Abstract
Context: Over 23 million Americans are afflicted with severe obesity, i.e. their body mass index (in kilograms per square meter) values exceed 35. Of even greater concern is the association of the adiposity with comorbidities such as diabetes, hypertension, cardiopulmonary failure, asthma, pseudotumor cerebri, infertility, and crippling arthritis.
Objective: Diets, exercise, behavioral modification, and drugs are not effective in these individuals. This article examines the effect of surgery on the control of the weight and the comorbidities, as well as the safety of these operations.
Interventions: Although the article focuses on the outcomes of the three most commonly performed operations, i.e. adjustable gastric banding, the gastric bypass, and the biliopancreatic bypass with duodenal switch, it aims for perspective with the inclusion of abandoned and current investigational procedures, a review of the complications, and an emphasis on the appropriate selection of patients.
Positions: Ample evidence, including controlled randomized studies, now document that bariatric surgery produces durable weight loss exceeding 100 lb (46 kg), full and long-term remission of type 2 diabetes in over 80% with salutary effects on the other comorbidities as well with significant reductions in all-cause mortality. Although the severely obese present with serious surgical risks, bariatric surgery is performed safely with a 0.35% 90-d mortality in Centers of Excellence throughout the United States—similar to the complication rates after cholecystectomy.
Conclusions: Until better approaches become available, bariatric surgery is the therapy of choice for patients with severe obesity.
Bariatric surgery, the only effective therapy for severe obesity, can also induce full remission of such co-morbidities as diabetes, hypertension, and sleep apnea with limited risk.
Pretty hard to believe. How can it be that one operation can produce full and durable remissions of our most resistant chronic diseases? How can one procedure reverse obesity, diabetes, hypertension, cardiopulmonary failure, polycystic ovary disease, and pseudotumor cerebri with a reduction in mortality (Fig. 1)? And, finally, is it really true that the operation, a highly complex abdominal procedure performed in vulnerable, severely obese patients, is now delivered throughout the United States with operative mortalities and morbidities that are no greater than the risks for cholecystectomy? Surprisingly, these claims are well supported.
Figure 1.
Gastric bypass surgery schedule. Dx, Diognosis; OR, operating room.
The intent of this article is to review the history of bariatric surgery, to compare the various bariatric operations, to list the current indications for these procedures, to evaluate the outcomes, and to consider the risks.
The History of Bariatric Surgery
Bariatric surgery, similar to the other surgical disciplines, was developed in waves through the contributions of many (1). The first breakthrough was the recognition by a group of surgeons at the University of Minnesota, led by Drs. Arnold Kremen and Richard Varco, that severe obesity was a disease dangerous enough to warrant surgery. Based on the experience with the “short gut” syndrome, they developed the intestinal bypass, a procedure that excludes the majority of the small intestine from contact with food. Multiple variations finally demonstrated that an end-to-end anastomosis between 14 inches (36 cm) of jejunum and 4 inches (10 cm) of ileum with the excluded segment draining into the sigmoid colon provided the most predictable weight loss. It is one of the dark blots in the history of surgery that over 30,000 intestinal bypass operations were performed before it was recognized that although the operations produced significant weight loss, the complications were unacceptable. Eventually almost all had to be reversed because they produced acute hepatic failure, cirrhosis, renal failure, autoimmune disease, and severe mineral abnormalities.
The second major breakthrough came with the careful investigations by Dr. Edward Mason, also a member of the Minnesota group, who documented that weight loss could be achieved as effectively and far more safely through two gastric procedures, the gastric band, an operation that limited intake with a small gastric pouch and limited outlet, and the gastric bypass, a procedure that interfered with digestion as well as intake by excluding food from the stomach. These two basic operations continue to be the most widely performed bariatric procedures in the world today. Gastric banding has been improved with the invention of the adjustable gastric band; the gastric bypass was extended by Scopinaro et al. (2) with the biliopancreatic bypass and by Hess et al. (3) with the addition of a duodenal switch. More recently, the gastric sleeve (GS) (4), the initial step in the biliopancreatic bypass, is under investigation as another independent restrictive operation. Moo and Rubino (5), interested in extending the benefits of the gastric bypass to diabetic patients who are not obese, have stimulated trials of the duodeno-jejunal bypass. Others are testing ileal transposition, i.e. the translocation of a segment of ileum close to the Ligament of Treitz, as another approach to resolving type 2 diabetes without weight loss.
The third advance was the documentation by Pories et al. (6) and MacDonald et al. (7), with rigorous 95% follow-up of 608 patients for up to 16 yr, that the gastric bypass produced 1) durable weight loss greater than 100 lb; 2) control of the comorbidities, even including diabetes; and 3) a decrease in mortality.
The fourth major development was the demonstration in 1994 by Wittgrove and Clark (8) that the gastric bypass, one of the most difficult abdominal surgical operations, could be performed with the laparoscopic approach safely and with far less trauma.
The fifth singular innovation was quality control of bariatric surgery on a nationwide basis and the documentation that the operations could be done with minimal mortality and morbidity in centers with high volume and experience. Confronted by reports of disastrous clinical outcomes in hospitals with limited experience, an explosion of malpractice suits and unaffordable insurance premiums, the leadership of the American Society for Bariatric and Metabolic Surgery (ASMBS) founded a program for the certification of Centers of Excellence. The concept differed from previous attempts at surgical quality control by requiring standardization of care paths and focusing not only on process but primarily on surgical outcomes. To assure credibility and stakeholder participation, the Society founded the Surgical Review Corporation (SRC) (www.surgicalreview.org), an independent, nonprofit organization, to manage the program (9). As of July 1, 2008, a total of 339 hospitals throughout the United States were certified as ASMBS Centers of Excellence, delivering bariatric surgery with a 0.14% hospital and a 0.35% 90-d mortality, similar rates to those reported for cholecystectomy, although the severely obese represent far greater operative risks (10).
Variations on a Theme
Bariatric operations have traditionally been divided into three groups: 1) restrictive, i.e. procedures that produce weight loss solely by limiting intake (gastric banding, GS); 2) malabsorptive, i.e. operations that induce weight loss totally by interference with digestion and absorption (intestinal bypass); and 3) and mixed, i.e. procedures that limit intake and produce malabsorption (gastric bypass, duodenal switch). Despite this apparently clear classification, the mechanisms of action remain unclear. For example, whereas the GS is considered a restrictive procedure, limiting intake due to the low volume of the tube, the longitudinal gastrectomy also discards the source of ghrelin production.
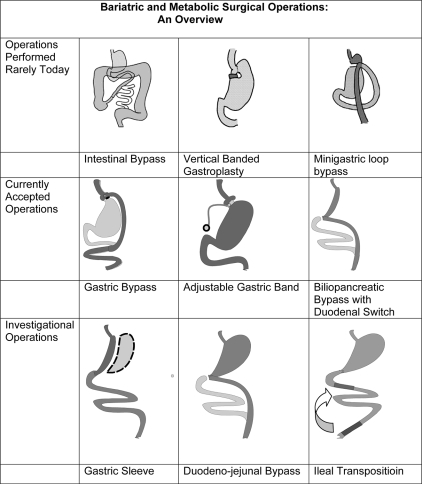
Figure 2 provides a diagrammatic overview of the various operations previously or currently in use. The list is only partial. Multiple variations of each of the operations have been performed and discarded over the last 50 yr with variations in the size of the gastric pouches, length of limbs, type and size of anastomoses, with or without vagotomy, the addition of constricting rings, and even wrapping the entire stomach in fabric.
Figure 2.
Overview of bariatric and metabolic surgical operations.
Operations No Longer Widely Performed
A listing of the operations that were once popular and are now no longer performed is important because patients with these procedures are still encountered in practice.
Intestinal bypass
This original bariatric surgical procedure excludes most of the small bowel by joining 14 in. (36 cm) of proximal jejunum beyond the Ligament of Treitz to 4 in. (10 cm) of terminal ileum. The excluded segment is drained into the distal colon (11). The disastrous outcomes of these procedures are noted in the introduction.
Vertical banded gastroplasty (VBG)
The VBG was the first successful restrictive procedure. The operation produced a 30-cc proximal gastric pouch with a vertical staple line from the Angle of His to a circular opening, measuring about 1 cm, punched out with a circular stapler. This opening provided the passage for a plastic band, usually made of Marlex, about 1 cm in width, that was tightened to narrow the outlet to about 1 cm. The operation is still performed by a few surgeons with excellent results but has been largely replaced by the adjustable gastric band, an operation that is far easier to perform, safer, and less likely to fail due to staple line breakdown.
Minigastric loop bypass
The minigastric loop bypass was the first version of the gastric bypass, but it was soon abandoned because of biliary regurgitation with bile gastritis and esophagitis. In addition, animal studies documenting dysplasia raised concerns about potential dangers of esophageal cancer. Although the operation is avoided by most, a few surgeons insist the procedure is safe and continue to promote it (12).
Currently Accepted Operations
Roux-en-Y gastric bypass (RYGB)
The RYGB, the most commonly performed bariatric operation in the United States, creates a proximal pouch about 30 cc in size, i.e. about the size of a golf ball, by segmentation of the stomach with staples or division. The proximal pouch is drained with a Roux-en-Y created by dividing the proximal jejunum about 30 cm below the Ligament of Treitz, bringing the distal segment up to form a gastroenterostomy of about 1 cm in diameter, and joining the proximal segment to the small bowel about 100 cm below the point of division. Although the procedure is becoming increasingly standardized, variations continue with differences in the size of the gastric pouches, construction of the gastrojejunostomies, use of a plastic ring at the gastroenterostomy to limit outflow, length of the Roux-en-Y limbs, and placement of the small bowel in front or behind the colon (13).
Adjustable gastric band (AGB)
The AGB has rapidly gained in popularity in the United States and abroad due to its safety and effectiveness. The AGB is a small bracelet-like device placed high in the stomach to produce a pouch of about 30 cm, similar to that in the RYGB. The band is lined by an inflatable cuff that is joined to a sc abdominal port to allow adjustment of the pouch outflow (14).
Biliopancreatic bypass with duodenal switch (BPDS)
The BPDS reduces the gastric reservoir by excising most of the stomach, leaving only a “gastric sleeve.” The duodenum, divided about 2 cm below the pylorus, is reconstituted by a Roux-en- Y anastomosis to the distal jejunum, excluding significantly more small bowel than the gastric bypass. The operation is not widely performed but is gaining adherents due to its effectiveness and the ease of creating the GS, which appears to offer a reasonable alternative to the gastric band (13).
Investigational Bariatric Surgical Procedures
Gastric sleeve (GS)
The GS creates a narrow gastric tube through the excision of most of the stomach. The operation does more than just limit intake; it also removes most and perhaps all of the ghrelin-producing cells of the gastric mucosa. The procedure, initially introduced as a first stage of the BPDS for use in superobese patients to reduce risk, appears to be an effective operation on its own and a potential competitor with the AGB. The early data are encouraging; some maintain that this operation should no longer be listed as investigational because of the excellent results reported (15).
Duodeno-jejunal bypass
The duodeno-jejunal bypass stomach-sparing operation was introduced as a procedure that could induce remission of diabetes without weight loss in lean patients with type 2 diabetes mellitus. The operation is based on the work of Rubino and colleagues (16) in Goto-Kakizaki genetically diabetic lean rats. Early human trials are encouraging.
Ileal transposition
Ileal transposition is still in the early stage of animal and human trials. It is mentioned here for completeness and to make the reader aware that there are early reports of satisfactory weight loss and remission of diabetes, but the evidence remains scant at this point, even in animal trials (17).
Effect of Bariatric Surgery on Weight
Bariatric surgery, the most effective treatment for severe obesity, produces dramatic and durable weight loss. Weight loss is most easily expressed in pounds or kilograms. In our series of 608 patients followed up to 16 yr with a 95% follow-up, the mean weight loss was 106 lb (48.2 kg.) [decreasing from 317 to 211 lb (144.1 to 95.9 kg)] (18).
Because of gender differences as well as the great variations in weight among the operated individuals, many prefer to express weight loss in terms of “excess weight,” i.e. current weight − ideal body weight = excess weight. For example, according to the Metropolitan Life Insurance tables of 1999, a 5-foot 3-in. woman with a medium frame has an “ideal body weight” of 121–135 lb (55–61 kg). The midpoint of this range is 128 lb (58.1 kg). At a weight of 300 lb, she has an excess weight of 172 lb (78.2 kg). If her weight after a gastric bypass is 180 lb (81.8 kg) upon stabilization, usually at about 18 months after surgery, she lost 120 lb (54.5 kg) or 69.7% of her excess body weight.
The use of percentage excess weight loss as a measure allows some comparison between the various bariatric operations. Buchwald et al. (19) used this metric in a meta-analysis of 2,738 citations in the English language from 1990–2002 that reviewed the results of bariatric surgery in 22,094 patients. These data showed the following outcomes, expressed in excess weight loss: AGB, 47.5%; VBG, 68.2%; RYGB, 61.6%; and BPDS, 79.1%. Although these figures are helpful and reasonably accurate, they reflect the outcomes of a rapidly changing technology, including improved stapling devices and bands that are less likely to fail as well as a sharp move from open to laparoscopic procedures.
The body mass index (BMI; in kilograms per square meter) is also frequently used as an index of obesity. However, although the BMI has been adopted widely, it is a badly flawed index for several reasons. As a unigender measure, it fails to reflect the differences in muscularity and body composition between the sexes. Because it only reflects weight and height, it fails to differentiate between the well-muscled athlete and the obese individual. One of our best running backs at East Carolina University had a BMI of 46.8, based on his height of 5-ft 8-in. (173 cm) and weight of 308 lb (140 kg), numbers that do not reflect the fact that his body fat represented only 7% of his body weight. The BMI measure is also unfavorable to some racial groups (20). Due to racial differences in body composition, African-American and Asian women suffer similar levels of comorbidities at a BMI of 32 that their Caucasian sisters encounter at a BMI of 35. Therefore, when the BMI of at least 35 is used as an exclusion index, it denies access to some of the population with the greatest need for bariatric surgery. Similarly, it can be argued that some patients currently undergo surgery based on their BMI, but possibly with a body fat percentage close to normal range. It is not a sufficiently precise measure on which to base life and death decisions.
Patients vary in their responses to bariatric operations. Most patients change their diets (21) with a tendency to avoid beef products and fibrous vegetables because they require a lot of chewing and may obstruct the narrow gastric outflow tracts. Some patients develop the symptoms of “dumping” when they eat sweets. Some learn to minimize or avoid alcohol because they get drunk much more easily; whereas still others report major changes in taste and food preference.
Other factors that influence weight loss include age, gender, race, body composition, education, emotional status, and level of activity (13). In general, younger patients, females, Caucasians, muscular and highly motivated individuals who follow an exercise program, patients who return for scheduled follow-up, and those who comply with the recommendations for vitamin/mineral supplements and do not snack will lose the most weight.
The surgical approach, i.e.“open” through a midline abdominal incision vs.“closed” with a laparoscopic approach, does not seem to affect the degree of weight loss (22,23), although the recovery is significantly faster after minimally invasive approaches.
Most patients reach their maximum weight loss by 2 yr and experience some increase of weight, perhaps 5–7%, by the fifth year with a gradual decrease again over the following years. Weight loss after the insertion of adjustable bands is generally less and slower than after gastric bypass and the duodenal switch, although there is early evidence that weight loss after banding may continue into the fifth year.
Failure of bariatric surgery remains to be defined. Failure, measured by the inadequate loss or the return of lost weight, varies by procedure and intensity of follow-up, ranging between 5 and 10% with higher rates for adjustable gastric banding. However, the lack of improvement of the comorbidities such as diabetes, asthma, stress incontinence, infertility, cardiopulmonary function, and pseudotumor represent more serious failures than inadequate weight loss. The developing large databases in the National Institutes of Health (NIH) Longitudinal Assessment of Bariatric Surgery (LABS) and the certification of Centers of Excellence for Bariatric Surgery should provide the information to develop objective measures.
If the weight gain is due to a technical failure of the operation from such factors as staple line breakdown, revisional surgery is usually successful. If, on the other hand, the failure is due to behavioral problems such as patients “out-eating” the operation, revision is usually not an effective approach.
Effect of Bariatric Surgery on Diabetes
The most remarkable effect of bariatric surgery is the full and rapid remission of type 2 diabetes mellitus, a disease previously considered unalterably progressive and minimally unresponsive to therapies except with a few demanding and unrealistic protocols. Despite the various medical advances, diabetes continues to be our most expensive disease. Diabetes, increasing at a faster rate than any other chronic disease, now affects over 24 million Americans and represents the main cause of blindness, renal failure, and amputations in the United States as well as a major cause of heart disease and stroke. The disease accounts for 11–13% of all health care expenses with an estimated annual cost of $73,000 per year for the recommended management of afflicted patients.
Diabetes clears in four out of five patients. Table 1 documents the long-term outcomes in a series of 608 severely obese individuals treated with the gastric bypass. Of these, 165 patients had type 2 diabetes and another 165 patients had impaired glucose tolerance (IGT). Durable resolution of diabetes with a return to euglycemia and normalization of glycosylated hemoglobin values occurred in 83% of the diabetic and 99% of the IGT patients. The diabetes clears rapidly, generally in a matter of days, to the degree that most diabetic bariatric surgical patients are discharged without any antidiabetic medications.
Table 1.
Full and durable remission of type 2 diabetes mellitus in 608 patients after gastric bypass with a mean follow-up of 9.4 yr
| Type 2 diabetes | IGT | |
|---|---|---|
| Total no. of patients | 165 | 165 |
| No. available for follow-up | 146 | 152 |
| Resolution of diabetes | 121 (83%) | 150 (99%) |
One paper indicates that the gastric bypass also reduces the mortality from diabetes. Although two major series (24,25) documented the reduction in mortality after gastric bypass, MacDonald et al. (26) showed that in diabetics the mortality decreased from 4.5 to 1% per year, based on a comparison group.
The finding that six different operations on the intestine can produce euglycemia has opened new avenues for diabetes research with a focus on the role of the intestine. Gastric inhibitory peptide, ghrelin, peptide YY3–36, and glucagon-like peptide-1 are only some of the gut hormones under intense investigation. In fact, exenatide, an analog of glucagon-like peptide-1, is already enjoying wide and successful clinical application (27).
In the long run, the hope for diabetes lies in the dissection of the metabolic pathways uncovered by bariatric surgery and applying the findings to the development of effective medical therapies. We can’t operate on 24 million Americans.
The Effect of Bariatric Surgery on the Other Comorbidities of Severe Obesity
Severe obesity affects virtually every system of the body with a broad expression of serious diseases, including pseudotumor cerebri, hypertension, diabetes, renal failure, immunoincompetence, asthma, gastroesophageal reflux disease, chronic obstructive pulmonary disease, cardiac failure, atherosclerosis, Pickwickian syndrome, arthritis of the weight bearing joints, infertility, skin breakdown, and an increased prevalence of cancers, especially colon, prostate, breast, and ovary.
All of these illnesses respond favorably to bariatric surgery, often with total and permanent remission. It is not unusual for patients who are restricted to wheelchairs before surgery to return to the surgeon 3 months later walking, often without even a cane. Patients diagnosed with asthma and gastroesophageal reflux disease, often related, usually find that they no longer need the various medications.
Most startling is the reduction in the prevalence of cancer in patients who have undergone bariatric surgery (28). Is this reduction, about 80% within 5 yr after the surgery, due to the decrease in inflammatory cytokines with the reduction of adipocytes? We can only speculate, but the implications are exciting.
Indications and Contraindications to Bariatric Surgery
The original indications and contraindications to bariatric surgery were established in 1991 by the NIH Consensus Conference on the Surgery for Obesity. In 2004, the American Society for Bariatric Surgery [ASBS, recently renamed as the American Society for Metabolic and Bariatric Surgery (ASMBS)] updated that statement with a follow-up Consensus Conference (29) that reached the following conclusions:
1. Bariatric surgery is the most effective therapy available for morbid obesity and can result in improvement or complete resolution of obesity comorbidities.
2. Types of operative procedures for morbid obesity have increased since 1991 and are continuously evolving. There are currently four types of procedures that can be used to achieve sustained weight loss: gastric bypass (standard, long-limb, and very long-limb Roux), alone or in combination with vertical banded gastroplasty; laparoscopic adjustable gastric banding; vertical banded gastroplasty; and biliopancreatic diversion and duodenal switch.
3. Both open and laparoscopic bariatric operations are effective therapies for morbid obesity and represent complementary state-of-the-art procedures.
4. Bariatric surgery candidates should have attempted to lose weight by nonoperative means, including self-directed dieting, nutritional counseling, and commercial and hospital-based weight loss programs, but should not be required to have completed formal nonoperative obesity therapy as a precondition for the operation.
5. The bariatric surgery patient is best evaluated and subsequently cared for by a multidisciplinary team.
6. Bariatric surgery candidates should have a comprehensive medical evaluation before the operation; evaluation by subspecialists (e.g. cardiologists, psychiatrists, and psychologists) is not routinely needed but should be available if indicated.
7. Bariatric surgery, performed only by experienced centers, should be considered in morbidly obese adolescents.
8. Extending bariatric surgery to patients with class I obesity (BMI, 30–34.9 kg/m2), who have a comorbid condition that can be cured or markedly improved by substantial and sustained weight loss, may be warranted and requires additional data and long-term risk and benefit analyses.
9. Bariatric surgery can be cost effective before the fourth year of follow-up.
10. Bariatric surgery offers rich opportunities for both basic and translational patient-oriented research to provide a better understanding of the factors involved in the regulation of food intake, pathophysiology of obesity, metabolic and clinical effects of sustained weight loss, and best treatment options for obese persons.
In practical terms, most physicians, surgeons, and carriers consider patients eligible for bariatric surgery if their BMI is at least 40 or if their BMI of at least 35 is accompanied by such comorbidities as diabetes, hypertension, arthritis limiting daily function, and cardiopulmonary failure. In the past, the age limits ranged from 18 to 65 yr, but recent data show that teenagers and patients older than 70 yr can benefit from the surgery with little or no increase in risk. Other inclusion criteria include the patient’s ability to understand the surgery and the consequences of the treatment, to comply with long-term follow-up, to agree to maintain vitamin and mineral supplementation, and to report problems promptly to specialists familiar with the complications of bariatric surgery. Contraindications include uncontrolled emotional disorders and drug or alcohol abuse. A relative contraindication observed by many surgeons is a lack of support or strong disagreement with the surgery by the family.
The choice of the individual procedure is not yet based on sound data, but many surgeons choose to favor gastric bypass or the duodenal switch over adjustable gastric banding in patients with diabetes. The rapidly growing databases of the NIH project, Longitudinal Assessment of Bariatric Surgery (LABS) and the Surgical Review Corporation (SRC), the independent, nonprofit organization that manages the ASMBS Centers of Excellence program, should soon facilitate these decisions.
Risks of Bariatric Surgery
Bariatric surgery is remarkably safe, especially given the large size of the patients as well as the frequency and seriousness of the comorbidities. Table 2 reflects the data reported in the applications of 272 ASMBS centers for certification as Centers of Excellence (30). The low operative mortality rate, while surprising, has been confirmed in two other series. It is similar to that reported by the NIH LABS group of six participating national centers, and it is not far removed from the 30-d mortality rates extracted in Buchwald’s meta-analysis: ABG, 0.1%; VBG, 0.1%; RYGB, 0.5%; and BPDS, 1.1%.
Table 2.
SRC data from 272 ASMBS Centers of Excellence with 495 surgeons reporting outcomes in more than 110,000
| n | % | |
|---|---|---|
| Hospital mortality | 76 | 0.14 |
| Operative mortality at 30 d (76 + 89 = 165) | 165 | 0.29 |
| Operative mortality at 90 d (76 + 89 + 31 = 196) | 196 | 0.35 |
| Readmissions | 1956 | 4.75 |
| Reoperations | 887 | 2.15 |
Data are based on applications.
Comparison with mortality rates reported from other common operations brings these very low rates into focus. The review of mortality by Dimick et al. (31) after common operations in U.S. hospitals revealed the following data: aortic aneurysm, 3.9%; coronary artery bypass graft, 3.5%, esophagectomy, 9%; and pancreatectomy, 8.3%. Only hip replacement with its mortality of 0.3% was as safe as bariatric surgery.
There is still no proven explanation for the significant mortality rates after discharge—in fact, more patients die after discharge than during hospitalization. Pulmonary emboli and arrhythmias are suspected but not yet proven. This is an important issue because these deaths may be preventable with the appropriate medication.
Although the mortality rates are low, probably due to the standardization of bariatric surgical care, the complications after bariatric surgery can be deadly and must be treated promptly by surgeons familiar with these problems. The complications fall into two groups: acute and long-term. The acute complications, which occur in 5–10% of the patients depending on the procedure, patient risk, age, and condition, mirror those after other abdominal operations, i.e. hemorrhage, obstruction, anastomotic leaks, infection, arrhythmias, and pulmonary emboli. Due to the patients’ weight, rhabdomyolysis is also seen occasionally, especially after prolonged operations.
Long-term complications may be baffling to those unfamiliar with bariatric surgery: neuropathies due to nutritional deficiencies, internal hernias, anastomotic stenoses, and emotional disorders. Although the nutritional deficits can be avoided with daily chewable multivitamin and mineral supplements and with calcium and iron for menstruating women, compliance with this recommendation is not universal. Unfortunately, we have seen full-blown cases of beri-beri, pellagra, kwashiorkor and severe neuropathies in patients who were treated for a variety of rare illnesses before the dietary deficiencies were recognized.
Another sometimes baffling complication is hypoglycemia (32), a condition that may appear as long as 14 yr after the surgery with plasma glucose levels as low as 30 mg%. Although some recommend surgical intervention for this syndrome, all of the 47 patients in our series recovered within 1 yr after conservative approaches, of which the most effective was the immediate availability of hard candy when the patients felt an “aura” of an oncoming attack.
The treatment of complications, both acute and long-term, requires someone familiar with the uncommon and baffling syndromes seen in the postbariatric surgical patient. Because some of these adverse outcomes require very prompt action, measured in hours, early consultation with a bariatric surgeon or a physician knowledgeable in this area is essential.
Summary
The best way to summarize the risks and benefits of bariatric surgery is to present the data from a representative ASMBS Center of Excellence, Dr. Robin Blackstone’s community practice, in Scottsdale, Arizona, in Table 3. The preoperative status of the patients, the degree of resolution of the comorbidities, and the low mortality rate (one patient = 0.08%) provides a good index of the quality of bariatric surgery delivered in the United States today.
Table 3.
Data from a bariatric surgical community practice (Dr. Robin P. Blackstone, FACS, Scottsdale Arizona, 2007)a
| Gastric bypass (n = 1104)
|
Adjustable gastric band (n = 84)
|
Revision (n = 37)
|
||||
|---|---|---|---|---|---|---|
| Pre-op status | Post-op resolution | Pre-op status | Post-op resolution | Pre-op status | Post-op resolution | |
| Type 2 diabetes | 24.8% | 80.5% | 16.7% | 78.6 | 24.3 | 66.7 |
| Hypertension | 51.3% | 63.3% | 44.0 | 35.1 | 43.2 | 50.0 |
| Sleep apnea | 45.1% | 68.9% | 32.1 | 59.2 | 27.0 | 80.0 |
| GERD | 57.9% | 87.6% | 39.3 | 54.5 | 62.1 | 73.9 |
| Venous insufficiency | 54.1% | 71.0% | 35.7 | 60.4 | 51.3 | 78.9 |
| Infertility | 4.7% | 6.0 | ||||
| Asthma | 26.1% | 66.0% | 19.0 | 37.5 | 16.2 | 83.3 |
| Stress incontinence | 55.0% | 84.0% | 33.3 | 57.1 | 43.2 | 75.0 |
| Depression | 18.9% | 31.4% | 32.1 | 27.2 | 16.2 | 33.3 |
| DJD | 95.9% | 67.1% | 98.8 | 49.3 | 91.9 | 50.0 |
| Hyperlipidemia | 47.8% | 61.4% | 52.4 | 25.0 | 37.8 | 50.0 |
| Average medications | 4.4 | 1.3 | 3.6 | 2.2 | 4.4 | 1.6 |
| Percent excess weight loss
|
||||||
|---|---|---|---|---|---|---|
| 3 mo | 6 mo | 9 mo | 12 mo | 24 mo | 36 mo | |
| Gastric bypass | 41.2% | 61.6% | 78.9% | 86.6% | 88.9% | 87.7% |
| n = 1002 | n = 934 | n = 839 | n = 754 | n = 414 | n = 126 | |
| Adjustable gastric band | 46.6% | 54.5% | 66.8% | 68.5% | 72.3% | |
| n = 30 | n = 28 | n = 25 | n = 19 | n = 8 | ||
| Revision | 29.6% | 40.1% | 45.7% | 56.9% | 62.3% | |
| n = 68 | n = 56 | n = 46 | n = 31 | n = 7 | ||
, Backstone R. Personal Communication 2008.
Footnotes
This work was supported by the National Institutes of Health, Johnson & Johnson, and the Brody Foundation.
Disclosure Statement: W.J.P. is Chairman Emeritus of the Surgical Review Corporation (Raleigh, NC), a nonprofit organization that certifies Centers of Excellence for the American Society for Bariatric Surgery, and an occasional consultant for Covidien Corporation and Johnson & Johnson Corporation.
Abbreviations: AGB, Adjustable gastric band; BMI, body mass index; BPDS, biliopancreatic bypass with duodenal switch; GS, gastric sleeve; IGT, impaired glucose tolerance; RYGB, Roux-en-Y gastric bypass; VBG, vertical banded gastroplasty.
References
- Buchwald H, Cowan, Cowan Jr GSM, Pories W 2007 Surgical management of obesity. Philadelphia: Saunders/Elsevier; 3–9 [Google Scholar]
- Scopinaro N, Papadia F, Camerini G, Marinari G, Civalleri D, Franco AG 2008 A comparison of a personal series of biliopancreatic diversion and literature data on gastric bypass help to explain the mechanisms of resolution of type 2 diabetes by the two operations. Obes Surg 18:1035–1038 [DOI] [PubMed] [Google Scholar]
- Hess DS, Hess DW, Oakley RS 2005 The biliopancreatic diversion with the duodenal switch: results beyond 10 years. Obes Surg 15:408–416 [DOI] [PubMed] [Google Scholar]
- Rubin M, Yehoshua RT, Stein M, Lederfein D, Fichman S, Bernstine H, Eidelman LA 15 Aug 2008 Laparoscopic sleeve gastrectomy with minimal morbidity early results in 120 morbidly obese patients. Obes Surg 10.1007/s11695-008-9652-2 [DOI] [PubMed] [Google Scholar]
- Moo TA, Rubino F 2008 Gastrointestinal surgery as treatment for type 2 diabetes. Curr Opin Endocrinol Diabetes Obes 15:153–158 [DOI] [PubMed] [Google Scholar]
- Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM 1995 Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg 222:339–350; discussion, 350–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald Jr KG, Long SD, Swanson MS, Brown BM, Morris P, Dohm GL, Pories WJ 1997 The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J Gastrointest Surg 1:213–220; discussion, 220 [DOI] [PubMed] [Google Scholar]
- Wittgrove AC, Clark GW 2000 Laparoscopic gastric bypass, Roux-en-Y- 500 patients: technique and results, with 3–60 month follow-up [see comment]. Obes Surg 10:233–239 [DOI] [PubMed] [Google Scholar]
- Pratt GM, McLees B, Pories WJ 2006 The ASBS Bariatric Surgery Centers of Excellence program: a blueprint for quality improvement. Surg Obes Relat Dis 2:497–503; discussion, 503 [DOI] [PubMed] [Google Scholar]
- Pratt GM Personal communication. July 2008 [Google Scholar]
- Griffen Jr WO, Bivins BA, Bell RM 1983 The decline and fall of the jejunoileal bypass. Surg Gynecol Obstet 157:301–308 [PubMed] [Google Scholar]
- Rutledge R, Walsh TR 2005 Continued excellent results with the mini-gastric bypass: six-year study in 2,410 patients. Obes Surg 15:1304–1308 [DOI] [PubMed] [Google Scholar]
- Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, Eid GM, Mattar S, Ramanathan R, Barinas-Mitchel E, Rao RH, Kuller L, Kelley D 2003 Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 238:467–484; discussion, 84–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald H 2008 International symposium on gastric banding. Supplement to Surgery for Obesity and Related Diseases. Cambridge, MA: Elsevier; 4:35; pp 71 [DOI] [PubMed] [Google Scholar]
- Himpens J, Dapri G, Cadiere GB 2006 A prospective randomized study between laparoscopic gastric banding and laparoscopic isolated sleeve gastrectomy: results after 1 and 3 years. Obes Surg 16:1450–1456 [DOI] [PubMed] [Google Scholar]
- Cohen RV, Schiavon CA, Pinheiro JS, Correa JL, Rubino F 2007 Duodenal-jejunal bypass for the treatment of type 2 diabetes in patients with body mass index of 22–34 kg/m2: a report of 2 cases. Surg Obes Relat Dis 3:195–197 [DOI] [PubMed] [Google Scholar]
- Wang TT, Hu SY, Gao HD, Zhang GY, Liu CZ, Feng JB, Frezza EE 2008 Ileal transposition controls diabetes as well as modified duodenal jejunal bypass with better lipid lowering in a nonobese rat model of type II diabetes by increasing GLP-1. Ann Surg 247:968–975 [DOI] [PubMed] [Google Scholar]
- Hickey MS, Pories WJ, MacDonald Jr KG, Cory KA, Dohm GL, Swanson MS, Israel RG, Barakat HA, Considine RV, Caro JF, Houmard JA 1998 A new paradigm for type 2 diabetes mellitus: could it be a disease of the foregut? Ann Surg 227:637–643; discussion, 643–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K 2004 Bariatric surgery: a systematic review and meta-analysis. JAMA [Erratum (2005) 293:1728] 292:1724–1737 [DOI] [PubMed] [Google Scholar]
- Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, Bae S, Cardarelli R 2008 Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring) 16:600–607 [DOI] [PubMed] [Google Scholar]
- Sarwer DB, Wadden TA, Moore RH, Baker AW, Gibbons LM, Raper SE, Williams NN 2008 Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surg Obes Relat Dis 4:640–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dielen FM, Soeters PB, de Brauw LM, Greve JW 2005 Laparoscopic adjustable gastric banding versus open vertical banded gastroplasty: a prospective randomized trial. Obes Surg 15:1292–1298 [DOI] [PubMed] [Google Scholar]
- Evans RK, Bond DS, Demaria EJ, Wolfe LG, Meador JG, Kellum JM 2004 Initiation and progression of physical activity after laparoscopic and open gastric bypass surgery. Surg Innov 11:235–239 [DOI] [PubMed] [Google Scholar]
- Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Agren G, Carlsson LM 2007 Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357:741–752 [DOI] [PubMed] [Google Scholar]
- Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC 2007 Long-term mortality after gastric bypass surgery. N Engl J Med 357:753–761 [DOI] [PubMed] [Google Scholar]
- MacDonald Jr KG, Long SD, Swanson MS, Brown BM, Morris P, Dohm GL, Pories WJ 1997 The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J Gastrointest Surg 1:213–220; discussion, 220 [DOI] [PubMed] [Google Scholar]
- Chia CW, Egan JM 15 July 2008 Incretin-based therapies in type 2 diabetes mellitus. J Clin Endocrinol Metab 10.1210/jc.2007–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP, MacLean LD 2004 Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg 240:416–423; discussion, 423–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald H, Consensus Conference Panel 2005 Consensus Conference Panel. Bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. J Am Coll Surg 200:593–604 [DOI] [PubMed] [Google Scholar]
- Hughes G, Pratt GM, Sugerman H, Wharthen M, Adams P, Pories WJ Bariatric Outcomes Longitudinal Database (BOLD): A national uniform database for quality control of bariatric surgery. Poster. The Obesity Society Annual Scientific Meeting, Phoenix, AZ, 2008 [Google Scholar]
- Dimick JB, Cowan Jr JA, Colletti LM, Upchurch Jr GR 2004 Hospital teaching status and outcomes of complex surgical procedures in the United States. Arch Surg 139:137–141 [DOI] [PubMed] [Google Scholar]
- Kellogg TA, Bantle JP, Leslie DB, Redmond JB, Slusarek B, Swan T, Buchwald H, Ikramuddin S 2008 Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Surg Obes Relat Dis 4:492–499 [DOI] [PubMed] [Google Scholar]