Google Scholar]
- Guillemant J, Le HT, Maria A, Allemandou A, Peres G, Guillemant S 2001 Wintertime vitamin D deficiency in male adolescents: effect on parathyroid function and response to vitamin D3 supplements. Osteoporos Int 12:875–879 [DOI] [PubMed] [Google Scholar]
- Dahifar H, Faraji A, Ghorbani A, Yassobi S 2006 Impact of dietary and lifestyle on vitamin D in healthy student girls aged 11–15 years. J Med Invest 53:204–208 [DOI] [PubMed] [Google Scholar]
- Matkovic V, Goel PK, Badenhop-Stevens NE, Landoll JD, Li B, Ilich JZ, Skugor M, Nagode LA, Mobley SL, Ha EJ, Hangartner TN, Clairmont A 2005 Calcium supplementation and bone mineral density in females from childhood to young adulthood: a randomized controlled trial. Am J Clin Nutr 81:175–188 [DOI] [PubMed] [Google Scholar]
- Holick MF 2006 High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 81:353–373 [DOI] [PubMed] [Google Scholar]
- Vieth R 2004 Why the optimal requirement for vitamin D3 is probably much higher than what is officially recommended for adults. J Steroid Biochem Mol Biol 89–90:575–579 [DOI] [PubMed] [Google Scholar]
- Lips P 2004 Which circulating level of 25-hydroxyvitamin D is appropriate? J Steroid Biochem Molec Biol 89–90:611–614 [DOI] [PubMed] [Google Scholar]
- Guillemant J, Cabrol S, Allemandou A, Peres G, Guillemant S 1995 Vitamin D-dependent seasonal variation of PTH in growing male adolescents. Bone 17:513–516 [DOI] [PubMed] [Google Scholar]
- Guillemant J, Taupin P, Le HT, Taright N, Allemandou A, Pérès G, Guillemant S 1999 Vitamin D status during puberty in French healthy male adolescents. Osteoporos Int 10:222–225 [DOI] [PubMed] [Google Scholar]
- Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ 2004 Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med 158:531–537 [DOI] [PubMed] [Google Scholar]
- Oliveri MB, Ladizesky M, Mautalen CA, Alonso A, Martinez L 1993 Seasonal variations of 25 hydroxyvitamin D and parathyroid hormone in Ushuaia (Argentina), the southernmost city of the world. Bone Miner 20:99–108 [DOI] [PubMed] [Google Scholar]
- Vieth R, McCarten K, Norwich KH 1990 Role of 25-hydroxyvitamin D3 dose in determining rat 1,25-dihydroxyvitamin D3 production. Am J Physiol 258(5 Pt 1):E780–E789 [DOI] [PubMed] [Google Scholar]
- Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R 1998 Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr 68:854–858 [DOI] [PubMed] [Google Scholar]
- Armas LA, Hollis BW, Heaney RP 2004 Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 89:5387–5391 [DOI] [PubMed] [Google Scholar]
- Houghton LA, Vieth R 2006 The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr 84:694–697 [DOI] [PubMed] [Google Scholar]
- Chel V, Wijnhoven HA, Smit JH, Ooms M, Lips P 2008 Efficacy of different doses and time intervals of oral vitamin D supplementation with or without calcium in elderly nursing home residents. Osteoporos Int 19:663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis BW 2004 The determination of circulating 25-hydroxivitamin D: no easy task. J Clin Endocrinol Metab 89:3149–3151 (Editorial) [DOI] [PubMed] [Google Scholar]
- Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, DeLuca HF, Drezner MK 2004 Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab 89:3152–3157 [DOI] [PubMed] [Google Scholar]
- Lips P, Chapuy MC, Dawson-Hughes B, Pols HA, Holick MF 1999 An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos Int 9:394–397 [DOI] [PubMed] [Google Scholar]
- Hathcock J, Shao A, Vieth R, Heaney R 2007 Risk assessment for vitamin D. Am J Clin Nutr 85:6–18 [DOI] [PubMed] [Google Scholar]
- Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, McGrath JJ, Norman AW, Scragg R, Whiting SJ, Willett WC, Zittermann A 2007 The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 85:649–650 [DOI] [PubMed] [Google Scholar]
- Knight JA, Lesosky M, Barnett H, Raboud JM, Vieth R 2007 Vitamin D and reduced risk of breast cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev 16:422–429 [DOI] [PubMed] [Google Scholar]
- Luscombe CJ, Fryer AA, French ME, Liu S, Saxby MF, Jones PW, Strange RC 2001 Exposure to ultraviolet radiation: association with susceptibility and age at presentation with prostate cancer. Lancet 358:641–642 [DOI] [PubMed] [Google Scholar]
- Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, Manson JE, Hu FB 2006 Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care 29:650–656 [DOI] [PubMed] [Google Scholar]
- Heaney RP 2003 Long-latency deficiency disease: insights from calcium and vitamin D. Am J Clin Nutr 78:912–919 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.