Cyclosporin A, the new immunosuppressive agent developed by Borel and associates (2), should permit major advances in whole organ transplantation. When cyclosporin A was first used in patients by Calne and co-workers (3, 5), it was hoped that no other drug would be routinely required. Our dissenting opinion has been that cyclosporin A should be combined with steroid therapy from the outset (14–16). In this report, observations in 42 more recent instances of cadaveric renal transplantation will be used to examine that view.
Cyclosporine A will be released soon for general use. When that time comes, physicians and surgeons who are unfamiliar with the combination therapy of cyclosporin A and steroids will need to have accurate foreknowledge of the convalescence patterns following renal transplantation and of how treatment with a standardized approach should be modified if recovery is unsatisfactory. The provision of such information is the second objective of this report.
METHODS
Case Material
Forty-two patients had cadaveric renal transplantation from two to eight months ago. Twenty-two were receiving their first grafts and 20 were undergoing transplantation for the second or third time. The recipients were consecutive and without exclusion, except for a woman from a previously reported series who under therapy with cyclosporin and steroids had previously lost a first kidney to primary hyperoxalosis (15). The primary hyperoxalosis could not be effectively treated because of pre-existing surgically produced hypoparathyroidism. By compassionate agreement, cyclosporin A was continued for a second transplantation with the proviso that she not be considered part of a new study. The second graft was even more promptly ruined by primary hyperoxalosis than the first.
Patient ages ranged from eight to 64 years, average 33.4±13.0, S.D. The primary recipients were deliberately transfused at least three times preoperatively, a preparatory step which was omitted for retransplantation. High risk medical factors, including diabetes mellitus, did not preclude candidacy.
HLA antigen matches at the A and B loci averaged 1.3 of a possible 4.0. Only seven donors were matched with their recipients for 3 or 4 HLA antigens, and at the other extreme, 15 had zero matches. Matching at the D locus was random, and there were no examples of complete D-related locus compatibility.
The serums of 13 of the 42 recipients contained antibodies against more than one-half of a lymphocyte panel contributed by 48 healthy donors. Ten of the 13 patients with widely reacting antibodies were among the 20 undergoing retransplantation, an adverse situation found only three times in the group of 22 recipients undergoing primary transplantation. In seven of the 13 patients, the preformed antibodies were warm anti-T lymphocyte cytotoxins which reacted against more than 90 per cent of the lymphocyte panel.
In four of the latter seven instances, the recipient serums that had been collected and stored as recently as three weeks earlier completely killed the lymphocytes of the actual donor, although cross matches were negative with serums drawn on the day of operation. In most centers, this finding is construed as a positive cross match and precludes transplantation.
Postoperative Diagnostic Studies
A noninvasive and noninstrumental approach to management was taken. Simple measures were the most important, including routine determinations of blood urea nitrogen and serum creatinine. Close attention to urinary output often allowed the diagnosis of rejection to be made before the results of blood chemistry analyses became available. Because wound tenderness, graft swelling and fever were common during rejection (16), the patients were examined at least once a day by experienced observers.
A day or two after transplantation, the grafts were studied with ultrasound to be sure that ureteral obstruction or perinephric fluid collection was not present. A radionuclide scan of the new kidney was obtained as a base line in the event that later differentiation of rejection from cyclosporin A nephrotoxicity might become necessary. Stables and colleagues (13) have shown that severely retarded renal blood flow in a poorly functioning graft is most characteristic of rejection, and we (10, 16) have shown that a normal or nearly normal renal flow scan with reduced excretion is most compatible with a differential diagnosis between cyclosporin A nephrotoxicity and nonspecific acute tubular necrosis.
Adjustment of drug dosages was a supremely important diagnostic tool. If manipulation of the steroid and cyclosporin A did not restore failing graft function, instrumentation was undertaken in six of the 42 recipients, including five, two and two examples each of renal biopsy, angiography and retrograde catheterization.
Monitoring of the immunologic status of the recipients and measurement of plasma concentrations of cyclosporin A were frequently carried out, but the results had no influence on day to day management and will not be reported herein.
Classification of Convalescence
Recovery of recipients who achieved prompt, sustained and satisfactory graft function was termed Class 1. If an initial diuresis, however brief, was followed by a secondary decline of renal function, the course was designated Class 2. The period of initial diuresis sometimes lasted for less than six hours before shut down ensued. In a Class 3 convalescence, anuria was present at the outset.
Convalescence Classification and Immunosuppressive Therapy
Treatment with cyclosporin A was given as previously recommended (14, 15), beginning with an initial oral dose of 17.5 milligrams per kilogram four to six hours before operation (Fig. 1). Twenty-four hours postoperatively, the same dosage was given by mouth. Intramuscular or intravenous administration was not necessary since all of the patients were able to resume a diet promptly. Thereafter, the daily dose of 17.5 milligrams per kilogram was divided, half being given every 12 hours. If there was no subsequent reason for change, the same daily drug dosages were continued for at least two months.
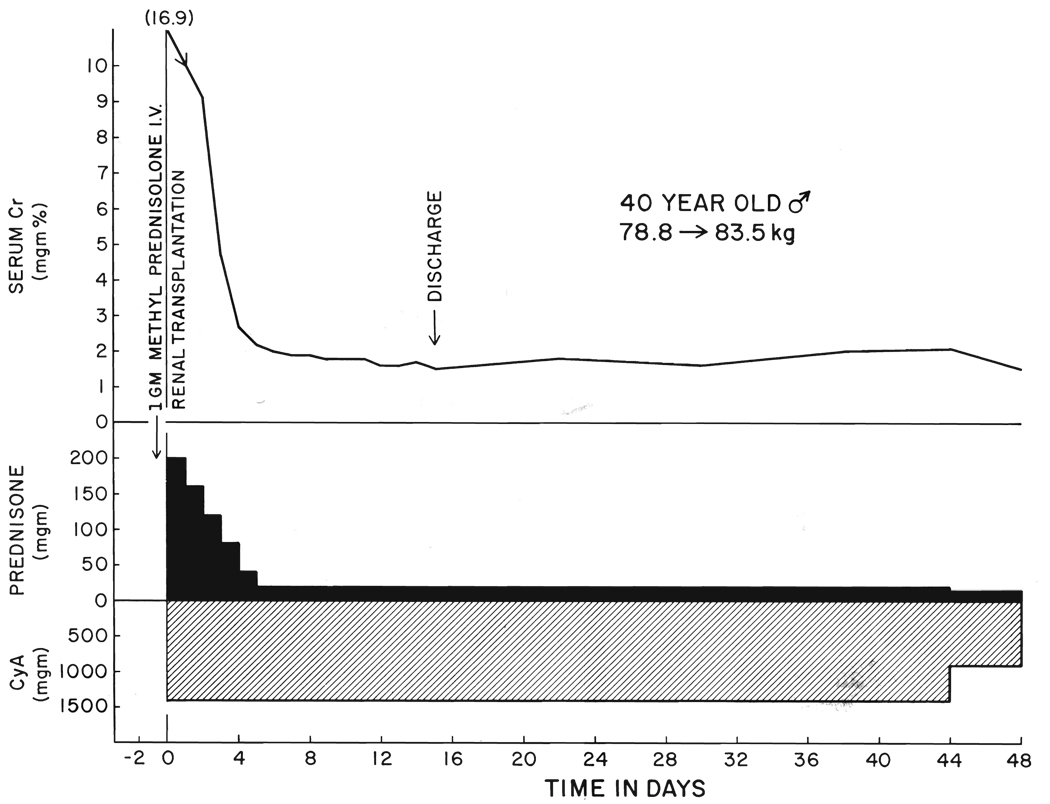
FIG. 1.
Benign convalescence under cyclosporin A, CyA, steroid therapy, Class 1. Cr, Serum creatinine.
Steroid therapy in adults was begun just before or during operation with an intravenous bolus of 1 gram methyl prednisolone. Afterward, the adult patients were given a five day course of prednisone, beginning with 200 milligrams the first day in six hourly divided intravenously administered doses. With subsequent daily reductions of 40 milligrams, the daily oral amount of prednisone had reached 40 milligrams by the fifth postoperative day. On the sixth day, the daily dose was reduced to a maintenance level of 20 milligrams from which further weaning was gradual (Fig. 1). The same steroid schedule was used for all adults without consideration of body weight, but for children, the peak and maintenance dosages were set lower (Fig. 2).
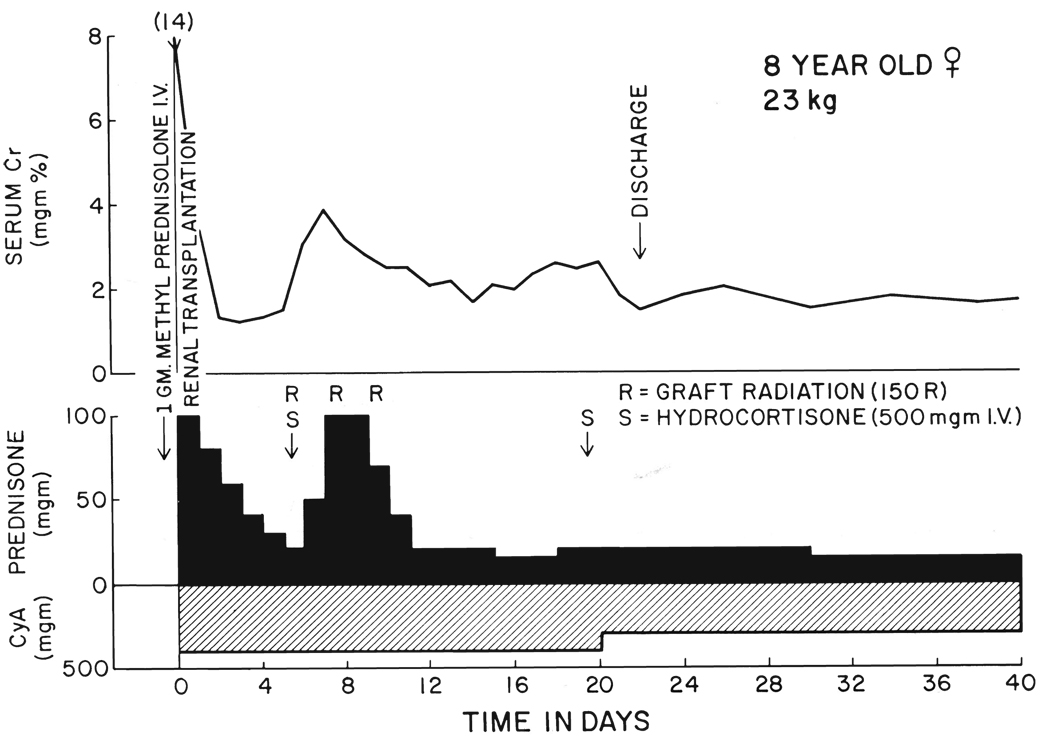
FIG. 2.
Class 2 recovery, with secondary deterioration of graft function which was immediately responsive to increased steroid therapy. The clinical diagnosis was rejection.
Class 1 recovery
This standardized therapeutic regimen was followed when early recovery was uncomplicated (Fig. 1).
Class 2 recovery
If an initial diuresis was followed by a secondary decline of renal function, rejection was assumed to be present. Urgent treatment was given intravenously with 1 gram of hydrocortisone. The same five day burst of prednisone that had been used at the outset was repeated (Fig. 2 and Fig 3), but the step-down of prednisone dosage was sometimes stopped at 30 milligrams per day. Local homograft irradiation with 450 rads at depth was often begun in three doses of 150 rads every other day (Fig. 2 and Fig 3). Some of the patients were treated with low dosages of heparin as was also irregularly done in patients with Class 1 and Class 3 courses.
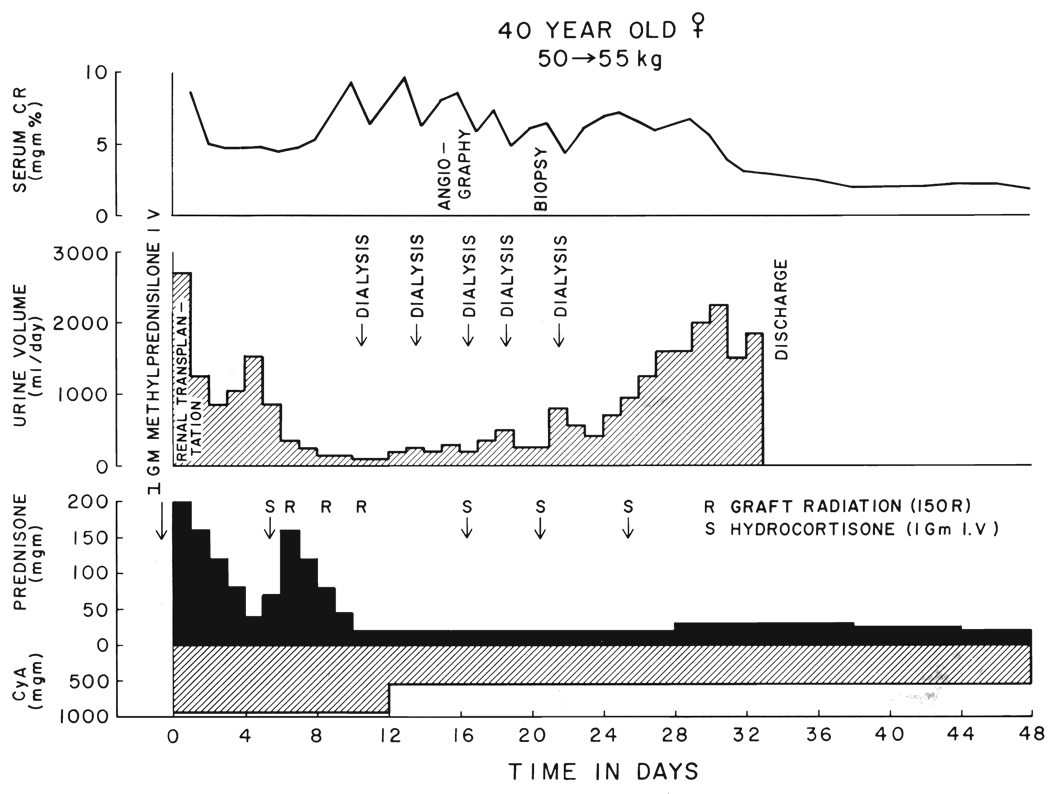
FIG. 3.
Class 2 recovery which was not promptly responsive to steroid therapy, prompting a reduction in the dosage of cyclosporin A. The angiogram showed a patent arterial system with a stripped tree appearance of the distal vessels. The biopsy showed glomerulitis. Although oliguria was prolonged, the eventual result was good.
Some of the patients who had renal function for only a few hours before the grafts shut down were just at the beginning of the initial burst therapy with prednisone. In this minority of Class 2 recipients, the original steroid schedule was not altered for several days, after which time a second steroid burst was considered if improvement had not occurred.
Whether secondary graft deterioration was very early or later, nephrotoxicity of cyclosporin A was kept in mind. This diagnosis became more likely if prompt and complete reversal was not obtained by the steroid increments already given and especially if repeat scans now showed maintenance of good renal blood flow. The daily doses of cyclosporin A were dropped to 10 milligrams per kilogram or occasionally lower (Fig. 3). Maintenance prednisone doses in adults were not kept above 30 milligrams per day, and usually, they were 20 milligrams.
For patients who did not respond, bolus therapy with 1 gram of intravenously given hydrocortisone was added every four or five days (Fig. 3). With the relatively low maintenance dosages of prednisone, the patients were not ill during the time they were placed back on dialysis while waiting recovery.
Class 3 recovery
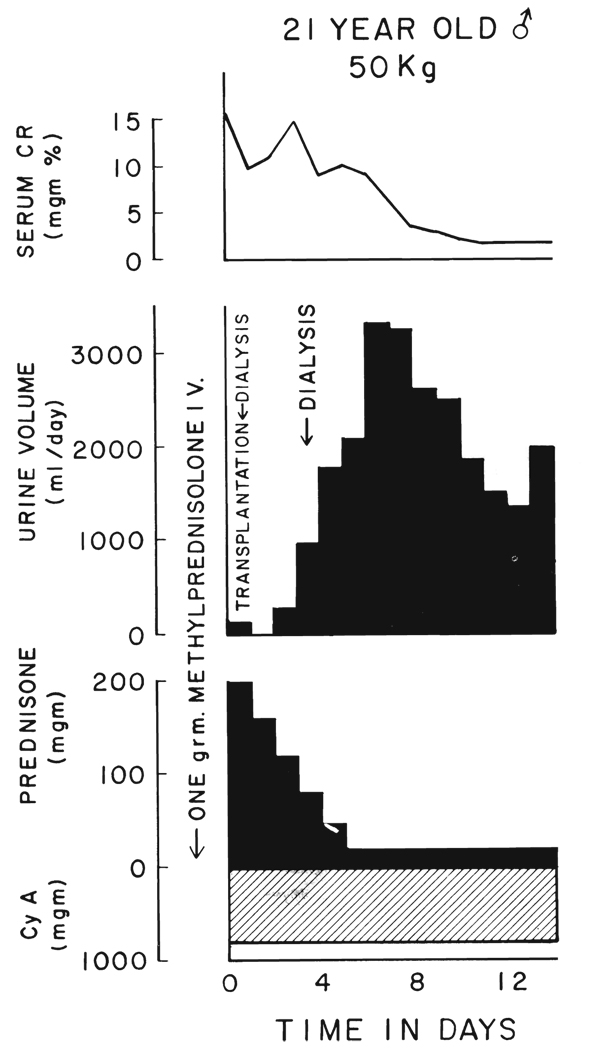
For patients who had immediate anuria postoperatively, the standardized immunosuppressive therapy was given (Fig. 4) with the assumption that the diagnosis was non-specific acute tubular necrosis. If recovery had not occurred within ten to 14 days, drug manipulation would have been instituted as for Class 2 patients, but this was not necessary since recovery occurred spontaneously.
FIG. 4.
Class 3 convalescence with immediate anuria. The graft was from a 13 year old donor and had warm ischemia for an unknown time, estimated 20 to 60 minutes, before cooling. Therapy with cyclosporin A and prednisone was by the standard schedule.
RESULTS
Mortality and Morbidity
None of the 42 recipients has died. Life-threatening infections were not seen, although annoying virus infections were frequent, most commonly caused by herpes simplex. One patient was treated for a mild Pncumocystis carinii pneumonitis, and another had a wound abscess after homograft nephrectomy.
An 18 year old man had a perforation of the ileum six months after transplantation. The resected specimen contained two discrete masses which were called lymphomas by conventional histopathologic criteria. The daily cyclosporin A dose was reduced from 17.5 to 9.0 milligrams per kilogram with a maintenance prednisone dose of 15 milligrams per day. He has not had further difficulties in the ensuing four months. The results of special studies of the tumors demonstrated Epstein-Barr nuclear antigen in the B lymphoid cells. Further studies are in progress to characterize the lymphoid tissue which was similar to that in another patient who was eventually diagnosed as having a polyclonal lymphoproliferative reaction (15).
Graft Losses
Primary transplantation
Twenty-one or 95.5 per cent of the 22 primary grafts are functioning. One kidney was lost promptly when, after an ABO blood group typing error, an A kidney was transplanted into an O recipient in violation of well accepted compatibility guidelines.
Retransplantation
Seventeen or 85 per cent of the 20 kidneys used for retransplantation are functioning. One graft was lost within 48 hours from a technical error and thrombosis of the renal artery. Two other grafts were rejected and removed after three and 11 weeks.
Incidence of Convalescent Classes
Excluding the two patients in whom transplantations were flawed by ABO typing or technical surgical errors, 40 were available for classification (Table I). Only about one-third of these 40 recipients had the completely uneventful recovery of Class 1, whereas the other two-thirds had either secondary deterioration of initial function, Class 2, or the immediate anuria of Class 3 (Table I).
TABLE I.
Classification of early recovery after 40 cadaveric transplantations
| Features | No | Good outcome |
|---|---|---|
| Class 1, uneventful recovery | 13 (32.5) | 13/13 |
| Class 2, secondary deterioration of early function | 24* (60) | 22/24 |
| Class 3, anuria at outset | 3 (7.5) | 3/3 |
Follow-up is two to eight months.
Six of these patients had an initial diuresis which began to shut down within a few hours. They would have been excluded from cyclosporin therapy if six or 12 hour urine output of 50 milliliters per hour were required as originally recommended for inclusion by Calne and others (3, 5).
Numbers in parentheses signify percentages.
The incidence of the convalescence classes was different after primary grafting versus retransplantation (Table II). Twice as many primary transplant recipients had the benign recovery of those in Class 1 as was observed in the immunologically more difficult patients undergoing retransplantation (Table II). Three-quarters of the recipients of second or third grafts had secondary deterioration of renal function from whatever level was initially observed. Commonly, this deterioration was very early, even within four to six hours.
TABLE II.
Incidence of convalescence classes after primary grafting and retransplantation
| Class 1, | Class 2, | Class 3, | |
|---|---|---|---|
| sustained | secondary graft | immediate | |
| function | deterioration | anuria | |
| 21 Primary grafts | 9 (42.8) | 10 (47.6) | 2 (9.6) |
| 19 Retransplants | 4 (21) | 14 (73.7) | 1 (5.3) |
Numbers in parentheses are percent age.
There were only three patients with the immediate anuria of Class 3 (Table I and Table II). These recipients recovered in the usual way after an acute tubular necrosis and were managed thereafter in the same way as Class 1 patients.
Renal Function
Difficulties in the early postoperative period tended to be transient and usually did not effect the ultimate renal function. All but four of the 38 recipients still bearing grafts have serum creatinine concentrations of less than 3 milligrams per cent. The other four have stable values of 3.0 to 3.5 milligrams per cent. The average creatinine level for the entire group is 2.0±0.7, S.D., milligrams per cent.
Incidence of Postoperative Dialysis
Of the total group of 42 patients, four or 9.5 per cent were returned to chronic dialysis and the transplants were removed. Two of the four grafts were lost because of the ABO typing or surgical technical errors already cited, and the two others were rejected. Fifteen of the 38 patients who eventually recovered required one to ten dialysis in the early postoperative period. The two patients who had kidney rejection were maintained on dialysis for three and 11 weeks before giving up.
DISCUSSION
The safety and efficacy of combination therapy with cyclosporin A and steroids were noted before (14–16) and confirmed in the more recent experience with 42 patients recorded herein. None of these patients has died. Ninety-six per cent of the primary grafts are functioning, as well as 85 per cent of the organs used for retransplantation. Although the follow-up periods are short, longer follow-up studies of our earlier series (15) and that of Calne and co-authors (4) have not revealed a tendency for patients under cyclosporin A to have later catch-up graft losses or to have major delayed morbidity from other causes. We and Caine and others (4) have not had the disillusionment reported by Carpenter (6) and Sweny (17) and their associates in their first trials with cyclosporin A.
As new teams begin using cyclosporin A, it will be important to avoid the unrealistic expectations about early convalescence that could be engendered by the high long term success rates which already have been achieved by Caine and co-workers (4) and by us (15). Of the 42 recently treated patients reported herein, only a third had a completely uneventful recovery. More than 40 per cent required some support with dialysis in the early postoperative period, because of either immediate anuria or secondary deterioration of initial graft function. The exceptionally high incidence of early renal failure after retransplantation was evidence for major adverse immunologic factors in these troublesome, but eventually manageable, patients whom Ascher and colleagues (1) have shown to be at high risk under conventional immunosuppression from both rejection and lethal complications.
The two principal factors which have influenced the convalescence of our previously (15, 16) and more recently treated patients have been rejection and nephrotoxicity from cyclosporin A. We (15, 16) and Carpenter and co-authors (6) have reported an extremely high incidence of clinically diagnosed or biopsy proved rejection. Control of the rejections with steroid therapy has been surprisingly easy (16), even in patients undergoing retransplantation. The nephrotoxicity first noted by Calne (3) and Powles (11) and their colleagues has been confirmed and characterized in our patients (9, 16).
The therapeutic regimen which we are recommending was designed to minimize diagnostic confusion between these two factors without resorting to routine biopsy or other instrumentation, to prevent permanent damage to the new kidney, to avoid jeopardizing the patient with over immunosuppression and to achieve these ends without the kinds of sophisticated immunologic and pharmacologic monitoring which is available in only a few centers. The last objective which we were able to achieve will be an important determinant in making cyclosporin A valuable for eventual widespread clinical use.
The use of cyclosporin A and steroids has had analogies to conventional combined therapy with azathioprine and prednisone but with greater efficiency in the control of rejection at much lower average steroid dosages. The conservative use of steroids with cyclosporin A from the time of transplantation has allowed protection of eventual graft function and has allowed stepwise adjustments of both agents to be a keen diagnostic tool with the main criterion of differential diagnosis between rejection and nephrotoxicity being the therapeutic response to change. However, effective treatment has not depended upon early graft function. Many of the patients in this series did not have the minimum early postoperative diuresis which Calne and associates (3, 5) have recommended as a condition for therapy with cyclosporin A. Although the luxury was lost of correlating graded drug changes with therapeutic response, these recipients were effectively treated without the use of the immunologic or pharmacologic monitoring techniques advocated by Keown (8), Rynasiewicz (12) and Kahan, (7) and their co-authors.
It is possible, but unlikely, that monitoring techniques eventually will delineate exactly the cause of renal graft dysfunction in the early postoperative period or later. In this series, the rapid and sometimes simultaneous drug adjustments in Class 2 and 3 patients accommodated both of the possibilities of rejection and cyclosporin A nephrotoxicity. Often, a final precise differential diagnosis could not be made even in retrospect. It was never assumed that postoperative renal dysfunction was attributable solely to rejection or nephrotoxicity of cyclosporin A. The guiding assumption was that both factors could and probably did contribute. Thus, the eventual therapeutic adjustment in patients with persistent renal dysfunction often was to increase the dosages of steroids and temporarily to reduce the dosages of cyclosporin A.
SUMMARY
The postoperative convalescence period was analyzed for 42 consecutive patients who had cadaveric renal transplantation under therapy with cyclosporin A and steroids. Twenty-two of the patients underwent transplantation for the first time, and the other 20 had retransplantation. None of the recipients has died. With follow-up period of two to eight months, the survival rate of grafts is 96 per cent after first transplantation and 85 per cent after retransplantation. Immunosuppression with a standard regimen was used for all patients at the outset. Early convalescence was highly variable, often necessitating adjustments of cyclosporin A and steroid dosage to accommodate the possibilities of rejection or cyclosporin A nephrotoxicity, or both, simultaneously. Management problems were more frequent and complex in patients undergoing retransplantation. From the results, a classification of convalescence patterns was evolved, with recommendations about how standardized initial therapy should be adjusted if the renal graft does not function promptly or deteriorates later.
Acknowledgments
Supported by grants from the Veterans Administration, Pittsburgh, and by Grant Nos. AM-17260 and AM-07772 from the National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Ascher NL, Ahrenolz DH, Simmons RL, et al. 100 second renal allografts from a single transplantation institution. Transplantation. 1979;27:30. doi: 10.1097/00007890-197901000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Borel JF, Feurer C, Gubler HU, et al. Biological effects of cyclosporin A; a new antilymphocytic agent. Agents Actions. 1976;6:468. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- 3.Calne RY, Rolles K, White DJG, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs; 32 kidneys, 2 pan-creases, and 2 livers. Lancet. 1979;2:1033. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 4.Calne RY, White DJG, Evans DB, et al. Cyclosporin A in cadaveric organ transplantation. Br. Med. J. 1981;282:934. doi: 10.1136/bmj.282.6268.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calne RY, White DJG, Thiru S, et al. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet. 1978;2:1323. doi: 10.1016/s0140-6736(78)91970-0. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter BJ, Tilney NL, Strom TB, et al. Cyclosporin A in cadaver renal allografts. Kidney Int. 1981;19:265. [Google Scholar]
- 7.Kahan BD, Vanburen CT, Lin SN, et al. Immunopharmacologic monitoring of cyclosporin A treated recipients of cadaveric kidney allografts. Transplantation. doi: 10.1097/00007890-198207000-00007. in press. [DOI] [PubMed] [Google Scholar]
- 8.Keown PA, Stiller CR, Ulan RA, et al. Immunological and pharmacological monitoring in the clinical use of cyclosporin A. Lancet. 1981;1:686. doi: 10.1016/s0140-6736(81)91971-1. [DOI] [PubMed] [Google Scholar]
- 9.Klintmalm GBG, Iwatsuki S, Starzl TE. Nephrotoxicity of cyclosporin A in liver and kidney transplant patients. Lancet. 1981;1:470. doi: 10.1016/s0140-6736(81)91851-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klintmalm GBG, Klingensmith WC, Iwatsuki S, et al. TC-99m-DTPA and I-131-hippuran findings in liver transplant recipients treated with cyclosporin A. Radiology. doi: 10.1148/radiology.142.1.7031760. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powles RJ, Clink HM, Spence D. Cyclos-porin A to prevent graft-versus-host disease in man after allogenic bone-marrow transplantation. Lancet. 1980;1:327. doi: 10.1016/s0140-6736(80)90881-8. [DOI] [PubMed] [Google Scholar]
- 12.Rynasiewicz JJ, Sutherland DER, Simmons RL, et al. Cyclosporin A for the oliguric renal transplantation patient. Lancet. 1981;1:276. doi: 10.1016/s0140-6736(81)92115-2. [DOI] [PubMed] [Google Scholar]
- 13.Stables DP, Klingensmith CW, III, Johnson ML. Renal transplantation. Diagnostic imaging in renal disease. 1979;9:167. [Google Scholar]
- 14.Starzl TE, Iwatsuki S, Klintmalm GBG, et al. Liver trans plantation, 1980, with particular reference to cyclosporin A. Transplant Proc. 1981;13:281. [PMC free article] [PubMed] [Google Scholar]
- 15.Starzl TE, Klintmalm GBG, Iwatsuki S, et al. Cyclosporin A and steroid therapy in 66 cadaver kidney recipients. Surg. Gynecol. Obstet. 1981;153:486. [PMC free article] [PubMed] [Google Scholar]
- 16.Starzl TE, Weil R, III, Iwatsuki S, et al. The use of cyclosporin A and prednisone in cadaver kidney transplantation. Surg. Gynecol. Obstet. 1980;151:17. [PMC free article] [PubMed] [Google Scholar]
- 17.Sweny P, Farrington K, Younis F, et al. Sixteen months experience with cyclospor in A in human kidney transplantation. Transplant Proc. 1981;13:365. [PubMed] [Google Scholar]