Abstract
To investigate the pre-vacuolar secretory pathway in Candida albicans, we cloned and analyzed the C. albicans homolog of the Saccharomyces cerevisiae vacuolar protein sorting gene VPS1. C. albicans VPS1 encodes a predicted 694-aa dynamin-like GTPase that is 73.3% similar to S. cerevisiae Vps1p. Plasmids bearing C. albicans VPS1 complemented the temperature-sensitive growth, abnormal class F vacuolar morphology, and carboxypeptidase missorting of a S. cerevisiae vps1 null mutant. To study VPS1 function in C. albicans, a conditional mutant strain (tetR-VPS1) was generated by deleting the first allele of VPS1 and placing the second allele under control of a tetracycline-regulatable promoter. With doxycycline, the tetR-VPS1 mutant was hyper-susceptible to sub-inhibitory concentrations of fluconazole, but not amphotericin B, 5-fluorocytosine, or nonspecific osmotic stresses. The repressed tetR-VPS1 mutant was defective in filamentation, and secreted less extracellular protease activity. Biofilm production and filamentation within the biofilm were markedly reduced. These results suggest that C. albicans VPS1 has a key role in several important virulence-related phenotypes.
Keywords: Candida albicans, biofilm, protein secretion, vacuole, virulence, VPS1
INTRODUCTION
The fungus Candida albicans has emerged as an important opportunistic pathogen (Jarvis, 1995; Pfaller and Diekema, 2007). Despite its clinical significance, available methods for the diagnosis and treatment of invasive candidiasis remain suboptimal (Lee and Wong, 2004). A range of C. albicans virulence-related proteins have been identified and many of these are cell surface or secreted proteins (Haynes, 2001; Ruiz-Herrera et al., 2006; Schaller et al., 2005), but little is known about the intracellular pathways and sorting processes by which these proteins reach their final destinations. In Saccharomyces cerevisiae, distinct secreted marker proteins are trafficked differentially through a pre-vacuolar compartment (PVC) prior to exocytosis (Harsay and Bretscher, 1995). Mutants blocked in this pre-vacuolar pathway missort several proteins found in high density post-Golgi vesicles into low density vesicles (Gurunathan et al., 2002; Harsay and Schekman, 2002). To study the pre-vacuolar branch of exocytosis in C. albicans, we identified close structural homologs of several S. cerevisiae pre-vacuolar secretory genes, including the late-Golgi vacuolar protein sorting gene VPS1. The function(s) of this gene’s homolog in C. albicans have not been previously studied.
S. cerevisiae VPS1 was originally identified in a screen for mutants that missort the vacuolar protein carboxypeptidase Y (CPY) extracellularly (Vater et al., 1992). S. cerevisiae Vps1p is an 80 kDa member of the dynamin-GTPase family. Vps1p mediates budding of clathrin-coated vesicles from the late Golgi that are diverted from the general secretory pathway to the vacuole (Bryant and Stevens, 1998). Structurally, Vps1p and dynamin are 45% identical and share a two-domain organization: the N-terminal domain is a GTPase and the C-terminal domain is required for membrane association. In the absence of Vps1p, Golgi and vacuolar membrane proteins are not transported to the PVC, but are instead missorted to the plasma membrane (Nothwehr et al., 1995; Vater et al., 1992; Wilsbach and Payne, 1993). S. cerevisiae vps1 mutants display a temperature-sensitive growth defect and are characterized by a wild-type vacuole surrounded by abnormal vacuolar fragments, defined as a class “F” vacuolar morphology (Raymond et al., 1992).
A search of the C. albicans genome database identified a close structural homolog of S. cerevisiae VPS1. Thus, the experiments described below were designed to confirm whether this C. albicans VPS1 homolog is functionally homologous to S. cerevisiae VPS1 and to determine whether it contributes to virulence-related phenotypes in C. albicans.
MATERIALS AND METHODS
Strains and media
The S. cerevisiae vps1 null mutant strain YKR001C was from American Type Culture Collection (Manassas, VA). C. albicans strains used in this study are indicated in Table 1. Strains were grown at 30 °C in YPD (1% yeast extract, 2% peptone, 2% glucose) supplemented with uridine (80 μg ml-1), or in minimal glucose (0.67% yeast nitrogen base without amino acids [YNB], 2% glucose) supplemented with appropriate amino acids according to auxotrophic requirements. Uracil auxotrophs were selected on 5-fluoro-orotic acid (FOA) medium (minimal glucose, 0.1 mg uridine ml-1, and 0.7 mg FOA ml-1). Complete synthetic medium supplemented with uridine and buffered to pH 4.0 with 150 mM HEPES was used for growth in acidic medium. YPD medium supplemented with uridine and buffered to pH 8.0 with 50 mM sodium succinate and 50 mM NaH2PO4 was used for growth in alkaline medium. Doxycycline was added to a final concentration of 20 μg ml-1 where required. Solid media were prepared by adding 2% (w/v) agar.
Table 1.
Candida albicans strains used in this study.
| Strain | Parent | Relevant genotype | Source/Reference |
|---|---|---|---|
| SC5314 | wild-type | (Kurtz et al., 1986) | |
| BWP17 | SC5314 | ura3Δ/ura3Δ arg4Δ/arg4Δ hisΔ/his1Δ VPS1/VPS1 | (Wilson et al., 1999) |
| SLZK1 | BWP17 | ura3Δ/ura3Δ arg4Δ/arg4Δ hisΔ/his1Δ VPS1/vps1Δ∷dpl200-URA3-dpl200 | This study |
| THE1 | CAI8 | ade2Δ∷hisG/ade2Δ∷hisG ura3Δ∷imm434/ura3Δ∷imm434 ENO1/eno1Δ∷ENO1-tetR-ScHAP4AD-3xHA-ADE2 VPS1/VPS1 | (Nakayama et al., 2000) |
| THE1-CIp10 | THE1 | ade2Δ∷hisG/ade2Δ∷hisG ura3Δ∷imm434/ura3Δ∷imm434 ENO1/eno1Δ∷ENO1-tetR-ScHAP4AD-3xHA-ADE2 RP10/RP10∷URA3 VPS1/VPS1 | This study |
| SMB1 | THE1 | ura3Δ∷imm434/ura3Δ∷imm434 VPS1/vps1Δ∷dpl200-URA3-dpl200 ade2Δ∷hisG/ade2Δ∷hisG ENO1/eno1Δ∷ENO1-tetR-ScHAP4AD-3xHA-ADE2 | This study |
| SMB1F | SMB1 | ura3Δ∷imm434/ura3Δ∷imm434 VPS1/vps1Δ∷dpl200 ade2Δ∷hisG/ade2Δ∷hisG ENO1/eno1Δ∷ENO1-tetR-ScHAP4AD-3xHA-ADE2 | This study |
| tetR-VPS1 | SMB1F | ura3Δ∷imm434/ura3Δ∷imm434 vps1Δ∷dpl200/99t-VPS1-URA3 ade2Δ∷hisG/ade2Δ∷hisG ENO1/eno1Δ∷ENO1-tetR-ScHAP4AD-3xHA-ADE2 | This study |
Preparation of plasmid and genomic DNA
Plasmids were expanded in Escherichia coli DH5α or TOP10F’ competent cells (Invitrogen) grown in LB medium with ampicillin (100 μg ml-1) at 37 °C. Plasmid DNA was prepared from E. coli strains using the Fast Plasmid Mini Kit™ (Eppendorf) following the manufacturer’s instructions. Genomic DNA was extracted from yeast cells using the Masterpure™ Yeast DNA Purification Kit (Epicentre Biotechnologies) according to manufacturer’s instructions with the exception of an additional incubation step (1 hr on ice) performed after the addition of the MPC Protein Precipitation Reagent.
Preparation of RNA and transcriptional analysis
RNA was prepared from yeast cells grown to mid-exponential phase in the presence or absence of doxycycline following the hot acidic phenol extraction method (Ausubel et al., 1993). Equal amounts of total RNA were run on a denaturing agarose gel (1% (w/v) agarose, 10 mM sodium orthophosphate buffer, pH 7.0, 7.6% (v/v) formaldehyde) and transferred for 4 hr to a positively charged nylon membrane (Roche) with 50 mM sodium hydroxide. The membrane was probed with a 32P-labeled DNA fragment of the VPS1 gene (nt 737-1678) amplified from C. albicans strain SC5314 genomic DNA using primers VPS1-5NB and VPS1-3NB. Prehybridization and hybridization steps were performed in UltraHyb™ (Ambion) at 37 °C for 4 and 18 hr, respectively. The membrane was then washed twice in 2x SSC, 0.1% SDS for 5 minutes at 65 °C, followed by two 15-minute washes with 0.1x SSC, 0.1% SDS at 65 °C. Detection was performed by autoradiography (Kodak Biomax MR, Kodak) for 24 hr.
mRNA was prepared from total RNA using the PolyATract mRNA Isolation System (Promega). RT-PCR was performed using the Access RT-PCR System (Promega) according to the manufacturer’s instructions using primers VPS1-5NB and VPS1-3NB and 50 ng mRNA as template. The absence of contaminating DNA was tested in parallel reactions in which the reverse transcriptase enzyme was excluded.
Isolation and analysis of C. albicans VPS1
A C. albicans gene homologous to S. cerevisiae VPS1 was identified by searching the Candida Genome Database (http://www.candidagenome.org) and CandidaDB (http://genolist.pasteur.fr/CandidaDB). The coding sequence and approximately 300 bp of upstream and downstream flanking sequence was amplified from C. albicans SC5314 genomic DNA using Platinum TaqHiFi DNA polymerase (Invitrogen) and primers VPS1-CP5 and VPS1-CP3 (Table 2). PCR reactions were typically heated to 94 °C for 3 min, followed by 35 cycles of 94 °C for 30 sec, 50 °C for 30 sec, and 72 °C for 2 min 30 sec, with a 10 min final extension at 72 °C. DNA sequencing of the forward and reverse strands confirmed the accuracy of the C. albicans VPS1 open reading frame. Standard methods were used for restriction mapping, subcloning, DNA sequencing, and lithium acetate transformation of S. cerevisiae mutants (Ausubel et al., 1993).
Table 2.
Primer sequences used in this study.
| Primer | Primer sequence (5’ – 3’) |
|---|---|
| VPS1-CP5 | CGAACATTTACTGTTGGTTTAGTC |
| VPS1-CP3 | AACTGTCACTGTCAGTTTAGTGAG |
| ScVPS1-5 | GCTCGAGGGTAAACCACTTGTGC |
| ScVPS1-3 | GTATTGGACCATTGAGGTATTAGG |
| VPS1-5DR | GTCCCACCAAAATCAATATTTACCAAAAAACGATAACAAACGAGTACTTTATTGACAACCGTTTTCCCAGTCACGACGTT |
| VPS1-3DR | AAGATAAATATATACCACCGACTTTCTGAAATAAAAAGAATTACTCTACTCTATAATACCTGTGGAATTGTGAGCGGATA |
| VPS1-5DRB | GAATGCATAGAGACAGTGCCACCAAAATCAATATTTACCAAAAAACGATAACAAACGAGTACTTTATTGACAACCAATTGGTTTTCCCAGTCACGACGTT |
| VPS1-3DRB | ATAAACACACTTATATTTATAAATTGGTTCATTAACTAAGCAGAATTATTAAGAAGATAAATATATACCACCGACTTTCTGTGTGGAATTGTGAGCGGATA |
| VPS1-5DRC | GGTAGAAAAAAATATTCTTTTATATATATTCCCATACAAAGCAATTAATCAAACGATAGAAAGTTATATCAAAAATGGATGGTTTTCCCAGTCACGACGTT |
| VPS1-3DRC | AGAATTACTCTACTCTATAATACCTCTAAACACTAGAAACAATTTCACTAGCATTTCTCAACACCTCAACCATTCTAACACTGTGGAATTGTGAGCGGATA |
| VPS1-5DetA | GATCATAACCGATGGTCGTGTG |
| VPS1-3DetA | ATTGCTCACAAGTTCACAGGCCC |
| VPS1-3DetC | GTGGATCATTTCTTGTCTTGC |
| VPS1-5SOU | CAAACACCAGAACAAATTGTCCATTTCACC |
| VPS1-3SOU | AGCAAGACAAGAAATGATCCACTTACACACG |
| TetVPS1-5DR | TGTAAGTGGATCATTTCTTGTCTTGCTTAATTTTTTTTTTGAATGCATAGAGACAGTGCCACCAAAATCGTAATACGACTCACTATAGGG |
| TetVPS1-3DR | CCTCCACCTAAAGGAGCCAATGCATCTTGTAATTTGTTAATGGTGGCAATCAATGTCTCATCCATTTTCTAGTTTTCTGAGATAAAGCTG |
| TetVPS1-5Det | GTTCAATGGAGGCTGGGTTTTG |
| TetVPS1-3Det | CTAGTTTTCTGAGATAAAGCTG |
| VPS1-5NB | GGCTGGACGTGTCATCCCAT |
| VPS1-3NB | GGTGATGTGGCTTGTGCTGG |
Attempted disruption of C. albicans VPS1
To disrupt both chromosomal alleles of C. albicans VPS1, we used a PCR-based gene disruption strategy which employs cis-recombination to recycle a URA3 marker thus preserving uracil auxotrophy (Wilson et al., 2000). PCR amplicons were generated using primers VPS1-5DR and VPS1-3DR (Table 2) and plasmid pDDB57 bearing the dpl200-URA3-dpl200 gene disruption cassette (from A. Mitchell, Columbia University) as the template. C. albicans BWP17 was transformed directly with the PCR reaction mixtures using the lithium acetate method (Ausubel et al., 1993). Uridine prototrophs were selected and purified on synthetic media lacking uracil and uridine, and genomic DNA was extracted by vortexing the transformants with glass beads in phenol-chloroform. Homologous integration of each gene targeting cassette was first verified by allele-specific PCR, using primers flanking the open reading frame and outside of the targeting region of the disruption cassette (Table 2). Next, C. albicans genomic DNA was digested with BglII and PstI and transferred to nylon membranes. The membranes were hybridized in 4x SSC (1x SSC is 0.15M NaCl plus 0.015 M sodium citrate) at 65 °C with a 417 bp digoxigenin-labeled PCR product generated with primers VPS1-5SOU and VPS1-3SOU using SC5314 genomic DNA as a template, after which the membranes were washed in 0.2x SSC-0.1% sodium dodecyl sulfate (SDS) at 65 °C and analyzed using the DIG DNA detection kit (Roche) according to manufacturer’s instructions.
To disrupt the second VPS1 allele, selected C. albicans VPS1/vps1Δ∷dpl200-URA3-dpl200 mutants were expanded in YPD with uridine to permit loss of URA3 by cis-recombination between the flanking dpl200 repeats, uracil auxotrophs were selected on FOA medium, and the genotype was determined by PCR and by Southern hybridization as described above. The resulting C. albicans VPS1/vps1Δ∷dpl200 strains were transformed again with the PCR-generated gene disruption cassette, uracil prototrophs were selected, and their genotypes were analyzed by PCR and by Southern hybridization.
Additional approaches for attempted disruption of C. albicans VPS1
PCR-based gene disruption using primers VPS1-5DRB and VPS1-3DRB was also performed as described above, except that the deletion of the second allele was attempted using the ARG4 marker in plasmid pRS-ARG4ΔSpeI (from A. Mitchell, Columbia University) (Wilson et al., 1999). The genotype of the resulting mutants and their wild-type parents were verified by allele-specific PCR using genomic DNA as a template and primers VPS1-5DetA and VPS1-3DetC (Table 2). Additional primer pairs bearing differing regions of homology (Table 2) were also used for PCR-mediated gene disruption. Finally, transformants recovered after incubation at 25 °C were also analyzed using identical methods.
Construction of a tetracycline-regulated VPS1 gene in C. albicans
One allele of the C. albicans VPS1 gene was deleted in strain THE1 (Nakayama et al., 2000), using primers VPS1-5DR and VPS1-3DR, according to the PCR-based gene disruption method described by Wilson et al. (2000) to generate strain SMB1 (Table 1). To regenerate ura3 auxotrophy, strain SMB-1 was first plated onto solid FOA medium. Next, FOA-resistant strains were screened by PCR for the VPS1/vps1Δ∷dpl200 genotype (strain SMB-1F). The regulatable tetracycline promoter was inserted upstream of the remaining VPS1 allele in strain SMB-1F using a strategy described by Bates et al. (2006) and primers TetVPS1-5DR and TetVPS1-3DR. Transformants carrying the tetR-VPS1 allele were screened using primers TetVPS1-5Det and TetVPS1-3Det. All other transformants were screened for the genotype of interest using primers VPS1-5DetA and VPS1-3DetA. The correct genotype of these strains was subsequently confirmed by Southern blot analysis following standard protocols (Ausubel et al., 1993). In brief, genomic DNA prepared from candidate strains was digested with Cla I (New England Biolabs) and run on a 0.8% (w/v) agarose gel. A digoxigenin-labeled probe was prepared from genomic DNA isolated from strain SC5314 with primers VPS1-5SOU and VPS1-3SOU and reagents supplied in the PCR DIG Probe Synthesis Kit (Roche). Table 2 lists primers used in this study.
Analysis of growth and stress tolerance
Growth was assessed in liquid media by measuring OD600 at fixed intervals, after the strains were washed and transferred to fresh complete synthetic media with uridine (80 μg ml-1) and diluted to a starting OD600 of 0.1. 400 μl cultures of each strain were grown in triplicate in a microtiter plate at 30 or 37 °C for 30 h in an automated Bioscreen C Analyzer (Thermo Labsystems). Shaking of the micro-cultures was performed at high intensity with irregular rotation every 3 min for 20 s and optical densities were measured every half hour. Growth curves were generated automatically using BioLINK software (Thermo Labsystems). Strains were analyzed in conditions of high osmolar stress (2.5 M glycerol or 1 M NaCl), in acidic (pH 4.0) or alkaline (pH 8.0) growth conditions, and different temperatures (30 or 37 °C). Strains grown in complete synthetic media supplemented with uridine were also exposed to sub-inhibitory concentrations of fluconazole, amphotericin B, and 5-fluorocytosine. Pilot growth curves using the URA3-complemented wild-type strain, THE1-CIp10, were first generated to determine the working concentration of each antifungal reagent to be used. After analysis of the growth of strain THE1-CIp10 in varying concentrations of antifungals, the final concentrations of fluconazole, amphotericin B, and 5-fluorocytosine used for phenotypic analysis were 10, 0.5, and 10 μg ml-1, respectively.
Visualization of vacuolar morphology
The fluorescent dye FM4-64 (Molecular Probes) was used to visualize endocytic and vacuolar membranes, as previously described (Vida and Emr, 1995). Following incubation with FM4-64, the cells were harvested and viewed directly using differential interference contrast (DIC) and fluorescence microscopy with an Olympus BH50 epifluorescence microscope equipped with a Retiga digital camera (QImaging, Canada), and a 60 x PlanApo oil immersion objective. Red fluorescence filters (excitation filter 533-588 nm, barrier 608-683 nm) (Chroma) were used to visualize structures stained with FM4-64. Digitized images were processed with Adobe Photoshop 5.0.
Enzyme assays
Carboxypeptidase secretion in S. cerevisiae strains was detected using the colony immunoblot method, as described by Conibear and Stevens (2002). In brief, colonies from overnight liquid cultures were spotted onto agar plates, and after 24 hours of growth, the colonies were overlaid with a nylon filter. Next, Western blotting was performed using anti-carboxypeptidase Y monoclonal antibody (from Abcam, UK), and detection was performed using chemiluminescence (ECL Detection Kit, Amersham Biosciences) and autoradiography. Extracellular protease secretion was assayed on BSA plates (Crandall and Edwards, 1987). Phospholipase activity was assessed on egg-yolk agar medium, and lipase activity was visualized on YNB agar containing 2.5% (v/v) Tween 80 (Fu et al., 1997). BSA degradation in liquid media was assessed by SDS-PAGE, followed by analysis of Sap2p secretion by Western blot analysis as described (Lee et al., 2007), except that the medium used was 1.18 % Yeast Carbon Base (YCB, Difco™), 0.01 % yeast extract, and 0.1 % BSA.
Filamentation assays
Filamentation was assayed at 37 °C in the following media, in the presence or absence of doxycycline: Medium 199 containing Earle’s salts (Invitrogen) supplemented with L-glutamine and buffered with 150 mM HEPES to pH 7.5; 10% (v/v) fetal calf serum in YPD; RPMI-1640 supplemented with L-glutamine (US Biological) and buffered with 165mM MOPS to pH 7.0; and Spider agar medium (Liu et al., 1994). Liquid medium was inoculated with cells from overnight cultures to achieve a starting density of 5×106 cells/ml, followed by incubation with shaking at 200 rpm, and visualization by light microscopy at specified time intervals.
Analysis of fungal biofilms
Formation of C. albicans biofilms and the XTT-reduction assay were performed as previously described (Ramage and Lopez-Ribot, 2005). Scanning electron microscopy was performed on biofilm samples formed on a coverslip (Thermanox, Nalge Nunc International) after 24 h incubation of a 0.5 ml inoculum containing 1×106 cells ml-1 according to previously described methods (Ramage et al., 2002).
RESULTS
Properties of Candida albicans VPS1
We first identified the C. albicans homolog of S. cerevisiae VPS1 from a BLAST search of CandidaDB and the Candida Genome Database. C. albicans VPS1 contains a 2082 bp intronless open reading frame whose deduced protein product is 73.3% similar to S. cerevisiae Vps1p and includes GTP-binding regions that are conserved in members of the dynamin-like GTPase family of proteins. Next, we cloned VPS1 by PCR using C. albicans strain SC5314 genomic DNA as the template. Sequencing of the cloned PCR product confirmed that it was identical to the published VPS1 open reading frame.
Complementation of a Saccharomyces cerevisiae vps1 mutant
To determine if the C. albicans VPS1 homolog is functionally homologous to S. cerevisiae VPS1, C. albicans VPS1 was sub-cloned into the low-copy number yeast shuttle vector pRS316, to generate pCaVPS1. Since S. cerevisiae vps1 mutants display a temperature-sensitive growth phenotype, we next transformed a S. cerevisiae vps1 null mutant with pCaVPS1 or with a plasmid bearing S. cerevisiae VPS1 and assayed growth at permissive (30 °C) and restrictive (37 °C) temperatures. Strains bearing C. albicans VPS1 or wild-type S. cerevisiae VPS1 grew at the restrictive temperature, in contrast to strains bearing empty vector alone which did not (Fig. 1A).
Fig. 1. Complementation of a S. cerevisiae vps1 null mutant.
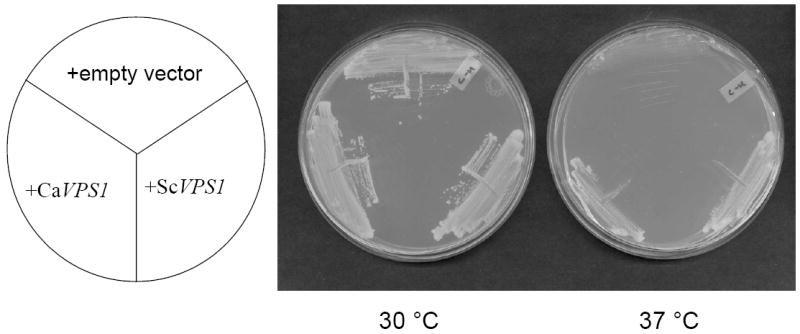
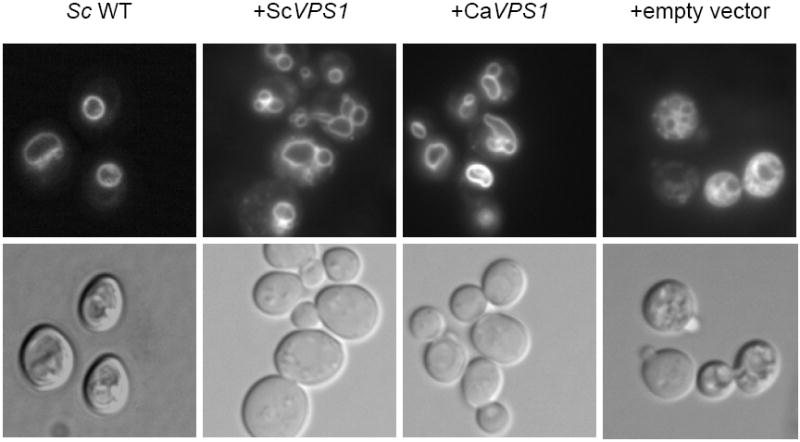

Over-expression of C. albicans VPS1 complements the temperature-sensitivity of a S. cerevisiae vps1 null mutant (A). Low copy number yeast shuttle plasmids (pRS316) containing C. albicans VPS1 (Ca VPS1) and S. cerevisiae VPS1 (Sc VPS1) conferred on a temperature-sensitive S. cerevisiae vps1 mutant the ability to grow at 37 °C, but empty vector alone did not. Over-expression of C. albicans VPS1 complements the S. cerevisiae vps1 null mutant vacuolar phenotype (B). Low copy number plasmids (pRS316) containing Sc VPS1, Ca VPS1, or no insert were introduced into a S. cerevisiae vps1 null mutant strain. Overnight cultures of strains grown in rich media were shifted to fresh media and examined during late exponential phase and stained with the vacuolar dye FM4-64. Live cells were examined by epifluorescence and DIC microscopy. The strain bearing vector alone displays a characteristic class “F” vacuolar morphology. Over-expression of C. albicans VPS1 complements carboxypeptidase (CPY) missorting of a S. cerevisiae vps1 null mutant (C). S. cerevisiae vps1 mutants missort CPY to the extracellular space, in contrast to wild-type strains, which do not. Therefore, overnight cultures of a S. cerevisiae wild-type strain and S. cerevisiae vps1 null mutant strains transformed with a vector bearing either Ca VPS1, Sc VPS1, or vector alone were spotted on solid media. Next, a colony immunoblot was performed by overlaying the spotted colonies with a nylon filter, followed by Western blotting using monoclonal antibody to S. cerevisiae CPY. A positive signal indicates missorting of CPY to the extracellular space.
We next examined vacuolar morphology in these strains. S. cerevisiae vps1 mutants bearing either C. albicans or S. cerevisiae VPS1 displayed wild-type vacuolar morphology. In contrast, S. cerevisiae vps1 null mutants transformed with empty vector exhibited a class “F” vacuolar morphology, characterized by a fragmented vacuole visible with light microscopy (Fig. 1B).
S. cerevisiae vps1 mutants missort the vacuolar enzyme carboxypeptidase (CPY) to the extracellular space (Vater et al., 1992), hence we assayed for CPY missorting using the colony immunoblot method. S. cerevisiae vps1 null mutants transformed with plasmids bearing C. albicans or S. cerevisiae VPS1 missorted much less CPY extracellularly compared to S. cerevisiae vps1 null mutants transformed with empty vector (Fig. 1C). Taken together, these results indicate that C. albicans VPS1 complements the S. cerevisiae vps1 mutant and therefore is functionally homologous to the corresponding S. cerevisiae gene.
Construction of a C. albicans VPS1 conditional mutant
We next sought to study VPS1 directly in C. albicans by generating a C. albicans vps1Δ null mutant, but our efforts were not successful. The first allele of C. albicans VPS1 was readily deleted in BWP17 using the PCR-based “mini-Urablaster” cassette (Wilson et al., 2000) to generate a VPS1/vps1Δ∷dpl200-ura3-dpl200 strain. Strains were next plated on FOA, and intrachromosomal recombination was confirmed by Southern analysis. However, efforts to generate a strain lacking the remaining wild-type VPS1 allele were unsuccessful, despite genotypic analysis of 80 independent transformants. Next, we attempted to disrupt the second allele using an alternative PCR-based gene disruption approach, using an ARG4 selection marker. Genotypic analysis of greater than 120 independent colonies again failed to identify any homozygous null mutants. Additionally, use of gene disruption primers bearing differing regions of homology, and analysis of transformants recovered at lower temperatures (i.e. 25 °C) failed to reveal a vps1Δ/vps1Δ null mutant.
Thus, in order to study C. albicans VPS1, we generated a tetracycline-regulated conditional mutant, strain tetR-VPS1 (Table 1). The correct genotype of all strains was confirmed using PCR and Southern blot analysis (Supplemental Figures 1 and 2). RT-PCR and Northern analysis indicated that in the presence of doxycycline, the VPS1 transcript was absent in all tetR-VPS1 isolates tested (Fig. 2). In non-repressing conditions, we observed higher levels of VPS1 transcript in the tetR-VPS1 strains compared to transcript levels in the wild-type strain (Fig. 2), since the Tet-promoter cassette in p99CAU1 provides strong induction of gene expression (Nakayama et al., 2000).
Fig. 2. Transcriptional analyses of VPS1 in the tetR-VPS1 strains.
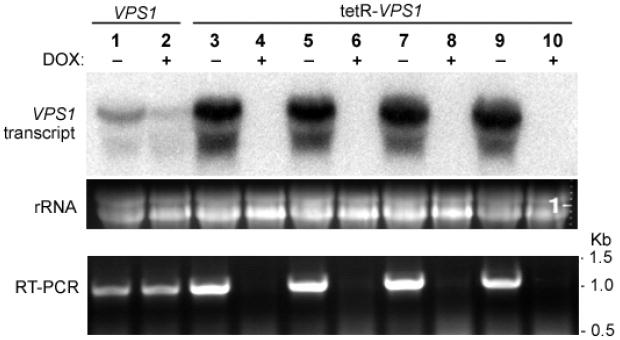
A Northern blot was probed with a 32P-labeled PCR product amplified from strain SC5314 using primers VPS1-5NB and VPS1-3NB (Table 2). There is complete repression of the VPS1 transcript in the tetR-VPS1 strains when doxycycline is present. RT-PCR on mRNA isolated from the same total RNA used in the Northern blot also showed complete repression of VPS1 transcription in the tetR-VPS1 strains in the presence of doxycycline. An ethidium bromide stained gel shows normalized loading of the RNA samples used in the Northern blot.
Growth and response to osmotic stress in vitro
Lack of VPS1 gene expression did not affect growth in complete synthetic medium at either 30 or 37 °C (Fig. 3A). No difference in growth was observed between the wild-type and tetR-VPS1 mutant strains, with or without doxycycline, when grown under alkaline (pH 8.0) or acidic (pH 4.0) conditions, conditions of osmotic stress (in media containing high concentrations of salt and glycerol) or at temperatures of 30 and 37 °C (data not shown).
Fig. 3. Characterization of growth of the tetR-VPS1 strain.
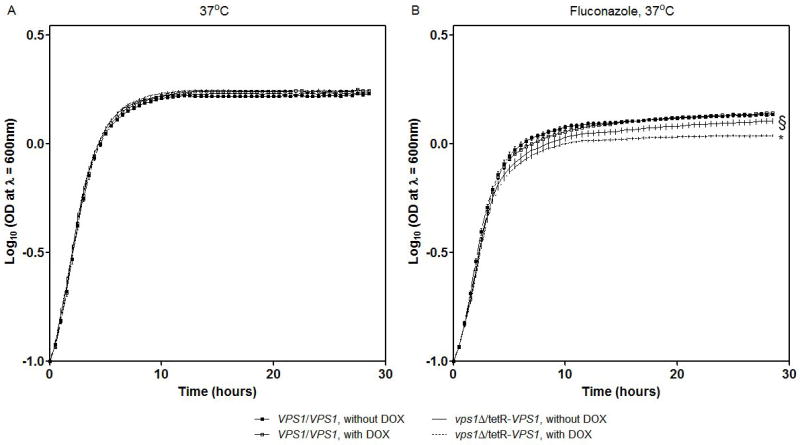
Growth of the tetR-VPS1 mutant strain was tested in the presence of sub-inhibitory concentrations of fluconazole. In complete synthetic medium supplemented with uridine at 37 °C, growth of the tetR-VPS1 strain was similar to the parental strain THE1 (A). However, growth of the tetR-VPS1 strain was inhibited in the presence of 10 μg ml-1 fluconazole (B). This sensitivity to fluconazole was increased when transcription of VPS1 was completely repressed with the addition of doxycycline. Growth curves were performed in triplicate, and corresponding error bars are indicated. The differences in growth between strain tetR-VPS1 without doxycycline and with doxycycline is statistically significant (* p < 0.0001). Similarly, the differences in growth between strain tetR-VPS1 without doxycycline and THE1-CIp10 is statistically significant (§ p < 0.0001). Statistical significance was tested by fitting growth curves to lognormal models which yielded R-squares of 99%.
Growth and response to antifungal agents in vitro
Next we tested growth in the presence of sub-inhibitory concentrations of several antifungal agents at 30 and 37 °C. No growth difference between the wild-type and tetR-VPS1 strain was seen when either amphotericin B or 5-fluorocytosine was used, in the presence or absence of doxycycline (data not shown). However, a clear growth difference occurred when these strains were grown in medium containing fluconazole (Fig. 3B). The heterozygous strain SLZK1 (VPS1/vps1Δ) grew less well compared to the parental strain BWP17 (data not shown). Similarly, the tetR-VPS1 strain expressing a single copy of VPS1 grew less well than the wild-type strain THE1-CIp10 (Fig. 3B). This growth defect increased even more when VPS1 expression was repressed in the presence of doxycycline, at both 30 and 37 °C (Fig. 3B).
Characterization of vacuolar morphology
The S. cerevisiae vps1 mutant exhibits a class F vacuolar morphology which is described as having a large central vacuole surrounded by smaller vacuole-like fragments (Raymond et al., 1992). Vacuolar staining of the tetR-VPS1 mutant strain with FM4-64 showed a single large vacuole (Fig. 4A) similar to the vacuole seen in the S. cerevisiae vps1 mutant described by Mullins and Bonifacino (2001). We also visualized the vacuolar morphology of the tetR-VPS1 strain using thin-section electron microscopy. Unexpectedly, no major differences in vacuolar morphology were apparent between strain THE1-CIp10 and the tetR-VPS1 mutant strain, in either the presence or absence of doxycycline (Fig. 4B).
Fig. 4. Vacuolar morphology of the tetR-VPS1 strain.
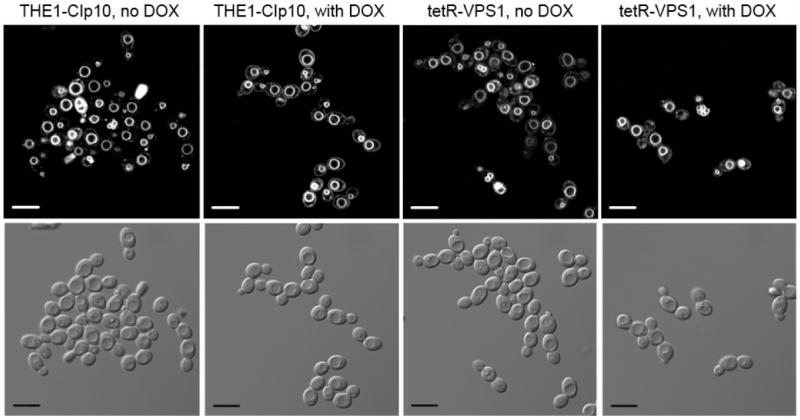
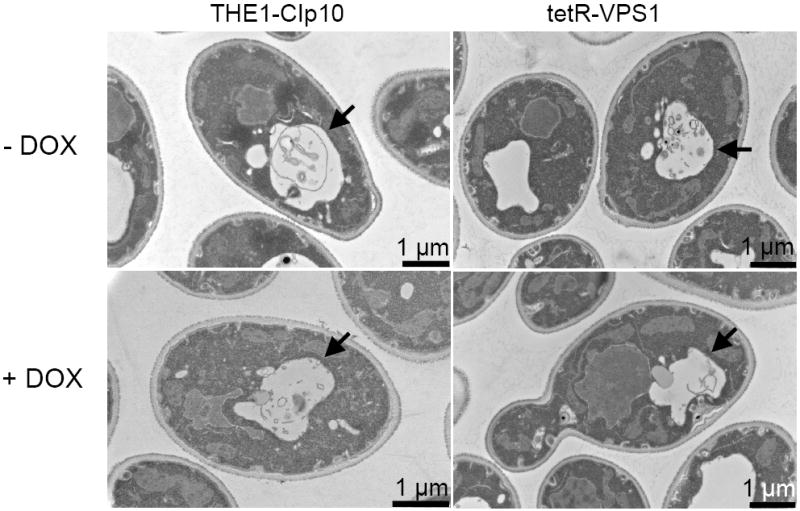
Vacuolar morphology was visualized by FM4-64 staining (A). A 10 μm bar is shown in either white or black. Thin-section electron microscopy images are shown in (B). A solid arrowhead is used to designate the vacuole. In either set of images, no major differences in vacuolar morphology were seen between the URA3-complemented wild-type strain THE1-CIp10 and the tetR-VPS1 strain, with or without doxycycline.
Secretion of extracellular proteases in the tetR-VPS1 mutant
Since S. cerevisiae VPS1 plays an important role in pre-vacuolar secretion (Gurunathan et al., 2002; Rothman et al., 1990), we sought to determine the effect of the loss of function of C. albicans VPS1 on secretion of extracellular proteases. When inoculated on medium containing BSA as the sole nitrogen source, wild-type C. albicans secretes aspartyl proteinases which result in a halo surrounding the colony due to extracellular proteolysis. The strains carrying a single copy of the VPS1 gene (SMB1 and SMB1F) produced a similar amount of extracellular BSA degradation as the wild-type strain (data not shown). Also, the addition of doxycycline did not affect the levels of BSA degradation in these strains (data not shown). Under non-repressing conditions, the tetR-VPS1 mutant strain produced the same amount of BSA degradation as the wild-type strain (Fig. 5A). However, when transcription of the VPS1 gene was repressed in the tetR-VPS1 strain by addition of doxycycline, a clear decrease in the amount of proteolysis was observed.
Fig. 5. Secretion of proteolytic enzymes.
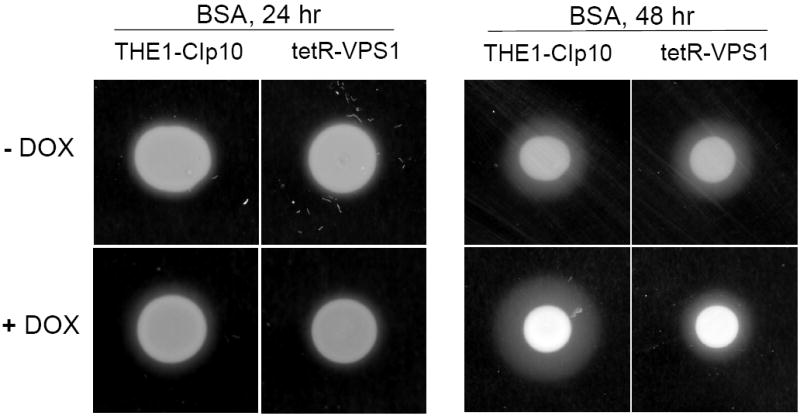
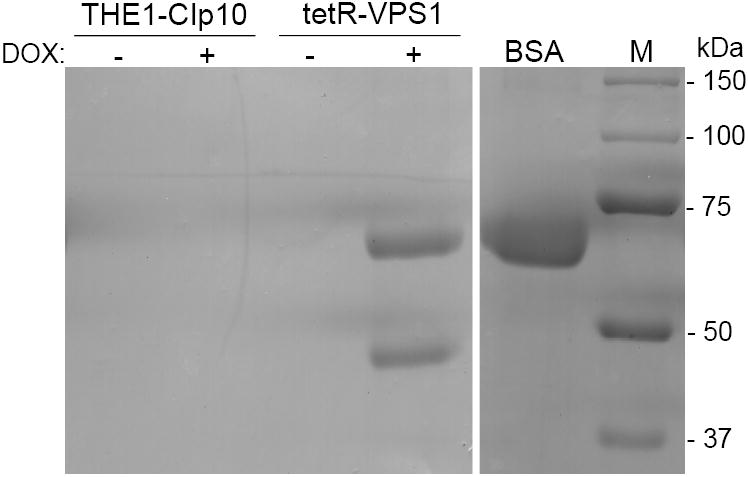
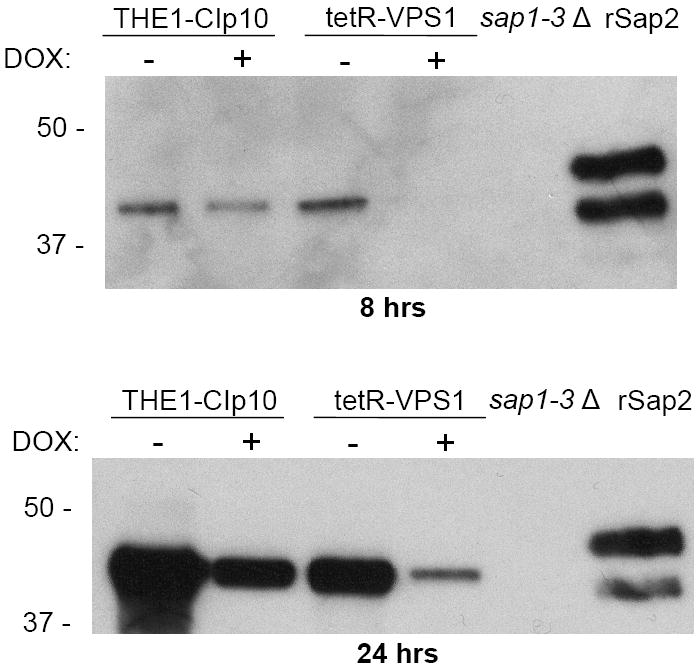
Proteolysis assay on BSA plates (A). Overnight cultures were spotted onto BSA plates and incubated at 30 °C for 24 and 48 hours. The relative amount of extracellular protease activity is indicated by the halo surrounding the fungal colony. In the absence of doxycycline, strains THE1-CIp10 and tetR-VPS1 produced similar amounts of extracellular BSA degradation. In the presence of doxycycline, the tetR-VPS1 strain produced much less BSA degradation than strain THE1-CIp10. Proteolysis assay in liquid BSA (B). Overnight cultures were shifted to medium containing BSA as a sole nitrogen source and incubated at 30 °C for 24 hours, with and without doxycycline. The degree of proteolysis of BSA was analyzed by reducing SDS-PAGE and Coomassie blue staining. The tetR-VPS1 strain produced much less proteolysis in the presence doxycycline compared to tetR-VPS1 without doxycycline or the control strain. Lanes from the same gel containing standard protein markers (M) and intact BSA are shown for reference. Western analysis of Sap2p secretion (C). Cell-free supernatants prepared as described in (B) were analyzed by Western blotting using anti-Sap2p antibodies (from M. Monod). Compared to the parental strain THE1-CIp10 (with and without doxycycline) and tetR-VPS1 (without doxycycline), much less Sap2 protein was secreted by the tetR-VPS1 strain when doxycycline was present. The triple deletion mutant strain sap1-3Δ (from B. Hube) was used as a negative control. rSap2 indicates purified recombinant Sap2p (from M. Monod) used as a positive control. Analysis of lipase secretion (D). Overnight cultures were spotted onto YNB-Tween 80 agar plates and incubated at 37 °C. The relative amount of lipolytic degradation after 3 and 6 days is indicated by the halo surrounding the fungal colony. The tetR-VPS1 strain produced much less extracellular lipolytic activity in the presence of doxycycline than corresponding controls. In contrast, no major differences in phospholipase activity were seen when assayed on egg-yolk agar plates (data not shown).
Further analysis of protease secretion using liquid BSA degradation and SDS-PAGE also produced similar results as the plate assay (Fig. 5B). When analyzed by Western blotting using anti-Sap2p polyclonal antibodies (gift of B. Hube, International Leibniz Research School), the tetR-VPS1 strain with doxycycline produced much less extracellular Sap2p than the tetR-VPS1 strain without doxycycline (Fig. 5C).
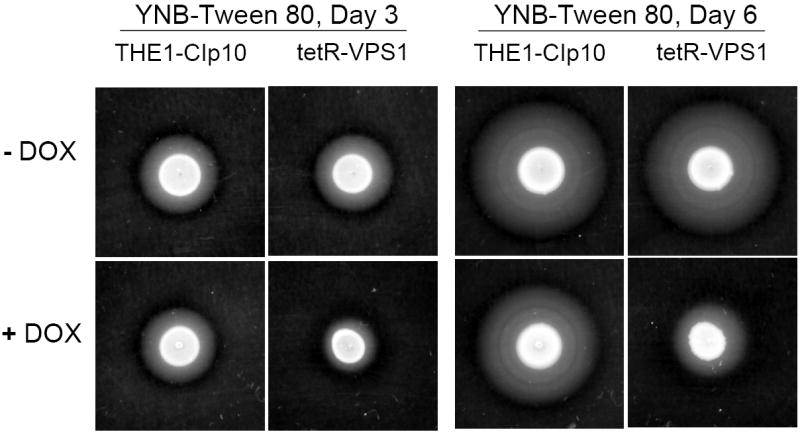
We next assayed for phospholipase secretion using egg yolk agar plates, however, no difference was seen among these strains with or without doxycycline (data not shown). In contrast, on YNB-Tween 80 plates, which have been used to assay for lipase secretion, the tet-VPS1 strain with doxycycline produced much less lipolysis than the tet-VPS1 strain without doxycycline (Fig. 5D). Taken together, these results suggest that lack of VPS1 expression reduces secretion of extracellular proteases and lipases.
Filamentation in the tetR-VPS1 mutant
Another important virulence attribute in C. albicans is the ability to switch between yeast, pseudohyphal, and filamentous forms (Braun and Johnson, 2000; Brown and Gow, 1999). We observed that wild-type C. albicans colonies inoculated onto BSA plates begin to produce filamentous structures on day 3. Interestingly, when expression of the VPS1 gene was repressed, the tetR-VPS1 strain did not produce filaments on BSA, even after 8 days (Fig. 6A). Similar results were also observed on conventional filamentation medium, i.e. Spider agar, M199 agar, and YPD agar with 10% fetal calf serum (Fig. 6A and 6B). On these agar plates, in derepressing conditions the tetR-VPS1 strain produced the same filamentous morphology as the wild-type parental strain. In repressing conditions (with doxycycline), the tetR-VPS1 mutant strain produced much less filamentation compared to the wild-type or derepressed tetR-VPS1 strains. A clear lag in filamentation was also seen when the tetR-VPS1 strain was grown in liquid RPMI-1640 and doxycycline at 37 °C (Fig. 6C). When assayed in liquid 10% fetal calf serum, similar results were also noted, although the difference in filamentation was less pronounced (Fig. 6D). These results suggest that VPS1 expression is required for normal filamentation.
Fig. 6. Filamentation of strains grown in hyphal-inducing media.
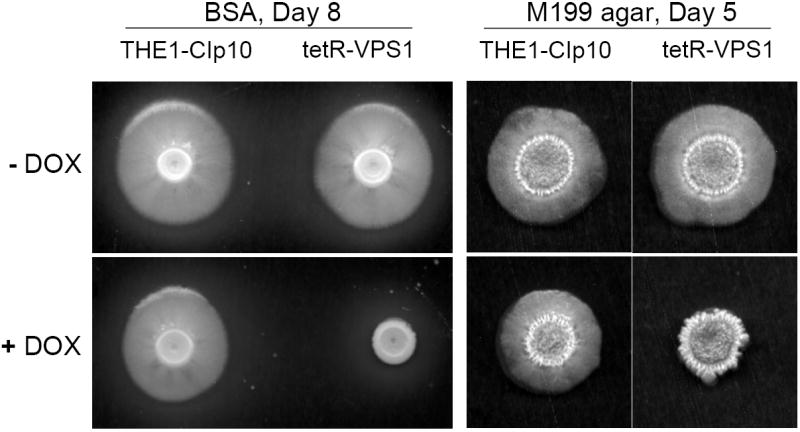
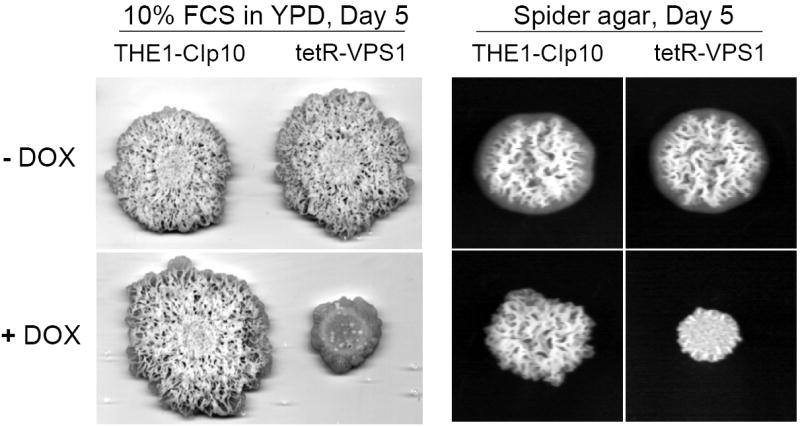
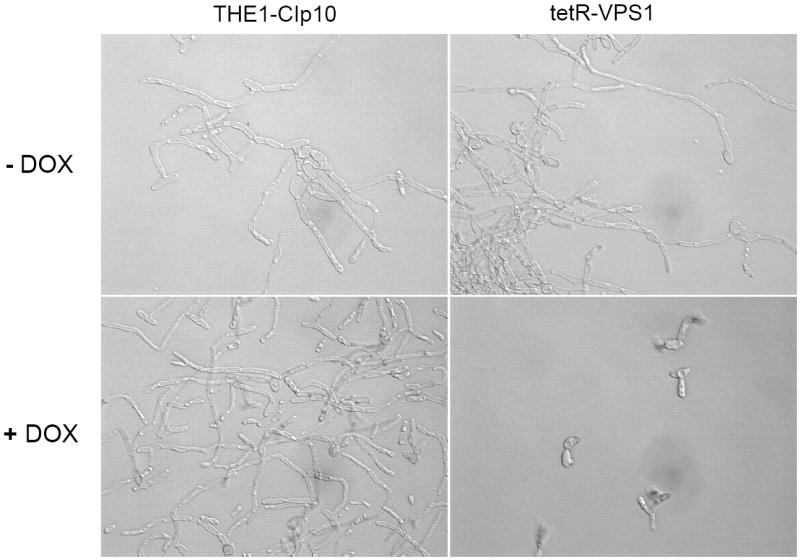

Filamentation on BSA and M199 agar plates (A). Overnight cultures of strains THE1-CIp10 and tetR-VPS1 were spotted onto BSA plates and incubated at 30 °C for 8 days. Robust filamentous structures emerging from the edge of each colony were observed in all strains, except in the tetR-VPS1 strain in the presence of doxycycline. Similar results were observed when these strains were spotted onto Medium 199 agar containing Earle’s salts, buffered with 150 mM HEPES to pH 7.5, and incubated at 37 °C for 5 days. Filamentation on Spider and 10% FCS agar plates (B). Overnight cultures were spotted onto either YPD containing 10% fetal calf serum (FCS) or Spider agar medium and incubated at 37 °C for 5 days. On Spider agar, robust filamentation extending above the surface of the plate can be seen in all strains except for the repressed tetR-VPS1 strain, which did not produce a raised colony. Similarly, on YPD agar with 10% FCS, filamentation was robust in all strains except in the tetR-VPS1 strain grown in the presence of doxycycline. Filamentation in liquid RPMI-1640 (C). Filamentation was assessed in RPMI-1640 medium buffered to pH 7.3 with 165 mM MOPS, with or without doxycycline. Medium was inoculated with 5 × 106 cells/ml and incubated at 37 °C for 24 hr with constant shaking at 200 rpm. Strain THE1-CIp10 produced hyphae when grown in the presence or absence of doxycycline. In contrast, strain tetR-VPS1 produced masses of hyphae when grown under derepressing conditions, but not under repressing conditions. Filamentation in YPD containing 10% FCS (D). Pre-warmed medium was inoculated with 5 × 106 cells/ml and incubated at 37 °C with constant shaking at 200 rpm. Induction of filamentation was followed by light microscopy at fixed time points from the time of inoculum, with a four hour time point shown. The tetR-VPS1 strain remained predominantly in the yeast form when grown in the presence of doxycycline, whereas, in contrast the other strains produced longer and greater numbers of filamentous structures.
Biofilm formation in the tetR-VPS1 mutant
We next hypothesized that the pre-vacuolar secretory defect of the tetR-VPS1 strain could affect fungal biofilm formation, since both secretion and filamentation were abnormal. We first compared the structure of Candida biofilms formed after 24 h using light microscopy. Biofilm morphology of the wild-type strains (in the absence or presence of doxycycline) and the derepressed tetR-VPS1 mutant was similar. However, in the absence of a functional VPS1 expression, the biofilm formed was markedly less dense (Fig. 7A). Scanning electron microscopy demonstrated that the tetR-VPS1 strain produced a sparse biofilm that was composed predominantly of yeast cells and pseudohyphal structures when doxycycline was present (Fig. 7B). In contrast, without doxycycline, the biofilm structure formed by the tetR-VPS1 strain was composed of a dense population of predominantly mature filamentous forms.
Fig. 7.
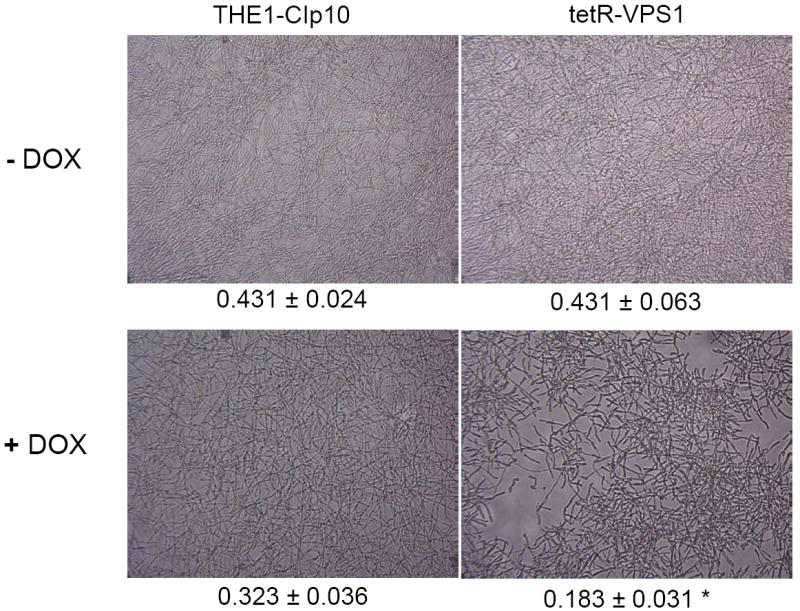
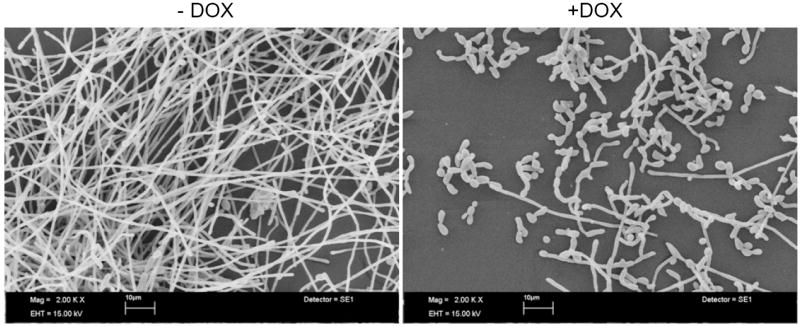
Light microscopy of biofilms formed by strains THE1-CIp10 and tetR-VPS1 (A). The gross morphology of biofilms formed by strains THE1-CIp10 and tetR-VPS1, grown with and without doxycycline, were visualized with light microscopy. Relative values from the XTT-reduction assay are indicated. A statistically significant reduction (* p < 0.001) in biofilm mass was observed in the tetR-VPS1 strain grown under repressed conditions (with doxycycline). Scanning electron microscopy of biofilms formed by the tetR-VPS1 strain (B). Biofilms formed by the tetR-VPS1 strain were visualized using scanning electron microscopy. Under derepressing conditions (without doxycycline), the tetR-VPS1 strain produced a thick biofilm that consisted predominantly of mature hyphae. Under repressing conditions (with doxycycline) the tetR-VPS1 strain produced a biofilm that was markedly less dense, and composed predominantly of yeast and pseudohyphal cells. The figures shown depict representative structures at 2,000x magnification with a 10 μm bar indicated.
Biofilm mass measured by the XTT reduction assay was similar for the derepressed tetR-VPS1 mutant strain and the wild-type control (Fig. 7A). A modest reduction in metabolic activity was seen in the wild-type strain when doxycycline was present. However, a significantly greater reduction in metabolic activity was measured in the tetR-VPS1 strain when doxycycline was added (p-value < 0.001). Thus, these results suggest that VPS1 contributes to normal biofilm formation in C. albicans.
DISCUSSION
The major goals of this study were to determine if the pathogenic fungus C. albicans has a pre-vacuolar exocytic pathway analogous to the pathway described in S. cerevisiae (Harsay and Schekman, 2002), and to determine the contribution of C. albicans VPS1 to key virulence-related phenotypes in vitro. When a search of the C. albicans genome database revealed a close structural homolog of the S. cerevisiae vacuolar protein sorting gene VPS1, we used a complementation approach to study gene function. First, the temperature-sensitivity, vacuolar morphology, and protein sorting phenotypes of a S. cerevisiae vps1 null mutant were corrected by plasmids bearing C. albicans VPS1, but not by empty vector. Taken together, these observations confirmed that the gene designated C. albicans VPS1 is both a structural and functional homolog of S. cerevisiae VPS1.
Our next objective was to study VPS1 directly in C. albicans. However, despite screening a large number of transformants, our efforts to generate a C. albicans vps1Δ null mutant were unsuccessful. Although we initially hypothesized that C. albicans VPS1 may be an essential gene, subsequent phenotypic analysis of the conditional tet-regulated vps1 mutant has indicated otherwise. Other possibilities that could account for our inability to generate a vps1Δ null mutant include an allele-specific reduction in the efficiency of homologous recombination, or reduction in the overall fitness of a potential null mutant.
Unexpectedly, the repressed C. albicans tetR-VPS1 mutant did not display a characteristic class “F” vacuolar morphology. It should be noted that the S. cerevisiae vps1 mutant has a vacuolar morphology that is intermediate between wild-type strains and that of class “B” vacuolar mutants (Raymond et al., 1992). The class “B” vacuole typically consists of a large central vacuole, which we also observed in the C. albicans tetR-VPS1 mutant (with doxycycline), often surrounded by smaller vacuole-like compartments, which we were not able to discern in the tetR-VPS1 mutant. Thus, it is possible that our thin-section electron microscopic approach was not ideally suited for visualizing these vacuolar fragments, or perhaps that C. albicans VPS1 does not perform an identical role as S. cerevisiae VPS1 in vacuolar biogenesis.
Proteins encoded by a number of vacuolar protein sorting (VPS) genes have been shown to play a role in several virulence-related properties in C. albicans in vitro. These proteins include the phosphatidylinositol 3-kinase Vps34p (Bruckmann et al., 2000), the vacuolar sorting protein Vps11p (Palmer et al., 2003), components of the endosomal sorting complex required for transport (ESCRT complexes) Vps28p and Vps32p (Cornet et al., 2005), and the AAA-ATPase Vps4p (Lee et al., 2007). Attenuated virulence in the vps4Δ, vps28Δ, vps32Δ, and vps34Δ null mutants has also been demonstrated in vivo (Bruckmann et al., 2000; Cornet et al., 2005; Lee et al., unpublished data). We thus sought to characterize virulence-related phenotypes in our tetR-VPS1 conditional mutant strain.
In the presence of doxycycline, transcription of the VPS1 gene is tightly repressed in the tetR-VPS1 mutant strain. We have demonstrated that loss of function of C. albicans VPS1 does not affect growth in complete synthetic or rich medium. Hence any phenotypic differences from the wild-type or derepressed tetR-VPS1 can be attributed to the loss of function of the VPS1 gene. Next, we have shown that the tetR-VPS1 mutant strain was hyper-susceptible to sub-inhibitory concentrations of fluconazole. Strains expressing only one copy of the VPS1 gene, i.e. heterozygous strain SLZK1 (VPS1/vps1Δ) and the tetR-VPS1 (tetR-VPS1/vps1Δ) strain, were sensitive to sub-inhibitory concentrations of fluconazole. In the repressed tetR-VPS1 strain (i.e., with the addition of doxycycline), the sensitivity to fluconazole was even greater. Thus we have demonstrated a gene dosage effect of VPS1 on the tolerance of C. albicans to sub-inhibitory concentrations of fluconazole.
Whereas growth of the tetR-VPS1 mutant strain was inhibited by fluconazole, it was not affected by either amphotericin B or 5-fluorocytosine. This phenotype is similar to the vps28Δ and vps32Δ homozygous knockout mutants which are hyper-susceptible to azoles, but not amphotericin B and 5-fluorocytosine, in a RIM101-dependent manner (Cornet et al., 2006). Vps28p and Vps32p regulate the expression of RIM101, PHR1, and PHR2 (Cornet et al., 2005), which are genes involved in the alkaline response (Davis et al., 2000). Cornet et al. (2006) showed that in the presence of a functional Rim101 protein, vps28Δ and vps32Δ null mutants were no longer hyper-susceptible to either fluconazole or voriconazole. In contrast to the vps28Δ and vps32Δ null mutants, growth of the tetR-VPS1 strain was not affected when grown under repressing conditions in alkaline medium (data not shown) suggesting that the pH-sensing mechanism in this strain is intact. However, it should be noted that a RIM101-independent pH-sensing mechanism exists in C. albicans, which allows the organism to survive when grown in alkaline conditions through induction of the alkaline-repressed PHR2 gene (Davis et al., 2000).
Secreted hydrolytic enzymes of C. albicans, such as the family of secreted aspartyl proteases (Saps), lipases, and phospholipase B have been shown to play a role in tissue invasion and evasion of the host-immune response (Ghannoum, 2000; Naglik et al., 2003; Schaller et al., 2005). Deletion of the VPS11 gene results in a strain that is deficient in secretion of Saps and lipases (Palmer et al., 2003). The class E type of vacuolar mutants vps34Δ and vps4Δ are also unable to secrete Sap2 protein in C. albicans (Kitanovic et al., 2005; Lee et al., 2007). Interestingly, the C. albicans vps4Δ null mutant secretes an increased amount of extracellular proteolytic activity on BSA plates, which has been identified as missorted carboxypeptidase. However, specific Western blotting reveals a complete absence of extracellular Sap2p (Lee et al., 2007). Here we report a role for the late-Golgi vacuolar protein sorting gene VPS1 in the secretion of proteases and lipases. We have demonstrated that the C. albicans tetR-VPS1 mutant secretes less extracellular proteolytic activity when grown on BSA agar and reduced lipolytic activity on YNB-Tween agar in repressing conditions. Western blotting of culture supernatants has indicated a reduction in extracellular Sap2p, suggesting that reduced Sap2p secretion may be the origin of this reduced extracellular proteolytic activity. This reduced proteolytic phenotype is similar to that seen with the C. albicans vps11Δ null mutant. VPS11 is thought to act not only at a trafficking step immediately prior to vacuolar fusion, but in the late-Golgi as well (Rieder and Emr, 1997; Robinson et al., 1991; Sato et al., 2000). Perhaps interruption of the pre-vacuolar secretory pathway at an immediate post-Golgi step reduces the overall amount of secreted proteases, whereas at a later pre-vacuolar step, Sap2p secretion is interrupted, but not secretion of missorted vacuolar enzymes such as carboxypeptidase. It will be interesting to examine the phenotypes of late-vacuolar vps mutants, such as class “D” vacuolar mutants, in order to determine if Saps and other secreted hydrolytic enzymes are trafficked through a pre-vacuolar pathway, or if some other post-translational regulatory mechanism exists.
In addition to the altered levels of protease secretion, some of the previously characterized vps mutants have also been shown to be defective in filamentation. There are several regulatory pathways mediating yeast-to-hyphal morphogenesis in C. albicans [reviewed in Liu (2001), Dhillon et al., (2003), and Monge et al (2006)]. These include a cAMP-protein kinase A pathway in which Efg1p is involved (Stoldt et al., 1997), a MAP-kinase pathway in which Cph1p plays a key role (Liu et al., 1994), the Tup1p-mediated pathway (Braun and Johnson, 1997), a Rim101p-dependent pH-sensing pathway (Davis et al., 2000), and Czf1p-mediated filamentation which is described when C. albicans is embedded in solid medium (Brown et al., 1999). C. albicans vps28Δ and vps34Δ null mutants exhibit defects in filamentation that are dependent on the RIM101 pathway (Cornet et al., 2005). The C. albicans vps34Δ null mutant produces more hyphae than the wild-type control when embedded in solid agar matrix at 23 °C (Kitanovic et al., 2005), but when grown in liquid hyphae-inducing medium at 37 °C, filamentation is considerably delayed in the mutant strain (Bruckmann et al., 2000). The C. albicans vps11Δ null mutant is unable to produce filaments and it has been suggested that VPS11 may play a role in vacuolar expansion required for yeast to hyphal morphogenesis (Palmer et al., 2003; Palmer et al., 2005). We have shown here that the loss of function of C. albicans VPS1 resulted in delayed filamentation when grown on strong or weak filamentation-inducing medium.
We hypothesize that the filamentation defect of the repressed tetR-VPS1 strain may result from mislocalization of Kex2p from the Golgi complex, where it is required for processing of proteins contributing to hyphal formation. In S. cerevisiae, Vps1p mediates retention of the endoprotease Kex2p in the Golgi membrane (Wilsbach and Payne, 1993). Mutations in VPS1 result in mislocalization of Kex2p to the vacuole where it is degraded by Pep4p (Wilsbach and Payne, 1993). Studies of the KEX2 homolog in C. albicans demonstrate that disruption of KEX2 results in a strain that is unable to produce hyphae when grown in filamentation-inducing conditions (Newport and Agabian, 1997). The authors hypothesized that Kex2p may be required for post-translational processing of components of the cell wall and other proteins. This hypothesis was supported by a subsequent study in which a genome-wide search identified a number of hydrolases, adhesins, cell wall components, and outer membrane proteins that contained putative Kex2p processing sites (Newport et al., 2003). Thus, a potential mechanism for the filamentation defect in the repressed tetR-VPS1 strain is that in the absence of Vps1p, Kex2p is mislocalized to the vacuole and degraded, resulting in abnormal processing of proteins required for filamentation.
Candida biofilms are composed of a mixed community of differentiated cells and extracellular matrix. The basic stages of biofilm formation include the (1) adhesion of cells to a surface conducive for biofilm formation, (2) formation of a basal layer that serves as an anchor, followed by (3) pseudohyphal and hyphal growth and the formation of the extracellular matrix (Nobile and Mitchell, 2006). We have shown that when transcription of VPS1 is repressed, the tetR-VPS1 strain formed a sparse biofilm composed predominantly of pseudohyphal and yeast cells. Interestingly, Marchais et al. (2005) observed increased expression of C. albicans VPS1 in germ tubes adherent to polystyrene, suggesting that C. albicans VPS1 may play a role in adhesion. Furthermore, VPS1 may also play a role in later stages of biofilm formation that are characterized by extensive differentiation and filamentation of cells. VPS1 may also contribute to biofilm formation in response to amino acid starvation. In genome-wide profiling studies, Tournu et al. (2005) showed increased expression of C. albicans VPS1 in a GCN2- and GCN4-dependent manner during amino acid starvation. GCN4 encodes a transcriptional activator within the general amino acid control response that is over-expressed during biofilm formation, which is in turn associated with amino acid starvation (Garcia-Sanchez et al., 2004).
Thus, we have shown that C. albicans VPS1 is involved in tolerance of azoles, protease secretion, filamentation, and biofilm formation, suggesting an important role of the pre-vacuolar secretory pathway in C. albicans virulence. We are currently undertaking further investigations on the mechanism by which VPS1 effects control on secretion, filamentation, and biofilm formation in vitro, and studies of virulence in vivo using fly and murine models of invasive candidiasis.
Supplementary Material
A. Transformants were screened for the genotype of interest by allele-specific PCR using primers VPS1-5DetA and VPS1-3DetA. Strains carrying the VPS1, vps1Δ∷dpl200-URA3-dpl200, and vps1Δ∷dpl200 alleles yield PCR products that are 2.5, 2.2, and 0.8 kb in size, respectively. PCR product from strains THE1 (VPS1/VPS1), SMB-1 (VPS1/vps1Δ∷dpl200-URA3-dpl200), and SMB-1F (VPS1/vps1Δ∷dpl200) are indicated as “WT”, “1st allele”, and “FOA”, respectively.
B. Transformants carrying the tetR-VPS1 allele were identified using primers TetVPS1-5Det and TetVPS1-3Det. Only those strains with the correct genotype (vps1Δ∷dpl200/99t-VPS1-URA3) yield a PCR product that is 2.0 kb in size. As expected, no PCR product is amplified from strains THE1 (WT) and SMB-1F (FOA).
Southern hybridization was performed on Cla I digests of genomic DNA from screened transformants using a digoxigenin-labeled DNA probe that hybridizes to a region upstream of C. albicans VPS1. The expected sizes of the restriction fragments are: wild-type (VPS1) allele 6.0 kb, 1st allele gene replacement (vps1Δ∷dpl200-URA3-dpl200) 2.5 kb, 1st allele FOA “loop-out” (vps1Δ∷dpl200) 1.0 kb, and tetR-VPS1 allele 0.8 kb. Two independent transformants are shown: parental strain THE1 is indicated as “WT”, SMB-1 is indicated as “1st allele knockout”, SMB-1F indicated as “FOA”, and tetR-VPS1 is indicated as “tetR-VPS1 transformant”.
Acknowledgments
We thank Peter Novick (Yale University) for providing plasmid pRS316 and valuable advice; Brian Wong (Oregon Health and Science University) for valuable advice; William Fonzi (Georgetown University) for providing strain SC5314; Aaron Mitchell (Columbia University) for providing strain BWP17 and plasmids pDDB57 and pRS-ARG4ΔSpeI; Hironobu Nakayama (Nippon Roche K. K. Research Center, Japan) for providing strain THE1 and plasmid p99CAU1; and Alistair Brown (University of Aberdeen, UK) for providing plasmid CIp10.
We thank Victoria Frohlich (University of Texas Health Science Center at San Antonio) for assistance with the fluorescence imaging of the tetR-VPS1 strain. Images were generated at the UTHSCSA Core Optical Imaging Facility which is supported by UTHSCSA, NIH-NCI P30 CA54174 (San Antonio Cancer Institute), NIH-NIA P30 AG013319 (Nathan Shock Center) and NIH-NIA P01AG19316. We thank Barbara Hunter (UTHSCSA) for assistance with scanning electron microscopy. We also thank Christopher Pierce and Jose Lopez-Ribot (University of Texas at San Antonio) for assistance with the biofilm studies, and Sarah Hardison (UTSA) for assistance with preparation of the manuscript. We would also like to thank Austin F. S. Lee (Massachusetts General Hospital) for assistance with statistical analyses.
Sequence data for C. albicans was obtained from the Stanford DNA Sequencing and Technology Center website at http://www-sequence.stanford.edu/group/candida. Sequencing of C. albicans was accomplished with the support of the NIDCR, NIH, and the Burroughs Wellcome Fund.
This work was supported in part by grants from the Department of Veterans’ Affairs (Career Development Award and MERIT Award to SAL), the NIDCR, Grant #DE14318 for the UTHSCSA CO*STAR Program (SMB) and the Biomedical Research Institute of New Mexico (SMB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. Wiley; New York: 1993. [Google Scholar]
- Bates S, Hughes HB, Munro CA, Thomas WP, MacCallum DM, Bertram G, Atrih A, Ferguson MA, Brown AJ, Odds FC, Gow NA. Outer chain N-glycans are required for cell wall integrity and virulence of Candida albicans. J Biol Chem. 2006;281:90–98. doi: 10.1074/jbc.M510360200. [DOI] [PubMed] [Google Scholar]
- Braun BR, Johnson AD. Control of filament formation in Candida albicans by the transcriptional repressor TUP1. Science. 1997;277:105–109. doi: 10.1126/science.277.5322.105. [DOI] [PubMed] [Google Scholar]
- Braun BR, Johnson AD. TUP1, CPH1 and EFG1 make independent contributions to filamentation in Candida albicans. Genetics. 2000;155:57–67. doi: 10.1093/genetics/155.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ, Gow NA. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 1999;7:333–338. doi: 10.1016/s0966-842x(99)01556-5. [DOI] [PubMed] [Google Scholar]
- Brown DH, Jr, Giusani AD, Chen X, Kumamoto CA. Filamentous growth of Candida albicans in response to physical environmental cues and its regulation by the unique CZF1 gene. Mol Microbiol. 1999;34:651–662. doi: 10.1046/j.1365-2958.1999.01619.x. [DOI] [PubMed] [Google Scholar]
- Bruckmann A, Kunkel W, Hartl A, Wetzker R, Eck R. A phosphatidylinositol 3-kinase of Candida albicans influences adhesion, filamentous growth and virulence. Microbiology. 2000;146:2755–2764. doi: 10.1099/00221287-146-11-2755. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Stevens TH. Vacuole biogenesis in Saccharomyces cerevisiae: Protein transport pathways to the yeast vacuole. Microbiol Mol Biol Rev. 1998;62:230–247. doi: 10.1128/mmbr.62.1.230-247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conibear E, Stevens TH. Studying yeast vacuoles. Methods Enzymol. 2002;351:408–432. doi: 10.1016/s0076-6879(02)51861-9. [DOI] [PubMed] [Google Scholar]
- Cornet M, Bidard F, Schwarz P, Da Costa G, Blanchin-Roland S, Dromer F, Gaillardin C. Deletions of endocytic components VPS28 and VPS32 affect growth at alkaline pH and virulence through both RIM101-dependent and RIM101-independent pathways in Candida albicans. Infect Immun. 2005;73:7977–7987. doi: 10.1128/IAI.73.12.7977-7987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet M, Gaillardin C, Richard ML. Deletions of the endocytic components VPS28 and VPS32 in Candida albicans lead to echinocandin and azole hypersensitivity. Antimicrob Agents Chemother. 2006;50:3492–3495. doi: 10.1128/AAC.00391-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall M, Edwards JE., Jr Segregation of proteinase-negative mutants from heterozygous Candida albicans. J Gen Microbiol. 1987;133:2817–2824. doi: 10.1099/00221287-133-10-2817. [DOI] [PubMed] [Google Scholar]
- Davis D, Wilson RB, Mitchell AP. RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol Cell Biol. 2000;20:971–978. doi: 10.1128/mcb.20.3.971-978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon NK, Sharma S, Khuller GK. Signaling through protein kinases and transcriptional regulators in Candida albicans. Crit Rev Microbiol. 2003;29:259–275. doi: 10.1080/713610451. [DOI] [PubMed] [Google Scholar]
- Fu Y, Ibrahim AS, Fonzi W, Zhou X, Ramos CF, Ghannoum MA. Cloning and characterization of a gene (LIP1) which encodes a lipase from the pathogenic yeast Candida albicans. Microbiol. 1997;143:331–340. doi: 10.1099/00221287-143-2-331. [DOI] [PubMed] [Google Scholar]
- Garcia-Sanchez S, Aubert S, Iraqui I, Janbon G, Ghigo JM, d’Enfert C. Candida albicans biofilms: A developmental state associated with specific and stable gene expression patterns. Eukaryot Cell. 2004;3:536–545. doi: 10.1128/EC.3.2.536-545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum MA. Potential role of phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev. 2000;13:122–143. doi: 10.1128/cmr.13.1.122-143.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan S, David D, Gerst JE. Dynamin and clathrin are required for the biogenesis of a distinct class of secretory vesicles in yeast. EMBO J. 2002;21:602–614. doi: 10.1093/emboj/21.4.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay E, Bretscher A. Parallel secretory pathways to the cell surface in yeast. J Cell Biol. 1995;131:297–310. doi: 10.1083/jcb.131.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay E, Schekman R. A subset of yeast vacuolar protein sorting mutants is blocked in one branch of the exocytic pathway. J Cell Biol. 2002;156:271–285. doi: 10.1083/jcb.200109077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes K. Virulence in Candida species. Trends Microbiol. 2001;9:591–596. doi: 10.1016/s0966-842x(01)02237-5. [DOI] [PubMed] [Google Scholar]
- Jarvis WR. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin Infect Dis. 1995;20:1526–1530. doi: 10.1093/clinids/20.6.1526. [DOI] [PubMed] [Google Scholar]
- Kitanovic A, Nguyen M, Vogl G, Hartmann A, Gunther J, Wurzner R, Kunkel W, Wolfl S, Eck R. Phosphatidylinositol 3-kinase VPS34 of Candida albicans is involved in filamentous growth, secretion of aspartic proteases, and intracellular detoxification. FEMS Yeast Res. 2005;5:431–439. doi: 10.1016/j.femsyr.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Kurtz MB, Cortelyou MW, Kirsch DR. Integrative transformation of Candida albicans, using a cloned Candida ade2 gene. Mol Cell Biol. 1986;6:142–149. doi: 10.1128/mcb.6.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Jones J, Khalique Z, Kot J, Alba M, Bernardo S, Seghal A, Wong B. A functional analysis of the Candida albicans homolog of Saccharomyces cerevisiae VPS4. FEMS Yeast Res. 2007;7:973–985. doi: 10.1111/j.1567-1364.2007.00253.x. [DOI] [PubMed] [Google Scholar]
- Lee SA, Wong B. Advances in diagnostic methods for invasive Candida and Aspergillus infections. In: Domer J, Kobayashi G, editors. The Mycota: A comprehensive treatise on fungi as experimental systems for basic and applied research. Springer; Berlin, Germany: 2004. pp. 97–114. [Google Scholar]
- Liu H. Transcriptional control of dimorphism in Candida albicans. Curr Opin Microbiol. 2001;4:728–735. doi: 10.1016/s1369-5274(01)00275-2. [DOI] [PubMed] [Google Scholar]
- Liu H, Kohler J, Fink GR. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- Marchais V, Kempf M, Licznar P, Lefrancois C, Bouchara JP, Robert R, Cottin J. DNA array analysis of Candida albicans gene expression in response to adherence to polystyrene. FEMS Microbiol Lett. 2005;245:25–32. doi: 10.1016/j.femsle.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Monge RA, Roman E, Nombela C, Pla J. The MAP kinase signal transduction network in Candida albicans. Microbiol. 2006;152:905–912. doi: 10.1099/mic.0.28616-0. [DOI] [PubMed] [Google Scholar]
- Mullins C, Bonifacino JS. Structural requirements for function of yeast GGAs in vacuolar protein sorting, alpha-factor maturation, and interactions with clathrin. Mol Cell Biol. 2001;21:7981–7994. doi: 10.1128/MCB.21.23.7981-7994.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev. 2003;67:400–428. doi: 10.1128/MMBR.67.3.400-428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Mio T, Nagahashi S, Kokado M, Arisawa M, Aoki Y. Tetracycline-regulatable system to tightly control gene expression in the pathogenic fungus Candida albicans. Infect Immun. 2000;68:6712–6719. doi: 10.1128/iai.68.12.6712-6719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport G, Agabian N. KEX2 influences Candida albicans proteinase secretion and hyphal formation. J Biol Chem. 1997;272:28954–28961. doi: 10.1074/jbc.272.46.28954. [DOI] [PubMed] [Google Scholar]
- Newport G, Kuo A, Flattery A, Gill C, Blake JJ, Kurtz MB, Abruzzo GK, Agabian N. Inactivation of Kex2p diminishes the virulence of Candida albicans. J Biol Chem. 2003;278:1713–1720. doi: 10.1074/jbc.M209713200. [DOI] [PubMed] [Google Scholar]
- Nobile CJ, Mitchell AP. Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol. 2006;8:1382–1391. doi: 10.1111/j.1462-5822.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- Nothwehr SF, Conibear E, Stevens TH. Golgi and vacuolar membrane proteins reach the vacuole in vps1 mutant yeast cells via the plasma membrane. J Cell Biol. 1995;129:35–46. doi: 10.1083/jcb.129.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer GE, Cashmore A, Sturtevant J. Candida albicans VPS11 is required for vacuole biogenesis and germ tube formation. Eukaryot Cell. 2003;2:411–421. doi: 10.1128/EC.2.3.411-421.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer GE, Kelly MN, Sturtevant JE. The Candida albicans vacuole is required for differentiation and efficient macrophage killing. Eukaryot Cell. 2005;4:1677–1686. doi: 10.1128/EC.4.10.1677-1686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: A persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G, Lopez-Ribot JL. Techniques for antifungal susceptibility testing of Candida albicans biofilms. Methods Mol Med. 2005;118:71–79. doi: 10.1385/1-59259-943-5:071. [DOI] [PubMed] [Google Scholar]
- Ramage G, Saville SP, Wickes BL, Lopez-Ribot JL. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol. 2002;68:5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CK, Howald-Stevenson I, Vater CA, Stevens TH. Morphological classification of the yeast vacuolar protein sorting mutants: Evidence for a prevacuolar compartment in class E vps mutants. Mol Biol Cell. 1992;3:1389–1402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder SE, Emr SD. A novel RING finger protein complex essential for a late step in protein transport to the yeast vacuole. Mol Biol Cell. 1997;8:2307–2327. doi: 10.1091/mbc.8.11.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JS, Graham TR, Emr SD. A putative zinc finger protein, Saccharomyces cerevisiae Vps18p, affects late Golgi functions required for vacuolar protein sorting and efficient alpha-factor prohormone maturation. Mol Cell Biol. 1991;11:5813–5824. doi: 10.1128/mcb.11.12.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JH, Raymond CK, Gilbert T, O’Hara PJ, Stevens TH. A putative GTP binding protein homologous to interferon-inducible Mx proteins performs an essential function in yeast protein sorting. Cell. 1990;61:1063–1074. doi: 10.1016/0092-8674(90)90070-u. [DOI] [PubMed] [Google Scholar]
- Ruiz-Herrera J, Elorza MV, Valentin E, Sentandreu R. Molecular organization of the cell wall of Candida albicans and its relation to pathogenicity. FEMS Yeast Res. 2006;6:14–29. doi: 10.1111/j.1567-1364.2005.00017.x. [DOI] [PubMed] [Google Scholar]
- Sato TK, Rehling P, Peterson MR, Emr SD. Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol Cell. 2000;6:661–671. doi: 10.1016/s1097-2765(00)00064-2. [DOI] [PubMed] [Google Scholar]
- Schaller M, Borelli C, Korting HC, Hube B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses. 2005;48:365–377. doi: 10.1111/j.1439-0507.2005.01165.x. [DOI] [PubMed] [Google Scholar]
- Stoldt VR, Sonneborn A, Leuker CE, Ernst JF. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 1997;16:1982–1991. doi: 10.1093/emboj/16.8.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournu H, Tripathi G, Bertram G, Macaskill S, Mavor A, Walker L, Odds FC, Gow NA, Brown AJ. Global role of the protein kinase GCN2 in the human pathogen Candida albicans. Eukaryot Cell. 2005;4:1687–1696. doi: 10.1128/EC.4.10.1687-1696.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater CA, Raymond CK, Ekena K, Howald-Stevenson I, Stevens TH. The Vps1 protein, a homolog of dynamin required for vacuolar protein sorting in Saccharomyces cerevisiae, is a GTPase with two functionally separable domains. J Cell Biol. 1992;119:773–786. doi: 10.1083/jcb.119.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsbach K, Payne GS. Vps1p, a member of the dynamin GTPase family, is necessary for Golgi membrane protein retention in Saccharomyces cerevisiae. EMBO J. 1993;12:3049–3059. doi: 10.1002/j.1460-2075.1993.tb05974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RB, Davis D, Enloe BM, Mitchell AP. A recyclable Candida albicans URA3 cassette for PCR product-directed gene disruptions. Yeast. 2000;16:65–70. doi: 10.1002/(SICI)1097-0061(20000115)16:1<65::AID-YEA508>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Wilson RB, Davis D, Mitchell AP. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol. 1999;181:1868–1874. doi: 10.1128/jb.181.6.1868-1874.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Transformants were screened for the genotype of interest by allele-specific PCR using primers VPS1-5DetA and VPS1-3DetA. Strains carrying the VPS1, vps1Δ∷dpl200-URA3-dpl200, and vps1Δ∷dpl200 alleles yield PCR products that are 2.5, 2.2, and 0.8 kb in size, respectively. PCR product from strains THE1 (VPS1/VPS1), SMB-1 (VPS1/vps1Δ∷dpl200-URA3-dpl200), and SMB-1F (VPS1/vps1Δ∷dpl200) are indicated as “WT”, “1st allele”, and “FOA”, respectively.
B. Transformants carrying the tetR-VPS1 allele were identified using primers TetVPS1-5Det and TetVPS1-3Det. Only those strains with the correct genotype (vps1Δ∷dpl200/99t-VPS1-URA3) yield a PCR product that is 2.0 kb in size. As expected, no PCR product is amplified from strains THE1 (WT) and SMB-1F (FOA).
Southern hybridization was performed on Cla I digests of genomic DNA from screened transformants using a digoxigenin-labeled DNA probe that hybridizes to a region upstream of C. albicans VPS1. The expected sizes of the restriction fragments are: wild-type (VPS1) allele 6.0 kb, 1st allele gene replacement (vps1Δ∷dpl200-URA3-dpl200) 2.5 kb, 1st allele FOA “loop-out” (vps1Δ∷dpl200) 1.0 kb, and tetR-VPS1 allele 0.8 kb. Two independent transformants are shown: parental strain THE1 is indicated as “WT”, SMB-1 is indicated as “1st allele knockout”, SMB-1F indicated as “FOA”, and tetR-VPS1 is indicated as “tetR-VPS1 transformant”.


