Abstract
Context: Surgical treatments of obesity have been shown to induce rapid and prolonged improvements in insulin sensitivity.
Objective: The aim of the study was to investigate the effects of gastric bypass surgery and the mechanisms that explain the improvement in insulin sensitivity.
Design: We performed a cross-sectional, nonrandomized, controlled study.
Setting: This study was conducted jointly between the Departments of Exercise Science and Physiology at East Carolina University in Greenville, North Carolina.
Subjects: Subjects were recruited into four groups: 1) lean [body mass index (BMI) < 25 kg/m2; n = 93]; 2) weight-matched (BMI = 25 to 35 kg/m2; n = 310); 3) morbidly obese (BMI > 35 kg/m2; n = 43); and 4) postsurgery patients (BMI ≈ 30 kg/m2; n = 40). Postsurgery patients were weight stable 1 yr after surgery.
Main Outcome Measures: Whole-body insulin sensitivity, muscle glucose transport, and muscle insulin signaling were assessed.
Results: Postsurgery subjects had insulin sensitivity index values that were similar to the lean and higher than morbidly obese and weight-matched control subjects. Glucose transport was higher in the postsurgery vs. morbidly obese and weight-matched groups. IRS1-pSer312 in the postsurgery group was lower than morbidly obese and weight-matched groups. Inhibitor κBα was higher in the postsurgery vs. the morbidly obese and weight-matched controls, indicating reduced inhibitor of κB kinase β activity.
Conclusions: Insulin sensitivity and glucose transport are greater in the postsurgery patients than predicted from the weight-matched group, suggesting that improved insulin sensitivity after bypass is due to something other than, or in addition to, weight loss. Improved insulin sensitivity is related to reduced inhibitor of κB kinase β activity and enhanced insulin signaling in muscle.
Insulin sensitivity is greater than expected in regards to body mass index in patients following gastric bypass surgery, suggesting improved skeletal muscle insulin signaling as a likely mechanism.
Gastric bypass surgery has been employed as a tool to assist in weight loss in morbidly obese individuals. For over 20 yr, our group has been performing gastric bypass surgery, which consists of surgical reduction in the size of the stomach and bypassing a portion of the proximal small intestine. We were the first to report that gastric bypass surgery results in rapid and long-term improvement in insulin sensitivity and an ultimate reversal of diabetes (1,2,3). More recently, Dixon et al. (4) observed that laparoscopic adjustable gastric banding resulted in a higher remission rate of type 2 diabetes compared with conventional medical treatment. Additionally, the reversal of diabetes by various surgical treatments of obesity was confirmed by meta-analysis, wherein gastric bypass surgery and laparoscopic adjustable gastric banding were found to resolve diabetes in approximately 84 and 48% of postsurgery patients, respectively (5).
Although an increase in whole-body insulin sensitivity after gastric bypass surgery is well established, the mechanisms involved are not fully understood. A potential signaling defect in the insulin signaling cascade in the muscle of obese and type 2 diabetics may be a cause for reduced insulin sensitivity. We have shown previously that insulin-stimulated muscle glucose transport is blunted in obese individuals and patients with type 2 diabetes and that insulin-receptor tyrosine kinase activity is diminished in obese and diabetic skeletal muscle (6,7). Serine phosphorylation of the insulin receptor substrate (IRS)-1 has been implicated in inhibiting insulin signal transduction (8,9), and although there are many potential serine phosphorylation sites on IRS-1 (10), the IRS1-Ser312(human)/Ser307(rodent) residue is of particular interest given its proximity to the phosphotyrosine-binding domain in IRS-1. The inhibitor of κB kinase β (IKKβ), a serine kinase, has been suggested as a potential culprit that results in deficient insulin signal transduction, given that it has been shown to phosphorylate IRS-1 on Ser312/307 (8,9,11,12). Moreover, IKKβ is activated by fatty acid metabolites, such as diacylglycerol and ceramide (13,14), which have been shown to be higher in insulin-resistant states, such as obesity (15).
Obesity is associated with an increased low-grade inflammatory tone, as evidenced by increased levels of proinflammatory cytokines as well as greater activity of the inflammatory factor, nuclear factor-κB (NF-κB) (16,17,18,19). NF-κB is a nuclear transcription factor that is responsible for the transcription of a multitude of cytokines (including TNF-α and IL-1β) (20,21). NF-κB is prevented from reaching the nucleus by an inhibitor, known as inhibitor κBα (IκBα). IκBα is phosphorylated by IKKβ, which results in the ubiquitination and degradation of IκBα, and NF-κB is free to migrate into the nucleus. In addition to regulating NF-κB activity, IKKβ has been shown to directly inhibit insulin signaling (8,9,22). To highlight the role of NF-κB pathway activity in inhibiting insulin signaling, thiazolidinediones, a known insulin-sensitizing drug, inhibit NF-κB activity (22,23). Similarly, Yin et al. (24) discovered that the mechanism of action for salicylate in improving insulin sensitivity was its inhibitory action on IKKβ.
We are interested in the causes of insulin resistance in muscle of obese individuals and the mechanisms that improve insulin resistance after patients have gastric bypass surgery. The purpose of this study was to investigate gastric bypass surgery-induced increases in insulin sensitivity at the whole body and muscle level. We also sought to determine a potential mechanism for the rapid and sustained improvement in insulin sensitivity as a result of gastric bypass surgery.
Subjects and Methods
Subjects and study design
To investigate the reversal of insulin resistance after bypass surgery, three experiments were performed. In the first experiment, whole-body insulin sensitivity was measured in postsurgery patients and nonsurgical controls by an iv glucose tolerance test (IVGTT) as outlined below. The second experiment involved glucose transport measured in rectus abdominis muscle strips from patients having elective surgery. These values were compared with previous data published for gastric bypass patients after weight loss (1). The activity of IKKβ and IRS-1 serine312 phosphorylation was assayed in vastus lateralis muscle biopsies of postsurgery patients and nonsurgery controls in the third experiment. All subjects were female and considered to be in good health after filling out a medical history questionnaire. Subjects were informed of potential risks associated with the study and signed an informed consent document, which was approved by the East Carolina University Institutional Review Board.
Whole-body insulin sensitivity experiment
The first experiment consisted of 128 female subjects who were divided into four groups. One group consisted exclusively of post-gastric bypass surgery patients who were weight stable at least 12 months after surgery (BMI ≈ 30 kg/m2). The other three groups of nonsurgery subjects were divided according to BMI: 1) lean (BMI < 25 kg/m2), 2) weight-matched to postsurgery patients (BMI = 25 to 35 kg/m2), and 3) morbidly obese (BMI > 35 kg/m2). As mentioned, this first cohort of subjects was used to obtain insulin sensitivity data, including fasting insulin and glucose, as well as insulin sensitivity with a 3-h IVGTT (outlined below).
Muscle glucose transport experiment
In the second experiment, glucose transport was measured in incubated muscle strips obtained during elective abdominal surgery. Subjects were recruited into similar BMI groups, namely, lean (BMI < 25 kg/m2), controls weight-matched to postsurgery patients (BMI = 25–35 kg/m2), and morbidly obese (BMI > 35 kg/m2). The data from these groups were then compared with data previously reported (1) for five postsurgery subjects to compare the given groups with postsurgery patients.
IKKβ activity and IRS1-Ser312 experiment
The third experiment included 36 subjects who were evenly recruited into four groups, similar to the BMI groups mentioned above. This cohort of subjects had a muscle biopsy (vastus lateralis) that was used to obtain all muscle protein data.
Roux-en-Y gastric bypass surgery
The gastric bypass surgery was performed as described previously (3). Briefly, Roux-en-Y gastric bypass surgery consists of surgical reduction in the size of the stomach and bypassing of a portion of the proximal small intestine (foregut).
Anthropometrics
Body mass was measured to the nearest 0.1 kg on a digital electronic scale. Height was measured to the nearest 0.5 cm with a stadiometer, and BMI was calculated [mass (in kilograms)/height2 (in meters)].
IVGTT
Insulin sensitivity was determined by a minimal model analysis using a 3-h IVGTT (25), as described previously (26). After fasting, samples were obtained and glucose (50%) was injected into a catheter placed in an antecubital vein at a dose of 0.3 g/kg body mass. Insulin, at a dose of 0.025 U/kg body mass was injected at min 20. Blood samples were obtained at min 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 25, 30, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, and 180; the samples were then centrifuged; and plasma was transferred and frozen at −80 C for the subsequent determination of insulin and glucose. Insulin was determined with immunoassay (Access Immunoassay System; Beckman Coulter, Fullerton, CA), and glucose was determined with an oxidation reaction (YSI model 2300 Stat Plus; Yellow Springs Instruments, Yellow Springs, OH). Insulin sensitivity index (SI) was calculated by using the minimal model (25); a higher SI indicates greater insulin sensitivity.
Muscle strip incubations
Human rectus abdominis muscle strip preparation and incubation was performed according to the procedure previously described (6). Muscle strips were incubated in the presence (100 nm) or absence of insulin. 2-Deoxy-d-glucose was used at a concentration of 5 mm for 60 min, and glucose transport was measured as described previously (6). 2-[1,2-3H(N)]Deoxy-d-glucose (30.2 Ci/mmol) was obtained from DuPont NEN Life Science Products (Boston, MA).
Muscle IRS-1, pSer312, and IκBα protein content
A muscle sample was obtained from the vastus lateralis with the percutaneous needle biopsy technique. Biopsy samples were immediately frozen in liquid nitrogen. Frozen muscle samples were homogenized in ice-cold lysis buffer [50 mm HEPES, 50 mm Na+ pyrophosphate, 100 mm Na+ fluoride, 10 mm EDTA, 10 mm Na+ orthovanadate, and protease and phosphatase (1 and 2) inhibitor cocktails (Sigma, St. Louis, MO)], followed by addition of 1% triton and brief sonication. After centrifugation for 1 h at 45,000 g at 4 C, supernatants were extracted (cytosolic portion), protein content was detected using a BCA protein assay (Pierce, Rockford, IL), and individual homogenate volumes were adjusted so that precisely 50 μg of protein was loaded into each lane. For IRS1-Ser312 and total IRS-1 analysis, 200 μg of homogenates was subjected to 10 μl IRS-1 monoclonal IP antibody (Santa Cruz Biotech, Santa Cruz, CA) overnight, then coupled to protein A Sepharose beads, rotated for 2 h at 4 C (Amersham Biosciences, Uppsala, Sweden), and washed. After addition of sample buffer, samples were separated by SDS-PAGE using 7.5 or 10% Tris-HCl gels and then transferred to polyvinylidene difluoride membranes for probing by the following antibodies: rabbit polyclonal IRS-1 and IRS-1 phospho serine312, purchased from Millipore (Billerica, MA); and IκBα antibodies, purchased from Cell Signaling (Beverly, MA). After incubation with primary antibodies, blots were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies. Horseradish peroxidase activity was assessed with ECL solution (Thermo Scientific, Rockford, IL) and exposed to film. The image was scanned, and band densitometry was assessed with Gel Pro Analyzer software, version 4.2 (Media Cybernetics, Silver Spring, MD). Content of phospho-proteins (using phospho-specific antibodies) was calculated from the density of the band of the phospho-protein divided by the density of the protein using the appropriate antibody.
Statistics
All data are shown as mean ± se. Comparisons between the groups with insulin sensitivity, muscle protein levels, and muscle glucose transport rates were made using one-way ANOVA, followed by post hoc analyses where appropriate. Correlations between variables were determined by Dunnette T3 post hoc analysis.
Results
Subject characteristics are presented in Table 1. None of the subjects were taking medications that would interfere with metabolism, all were free from known cardiovascular disease and diabetes mellitus, and a few were glucose tolerance impaired (fasting glucose >110 mg/dl; two in the lean group, two in the obese group, and five in the weight-matched group). The mean weight of all gastric bypass subjects decreased from 136.6 ± 21.5 to 81.9 ± 13.2 kg (mean ± sd; P < 0.001), resulting in a mean weight loss of 54.7 ± 18.7 kg. Similarly, BMI fell from 48.6 ± 5.7 to 28.8 ± 4.6 kg/m2 (P = 0.001). The BMI of the postsurgery groups was not significantly different than the weight-matched control groups.
Table 1.
Characteristics of patients/subjects in the three experiments
| Subject characteristics | Age (yr) | BMI (kg/m2) | No. of observations |
|---|---|---|---|
| Experiment 1: Whole-body insulin sensitivity, fasting glucose and insulin | |||
| Lean [BMI < 25 kg/m2 (nonsurgery)] | 38.9 ± 15.0 | 23.4 ± 1.5 | 33 |
| Weight-matched [BMI 25–35 kg/m2 (nonsurgery)] | 41.8 ± 14.1 | 28.5 ± 1.7 | 50 |
| Morbidly obese [BMI > 35 kg/m2 (nonsurgery)] | 40.3 ± 16.1 | 47.7 ± 9.5 | 14 |
| After surgery | 43.5 ± 9.8 | 29.4 ± 4.9 | 31 |
| Experiment 2: Muscle glucose transport from rectus abdominis muscle taken during surgery | |||
| Lean (BMI < 25 kg/m2) | 39.3 ± 6.4 | 22.4 ± 2.1 | 51 |
| Weight-matched (BMI 25–35 kg/m2) | 42.6 ± 19.0 | 28.9 ± 2.8 | 251 |
| Morbidly obese (BMI > 35 kg/m2) | 41.6 ± 4.2 | 46.2 ± 4.0 | 20 |
| Experiment 3: Vastus lateralis muscle biopsy for IRS1-Ser312 and IκBα | |||
| Lean [BMI < 25 kg/m2 (nonsurgery)] | 35.1 ± 14.4 | 21.7 ± 1.9 | 9 |
| Weight-matched [BMI 25–35 kg/m2 (nonsurgery)] | 35.5 ± 10.1 | 30.9 ± 2.7 | 9 |
| Morbidly obese [BMI > 35 kg/m2 (nonsurgery)] | 32.8 ± 13.3 | 44.6 ± 4.2 | 9 |
| After surgery | 40.8 ± 8.1 | 29.9 ± 4.1 | 9 |
Data represent mean ± sd. There is no statistical difference in BMI between the weight-matched and postsurgery groups.
Fasting glucose, insulin, and insulin resistance (HOMA)
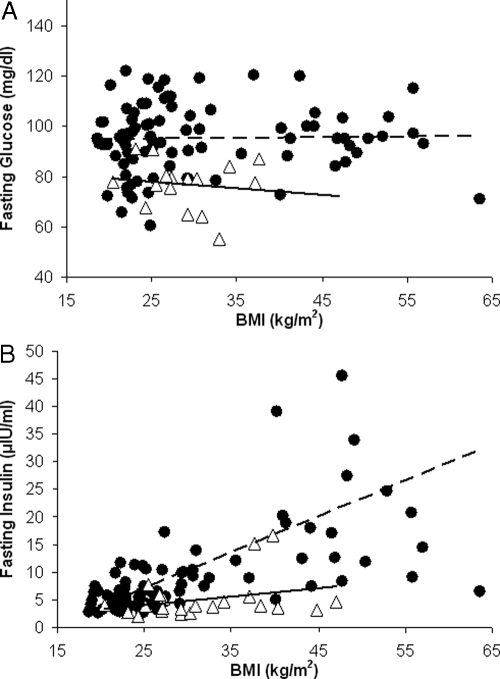
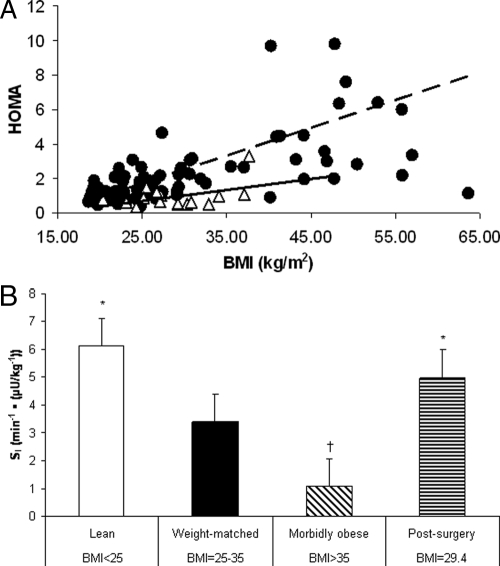
Average plasma glucose concentration for the postsurgery subjects (76 ± 10.2 mg/dl) was lower when compared with all nonsurgery control subjects (95.3 ± 13.8 mg/dl) (Fig. 1A; P < 0.001). There was no significant change in fasting glucose with increasing BMI in either control or postsurgery groups. Fasting insulin concentration and BMI were positively correlated (r = 0.598, P < 0.001) for all nonsurgery control subjects. In contrast, the same correlation in postsurgery subjects revealed no significant relationship (r = 0.304, P = 0.169; Fig. 1B). Fasting glucose and insulin values were used to calculate homeostasis model assessment (HOMA) values, an index of insulin resistance. HOMA values were significantly related to BMI in the nonsurgery subjects (P < 0.01, r = 0.283; Fig. 2A), whereas the postsurgery patients revealed no significant correlation.
Figure 1.
The correlations between BMI and glucose (A) and insulin (B) for nonsurgery control subjects (black circles) and postsurgery patients (white triangles). The data are from experiment 1 patients (Table 1). A, The comparison of fasting glucose values between nonsurgery control subjects (n = 97) and postsurgery subjects (n = 31). B, Fasting insulin values between nonsurgery subjects and postsurgery subjects. A positive and significant correlation was found (P < 0.001, r = 0.598) for the nonsurgery subjects. The same correlation in postsurgery subjects revealed no significant relationship (P = 0.169, r = 0.304).
Figure 2.
Whole-body insulin sensitivity determined by HOMA correlated with BMI (A) and IVGTT for four BMI groups (B). The data for the HOMA and insulin sensitivity analysis were taken from experiment 1 (Table 1). A, The comparison of HOMA values for nonsurgery control subjects (black circles) and postsurgery patients (white triangles). A positive and significant correlation between HOMA and BMI was found (P < 0.01, r = 0.283) for the nonsurgery subjects. The same correlation in postsurgery subjects revealed no significant relationship (P = 0.083, r = 0.446). B, The difference in whole-body insulin sensitivity between BMI groups. *, The postsurgery and lean groups are statistically higher (P < 0.005) than the morbidly obese and weight-matched groups. †, P < 0.05 for the comparison between the morbidly obese and all other groups. Data are presented as mean ± sd.
Whole-body insulin sensitivity
The postsurgery group had insulin SI values that were 361% higher than the morbidly obese and 47% higher than weight-matched groups (P < 0.005 for both; Fig. 2B). The postsurgery group’s SI levels were not significantly different from the lean group, despite having a greater BMI. The SI value of the morbidly obese was 68% lower than the weight-matched controls, suggesting an inverse relationship between whole-body insulin sensitivity and BMI (P < 0.05). This is consistent with the positive correlation between BMI and HOMA from all nonsurgery subjects (Fig. 2A; P < 0.01, r = 0.283).
Muscle glucose transport
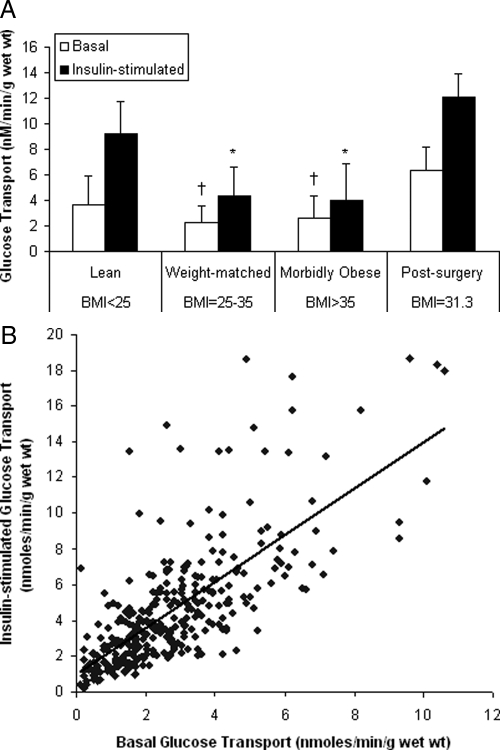
We previously reported that muscle glucose transport was significantly lower in morbidly obese patients than in lean controls (1,6). Additionally, in five gastric bypass patients who subsequently had elective abdominal surgery after weight loss, muscle glucose transport was not different than that of lean subjects, although the postsurgery group had an average BMI of 31 kg/m2 (1). To determine how postsurgery muscle glucose transport compared with a weight-matched control group, we retrieved muscle glucose transport measurements from a database of our previous experiments (Fig. 3A). Previously published postsurgery glucose transport values (1) are shown in Fig. 3A for comparison. Insulin-stimulated glucose transport values were lower in the weight-matched group and morbidly obese groups, compared with the lean control groups (P < 0.05). There were no differences in insulin-stimulated glucose transport between the morbidly obese and weight-matched groups. This is in agreement with our previous report that muscle glucose transport is related to BMI up to a BMI of 30 kg/m2, after which there is no further change (27); i.e. muscle insulin resistance is maximal at a BMI of 30 kg/m2. Postsurgery glucose transport values were similar to the lean group and greater than morbidly obese and weight-matched subjects.
Figure 3.
2-Deoxy-d-glucose uptake in incubated muscle strips in the presence (100 nm) or absence of insulin (A) and the correlation between basal and insulin-stimulated glucose transport (B). The data were obtained from subjects in experiment 2 (Table 1). A, A comparison of basal (empty bars) and insulin-stimulated (filled bars) glucose transport. *, Insulin-stimulated glucose transport in the morbidly obese and weight-matched groups was statistically lower (P < 0.05) than the lean and postsurgery groups. †, Basal glucose transport in the morbidly obese and weight-matched groups was statistically lower (P < 0.05) than the lean and postsurgery groups. Basal and insulin-stimulated glucose transport in muscle from morbidly obese subjects did not differ from the weight-matched subjects. B, The correlation between basal and insulin-stimulated glucose transport revealed a highly significant relationship (P < 0.001; r =0.726). Data are presented as mean ± sd.
We previously reported that insulin-stimulated glucose transport was depressed in muscle of morbidly obese patients, compared with lean controls (6). With the larger number of observations in the present data set, we extend this observation by reporting that basal glucose transport (in the absence of insulin) is also lower in muscle of morbidly obese patients. Also, a significant positive relationship was found between the rates of basal and insulin-stimulated glucose transport (P < 0.001, r = 0.726; Fig. 3B). This suggests that the factors that regulate basal glucose might also be those that regulate the rate in the presence of insulin. This was unexpected because we had predicted that insulin resistance might only have an effect on insulin-stimulated glucose transport.
Insulin sensitivity and IRS1-Ser312
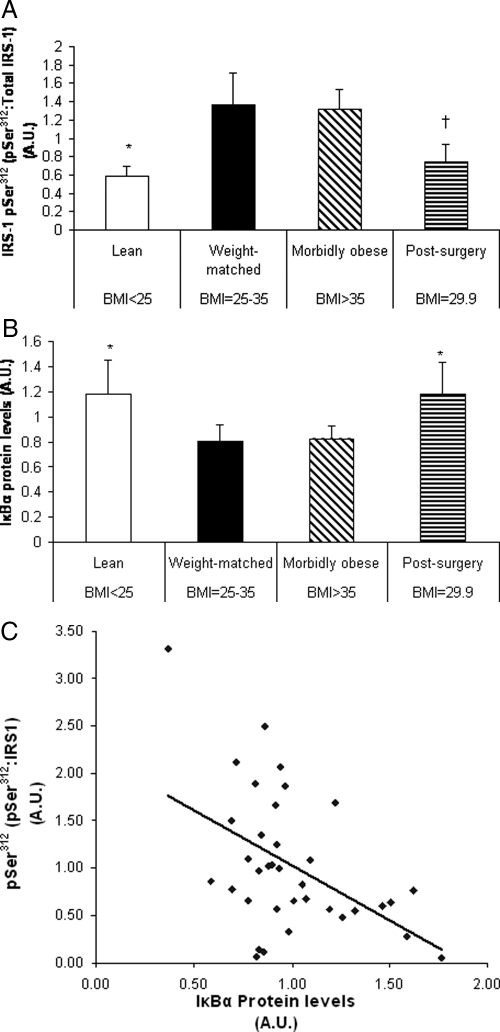
Inasmuch as serine312 phosphorylation of the IRS-1 protein has been implicated in inhibiting insulin signaling, this parameter was investigated in vastus lateralis muscle biopsies of lean, weight-matched, morbidly obese, and postsurgery subjects (group 3 in Table 1). Ser312 phosphorylation of IRS-1 was significantly greater in the morbidly obese and weight-matched subjects vs. the lean and postsurgery subjects (P < 0.05). IRS1-Ser312 phosphorylation followed a similar pattern to the insulin-stimulated glucose transport data. In particular, IRS1-pSer312 did not differ between the morbidly obese and weight-matched groups (Fig. 4A). Moreover, IRS1-Ser312 phosphorylation in postsurgery subjects was significantly reduced by 47% compared with morbidly obese (P = 0.04) and tended to be lower when compared with the weight-matched subjects (P = 0.057).
Figure 4.
Muscle levels of IRS1-phospho-Ser312 (A) and IκBα (B), and the correlation between IRS1-pSer312 and IκBα (C). Nonsurgery control subjects and postsurgery patients were from experiment 3 (Table 1). A, Western blot analysis of serine312 phosphorylation of IRS1. *, P < 0.05 for the comparison between the lean group and the morbidly obese and weight-matched. †, P < 0.05 for the comparison between the postsurgery and the morbidly obese groups. The postsurgery group tended to have a lower level compared with the weight-matched group (P = 0.057). B, Western blot analysis of IκBα in skeletal muscle between groups. *, P < 0.05 for the observation that the lean and postsurgery groups were statistically higher than the morbidly obese and weight-matched groups. IκBα levels were similar between postsurgery subjects and lean subjects. C, The correlation between IκBα and IRS1-phospho-Ser312 levels between nonsurgery and postsurgery subjects (P = 0.003, R = −0.47). Data are presented as mean ± sd.
IκBα and IRS1-Ser312
Due to the implicated role of IKKβ as a serine kinase of IRS1-Ser312, muscle levels of IκBα, a substrate of IKKβ that is rapidly ubiquitinated and degraded upon phosphorylation, were measured in an effort to determine IKKβ activity (28). Greater IKKβ activity would be evident in reduced levels of IκBα. Similar to our observations with IRS1-phospho-Ser312 and insulin-stimulated glucose uptake in muscle, IκBα levels in the postsurgery subjects were similar to lean subjects and were significantly elevated by 44% compared with the morbidly obese (P < 0.05) and 47% compared with the weight-matched controls (P < 0.05), which demonstrated increased IKKβ activity in the weight-matched and morbidly obese compared with the lean and post-bypass subjects (Fig. 4B). With correlational analysis, we discovered a highly significant inverse relationship between IκBα and phospho-Ser312 levels (P = 0.003, r = −0.47; Fig. 4C).
Discussion
The primary effect of gastric bypass surgery in the obese is significant weight loss, but an important side effect of the procedure is the dramatic and rapid rescuing of insulin sensitivity. We observed lower fasting glucose and insulin values from the postsurgery group compared with all other subjects. Furthermore, we found that whole-body insulin sensitivity, as measured by IVGTT, is significantly greater in the postsurgery subjects compared with BMI-matched control subjects. Additionally, despite having a significantly higher BMI than the lean subjects, postsurgery subjects enjoy comparable SI values.
We have previously shown that muscle insulin resistance in obese individuals is related to depressed insulin signal transduction (7). Because phosphorylation of the IRS1-Ser312(human)/307(rodent) residue has been implicated in attenuating insulin signal transduction, we measured IRS1-pSer312 and IκBα protein levels, as a surrogate for IKKβ activity, and found that both IκBα and IRS1-phospho-Ser312 protein levels in skeletal muscle from postsurgery patients were similar to that seen in muscle from lean subjects despite the postsurgery group having a significantly higher BMI (Fig. 4, A and B). The NF-κB pathway has garnished a great deal of attention recently in regards to its novel effects on metabolic function and substrate utilization. In particular, IKKβ has been studied in a variety of cells, including endothelial, hepatic, and skeletal muscle. Research into the role of the NF-κB pathway in endothelial cells has been conducted due, in part, to the pathological consequences of inflammation in the vascular system (29,30,31,32). Recent work by Wu et al. (33) determined that activation of IKKβ by TNF-α is suppressed by adiponectin. This is particularly interesting due to the ability of adiponectin to improve insulin sensitivity (34). Furthermore, studying the role of NF-κB pathway proteins in the liver, Cai et al. (8) revealed that diet-induced obesity in mice led to increased NF-κB pathway signaling in the liver. Mice with a liver-specific constitutively active IKKβ transgene suffered from systemic insulin resistance, and further analysis revealed a dose-dependent relationship between NF-κB activity in the liver and the onset of system insulin resistance. Moreover, in human hepatocytes, Gao et al. (35) treated cells with TNF-α and found increased IRS-1 phosphorylation at Ser312. This IRS1-Ser312 phosphorylation correlated with the disappearance of IκBα, which represents increased IKKβ activity.
Because skeletal muscle is the main depot for insulin-stimulated glucose uptake, studying the effects of IKKβ on insulin signaling in this tissue is worthwhile. A study by de Alvaro et al. (28) reported that IKKβ activation in rat skeletal muscle cell culture resulted in a significant increase in IRS1-Ser307 phosphorylation. They also explored the role of a pharmacological IKKβ inhibitor, salicylate, in preventing IKKβ-induced insulin resistance. Targeted disruption of the Ikkβ locus in mice results in lower fasting glucose and insulin concentrations, as well as preventing insulin resistance from a high-fat diet. Similar results have been reported with salicylate treatment (9). Additionally, Kim et al. (12) employed lipid infusions in rodents to cause acute insulin resistance. They observed that this effect was attenuated with pretreatment with salicylates. Furthermore, lipid infusions performed in Ikkb heterozygous knockout mice revealed improvements in insulin sensitivity during hyperinsulinemic-euglycemic clamps when compared with controls.
IKKβ activation, and subsequent IRS1-Ser312 phosphorylation, has been shown to be a result of the accumulation of excess fatty acids within the muscle that are subsequently partitioned toward storage or the formation of fatty acid metabolites, resulting in insulin resistance (13,14,15,23,36). We have previously shown that intramyocellular lipid (IMCL) levels are significantly reduced after gastric bypass surgery (37). Moreover, Greco et al. (38) determined that surgery-induced weight loss results in a greater-than-expected reduction in IMCL when compared with diet-induced weight loss alone. IMCL depletion is certainly a likely explanation for the improvements we observed in postsurgery patients in regards to levels of IκBα and IRS1-pSer312 due to their role in mediating lipid-induced insulin resistance, and it may partly explain the differences in whole-body insulin sensitivity.
Not surprisingly, in control subjects fasting insulin levels and insulin resistance (HOMA) have positive relationships with BMI. However, fasting insulin and insulin resistance are not associated with BMI in the postsurgery subjects. BMI was used as a global assessment of body fat and weight loss in the current study. Whereas BMI is not an accurate indicator of body fat in certain populations with relatively greater muscle mass, given that all subjects in the current study were sedentary, we feel it is a legitimate measurement. Moreover, in regards to the weight loss and reduction in BMI associated with bypass surgery, it has been shown that the tremendous fat loss associated with gastric bypass surgery is mostly a result of a reduction in truncal fat (39).
A possible concern may be the possible confounding effect of a change in food intake as a result of gastric bypass surgery, which our group has previously explored (40). Although patients experience an acute and meaningful decrease in caloric intake after surgery that results in significant weight loss, the outcomes we explored in the current study were measured approximately 12 months after surgery in patients who were weight stable on a eucaloric diet, which would seemingly negate any dietary effect.
We interpret our data to suggest that the improvement in insulin sensitivity after gastric bypass is not exclusively due to weight loss and may implicate an effect of the surgery itself (2). A comparison of various surgical interventions lends support for this hypothesis. Namely, gastric banding surgery restricts food intake but does not change the channel of food flow. The remission rate for gastric banding is 48%, whereas that for gastric bypass is 84% (5). In addition, Dixon et al. (4) reported that the remission of diabetes was correlated with the degree of weight loss, which is in contrast with the data we present here for gastric bypass. Moreover, Laferrere et al. (41) compared subjects who experienced comparable levels of weight loss due to hypocaloric diet and surgery. Whereas both interventions result in improved diabetes control, they found that patients who underwent bypass surgery enjoyed a better clinical outcome and no longer required diabetes medication.
If the improvement in insulin sensitivity after gastric bypass is not related to weight loss, an attractive alternative hypothesis is that increased insulin sensitivity and remission of diabetes after gastric bypass are due to restructuring of the gastrointestinal tract (2). According to this hypothesis, bypassing a portion of the duodenum and jejunum causes either: 1) decreased secretion of a diabetes-inducing peptide (anti-incretin) from the foregut; or 2) increased secretion of an antidiabetes peptide (incretin) from the hindgut. This “foregut bypass hypothesis” was first suggested Pories (2) but has received recent attention by Rubino (42), who, with his colleagues, has extensively studied this hypothesis in a nonobese animal model of diabetes (GK rat). They found that a duodenal-jejunal bypass, which does not result in food restriction or weight loss, leads to improved glucose tolerance in the diabetic Goto-Kakisaki rat.
In summary, whole-body insulin sensitivity, as measured by IVGTT, and muscle glucose transport after gastric bypass are greater than predicted from the weight-matched control group. We interpret these findings to suggest that the improvement in insulin sensitivity is not a result of weight loss, but is more likely related to bypass of the foregut. Based on our results, we believe that IRS1-Ser312 phosphorylation is a potential cause for the insulin resistance seen in the weight-matched and morbidly obese compared with the lean group, and that gastric bypass appears to result in reduced IRS1-Ser312 phosphorylation to levels similar to lean controls. Finally, we have implicated IKKβ, an NF-κB pathway serine kinase, as a potential culprit responsible for phosphorylating IRS1-Ser312, and we find that bypass surgery may indirectly result in reduced IKKβ activity in human skeletal muscle.
Acknowledgments
We thank the nurses for their valuable assistance and the hundreds of subjects who participated in this study.
Footnotes
This work was supported by National Institutes of Health Grant R01 DK046121 (to G.L.D.) and R01 DK56112 (to J.A.H.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 2, 2008
Abbreviations: BMI, Body mass index; HOMA, homeostasis model assessment; IκBα, inhibitor κBα; IKKβ, inhibitor of κB kinase β; IMCL, intramyocellular lipid; IRS, insulin receptor substrate; IVGTT, iv glucose tolerance test; NF-κB, nuclear factor-κB; SI, sensitivity index.
References
- Friedman JE, Dohm GL, Leggett-Frazier N, Elton CW, Tapscott EB, Pories WP, Caro JF 1992 Restoration of insulin responsiveness in skeletal muscle of morbidly obese patients after weight loss. Effect on muscle glucose transport and glucose transporter GLUT4. J Clin Invest 89:701–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pories WJ 1992 Why does the gastric bypass control type 2 diabetes mellitus? Obes Surg 2:303–313 [DOI] [PubMed] [Google Scholar]
- Pories WJ, MacDonald Jr KG, Flickinger EG, Dohm GL, Sinha MK, Barakat HA, May HJ, Khazanie P, Swanson MS, Morgan E, Leggett-Frazier N, Long SD, Brown BM, O'Brien K, Caro JF 1992 Is type II diabetes mellitus (NIDDM) a surgical disease? Ann Surg 215:633–642; discussion, 643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JB, O'Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M 2008 Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 299:316–323 [DOI] [PubMed] [Google Scholar]
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K 2004 Bariatric surgery: a systematic review and meta-analysis. JAMA 292:1724–1737 [DOI] [PubMed] [Google Scholar]
- Dohm GL, Tapscott EB, Pories WJ, Dabbs DJ, Flickinger EG, Meelheim D, Fushiki T, Atkinson SM, Elton CW, Caro JF 1988 An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J Clin Invest 82:486–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Dolan PL, Dohm GL 1999 Dephosphorylation increases insulin-stimulated receptor kinase activity in skeletal muscle of obese Zucker rats. Mol Cell Biochem 194:209–216 [DOI] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE 2005 Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med 11:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE 2001 Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science 293:1673–1677 [DOI] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Shulman GI 2006 Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55(Suppl 2):S9–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M 2005 IKK-β links inflammation to obesity-induced insulin resistance. Nat Med 11:191–198 [DOI] [PubMed] [Google Scholar]
- Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI 2001 Prevention of fat-induced insulin resistance by salicylate. J Clin Invest 108:437–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Peiffer C 2002 Protein kinase C and lipid-induced insulin resistance in skeletal muscle. Ann NY Acad Sci 967:146–157 [DOI] [PubMed] [Google Scholar]
- Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI 2002 Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277:50230–50236 [DOI] [PubMed] [Google Scholar]
- Itani SI, Ruderman NB, Schmieder F, Boden G 2002 Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes 51:2005–2011 [DOI] [PubMed] [Google Scholar]
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B 2006 Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17:4–12 [PubMed] [Google Scholar]
- Festa A, D'Agostino Jr R, Williams K, Karter AJ, Mayer-Davis EJ, Tracy RP, Haffner SM 2001 The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord 25:1407–1415 [DOI] [PubMed] [Google Scholar]
- Permana PA, Menge C, Reaven PD 2006 Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochem Biophys Res Commun 341:507–514 [DOI] [PubMed] [Google Scholar]
- Warnberg J, Marcos A 2008 Low-grade inflammation and the metabolic syndrome in children and adolescents. Curr Opin Lipidol 19:11–15 [DOI] [PubMed] [Google Scholar]
- Granger DN, Vowinkel T, Petnehazy T 2004 Modulation of the inflammatory response in cardiovascular disease. Hypertension 43:924–931 [DOI] [PubMed] [Google Scholar]
- Piva R, Belardo G, Santoro MG 2006 NF-κB: a stress-regulated switch for cell survival. Antioxid Redox Signal 8:478–486 [DOI] [PubMed] [Google Scholar]
- Ghanim H, Garg R, Aljada A, Mohanty P, Kumbkarni Y, Assian E, Hamouda W, Dandona P 2001 Suppression of nuclear factor-κB and stimulation of inhibitor κB by troglitazone: evidence for an anti-inflammatory effect and a potential antiatherosclerotic effect in the obese. J Clin Endocrinol Metab 86:1306–1312 [DOI] [PubMed] [Google Scholar]
- Todd MK, Watt MJ, Le J, Hevener AL, Turcotte LP 2007 Thiazolidinediones enhance skeletal muscle triacylglycerol synthesis while protecting against fatty acid-induced inflammation and insulin resistance. Am J Physiol Endocrinol Metab 292:E485–E493 [DOI] [PubMed] [Google Scholar]
- Yin MJ, Yamamoto Y, Gaynor RB 1998 The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(κ)B kinase-β. Nature 396:77–80 [DOI] [PubMed] [Google Scholar]
- Bergman RN, Finegood DT, Ader M 1985 Assessment of insulin sensitivity in vivo. Endocr Rev 6:45–86 [DOI] [PubMed] [Google Scholar]
- Houmard JA, Weidner MD, Dolan PL, Leggett-Frazier N, Gavigan KE, Hickey MS, Tyndall GL, Zheng D, Alshami A, Dohm GL 1995 Skeletal muscle GLUT4 protein concentration and aging in humans. Diabetes 44:555–560 [DOI] [PubMed] [Google Scholar]
- Elton CW, Tapscott EB, Pories WJ, Dohm GL 1994 Effect of moderate obesity on glucose transport in human muscle. Horm Metab Res 26:181–183 [DOI] [PubMed] [Google Scholar]
- de Alvaro C, Teruel T, Hernandez R, Lorenzo M 2004 Tumor necrosis factor α produces insulin resistance in skeletal muscle by activation of inhibitor κB kinase in a p38 MAPK-dependent manner. J Biol Chem 279:17070–17078 [DOI] [PubMed] [Google Scholar]
- Cacicedo JM, Yagihashi N, Keaney Jr JF, Ruderman NB, Ido Y 2004 AMPK inhibits fatty acid-induced increases in NF-κB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun 324:1204–1209 [DOI] [PubMed] [Google Scholar]
- Hattori Y, Suzuki K, Hattori S, Kasai K 2006 Metformin inhibits cytokine-induced nuclear factor κB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 47:1183–1188 [DOI] [PubMed] [Google Scholar]
- Partridge J, Carlsen H, Enesa K, Chaudhury H, Zakkar M, Luong L, Kinderlerer A, Johns M, Blomhoff R, Mason JC, Haskard DO, Evans PC 2007 Laminar shear stress acts as a switch to regulate divergent functions of NF-κB in endothelial cells. FASEB J 21:3553–3561 [DOI] [PubMed] [Google Scholar]
- Zhang WJ, Frei B 2001 α-Lipoic acid inhibits TNF-α-induced NF-κB activation and adhesion molecule expression in human aortic endothelial cells. FASEB J 15:2423–2432 [DOI] [PubMed] [Google Scholar]
- Wu X, Mahadev K, Fuchsel L, Ouedraogo R, Xu SQ, Goldstein BJ 2007 Adiponectin suppresses IκB kinase activation induced by tumor necrosis factor-α or high glucose in endothelial cells: role of cAMP and AMP kinase signaling. Am J Physiol Endocrinol Metab 293:E1836–E1844 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T 2001 The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:941–946 [DOI] [PubMed] [Google Scholar]
- Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J 2002 Serine phosphorylation of insulin receptor substrate 1 by inhibitor κB kinase complex. J Biol Chem 277:48115–48121 [DOI] [PubMed] [Google Scholar]
- Lee JS, Pinnamaneni SK, Eo SJ, Cho IH, Pyo JH, Kim CK, Sinclair AJ, Febbraio MA, Watt MJ 2006 Saturated, but not n-6 polyunsaturated, fatty acids induce insulin resistance: role of intramuscular accumulation of lipid metabolites. J Appl Physiol 100:1467–1474 [DOI] [PubMed] [Google Scholar]
- Gray RE, Tanner CJ, Pories WJ, MacDonald KG, Houmard JA 2003 Effect of weight loss on muscle lipid content in morbidly obese subjects. Am J Physiol Endocrinol Metab 284:E726–E732 [DOI] [PubMed] [Google Scholar]
- Greco AV, Mingrone G, Giancaterini A, Manco M, Morroni M, Cinti S, Granzotto M, Vettor R, Camastra S, Ferrannini E 2002 Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes 51:144–151 [DOI] [PubMed] [Google Scholar]
- Olbers T, Bjorkman S, Lindroos A, Maleckas A, Lonn L, Sjostrom L, Lonroth H 2006 Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg 244:715–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EK, Settle EA, Van Rij AM 1982 Food intake patterns of gastric bypass patients. J Am Diet Assoc 80:437–443 [PubMed] [Google Scholar]
- Laferrere B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B 2008 Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 93:2479–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino F 2008 Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care 31(Suppl 2):S290–S296 [DOI] [PubMed] [Google Scholar]