Abstract
Context: Hypovitaminosis D appears to be on the rise in young children, with implications for skeletal and overall health.
Objective: The objective of the study was to compare the safety and efficacy of vitamin D2 daily, vitamin D2 weekly, and vitamin D3 daily, combined with supplemental calcium, in raising serum 25-hydroxyvitamin D [25(OH)D] and lowering PTH concentrations.
Design: This was a 6-wk randomized controlled trial.
Setting: The study was conducted at an urban pediatric clinic in Boston.
Subjects: Forty otherwise healthy infants and toddlers with hypovitaminosis D [25(OH)D < 20 ng/ml] participated in the study.
Interventions: Participants were assigned to one of three regimens: 2,000 IU oral vitamin D2 daily, 50,000 IU vitamin D2 weekly, or 2,000 IU vitamin D3 daily. Each was also prescribed elemental calcium (50 mg/kg·d). Infants received treatment for 6 wk.
Main Outcome Measures: Before and after treatment, serum measurements of 25(OH)D, PTH, calcium, and alkaline phosphatase were taken.
Results: All treatments approximately tripled the 25(OH)D concentration. Preplanned comparisons were nonsignificant: daily vitamin D2 vs. weekly vitamin D2 (12% difference in effect, P = 0.66) and daily D2 vs. daily D3 (7%, P = 0.82). The mean serum calcium change was small and similar in the three groups. There was no significant difference in PTH suppression.
Conclusions: Short-term vitamin D2 2,000 IU daily, vitamin D2 50,000 IU weekly, or vitamin D3 2,000 IU daily yield equivalent outcomes in the treatment of hypovitaminosis D among young children. Therefore, pediatric providers can individualize the treatment regimen for a given patient to ensure compliance, given that no difference in efficacy or safety was noted among these three common treatment regimens.
Vitamin D2 or vitamin D3, each administered with supplemental calcium, are equally safe and efficacious for the treatment of hypovitaminosis D among young children based on a 6-week randomized controlled trial.
Vitamin D deficiency, or hypovitaminosis D, appears to be on the rise in young children, with an increased prevalence noted among African-American breast-fed infants residing in northern latitudes (1). This deficiency has been identified as the leading cause of rickets among infants, as breast milk contains inadequate amounts of vitamin D to support skeletal health in this age group (2,3). Furthermore, numerous sources of evidence now indicate that vitamin D (cholecalciferol) has several important physiological effects beyond calcium absorption and bone maintenance (4,5), and early vitamin D repletion through supervised supplementation may have a positive impact on later neurologic health (6,7), immune function (8,9), and chronic disease risk (10,11). With the reemergence of hypovitaminosis D among infants and toddlers, questions regarding the most appropriate treatment regimen require clarification.
Multiple treatment regimens have been proposed to treat hypovitaminosis D in young children, including daily or weekly dosing for varying periods of time. Common recommendations include 200,000 IU vitamin D3 every 3 months (12), 1,000–2,000 IU vitamin D2 or vitamin D3 daily for several weeks (13), or the administration of a single im injection of 600,000 IU of vitamin D2, repeated after 12 wk (14,15,16). A regimen that has been used commonly in treating adults is 5,000–15,000 IU of vitamin D2 weekly for 4–8 wk (17,18). Another effective method for treating hypovitaminosis D in adult patients is an oral dose of 50,000 IU of vitamin D2 weekly for 8 wk, with subsequent increases in serum 25-hydroxyvitamin D [25(OH)D] and decreases in PTH concentrations noted (19). Little information currently exists regarding the safety or efficacy of these vitamin D treatment doses in a pediatric population. The form of vitamin D administered during treatment also remains as an area of debate, and again little information is available in children. Recent reports citing data in adults suggest that vitamin D3 may provide a more efficacious treatment regimen than vitamin D2, in both terms of potency and duration of action (20,21,22). However, there is controversy surrounding this point because one recent study in adults noted the two treatment methods to be equally effective (23). Additionally, a study of infants and their mothers showed that vitamin D2 and vitamin D3 accounted for a similar proportion of total 25(OH)D concentration in neonates (cord blood) and maternal serum (24).
The aim of the present study was to examine prospectively three common treatment short-term regimens for correction of hypovitaminosis D in infants and toddlers. We conducted a randomized clinical trial, treating participants with either a daily low dose of vitamin D2, a higher dose of vitamin D2 once weekly, or a low dose of vitamin D3 once daily. This study examined: 1) the efficacy of each treatment in raising serum 25(OH)D and lowering PTH concentrations; and 2) the safety and tolerance of each regimen in infants and toddlers, as evaluated through documentation of hypo- or hypercalcemia and reported symptoms.
Subjects and Methods
Subjects
A complete description of the referral sample for this treatment trial has been published previously (25). Briefly, during the cross-sectional screening portion of the study, 380 infants and toddlers, aged 8–24 months, were enrolled consecutively throughout the calendar year from the Children’s Hospital Boston Primary Care Center between October 2005 and June 2007. Subjects completed a nutritional survey, and serum measurements of 25(OH)D, alkaline phosphatase, PTH, calcium, phosphorus, and magnesium were obtained. Skin pigmentation and sun sensitivity were evaluated by a research assistant using established methods (26,27). Exclusion criteria for the study included having a chronic disease (e.g. asthma, seizure disorder, sickle cell disease), the use of oral glucocorticoid over the previous 3 months, or other therapy known to affect vitamin D metabolism. Patients found to be vitamin D deficient [25(OH)D ≤ 20 ng/ml (50 nmol/liter)] were invited to participate in the randomized clinical trial, which included randomization to one of three vitamin D treatment regimens. The Committee on Clinical Investigation, Children’s Hospital Boston, approved the study protocol, and parents or guardians of all participants provided written informed consent.
Treatment protocol
Patients identified to have hypovitaminosis D were randomly assigned to one treatment protocol. The randomization list was stratified by age at screening (9 or 18 months) and blocked in randomly permuted sequences of 3 or 6, ensuring that no treatment would be disproportionately represented in any season or age group. The vitamin D treatments included one of three regimens: 2,000 IU oral ergocalciferol (vitamin D2) daily, 50,000 IU vitamin D2 weekly, or 2,000 IU cholecalciferol (vitamin D3) daily. Each group was also prescribed 50 mg/kg·d of elemental calcium to prevent hypocalcemia associated with hungry bone syndrome (14). Infants received the combined vitamin D and calcium treatment for a course of 6 wk.
Vitamin D and calcium supplements were each provided in a liquid suspension that was administered orally from a vial directly onto the tongue. Parents were instructed to shake the vial before administration. The vitamin D2 preparation (200 IU per drop or 0.025 ml) was manufactured by Sanofi-Synthelabo Inc. (Bridgewater, NJ), and doses were provided as 10 drops or 0.25 ml daily for the 2,000 IU dose and 6.25 ml weekly for the 50,000 IU dose; for each vitamin D2 dose, the suspension was administered via a provided dropper onto the tongue. The vitamin D3 (2000 IU per drop, oil and water emulsion) was provided by Biotics Research Corp. (Rosenberg, TX), and one drop or 0.025 ml was administered daily from the vial directly onto the child’s tongue. Assays of products ensured potency. The administration of the vitamin and mineral preparations in liquid form prevented problems with swallowing pills that may have presented a choking or compliance risk in our young patient population.
After approximately 3 wk of treatment, a research assistant contacted each family to ensure that the child was receiving both the calcium and vitamin D without difficulty. Within 1 wk after completing the treatment regimen, repeat serum 25(OH)D concentrations along with PTH, calcium, magnesium, phosphorus, and alkaline phosphatase were measured. To assess compliance, the amount of vitamin D and calcium syrups remaining in the respective bottles was measured by a technician in the Central Pharmacy at Children’s Hospital Boston. Outcome measures included changes in serum 25(OH)D, PTH, and alkaline phosphatase levels between baseline and follow-up. Two comparisons were formally designated as being of primary interest: daily vitamin D2 vs. weekly vitamin D2 and daily vitamin D2 vs. daily vitamin D3.
Laboratory measurements
During the baseline and follow-up visits, one blood sample (15 ml) was obtained from each subject. Laboratory samples were processed immediately at both Children’s Hospital Boston and ARUP Laboratories (Salt Lake City, UT). Serum 25(OH)D levels were measured at ARUP Laboratories, using a Diasorin chemiluminescent assay (LIAISON; DiaSorin Inc., Stillwater, MN). This assay accurately quantifies the sum of both 25(OH)D3 and 25(OH)D2. A multichannel analyzer (Roche Diagnostics, Indianapolis, IN) was used to measure serum calcium, phosphorus, magnesium, and alkaline phosphatase levels on site. Intact PTH was measured by a two-site chemiluminescence immunoassay (Nichols Institute, San Clemente, CA).
Interassay coefficients of variation were 5.4–7% for PTH, 8.6–10.0% for 25(OH)D, 0.67% for alkaline phosphatase, and 1.5–2.2% for the cations. The definition of hypovitaminosis D correlated with the lowest end of the normal reference range as provided by the manufacturer (Diasorin). Identification of severe hypovitaminosis D was consistent with the 25(OH)D Diasorin LIAISON assay sensitivity (7 ng/ml).
Statistics
We conducted an intention-to-treat analysis, attributing the assigned treatment to all randomized subjects regardless of compliance. To compare baseline characteristics among the three trial arms, we used Fisher’s extract test for dichotomous variables and one-way ANOVA for continuous measures, the latter corroborated in cases of skewed distribution by the Kruskal-Wallis test.
Changes in 25(OH)D, PTH, alkaline phosphatase, and cation levels were assessed and compared among trial arms by repeated-measures analysis of covariance (ANCOVA), adjusted for age, weight, sex, skin tone, and sun sensitivity. All concentrations showed mildly skewed distributions and were log transformed for analysis. For reporting, levels were retransformed to concentration units. Mean changes on the log scale were constructed from parameters of the fitted ANCOVA and expressed as percentages, e.g. 100% × [exp(ΔlogPTH) − 1]. The two contrasts of primary interest, daily D2-weekly D2 and daily D2-daily D3, were constructed from parameters of the ANCOVA and compared with zero with a two-sided model, Bonferroni-adjusted critical P value of 0.025.
Pretrial power calculations for the two primary comparisons indicated that a sample of 15 subjects per arm would provide 80% power to detect a difference between treatments on the order of 60% for the 6-wk change in 25(OH)D. These calculations were based on cross-sectional data from our adolescent clinic (28) and an estimated intraclass correlation of 0.5 (not obtainable from the cross-sectional data).
Statistical computations and generation of the randomization list were performed with SAS software version 9.1 (SAS Institute, Cary, NC).
Results
Subjects
Within our clinical sample of 380 infants and toddlers (25), we identified 40 infants and toddlers to have hypovitaminosis D [25(OH)D ≤ 20 ng/ml (50 nmol/liter)]. Within this sample of 40 participants, 35 completed the course of therapy (87.5%). The baseline characteristics of participants in the three treatment arms are illustrated in Table 1. There were no significant differences between groups with respect to gender, skin pigmentation, skin sensitivity, or season of year at baseline, before randomization. Biochemically, participants were also similar, and weight and age did not significantly differ across treatment groups.
Table 1.
Baseline characteristics of participants, compared across randomly assigned treatment groups
| D2 daily (n = 12), n (%) | D2 weekly (n = 14), n (%) | D3 daily (n = 14), n (%) | Pa | |
|---|---|---|---|---|
| Gender | ||||
| Male | 4 (33) | 6 (43) | 8 (57) | 0.45 |
| Female | 8 (67) | 8 (57) | 6 (43) | |
| Skin pigmentation | 0.56 | |||
| 1 (heaviest) | 7 (58) | 9 (64) | 9 (64) | |
| 2 | 4 (33) | 3 (21) | 3 (21) | |
| 3 | 1 (8) | 2 (14) | 0 (0) | |
| 4 (lightest) | 0 (0) | 0 (0) | 2 (14) | |
| Skin sensitivity | 0.35 | |||
| 1 (burn easily) | 0 (0) | 0 (0) | 1 (7) | |
| 2 (burn always) | 0 (0) | 0 (0) | 0 (0) | |
| 3 (burn moderately) | 0 (0) | 2 (14) | 2 (14) | |
| 4 (burn minimally) | 3 (25) | 2 (14) | 2 (14) | |
| 5 (burn rarely) | 7 (58) | 6 (43) | 9 (64) | |
| 6 (never burn) | 2 (17) | 4 (29) | 0 (0) | |
| Month of enrollment | 0.94 | |||
| Darkest (November-January) | 5 (42) | 4 (29) | 3 (21) | |
| Intermediate (February-April) | 2 (17) | 2 (14) | 3 (21) | |
| Lightest (May-July) | 2 (17) | 4 (29) | 5 (36) | |
| Intermediate (August-October) | 3 (25) | 4 (29) | 3 (21) | |
| Median (QD), minimum-maximumb | ||||
| Age, months | 10.0 (3.5), 9.0–21.6 | 9.8 (1.5), 7.6–22.6 | 10.1 (1.9), 8.0–22.9 | 0.63 |
| Weight, kg | 10.1 (0.8), 8.2–12.0 | 9.2 (0.6), 7.5–11.4 | 9.5 (1.0), 7.2–12.4 | 0.41 |
| 25(OH)D, ng/ml | 18 (3), 7–20 | 17 (4), 7–20 | 17 (4), 7–20 | 0.75 |
| PTH, pg/ml | 33 (16), 12–166 | 27 (45), 7–508 | 37 (14), 16–72 | 0.22 |
| Alkaline phosphatase, U/liter | 267 (51), 144–553 | 329 (97), 192–708 | 241 (58), 172–537 | 0.14 |
| Calcium, mg/dl | 10.4 (0.2), 9.7–11.2 | 10.4 (0.3), 7.0–11.1 | 10.3 (0.3), 9.9–10.8 | 0.59 |
| Magnesium, mg/dl | 2.5 (0.1), 2.2–2.7 | 2.3 (0.2), 2.0–2.7 | 2.3 (0.2), 2.0–2.8 | 0.30 |
| Phosphorus, mg/dl | 5.6 (0.5), 3.7–6.8 | 5.9 (0.5), 2.5–7.0 | 5.8 (0.5), 4.3–6.4 | 0.80 |
Testing for equal distribution in the three treatment arms. For binary and polytomous variables, Fisher exact test; for continuous measures, one-way ANOVA, corroborated by Kruskal-Wallis test in cases of skewed distribution.
QD equals half the interquartile range (75th percentile minus 25th percentile), analogous to sd.
Treatment effects on serum 25(OH)D
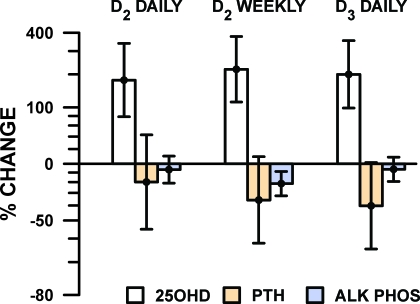
All three treatments virtually tripled the 25(OH)D concentration in these vitamin D-deficient children (Fig. 1; Table 2). The greatest effect was attained with weekly vitamin D2: from 13.8 to 44.0 ng/ml, an increase of 220%. The next greatest was the effect of D3 (13.7–41.2 ng/ml, 202%), followed by daily vitamin D2 (15.7–43.9 ng/ml, 182%). The preplanned comparisons were nonsignificant: daily vitamin D2 vs. weekly vitamin D2 (12% differences in effect, P = 0.66) and daily D2 vs. daily D3 (7%, P = 0.82). All participants achieved 25(OH)D concentrations of 20 ng/ml or greater except for three participants. Within this subgroup, one participant was receiving vitamin D3 daily, the other two, vitamin D2 weekly. For each case, the compliance of the family had been questioned at the follow-up visit.
Figure 1.
Change in serum 25(OH)D and related markers in infants and toddlers diagnosed with hypovitaminosis D [(25OH)D ≤ 20 ng/dl], after 6 wk of treatment with randomly assigned treatment regimens. Mean and 95% confidence interval from repeated-measures regression analysis of log-transformed concentration measures are shown.
Table 2.
Serum levels of 25(OH)D vitamin and related biochemical measurements (before and after treatment, all participants)
| Median (25th percentile to 75th percentile)
|
||
|---|---|---|
| Baseline | After treatment | |
| 25(OH)D, ng/ml | 17 (11–19) | 36 (23–70) |
| PTH, pg/ml | 34 (20–50) | 24 (18–35) |
| Alkaline phosphatase, U/liter | 283 (232–383) | 269 (211–350) |
| Calcium, mg/dl | 10.4 (10.1–10.7) | 10.3 (10.1–10.6) |
| Magnesium, mg/dl | 2.4 (2.2–2.5) | 2.3 (2.2–2.4) |
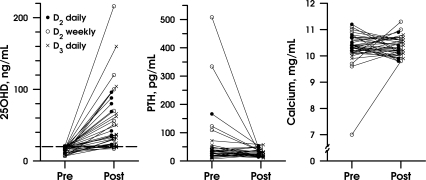
The two preplanned contrasts were small in comparison with the 200% pre-post change, and both were nonsignificant. Daily vitamin D2 showed an effect 12% lower than weekly vitamin D2 (P = 0.66) and 7% lower than daily D3 (P = 0.82). Post hoc power calculations using the attained sample size and ses showed that the conjectured 60% difference between arms was detectable with only 22% power, partly due to a lower correlation than anticipated (0.2 vs. 0.5) between baseline and the greatly increased posttreatment levels (Fig. 2).
Figure 2.
Absolute changes in serum 25(OH) D, PTH, and calcium are depicted from the baseline and follow-up visits for the three treatment groups: vitamin D2 (2,000 IU) daily (closed circle) and (50,000 IU) weekly (open circle), and vitamin D3 (2,000 IU) daily (x).
Calcium
We examined serum calcium concentrations from the larger cross-sectional sample of healthy infants and toddlers from which the trial participants were derived. Baseline calcium concentrations were compared with the current trial participants, each with hypovitaminosis D, to 329 vitamin D replete subjects. The median calcium level was slightly higher in the vitamin D replete subjects (10.50 vs. 10.35, P = 0.04 by Wilcoxon test).
The mean change in serum calcium levels was small and similar in the three treatment groups (−3% for vitamin D2 daily, +3% vitamin D2 weekly, +1% vitamin D3 daily).
PTH
Eight participants (20%) presented with elevated PTH at baseline (reference range 10–65 pg/ml). All cases returned to normal limits after treatment (Table 2). As illustrated in Fig. 1, the largest change in PTH was observed in the group receiving vitamin D2 weekly (down 40%, from 32.1 to 19.2 pg/ml, adjusted for covariates), compared with patients in the other treatment arms (vitamin D2 daily, down 20% from 38.5 to 30.8 pg/ml, and vitamin D3 daily, down 36% from 40.9 to 26.3 pg/ml). There was no significant difference in PTH suppression among the three groups (P = 0.74).
Alkaline phosphatase
There was no significant impact of treatment on alkaline phosphatase concentrations (Fig. 1).
Compliance
To assess compliance, we submitted vials containing the remaining vitamin D and calcium liquid preparations to the Children’s Hospital Boston Central Pharmacy. The amount remaining in the vials was compared with the expected amount consumed. No appreciable difference was noted in compliance among the three treatment groups.
Discussion
To our knowledge, this study is the first to compare the efficacy and safety of three common short-term treatment regimens to correct hypovitaminosis D in infants and toddlers. We report no difference in outcome between vitamin D2 daily, vitamin D2 weekly, or vitamin D3 daily for a sample of young children. These findings are consistent with one previous report in adults suggesting that these two formulations contribute equally to circulating 25(OH)D levels (23).
Three previous reports citing data from adults have advocated strongly for supplementation with vitamin D3 (cholecalciferol) over D2 (ergocalciferol) as the preferred treatment method for vitamin D deficiency (20,21,22). However, our study showed that each treatment regimen was equally effective as well as safe. These data are reassuring to providers because vitamin D2 daily or weekly, or vitamin D3 daily, combined with elemental calcium, appears to provide an effective and well-tolerated treatment for correcting hypovitaminosis D in infants and toddlers. Furthermore, the consistency of these data across the treatment arms will allow practitioners to tailor their specific treatment regimens to meet an individual patient’s needs, preferences, and probability of compliance.
In this study, we sought to examine the differences between PTH at baseline and after replacement therapy in each treatment group because suboptimal serum 25(OH)D levels can be associated with a secondary or compensatory hyperparathyroidism. Interestingly, the largest change in PTH between baseline and 7 wk was observed in the group receiving vitamin D2 weekly. At baseline, we found elevated PTH concentrations in eight subjects among the cohort, and all participants’ levels decreased to the normal range after treatment. These findings further support the similarities among these three treatment arms in reversing the secondary effects that associated biochemical markers may have on vitamin D homeostasis.
Our data provide clinical guidance regarding the appropriate dosage range of vitamin D to treat deficiency in this young population. Among infants, hypercalcemia has been reported with the administration of single high-dose therapy of 300,000 IU (29) or 600,000 IU (30) as well as daily doses exceeding 10,000 IU daily (31). Whereas a single 600,000 IU dose has been strongly advocated by one group as a safe regimen and one that eliminates the problem of noncompliance (12), this recommendation has been met with controversy and, specifically, concerns about hypercalcemia (29,32), especially in an outpatient setting. In our study, we report a surprising higher overall incidence of mild hypercalcemia at baseline in contrast to after treatment. All subjects were asymptomatic. There was no statistically significant correlation between the presence of hypercalcemia at baseline and after each tested course of treatment. Therefore, these more conservative regimens of vitamin D2 daily, vitamin D2 weekly, or vitamin D3 daily may provide the necessary treatment without the increased risk of hypercalcemia commonly associated with single large dose therapies (also known as stosstherapy) (12). The potential toxicity associated with stosstherapy is further underscored by a recent report that showed hypercalcemia in an infant treated with the equivalent of four daily stosstherapy doses (33).
Limitations of this study deserve acknowledgment and consideration. First, the sample size was small and power limited. The observed differences among the three treatment regimens in improvement of 25(OH)D level, although consistently small in comparison with the gross change achieved by treatment, were not precisely determined and thus admit the possibility of larger underlying effects. Our negative finding may therefore be due to the small sample size, although likely not to variability in age or weight, for which we controlled in our analyses. Second, it is more difficult to assess compliance among young infants and toddlers because they are unable to swallow pills, necessitating administration of a liquid vitamin preparation. Therefore, measurements of the exact amount of remaining vitamin D suspension administered during treatment were more difficult to assess, compared with pill counts that would be possible in an older patient population. To standardize our data acquisition, a single pharmacy technician completed all measurements throughout the course of the study. However, the potential inaccuracy of viscous liquid (vs. pill) measurement deserves acknowledgment, including the fact that the measurement involved extraction of the suspension from a vial. Lastly, it is possible that the participants’ parents provided increased amounts of dietary vitamin D (e.g. vitamin D-fortified milk, salmon, eggs) in addition to the supplementation upon hearing of their child’s deficiency. However, such low-potency dietary modification is not likely to have significantly affected one treatment group’s results, compared with another, and not in a way that would change the observed consistency noted among the treatment groups.
In summary, we demonstrate that 2,000 IU daily vitamin D2, 50,000 IU vitamin D2 weekly, or 2,000 IU daily vitamin D3 yield equivalent outcomes in the short-term treatment of hypovitaminosis D among otherwise healthy infants and toddlers. These results indicate that pediatric providers can determine the appropriate method of treatment for a given patient or family to ensure compliance, given that no difference in efficacy or safety was noted. The argument favoring large-dose depot therapies for correcting hypovitaminosis D must be reevaluated because more conservative lower dose therapies may provide a safer method of treatment, especially in the outpatient setting, without the associated risk of hypercalcemia. In addition, the case for vitamin D3 as the most effective treatment method must be reconsidered for young children because a weekly dose of vitamin D2 may yield a similar outcome without the inconvenience of a daily treatment. We recommend early treatment with one of these three treatment regimens, or subtle variations to the dosages studied, to prevent the potential skeletal and extraskeletal problems associated with hypovitaminosis D. Lastly, we do not endorse the use of the current relatively high doses of vitamin D for the long-term prevention of hypovitaminosis D in infants and young children. Further research is needed to clarify the appropriate daily vitamin D supplementation dose for the pediatric age group.
Acknowledgments
We acknowledge and thank our patients and their families, clinical providers within the Children’s Hospital Boston Primary Care Center, and Stephanie Bristol, Melissa Chittle, and Lisa Bartels for outstanding technical assistance.
Footnotes
This work was supported by grants from the Allen Foundation and McCarthy Family Foundation; National Institutes of Health Grant MO1-RR-2172 to the Children’s Hospital Boston General Clinical Research Center; and Project T71 MC00009 Maternal and Child Health Bureau, Human Resources and Services Administration.
Disclosure Summary: All authors have nothing to disclose.
First Published Online April 15, 2008
Abbreviations: ANCOVA, Analysis of covariance; 25(OH)D, 25-hydroxyvitamin D.
References
- Ziegler EE, Hollis BW, Nelson SE, Jeter JM 2006 Vitamin D deficiency in breastfed infants in Iowa. Pediatrics 118:603-610 [DOI] [PubMed] [Google Scholar]
- Pettifor JM 2004 Nutritional rickets: deficiency of vitamin D, calcium, or both? Am J Clin Nutr 80:1725-1729 [DOI] [PubMed] [Google Scholar]
- Hollis BW, Wagner CL 2004 Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr 80(Suppl 6):1752S-1758S [DOI] [PubMed] [Google Scholar]
- Zittermann A 2003 Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr 89:552-572 [DOI] [PubMed] [Google Scholar]
- Vasquez A, Manso G, Cannell J 2004 The clinical importance of vitamin D (cholecalciferol): a paradigm shift with implications for all healthcare providers. Altern Ther Health Med 10:28-36 [PubMed] [Google Scholar]
- McGrath JJ, Féron FP, Burne TH, Mackay-Sim A, Eyles DW 2004 Vitamin D3-implications for brain development. J Steroid Biochem Mol Biol 89-90:557-560 [DOI] [PubMed] [Google Scholar]
- McGrath J, Saari K, Hakko H, Jokelainen J, Jones P, Järvelin MR, Chant D, Isohanni M 2004 Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophr Res 67:237-245 [DOI] [PubMed] [Google Scholar]
- Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM 2001 Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 358:1500-1503 [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Mahon BD 2004 Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med 229:1136-1142 [DOI] [PubMed] [Google Scholar]
- Heaney RP 2003 Long-latency deficiency disease: insights from calcium and vitamin D. Am J Clin Nutr 78:912-919 [DOI] [PubMed] [Google Scholar]
- Holick MF 2004 Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 79:362-371 [DOI] [PubMed] [Google Scholar]
- Shah BR, Finberg L 1994 Single-dose therapy for nutritional vitamin D-deficiency rickets: a preferred method. J Pediatr 125:487-490 [DOI] [PubMed] [Google Scholar]
- Markestad T, Halvorsen S, Halvorsen KS, Aksnes L, Aarskog D 1984 Plasma concentrations of vitamin D metabolites before and during treatment of vitamin D deficiency rickets in children. Acta Padiatr Scand 73:225-231 [DOI] [PubMed] [Google Scholar]
- Thacher TD, Fischer PR, Pettifor JM, Lawson JO, Isichei CO, Reading JC, Chan GM 1999 A comparison of calcium, vitamin D, or both for nutritional rickets in Nigerian children. N Engl J Med 341:563-568 [DOI] [PubMed] [Google Scholar]
- Holick MF 2006 Resurrection of vitamin D deficiency and rickets. J Clin Invest 116:2062-2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF 2007 Vitamin D deficiency. N Engl J Med 357:266-281 [DOI] [PubMed] [Google Scholar]
- Pettifor JM 2003 Nutritional Rickets. In: Jüppner H, Glorieux FH, eds. Pediatric bone. 1st ed. San Diego: Academic Press; 541-560 [Google Scholar]
- Pettifor JM 1999 Nutritional and drug-induced rickets and osteomalacia. In: Favus MJ, ed. Primer on the metabolic bone diseases and disorders of mineral metabolism. 4th ed. Philadelphia: Lippincott Williams and Wilkins; 323-327 [Google Scholar]
- Malaban A, Veronikis IE, Holick MF 1998 Redefining vitamin D insufficiency. Lancet 351:805-806 [DOI] [PubMed] [Google Scholar]
- Trang HM, Cole DEC, Rubin LA, Pierratos A, Siu S, Vieth, R 1998 Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr 68:854-858 [DOI] [PubMed] [Google Scholar]
- Armas LAG, Hollis BW, Heaney RP 2004 Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 89:5387-5391 [DOI] [PubMed] [Google Scholar]
- Houghton LA, Vieth R 2006 The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr 84:694-697 [DOI] [PubMed] [Google Scholar]
- Rapuri PB, Gallager JC, Haynatzki G 2004 Effect of vitamin D2 and D3 supplement use on serum 25(OH)D concentration in elderly women in summer and winter. Calcif Tissue Int 74:150-156 [DOI] [PubMed] [Google Scholar]
- Markestad T, Aksnes L, Ulstein M, Aarskog D 1984 25-Hydroxyvitamin D and 1,25-dihydroxyvitamin D of D2 and D3 origin in maternal and umbilical cord serum after vitamin D2 supplementation in human pregnancy. Am J Clin Nutr 40:1057-1063 [DOI] [PubMed] [Google Scholar]
- Gordon CM, Feldman HA, Sinclair L, Williams AL, Kleinman PK, Perez-Rossello J, Cox J 2008 Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch Ped Adol Med 162:505-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak MA, Jimbow K, Szabo G, Fitzpatrick TB 1976 Sunlight and melanin pigmentation. In: Goldsmith K, ed. Photochemical and photobiological reviews. New York: Plenum Press; 211-239 [Google Scholar]
- Jimbow K, Fitzpatrick B, Wick MM 1991 Biochemistry and physiology of melanin pigmentation. In: Goldsmith LA, ed. Physiology, biochemistry, and molecular biology of the skin. New York: Oxford University Press; 873-909 [Google Scholar]
- Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ 2004 Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med 158:531-537 [DOI] [PubMed] [Google Scholar]
- Cesur Y, Caksen H, Gundem A, Kirimi E, Odabas D 2003 Comparison of low and high dose of vitamin D treatment in nutritional vitamin D deficiency rickets. J Pediatr Endocrinol Metab 16:1105-1109 [DOI] [PubMed] [Google Scholar]
- Markestad T, Hesse V, Siebenhuner M, Jahreis G, Aksnes L, Plenert W, Aarskog D 1987 Intermittent high-dose vitamin D prophylaxis during infancy: effect on vitamin D metabolites, calcium, and phosphorus. Am J Clin Nutr 46:562-568 [DOI] [PubMed] [Google Scholar]
- Jacobus CH, Holick MF, Shao Q, Chen TC, Holm IA, Kolodny JM, Fuleihan GE, Seely EW 1992 Hypervitaminosis D associated with drinking milk. N Engl J Med 326:1173-1177 [DOI] [PubMed] [Google Scholar]
- Mimouni F 1995 Single-day therapy for rickets. J Pediatr 126:1019-1020 [DOI] [PubMed] [Google Scholar]
- Barrueto Jr F, Wang-Flores HH, Howland MA, Hoffman RS, Nelson LS 2005 Acute vitamin D intoxication in a child. Pediatrics 116:e453 [DOI] [PubMed] [Google Scholar]