Summary
Asymmetric cell divisions produce new cell types during animal development. Studies in Caenorhabditis elegans have identified major signal-transduction pathways that determine the polarity of cell divisions. How these relatively few conserved pathways interact and what modulates them to ensure the diversity of multiple tissue types is an open question. The Wnt/β-catenin asymmetry pathway governs polarity of the epidermal T seam cell in the C. elegans tail. Here, we show that the asymmetry of T-seam-cell division and morphogenesis of the male sensory rays require NHR-25, an evolutionarily conserved nuclear receptor. NHR-25 ensures the neural fate of the T-seam-cell descendants in cooperation with the Wnt/β-catenin asymmetry pathway. Loss of NHR-25 enhances the impact of mutated nuclear effectors of this pathway, POP-1 (TCF) and SYS-1 (β-catenin), on T-seam-cell polarity, whereas it suppresses the effect of the same mutations on asymmetric division of the somatic gonad precursor cells. Therefore, NHR-25 can either synergize with or antagonize the Wnt/β-catenin asymmetry pathway depending on the tissue context. Our findings define NHR-25 as a versatile modulator of Wnt/β-catenin-dependent cell-fate decisions.
Keywords: Asymmetric cell division, Seam cells, Male tail, Wnt signaling, RUNX signaling, mab-5
Introduction
Asymmetric cell divisions produce daughter cells with distinct developmental fates, therefore representing a key mechanism of tissue and organ differentiation during animal development (Betschinger and Knoblich, 2004; Roegiers and Jan, 2004; Gönczy, 2008). Cell commitment to a particular fate depends on establishment of a polarity axis, orientation of mitotic spindle along this axis and asymmetric segregation of cell-fate determinants. These processes are ensured by precise spatial and temporal cellular signaling. The Caenorhabditis elegans model has been instrumental for understanding the genetics and molecular biology of cell-fate determination, because worm development relies heavily on asymmetric cell divisions (Sulston et al., 1983; Sulston and Horvitz, 1977). Signal-transduction pathways including Notch, Wnt/β-catenin and G-protein signaling regulate asymmetric cell divisions during early embryogenesis, differentiation of the epidermal stem cells, the somatic gonad and the germline, and morphogenesis of the vulva (Gönczy, 2008; Kimble and Crittenden, 2007; Mizumoto and Sawa, 2007b).
The epidermal stem cells in C. elegans, known as the seam cells, provide an excellent system in which to study asymmetric cell divisions during postembryonic development. The seam cells divide asymmetrically to produce a copy of themselves and a differentiated cell, either a hypodermal cell or a neuron, depending on the seam-cell position (Sulston and Horvitz, 1977). The posterior seam cells V5, V6 and T are extensively studied. Already at its first division the T seam cell generates anterior (T.a) and posterior (T.p) daughters with distinct fates. The T.a daughter assumes a hypodermal fate, whereas T.p gives rise to neural cells. Divisions of the T-seam-cell lineage differ between hermaphrodites and males. At the L2 stage in males, the T.ap (posterior daughter of T.a) cell together with posterior daughters of the V5 and V6 seam cells begins to generate male-specific sensory rays that are essential for mating.
Differentiation of the T seam cell relies on proper establishment of its polarity, which is controlled by the Wnt/β-catenin asymmetry pathway (Herman, 2002; Herman and Wu, 2004; Mizumoto and Sawa, 2007b). A LIN-44/Wnt (C. elegans/mammalian homolog) signal from the epidermal tail tip cells activates its receptor LIN-17/Frizzled, localized to the posterior membrane of the T seam cell (Wu and Herman, 2007). The signal ensures asymmetric distribution of APR-1/APC, PRY-1/Axin, LIT-1/NLK and WRM-1/β-catenin in the T seam cell (Mizumoto and Sawa, 2007a; Mizumoto and Sawa, 2007b), leading to uneven inheritance of these cell-fate determinants by the T.a and T.p daughters. In the T.p cell nucleus, abundant WRM-1/β-catenin and LIT-1/NLK facilitate export of the POP-1/TCF transcription factor from the nucleus. The remaining POP-1/TCF associates with its cofactor SYS-1/β-catenin (Kidd et al., 2005), which is enriched in the T.p nucleus, and activates neural-fate-promoting genes, exemplified by tlp-1. By contrast, the anterior T.a daughter is characterized by the absence of nuclear SYS-1/β-catenin and by abundant nuclear POP-1/TCF, a situation leading to the hypodermal fate. The tlp-1 gene encodes a transcription factor of the Sp1 family and its asymmetric expression in T.p is required for correct differentiation of the neural T.p lineage (Zhao et al., 2002). Similarly, mutations in LIN-17/Frizzled, WRM-1/β-catenin, LIT-1/NLK and POP-1/TCF abolish the asymmetry of the T-seam-cell division, rendering all T-seam-cell descendants hypodermal (Sternberg and Horvitz, 1988; Herman, 2001; Mizumoto and Sawa, 2007a). Loss of LIN-44/Wnt function reverses the polarity of the first T-seam-cell division, causing T.a to adopt the neural and T.p the hypodermal character (Herman and Horvitz, 1994).
In parallel to the Wnt/β-catenin asymmetry pathway, the asymmetric expression of TLP-1/Sp1 is also regulated by a transcription factor of the RUNX family, RNT-1 (Kagoshima et al., 2005). Impaired function of RNT-1/RUNX or its coactivator BRO-1/CBFβ abolishes the neural fate in the T.p lineage, i.e. the same T-seam-cell polarity defect as that caused by mutation of tlp-1 (Kagoshima et al., 2005; Kagoshima et al., 2007a; Kagoshima et al., 2007b). In addition to the T seam cell, RNT-1/RUNX and BRO-1/CBFβ also act in the V seam cells as rate-limiting regulators of proliferative divisions. Their loss- and gain-of-function effects are reciprocal, resulting in missing or extra seam cells, respectively (Ji et al., 2004; Kagoshima et al., 2005; Nimmo et al., 2005; Kagoshima et al., 2007a; Kagoshima et al., 2007b; Xia et al., 2007). This phenotype reflects the fact that RNT-1/RUNX and BRO-1/CBFβ promote cell-cycle progression by downregulating expression of a cyclin-dependent kinase inhibitor CKI-1 (Nimmo et al., 2005; Kagoshima et al., 2007a; Xia et al., 2007).
Nuclear receptors are important multifunctional transcription factors involved in many aspects of animal physiology, hormonal regulation, cell differentiation and development (Mangelsdorf et al., 1995; Kastner et al., 1995). Nuclear receptors also engage in numerous molecular interactions with other signaling pathways. Recent studies have demonstrated functional interactions of several nuclear receptors with the Wnt/β-catenin signaling pathway (Mulholland et al., 2005). C. elegans possesses a vastly expanded family of 284 nuclear hormone receptor (NHR) genes (Maglich et al., 2001; Sluder et al., 1999), the functions of which remain mostly unknown. Fifteen of the NHRs are evolutionarily conserved with homologs in other species.
We have investigated the function of the nuclear receptor nhr-25, which encodes a homolog of the Drosophila Fushi tarazu factor 1 (Ftz-F1) and of the mammalian steroidogenic factor 1 (SF-1) and liver receptor homolog 1 (LHR-1) proteins. We have previously demonstrated that NHR-25 antagonizes a β-catenin pathway in the process of cell-fate decision in the somatic gonad of C. elegans (Asahina et al., 2006). That was an intriguing observation, because SF-1, the mammalian homolog of NHR-25, acts in synergy with Wnt/β-catenin signaling (Gummow et al., 2003; Hossain and Saunders, 2003; Jordan et al., 2003; Mizusaki et al., 2003; Botrugno et al., 2004; Parakh et al., 2006; Salisbury et al., 2007). Here, we report that NHR-25 cooperates with both RUNX and Wnt/β-catenin asymmetry pathways to ensure the correct cell fate of the T-seam-cell descendants. Reduced NHR-25 function enhances phenotypes of mutated POP-1/TCF and SYS-1/β-catenin in the T seam cell, whereas it suppresses effects of these mutations in the somatic gonad. Therefore, NHR-25 can modulate the Wnt/β-catenin asymmetry pathway either positively or negatively depending on the tissue context.
Results
Absence of NHR-25 in epidermal cells causes extra seam cells in adults
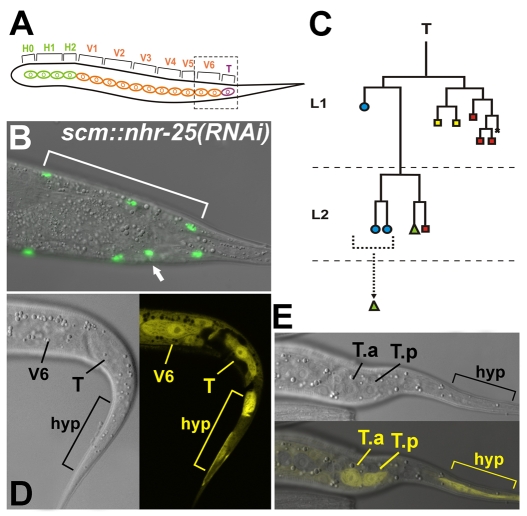
Adult worms normally possess two sets of 16 epidermal seam cells descending from H0 and embryonic blast cells H1, H2, V1-V6 and T (Table 1, Fig. 1A). Loss of nhr-25 function has been found to affect epidermal differentiation, leading to extranumerary seam cells (Chen et al., 2004; Silhankova et al., 2005). However, nhr-25 mutations or RNAi knockdown also cause lethality, molting defects and severe malformations of tail structures (Asahina et al., 2000; Chen et al., 2004; Gissendanner and Sluder, 2000; Silhankova et al., 2005), obscuring specific requirements for nhr-25 in epidermal cell differentiation. Therefore, we generated scm::nhr-25(RNAi) transgenic worms that produce nhr-25 dsRNA under the control of a seam-cell-specific promoter (SCM). The seam-cell-targeted nhr-25 RNAi eliminated all of the severe defects, yet it revealed the extra-seam-cell phenotype. In 55% of cases, scm::nhr-25(RNAi) adult hermaphrodites possessed more than 16 scm::gfp-positive seam cells (Table 1), indicating that some seam cells either did not divide asymmetrically or that they underwent further division. Although the additional seam cells occurred along the whole body axis, the head (data not shown) and tail (Fig. 1A-C) areas exhibited the highest incidence of ectopic seam cells. In agreement with the scm::nhr-25(RNAi) phenotype, we observed nhr-25 expression in V seam cells (see also Silhankova et al., 2005), in the posterior seam-cell T and its daughters (T.a, T.p), and in the hyp cells of wild-type animals (Fig. 1D,E).
Table 1.
Aberrant seam-cell numbers in scm::nhr-25(RNAi) adult hermaphrodites
|
Missing
|
Wild type
|
Ectopic
|
||||||
|---|---|---|---|---|---|---|---|---|
| Number of seam cells: | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 |
| wt(%; n=100) | 0 | 7 | 93 | 0 | 0 | 0 | 0 | 0 |
| scm::nhr-25(RNAi) (%; n=123) | 2 | 7 | 37 | 33 | 11 | 8 | 1 | 2 |
Frequency of seam-cell numbers. Wild-type adult hermaphrodites usually possess 16 seam cells. Ectopic seam cells occurred in 55% of scm::nhr-25(RNAi) adult worms
Fig. 1.
Silencing of nhr-25 in the seam cells results in increased seam-cell numbers. Seam cells were counted in scm::nhr-25(RNAi) adult hermaphrodites expressing scm::gfp. Also see Table 1. (A) A schematic drawing representing the 16 seam cells in an adult worm, originating from H0-H2 cells (green), from V1-V6 cells (orange), and from the T seam cell (purple). Dashed rectangle marks the tail area shown in microscope image (B). (B) Example of an extra seam cell (arrow) in the tail. The other side of the worm tail displays the wild-type situation, with three posterior-most seam cells (area indicated by bracket). (C) Lineage diagram of T-seam-cell differentiation. Blue circles indicate cells that fuse with the hyp7 syncytium, green triangles mark seam cells, and rectangles represent neural cells; specifically, yellow rectangles symbolize phasmid socket cells and red rectangles symbolize other neural cells. Bracket indicates hypodermal cells, one of which probably maintained a seam-cell potential. (D,E) Expression of the nhr-25::gfp transgene in L1 wild-type hermaphrodites. Strong reporter activity appears in the seam cells V6, T and in the tail hypodermis (hyp) (D). (E) nhr-25::gfp expression in the T-cell daughters (T.a, T.p) and the tail hypodermis is indicated. Anterior is to the left.
Loss of NHR-25 disrupts male tail morphology
Extra seam cells in the tails of scm::nhr-25(RNAi) worms prompted us to examine whether NHR-25 plays a role in male tail differentiation. In the wild-type male tail, the seam-cell lineages V5, V6 and T form nine finger-like sensory rays embedded in a cuticle fan (Fig. 2A,B), and each ray has a distinct set of morphogenetic and molecular features. Because systemic nhr-25 RNAi caused severe damage to the male tail morphology, ablating the typical shape and most of the rays (Fig. 2C), we used the scm::nhr-25(RNAi) line in most experiments.
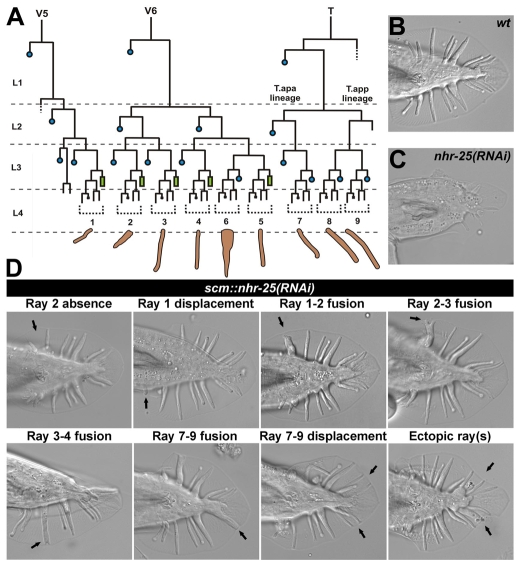
Fig. 2.
NHR-25 knockdown causes male tail abnormalities. (A) Lineage diagram of the posterior seam cells V5, V6 and T, which produce the nine male-specific sensory rays. Blue circles represent cells that fuse with the hyp7 syncytium; green rectangles indicate male tail seam cells. Dotted brackets denote ray cell groups that generate individual rays 1 to 9, numbered from anterior to posterior. X represents programmed cell death. (B) Wild-type male tail. (C) Severe male abnormal (Mab) phenotype with most rays absent upon systemic nhr-25 RNAi. (D) scm::nhr-25(RNAi) males display eight major classes of Mab phenotypes. Arrows indicate defective rays or abnormal ray positions. Some individuals often displayed combinations of distinct ray phenotypes. Individual rays were distinguished on the basis of morphological attributes, such as shape, thickness, and length, and according to their position relative to the other rays.
We characterized several classes of the most frequent ray phenotypes related to altered cell fate and/or migration in a total of 193 scm::nhr-25(RNAi) males (Fig. 2D). These phenotypes and additional defects (such as absence of rays other than ray 1 or 2) were also observed in a hypomorphic mutant nhr-25(ku217) (Fig. 3 and data not shown). Among specific tail defects, we could distinguish ray-1 displacement (13%), which was evidenced by dislocation of the ray cell cluster 1 as visualized with the ajm-1::gfp marker for adherens junctions (Fig. 4C). We often detected displacement of rays 7-9 (45%). Both of these displacements indicated that ray precursor cells failed to migrate properly. Another defect, ray fusion, was presumably a consequence of ray transformation (Chow and Emmons, 1994) and incomplete ray cell sorting (Baird et al., 1991). Among the different classes of ray fusions (Fig. 2D), fusions of rays 1-2 and 3-4 were the most frequent (41% and 33%, respectively).
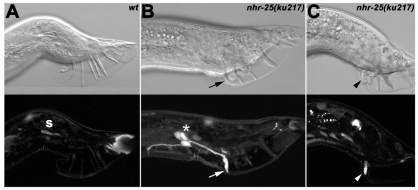
Fig. 3.
Loss of nhr-25 function leads to ectopic expression of mab-5 in ray 1. Nomarski images (top row) show male tail structures with sensory rays; below are confocal images of the mab-5::gfp activity. (A) In a wild-type adult male, none of the nine sensory rays show mab-5 expression; only autofluorescence of the spicule (s) and at the tail tip is apparent. (B,C) nhr-25(ku217) males display defective tail morphology (missing or fused rays) and abnormal mab-5::gfp expression that persists in ray 1 until the adult stage (arrowhead and arrow). Moreover, the ectopic mab-5 signal is also visible in cell bodies of the R1A, R1B and R1st (asterisk in B). Ray 1, which shows the mab-5 activity in C, is fused to ray 2 (arrowhead).
Fig. 4.
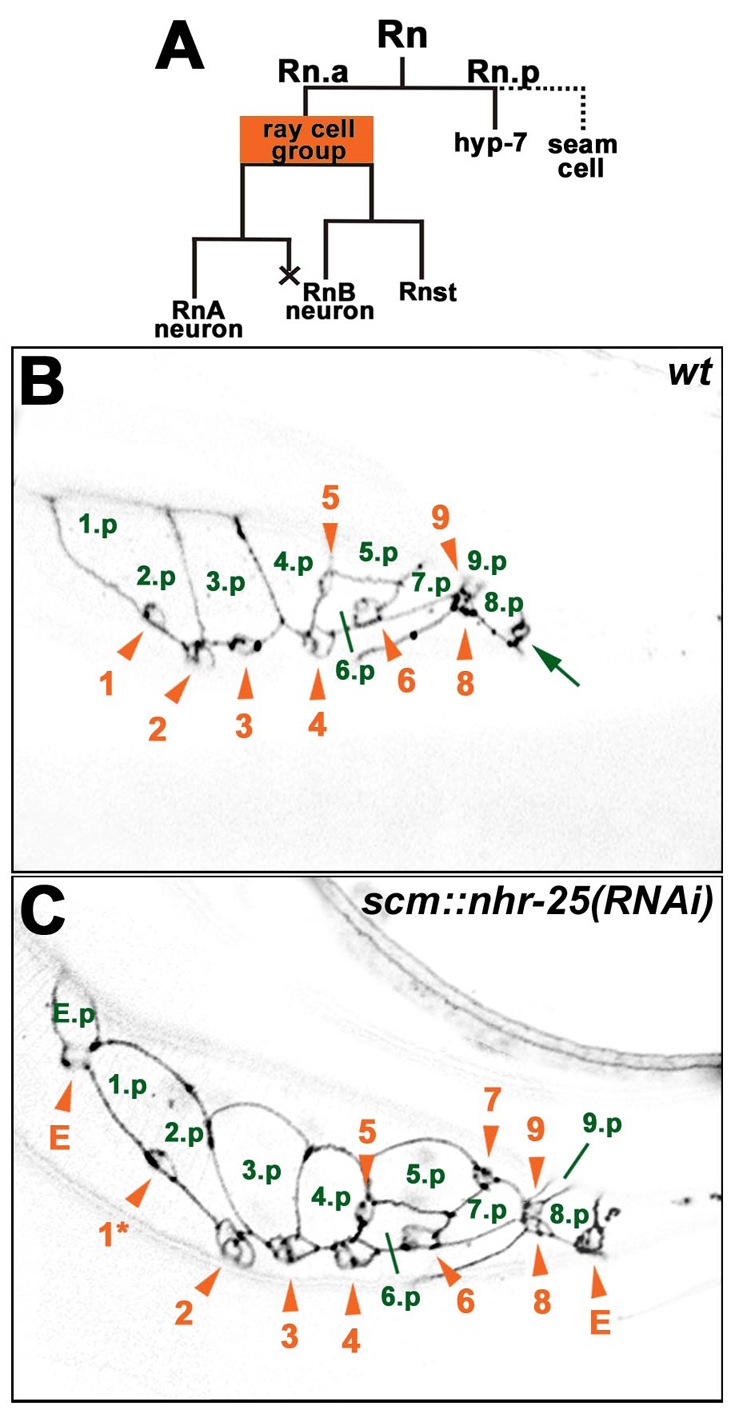
scm::nhr-25(RNAi) males display ectopic ray cell groups. (A) Lineage diagram of a ray cell cluster (Rn). An anterior Rn daughter produces a ray cell group, consisting of two neurons and one structural cell. The posterior daughter has a hypodermal character and it either fuses with the hyp7 syncytium (in the case of rays 6, 7, 8 and 9) or it fuses with other seam cells (1.p-5.p) to form a tail seam syncytium (SET). X represents programmed cell death. (B,C) Confocal sections show adherens junctions of ray cell clusters using the ajm-1::gfp transgene. The images of L4 males were taken approximately 40 hours after hatching. Signals were inverted for better clarity. Green numbers mark hypodermal Rn.p cells. The 1.p and 2.p cells are already fused, signifying formation of the SET syncytium. Ray cell groups are labeled with orange arrowheads and numbered in the order of the corresponding rays. Ray cell group 7 is out of the focal plane in wild type (B). (C) Males that are heterozygous for scm::nhr-25(RNAi) display an anterior ectopic ray cell cluster, which includes a ray cell group (E) and a hypodermal cell (E.p). Another ectopic ray cell group (E) is situated posterior to ray cell groups 8 and 9. Phasmid (arrow in B) is in another focal plane in C. In addition, displacement of ray cell group 1 is apparent (asterisk in C). Anterior is to the left.
It has been reported that ray-fusion phenotypes can result from mab-5 gain-of-function mutations or mab-5 mis-expression (Chow and Emmons, 1994; Salser and Kenyon, 1996). We therefore examined mab-5 expression in the tail of nhr-25(ku217) males. Tail morphology in these males exhibited missing or fused rays (Fig. 3B,C). Strong ectopic expression of mab-5::gfp was observed in ray 1 and also in the R1A, R1B and R1St cell bodies (Fig. 3B). Because the mab-5 mis-expression was also evident in the fused rays 1-2 (Fig. 3C), we investigated whether mab-5 loss-of-function mutation suppressed the incidence of ray fusion in nhr-25 mutants. Because mab-5(e1239) causes complete absence of V-derived rays (Salser and Kenyon, 1996), we scored fusion defects in mab-5(e1239)/+; nhr-25(ku217) males. We observed reduction of ray 1-2 fusion from 16% (n=90) in nhr-25(ku217) mutants to 5% in mab-5(e1239)/+; nhr-25(ku217) double mutants (n=110). Thus, mab-5 mis-expression is probably responsible for ray 1-2 fusion in the nhr-25 mutant background.
scm::nhr-25(RNAi) males also showed tail abnormalities that apparently arose from cell-fate transformation, such as absence of ray(s) 1 and/or 2 (13% and 19%, respectively), or extra rays (Fig. 2D). Interestingly, ectopic ray(s) occurred among those derived from the T seam cell (Fig. 2A,D). The number of extra rays ranged from 1 to 4, thus making a total of four to seven T-seam-cell-derived rays. Using the adherens-junction marker ajm-1::gfp, we detected ectopic ray primordial cells in L4 males (Fig. 4). The presence of ray defects indicated that NHR-25 was involved in the cell-fate decision of the V5, V6 and T seam-cell lineages as well as in the migration of ray cell groups.
Ectopic rays in scm::nhr-25(RNAi) males exhibit ray-9 identity
To discern which part of the T seam cell lineage was affected by NHR-25 deficiency to cause formation of extra rays, we determined the ectopic ray identity. Each ray represents an epidermal protrusion comprising a ray structural cell (Rnst) and two distinct neurons, A-type (RnA) and B-type (RnB) (Fig. 5A). The neurons use distinct sets of neurotransmitters. We chose three GFP transgenes that mark specific RnB neurons. flp-6 and flp-17 are members of the FMRFamide-related neuropeptide gene family and are expressed in R2B, R5B, R6B, R7B and R1B, R5B, R7B neurons, respectively (Kim and Li, 2004); tph-1 encodes a tryptophane hydroxylase and marks serotonergic neurons, including R1B, R3B and R9B (Lints et al., 2004). Expression of these markers was unaffected in scm::nhr-25(RNAi) males, showing that the individual rays maintained their features and thus could be identified. In addition, by using a pkd-2::gfp marker for all RnB neurons except R6B, we verified that B neurons were indeed present in most ectopic rays of scm::nhr-25(RNAi) males (data not shown).
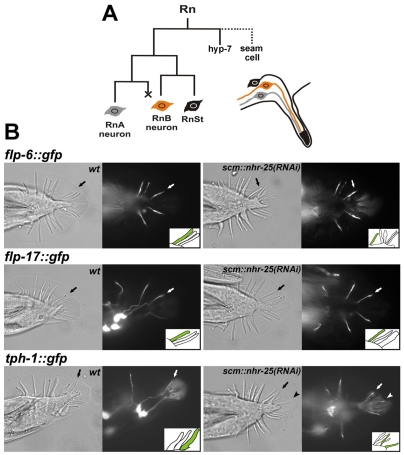
Fig. 5.
Ectopic rays in scm::nhr-25(RNAi) males exhibit ray-9 identity. (A) Lineage diagram of a ray cell group with two neurons and a structural cell. X represents programmed cell death. (B) Nomarski and fluorescence images illustrating the expression of three markers of specific RnB neurons. Schematic drawings (insets) of fluorescence images show expression of flp-6, flp-17 and tph-1 in T-seam-cell-derived rays. Arrows indicate wild-type signals in B neurons of the T-seam-cell-derived rays (R7B or R9B). flp-6::gfp is expressed in R2B (out of focal plane), R5B, R6B and R7B in the wild type (left). scm::nhr-25(RNAi) males (right) display flp-6::gfp signal in the same RnB neurons as wild-type males (R2B is out of the focal plane), but not in any of the ectopic rays. Similarly, flp-17 is expressed only in R1B, R5B and R7B (wild type), but not in the extra rays. In contrast to ray-7 markers, the tph-1::gfp signal is seen not only in R1B (out of focal plane in wild type), R3B and R9B neurons, but also in the B neuron of the ectopic ray (arrowhead). Anterior is to the left.
The majority of ectopic rays (89%) in scm::nhr-25(RNAi) males carrying the tph-1::gfp transgene displayed the R9B-specific signal (Fig. 5B; Table 2). The remaining 11% of ectopic rays, which lacked the R9B signal, probably assumed identity of rays 7 or 8. Consistent with these counts, only 13% and 8% of ectopic rays showed R7B-specific flp-6::gfp or flp-17::gfp expression, respectively (Fig. 5B; Table 2). These data indicated that extra rays derived from the T seam cell generally adopted the identity of ray 9. Hence, we speculated that differentiation of the T.app (posterior daughter of T.ap) lineage was more likely to be affected than divisions of the T.apa (anterior daughter of T.ap) lineage in the absence of NHR-25 (Fig. 2A).
Table 2.
Identity of ectopic ray(s) in scm::nhr-25(RNAi) males
| Transgenic marker | n | Ectopic ray(s) with positive signal (%) | Ray identity |
|---|---|---|---|
| tph-1::gfp | 51 | 89 | Ray 9 |
| flp-6::gfp | 60 | 13 | Ray 7 |
| flp-17::gfp | 60 | 8 | Ray 7 |
NHR-25-deficient worms display defective T-seam-cell polarity
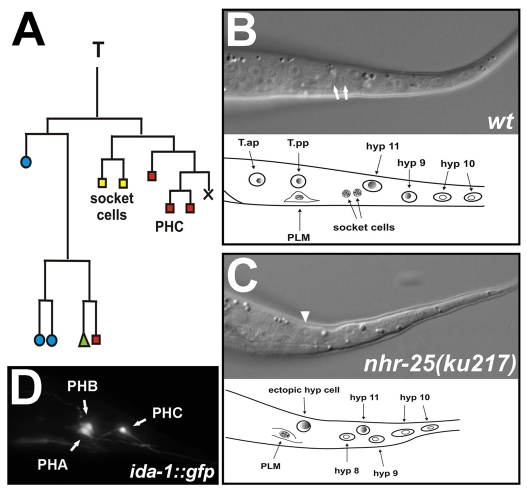
Obviously, the impaired function of NHR-25 affected the T-seam-cell lineage, leading to male abnormal (Mab) phenotypes. However, it remained unclear whether the lack of NHR-25 influenced the early asymmetric divisions of the T-seam-cell lineage. In addition, NHR-25 deficiency might disrupt the T-seam-cell lineage not only in males but also in hermaphrodites. In wild-type hermaphrodites, the T seam cell divides asymmetrically at early L1 stage, producing an anterior T.a daughter with hypodermal fate and a posterior daughter, T.p, that generates a neuronal branch including phasmid socket cells PHso1 and PHso2 (Fig. 6A). The socket cells are connected to phasmid neurons PHA and PHB that derive from the AB lineage. PHA and PHB can be filled with a fluorescent dye through the socket cells, which are open to the environment (Hedgecock et al., 1985; Herman and Horvitz, 1994). Absence of the socket cells causes a failure of phasmid staining (Pdy, phasmid dye-filling defect, also known as Dyf, dye-filling defect; see Materials and Methods) and thus serves as a test for disrupted T-seam-cell polarity.
Fig. 6.
T-seam-cell polarity defect in NHR-25-deficient worms. (A) Lineage diagram of the T seam cell. Blue circles indicate cells that fuse with the hyp7 syncytium; green triangle represents a seam cell; yellow squares mark phasmid socket cells, and red rectangles other neurons. X represents programmed cell death. (B,C) Nomarski images of L2 hermaphrodites demonstrating phasmid-socket absent (Psa) phenotype analysis. Socket cells (white arrows) are the most posterior neurons located between the hyp8 (hyp11) cells and the PLM neuron, which has a typical eye-like appearance (B). An ectopic hypodermal cell (arrowhead) appears instead of the socket cells in the nhr-25(ku217) mutant (C). Anterior is to the left, dorsal up. (D) Phasmids A, B and C marked with the ida-1::gfp transgene in wild type. Anterior is to the left.
Using the phasmid dye-filling technique in adults, we consistently observed the Pdy phenotype upon systemic nhr-25 RNAi, in scm::nhr-25(RNAi) animals and in nhr-25(ku217) mutants (Table 3). We next performed a complementary test, the Psa (phasmid socket absent) phenotype analysis (Sawa et al., 2000), to verify that the phasmid dye-filling defect was indeed caused by absent socket cells. The Psa phenotype was detected in 40% of nhr-25(ku217) worms (Fig. 6B,C; Table 3). Finally, to exclude the possibility that Pdy occurred because of missing phasmids, we used a transgenic marker ida-1::gfp to visualize phasmids PHA, PHB and PHC in nhr-25 RNAi and mutant worms (Fig. 6D). The vast majority of the worms had all three phasmids or at least one of the stainable cells (PHA or PHB) (Table 3). Therefore, the dye-filling defect could not result from phasmid absence.
Table 3.
T-seam-cell polarity defect in NHR-25-deficient worms
|
Presence of phasmids (%)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Pdy (%) | n | Psa (%) | n | PHA, PHB, PHC (wild type) | PHA and/or PHB | PHC | No phasmid | |
| N2 (wild type) | 120 | 3 | 60 | 0 | 112 | 93 | 4 | 2 | 1 |
| scm::nhr-25(RNAi) | 522 | 47 | 0 | ND | 0 | ND | ND | ND | ND |
| nhr-25(ku217) | 440 | 61 | 120 | 40 | 112 | 72 | 24 | 1 | 3 |
| Systemic nhr-25(RNAi) | 320 | 75 | 0 | ND | 236 | 62 | 38 | 0 | 0 |
ND, not determined; Psa, phasmid socket cells absent; Pdy, phasmid dye-filling defect
The above results demonstrate that NHR-25 function is not restricted to male-specific T-seam-cell divisions, but it is involved in proper T-seam-cell differentiation regardless of sex. We suggest that NHR-25 is also required in early divisions of the T-seam-cell lineage, where it ensures proper neuronal differentiation of the posterior T.p branch.
NHR-25 is essential for neural cell fate of the T-seam-cell lineage
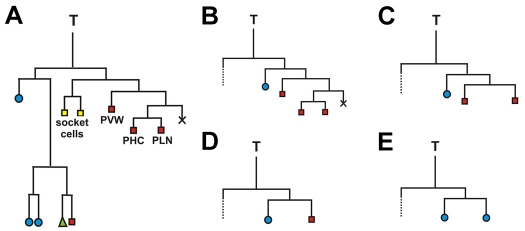
To directly reveal the role of NHR-25 during T-seam-cell differentiation, we performed analysis of the T-seam-cell lineage. In wild-type hermaphrodites, bilaterally symmetric T seam cells, TL and TR, produce T.a and T.p daughters with distinct fates: the anterior T.a generates four hypodermal cells and one neuron, whereas the posterior T.p yields five neural cells (Fig. 7A). We followed divisions of both the TL and TR cells in five nhr-25(ku217) mutant L1 larvae, thus evaluating ten T-seam-cell lineages in total. The lineage analysis showed that the cell fate of the posterior daughter T.p was defective in seven cases. All of these cases included a defect of the T.pa cell, which maintained hypodermal morphology and failed to divide further, thus resulting in the Psa phenotype. The defects in the T-seam-cell lineage differed in T.pp division, and we discerned four different patterns of anomalies (Fig. 7). In three cases, T.pp divided in a wild-type pattern to produce three neurons (Fig. 7B). In one case we observed the T.pp cell undergoing a division that generated two neural daughters, but further divisions of the T.ppp (posterior daughter of T.pp) that would differentiate the PHC and PLN neurons did not occur (Fig. 7C). Finally, three cases showed abolished division of the T.pp cell that exhibited either neural or hypodermal morphology (Fig. 7D,E). To summarize, the results suggest that nhr-25 mutation causes various defects of the posterior neural T.p lineage including the Psa phenotype (T.pa) and abnormal divisions of the T.pp cell. Clearly, NHR-25 is required to establish the neuronal fate in the posterior T.p lineage.
Fig. 7.
T-seam-cell lineages in wild-type and nhr-25(ku217)-mutant hermaphrodites. The directions of cell divisions are shown with anterior to the left. (A) Wild-type T-seam-cell lineage. Blue circles mark hypodermal cells that join the hyp7 syncytium; green triangle labels a seam cell; yellow rectangles indicate phasmid socket cells; and red rectangles show other neurons. X represents programmed cell death. (B-E) Both sides of five nhr-25(ku217) mutants were analyzed. Seven out of the ten T-seam-cell lineages exhibited abnormal cell division pattern (B-E). All of them showed a T.pa cell with hypodermal morphology and without subsequent division, resulting in Psa (phasmid-socket absent) phenotype. Three nhr-25(ku217) lineages displayed only Psa phenotype (B). In one case, T.pp underwent a division producing two neural cells, but without further division of the posterior daughter (C). Failure of T.pp cell division was noticed in three lineages, of which in one case the T.pp cell exhibited neural morphology (D), whereas in two cases it adopted hypodermal morphology (E). The anterior lineages, indicated by dotted lines, were followed until T.ap had divided, at which time all the cells showed hypodermal character.
nhr-25 genetically interacts with Wnt and RUNX signaling to specify neural fate of the T-seam-cell lineage
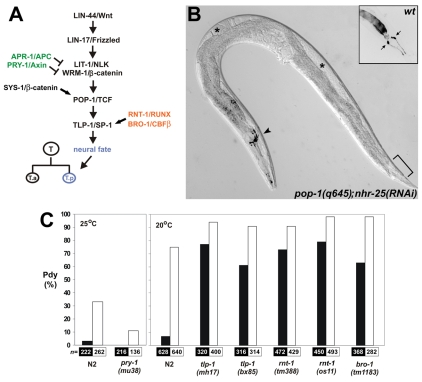
The loss of T.p-derived neural cell fates caused by NHR-25 deficiency was reminiscent of certain mutations in the Wnt/β-catenin asymmetry pathway, which controls the polarity of the T-seam-cell division (Sternberg and Horvitz, 1988; Herman, 2001; Mizumoto and Sawa, 2007a) (Fig. 8A). To test for a genetic interaction with this pathway, we applied nhr-25 RNAi to adults heterozygous for a POP-1/TCF mutation, pop-1(q645), and scored the Pdy phenotype in their progeny. Consistent with a previous report (Siegfried et al., 2004), the pop-1(q645) homozygotes displayed 42% incidence of Pdy, which was doubled by simultaneous nhr-25 knockdown (Fig. 8B; Table 4). An even stronger enhancement was observed for a SYS-1/β-catenin mutation, sys-1(q544) (Table 4). Both pop-1(q645) and sys-1(q544) worms were sterile owing to a Sys (symmetrical sisters) defect, a failure of the somatic gonad precursors to differentiate a distal tip cell and the consequent absence of the gonadal arms (Siegfried and Kimble, 2002). As we had shown previously (Asahina et al., 2006), nhr-25 RNAi suppressed the fully penetrant Sys (Table 4), restoring gonad differentiation and fertility in both mutants. Thus, we were able to visualize, in the same animals (Fig. 8B), both enhancement (Pdy in the T seam cell) and suppression (Sys in the gonad) of POP-1- or SYS-1-deficiency phenotypes. These data suggested that NHR-25 could cooperate with or counteract POP-1 and SYS-1 depending on cell type.
Fig. 8.
Genetic interaction of the RUNX and Wnt/β-catenin asymmetry pathways with NHR-25. (A) The Wnt/β-catenin asymmetry pathway is required for determination of the neural fate of the posterior T-seam-cell daughter, T.p (see Introduction for references). APR-1 and PRY-1 inhibit Wnt/β-catenin signaling, whereas RNT-1 and BRO-1 promote expression of the Wnt/β-catenin target tlp-1, a gene that is required for the neural fate. (B) nhr-25 RNAi suppresses the effect of pop-1(q645) mutation in the somatic gonad and enhances its effect in the tail. nhr-25 RNAi in a pop-1(q645) mutant restores differentiation of the gonadal arms (asterisks) and at the same time augments the Pdy (phasmid dye-filling defect). An inset shows positive staining of the phasmids (arrows) in wild type, whereas in the pop-1(q645);nhr-25(RNAi) worm the phasmid neurons (area indicated by bracket) remain unstained. Positive signal in the amphids (arrowhead) confirms successful dye filling. The fluorescent signal is shown as inverted. Also see Table 4. (C) NHR-25 positively modulates Wnt-RUNX signaling. The effect of systemic nhr-25 RNAi on T-seam-cell polarity as assessed by phasmid dye-filling is suppressed by a temperature-sensitive mutation of pry-1 (left, 25°C), and enhanced by mutations in tlp-1, rnt-1 and bro-1 genes. Black columns indicate control RNAi (empty vector); white columns signify nhr-25 RNAi. The suppression of nhr-25 RNAi-induced dye-filling defect by pry-1(mu38) mutation was statistically significant (P=0.007). The differences between Pdy penetrance in nhr-25(RNAi) alone and in tlp-1, rnt-1 and bro-1 mutant backgrounds were determined to be statistically significant at P<0.01. The paired Student's t-test with one-tailed probability values was used to assess significance of the differences. Results are shown as the mean of three to four independent RNAi experiments, and total numbers of animals scored are indicated below each column.
Table 4.
nhr-25 silencing enhances the Pdy defect, whereas it suppresses the gonadal Sys (symmetrical sisters) phenotype in pop-1 and sys-1 mutants
| n | RNAi | Pdy (%) | Sys (%) | |
|---|---|---|---|---|
| N2 | 332 | Control | 3 | 0 |
| 308 | nhr-25 | 44 | 0 | |
| pop-1 (q645) | 158 | Control | 42 | 100 |
| 226 | nhr-25 | 84 | 81 | |
| sys-1 (q544) | 178 | Control | 33 | 100 |
| 136 | nhr-25 | 93 | 70 |
To further explore the positive regulatory role of NHR-25, we treated mutants for two inhibitors of the Wnt/β-catenin asymmetry pathway, the PRY-1/Axin and APR-1/APC proteins (Korswagen et al., 2002; Mizumoto and Sawa, 2007a), with nhr-25 feeding RNAi. Neither pry-1(mu38) nor apr-1(ok2970) mutations caused Pdy alone. However, pry-1(mu38) suppressed the penetrance of the Pdy phenotype from 33% in nhr-25(RNAi) worms at 25°C to 11% (Fig. 8C). We did not observe such a suppression of Pdy in the apr-1(ok2970) mutant background (data not shown).
Next, we examined interaction between NHR-25 and the transcription factor TLP-1, which is essential for T-seam-cell polarity and its expression depends on the Wnt/β-catenin asymmetry pathway (Zhao et al., 2002). The frequency of Pdy induced by nhr-25 silencing via RNAi at 20°C was enhanced from 75% to 94% in tlp-1(mh17) and to 91% in tlp-1(bx85) mutants, respectively, and the enhancement was statistically significant (Fig. 8C). Together, these data suggested that NHR-25 positively modulated the Wnt/β-catenin asymmetry pathway to ensure proper T-seam-cell polarity.
In addition to Wnt signaling, TLP-1 expression also requires the transcription factor RNT-1, the loss of which causes abnormal male tail morphology and abolishes the asymmetry of the T-seam-cell division (Ji et al., 2004; Kagoshima et al., 2005; Nimmo et al., 2005). To specify T-seam-cell polarity, RNT-1 cooperates with BRO-1 (Kagoshima et al., 2007b; Xia et al., 2007). Because mutant phenotypes of rnt-1 or bro-1 resemble impaired function of nhr-25, we examined genetic interaction between nhr-25 and the two genes. Similarly to tlp-1 mutations, mutant alleles rnt-1(tm388) and rnt-1(os11) [also known as mab-2(os11)] also increased the incidence of Pdy caused by nhr-25 systemic RNAi from 75% to 91% and 98%, respectively (Fig. 8C). Almost full penetrance of Pdy also occurred in the bro-1(mu38) mutant background (Fig. 8C). On the basis of these genetic-interaction data, we conclude that nhr-25 cooperates with RNT-1 and BRO-1, and therefore that NHR-25 probably acts in parallel with the Wnt/β-catenin asymmetry pathway to establish the neural fate of the T-seam-cell lineage (Fig. 8A).
Discussion
We show in this study that the nuclear receptor NHR-25 is required for proper morphogenesis of the C. elegans tail. Loss of NHR-25 disrupts differentiation of the posterior seam cell T. Absence of the phasmid socket cells (Psa) and of other specific neurons in nhr-25-deficient worms, evidenced by lineage analysis, demonstrates the necessity of NHR-25 for the proper polarity of the T-seam-cell division and for differentiation of the neural branch of the T-seam-cell lineage. These results directly implicate NHR-25 in a specific cell-fate decision during animal development.
NHR-25 cooperates with the Wnt/β-catenin asymmetry pathway in the T seam cell but opposes it in the gonad
Loss of T-seam-cell polarity upon nhr-25 silencing was strongly enhanced by mutations affecting the transcriptional effectors of Wnt signaling – POP-1/TCF and its coactivator SYS-1/β-catenin. Strikingly, in the same animals, deficiency of NHR-25 suppressed the fully penetrant effect of either of these hypomorphic mutations on the somatic gonad. During asymmetric division of the somatic gonad precursor cells Z1 and Z4, NHR-25 promotes a proximal fate of the daughter cells and thus antagonizes a POP-1/TCF- and SYS-1/β-catenin-dependent activity, which ensures the alternative distal fate (Asahina et al., 2006). These results clearly demonstrate the capacity of NHR-25 to modulate the Wnt/β-catenin asymmetry pathway either positively or negatively depending on the tissue context. One intriguing difference between the T seam cell and the gonad is that the polarity of the somatic gonad precursors seems to be independent of known Wnt signals (Hitoshi Sawa, personal communication).
The correct fate of the T-seam-cell daughters is ensured by the reciprocal asymmetry of the POP-1/TCF and SYS-1/β-catenin proteins (Mizumoto and Sawa, 2007b), which leads to asymmetric expression of the pro-neural gene tlp-1 (Zhao et al., 2002). Therefore, regulation of the asymmetric distribution of these components is one possible mode of NHR-25 interaction with the Wnt/β-catenin asymmetry pathway. Nevertheless, partitioning of POP-1 to the T-seam-cell daughters seemed unaffected by impaired NHR-25 function (data not shown). The distribution of POP-1 was also normal in the V cells of nhr-25(RNAi) worms (M.A., unpublished observations). In fact, involvement of NHR-25 in POP-1 asymmetric distribution was not expected, because loss of NHR-25 function altered the POP-1-dependent cell fate in the somatic gonad precursor cells without perturbing POP-1 asymmetry (Asahina et al., 2006). Consistent with the normal asymmetric distribution of POP-1, expression of the tlp-1::gfp reporter was also unaffected by loss of NHR-25 (data not shown).
Because NHR-25 does not alter cell fate by redistribution of POP-1, it probably acts either by modulating the activity of genes downstream of the Wnt/β-catenin asymmetry pathway, other than tlp-1, or by regulating its own targets. An attractive possibility is that NHR-25 might cooperate with the transcriptional coactivator SYS-1/β-catenin, which is a limiting factor necessary for POP-1-dependent expression of neural fate-promoting genes (Mizumoto and Sawa, 2007b). This idea is supported by the strong genetic interaction between nhr-25 and sys-1 in the T seam cell (this study) and by previous evidence that NHR-25 binds SYS-1 to stimulate SYS-1- and POP-1-dependent gene expression (Asahina et al., 2006). Interestingly, mammalian orthologs of NHR-25, the SF-1 and LRH-1 have been shown to activate target genes in synergy with β-catenin (Gummow et al., 2003; Hossain and Saunders, 2003; Jordan et al., 2003; Mizusaki et al., 2003; Botrugno et al., 2004; Parakh et al., 2006; Salisbury et al., 2007). Therefore, the positive interaction between nhr-25 and sys-1 in the T seam cell resembles the scenarios known from the mammalian systems.
Alternatively, NHR-25 could influence the neural fate in the posterior T-seam-cell daughter by interacting with TLP-1. SF-1 and Sp1, the mammalian orthologs of TLP-1, co-regulate transcription of specific genes by binding to adjacent DNA elements in their promoter regions (Liu and Simpson, 1997; Kaiser et al., 2000; Sugawara et al., 2000). Whether NHR-25 and TLP-1 cooperate through a similar mechanism remains to be tested.
To ensure proper T-seam-cell differentiation, NHR-25 cooperates with RUNX signaling, which acts in parallel to the Wnt/β-catenin asymmetry pathway. The interaction of nhr-25 with the genes encoding the transcription factor RNT-1 and its cofactor BRO-1 results in almost a fully penetrant T-seam-cell polarity defect. In addition to their role in the T seam cell, RNT-1 and BRO-1 promote V-cell divisions, and therefore rnt-1 and bro-1 mutations reduce the total number of seam cells (Nimmo et al., 2005; Kagoshima et al., 2007b; Xia et al., 2007). Although this phenotype is opposite to the effect of NHR-25 deficiency (extra seam cells) (Chen et al., 2004; Silhankova et al., 2005) (and this study), we have not detected interaction between nhr-25 and rnt-1 or bro-1 genes that would restore the normal seam-cell number (data not shown). Thus, in contrast to the regulation of T-seam-cell polarity, the function of RUNX signaling in cell cycle of the V cells is independent of NHR-25.
Effects of NHR-25 on male tail morphogenesis
We have observed multiple defects of the sensory rays that were caused by loss of NHR-25, namely ray displacement or absence, ray fusions and ectopic rays. These diverse anomalies correspond to the pleiotropic nature of NHR-25 and might reflect involvement of NHR-25 in migration and cell-fate decision of the ray precursor cells. One of the defects, ray-1 displacement, resembles a specific phenotype of mutants in the semaphorin-plexin pathway (Fujii et al., 2002; Ginzburg et al., 2002; Nukazuka et al., 2008). Proper positioning of ray 1 is achieved by posterior attraction and subsequent migration of ray-1 precursors through transmembrane semaphorins, smp-1 and smp-2, and their receptor plexin, plx-1. It is not clear at this point whether NHR-25 cooperates with semaphorin-plexin signaling, because mutations in the smp-1, smp-2 and plx-1 genes, at least in the heterozygous condition, did not enhance the frequency of ray-1 displacement seen in scm::nhr-25(RNAi) males (data not shown). Recently, ray-1-precursor migration has been shown to depend on cooperation between the semaphorin-plexin pathway with Myc and Mondo-like proteins and Wnt/β-catenin signaling (Pickett et al., 2007). Because NHR-25 interacts with the Wnt/β-catenin asymmetry pathway (Asahina et al., 2006) (and this study), it is plausible that NHR-25 functions through this pathway to determine the position of ray 1.
The presence of fused rays in NHR-25-deficient males signifies that NHR-25 not only contributes to correct positioning of ray precursor cells but that it might also specify ray identity. Abnormal dosage of the Hox genes mab-5 and egl-5 (Chow and Emmons, 1994; Salser and Kenyon, 1996) can generate ray fusions due to ray-identity transformation. Interestingly, mab-5 becomes ectopically expressed in ray 1 and the fusion of ray 1 to ray 2 was frequently observed in nhr-25(ku217) mutants. Reduction of mab-5 dosage in these mutants suppressed the ray 1-2 fusion, suggesting that NHR-25 affects proper ray formation by regulating mab-5 activity. Although the gain of mab-5 expression and reduced mab-5 dosage both cause specific ray fusions, the complete absence of mab-5 function removes V5- and V6-derived rays (Salser and Kenyon, 1996). Similarly, varying degrees of loss of nhr-25 function lead to variably severe effects on ray morphology, ranging from specific ray fusions to ray deletion. These findings suggest that the process of male tail differentiation is highly sensitive to the levels of mab-5 and nhr-25 activities.
In summary, our study identifies a new role for the nuclear receptor NHR-25 in the differentiation of sex-specific tail structures and in the regulation of cell polarity during asymmetric division of the epidermal T seam cell. In contrast to previous results from the somatic gonad of C. elegans (Asahina et al., 2006), our present data establish NHR-25 as a positive regulator of Wnt/β-catenin-dependent cell-fate decisions, a situation that is consistent with synergy between NHR-25 homologs and β-catenin in mammals. We propose that NHR-25 is an evolutionarily conserved, versatile modulator of Wnt/β-catenin signaling.
Materials and Methods
Worm strains
The wild type C. elegans Bristol strain (N2) and the following transgenic lines and mutant strains were maintained according to the standard protocol (Brenner, 1974) at 20°C, except for temperature-sensitive mutants. Strains JR667 unc-119(e2498::Tc1); wIs51[unc-119(+),scm::gfp] (Terns et al., 1997), MH1955 nhr-25(ku217) (Chen et al., 2004), SU93 jcIs1[ajm-1::gfp,unc-29(+), rol-6(su1006)] (Mohler et al., 1998), DR466 him-5(e1490) (Hodgkin et al., 1979), NY2067 ynIs67[flp-6::gfp]; him-5(e1490) (Kim and Li, 2004), NY2064 ynIs64[flp-17::gfp]; him-5(e1490) (Kim and Li, 2004), GR1366 mgIs42[tph-1::gfp,rol-6(su1006)] (Sze et al., 2000), EM347 tlp-1(bx85); him-5(e1490) (Zhao et al., 2002), RB2190 apr-1(ok2970) (International C. elegans Gene Knockout Consortium), CF491 pry-1(mu38ts); him-5(e1490) (Maloof et al., 1999), BL5717 inIs179[ida-1::gfp]; him-8(e1489) (Zahn et al., 2001), JK2944 pop-1(q645)/hT2[qls48] (Siegfried and Kimble, 2002), JK3437 him-5(e1490); qIs74[POP-1::gfp] (Siegfried et al., 2004), CB3531 mab-5(e1239); him-5(e1490) (Kenyon, 1986) and CF453 muIs16[mab-5::gfp,dpy-20(+)]; dpy-20(e1282) (Hunter et al., 1999) were obtained from the Caenorhabditis Genetics Center. Other strains used in this study: KS115 tlp-1(mh17) (Zhao et al., 2002), KS246 unc-29(e1072); mhEx50[unc-29(+) tlp-1::gfp] (Zhao et al., 2002), HS147 rnt-1(os11) (originally described as mab-2) (Kagoshima et al., 2005) (a gift from H. Sawa, RIKEN Center for Developmental Biology, Kobe, Japan), YK101 rnt-1(tm388) and YK134 bro-1(tm1183) (Kagoshima et al., 2007b) (provided by Hiroshi Kagoshima, National Institute of Genetics, Mishima, Japan), PT1937 nIs133[pkd-2::gfp]; him-5(e1490) (Bae et al., 2006) (a gift from Maureen M. Barr, Rutgers University, Piscataway, NJ), HL252 jmEx33[nhr-25::gfp] (Silhankova et al., 2005), HL65 him-5(e1490); nhr-25 (ku217), HL66 inIs179[ida-1::gfp]; nhr-25(ku217).
RNA interference
Systemic RNAi was performed by feeding as described previously (Timmons et al., 2001). nhr-25 dsRNA was first induced in the liquid bacterial culture as described (Silhankova et al., 2005), and bacteria were centrifuged and seeded on the nematode growth medium (NGM) agarose plates containing 50 μg/ml carbenicillin, 12.5 μg/ml tetracycline and 0.4 mM isopropyl-β-D-thiogalactosidase (IPTG). Bacteria were further grown for approximately 16 hours at 25°C before L4 or young adult worms were added to the plates. Worms were then kept at 20°C. Bacteria carrying the empty pPD129.36 vector were used as a control.
To target nhr-25 RNAi to the seam cells, we constructed a plasmid for transgenic expression of nhr-25 dsRNA (Timmons et al., 2003). The seam-cell-specific SCM promoter (Terns et al., 1997) was excised from a pSCM3 (a gift from H. Sawa) plasmid and cloned into the pPD95.70 vector (provided by Andrew Fire, Stanford University School of Medicine, Stanford, CA) using PstI and SmaI sites. The nhr-25 RNAi construct was generated by removing the nuclear-localization sequence (NLS) and gfp sequence from pPD95.70 using KpnI and EcoRI sites and replacing them with a sequence consisting of two copies of a 185-bp fragment from the first nhr-25 exon in inverted orientation. The two halves of the palindrome were cloned by using KpnI-SalI and SalI-EcoRI sites, respectively, and were separated by 114 bp of the first nhr-25 intron to generate the pPD95.70{scm::nhr-25(RNAi)} vector. To obtain transgenic worms, 50 ng/μl of the pPD95.70{scm::nhr-25(RNAi)} plasmid were co-injected with 25 ng/μl of the pRF4 rol-6(su1009) transformation marker (Mello et al., 1991) and 25 ng/μl of pBluescript into the JR667 strain. Transformants were subjected to UV irradiation to chromosomally integrate the constructs. Integrated lines were backcrossed with the N2 strain twice. The resulting strain HL70 jmIs70[scm::nhr-25(RNAi), rol-6(su1009)]; wIs51[unc-119(+), scm::gfp] was used in all experiments. The HL70 strain was crossed with the NY2067, NY2064, GR1366, PT1937 and SU93 strains to introduce the desired transgenic markers into the scm::nhr-25(RNAi) background. For male tail analyses, the HL70 strain was also crossed with DR466 to increase the frequency of males. scm::nhr-25(RNAi) males did not mate effectively.
Phasmid dye-filling
The Dyf (dye-filling defect) phenotype was examined as previously described (Hedgecock et al., 1985; Herman and Horvitz, 1994). Adult hermaphrodites were washed out with S-basal buffer from NGM plates and were collected in a 1.5-ml tube. The worms were incubated in 1 ml of S-basal buffer containing 20 μg/μl of the 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO, Sigma) on a rotator for 2 hours at 20°C. After staining, worms were washed once in S-basal buffer and placed onto NGM plates for 1 hour for destaining of the gut. Worms were then observed under a microscope equipped with epifluorescence for GFP. While dye-filled amphid neurons served as a control of successful staining, the number of stained phasmids was scored. Because the dye-filling defect concerned only the phasmids but not the amphid neurons, we use the more specific term Pdy (phasmid dye-filling defect) in this study.
Phasmid socket-cell analysis
The absence of socket cells (phasmid socket absent, Psa phenotype) was analyzed at mid-L2 or early-L3 stage (20-25 hours after hatching). Using Nomarski optics, nuclear morphology of the cells located between the PLM neuron and hyp8 (hyp11) were evaluated. In wild-type L2 and L3 worms, the phasmid socket cells are the most posterior neurons positioned as specified above, and they exhibit neural morphology, i.e. a small nucleus with granular nucleoplasm. By contrast, a hypodermal cell typically possesses a large round nucleus with smooth nucleoplasm (Herman and Horvitz, 1994).
Cell-lineage analysis
Individual L1 larvae with the T seam cells approaching the time of division were placed on 5% agar pads with 2 μl of M9 buffer. A tiny amount of OP-50 bacteria was applied to the center of a coverslip to attract the worm. The edges of the coverslip were greased to prevent the dehydration of the worm (Sulston and Horvitz, 1977). Between individual observations, the agar pad with the worm was kept in a moist chamber to ensure continuation of development. The cell divisions were observed using Nomarski optics with 100× oil-immersion objectives. Fates of the T-seam-cell descendants were judged based on their nuclear morphology as described previously (Herman and Horvitz, 1994).
Microscopy
Worms were mounted on freshly made 2% agarose pads for fluorescence observation or on 5% agar pads for Nomarski imaging. 10 mM tetramisole hydrochloride (2,3,3,6 tetrahydro-6-phenylimidasol) in S-basal buffer was used to anesthetize the animals. Microscopy analyses were performed using Zeiss Axioplan 2 equipped with Nomarski optics and epifluorescence. An Olympus FV1000 Laser Scanning Confocal microscope was used for imaging of adherens junctions of the male tail.
We thank Hitoshi Sawa for providing materials and for sharing unpublished information. Strains from Hiroshi Kagoshima, Maureen M. Barr, the C. elegans Genetics Center and the International C. elegans Gene Knockout Consortium are greatly appreciated. We thank Yinhua Zhang for useful suggestions. This work was supported by projects 204/07/0948 and 204/09/H058 from the Czech Science Foundation, 2B06129 from the Czech Ministry of Education, Z60220518 from the Institute of Parasitology, and GM56339 and P20RR016475 from the NIH National Center for Research Resources (NCRR). Deposited in PMC for release after 12 months.
References
- Asahina, M., Ishihara, T., Jindra, M., Kohara, Y., Katsura, I. and Hirose, S. (2000). The conserved nuclear receptor Ftz-F1 is required for embryogenesis, moulting and reproduction in Caenorhabditis elegans. Genes Cells 5, 711-723. [DOI] [PubMed] [Google Scholar]
- Asahina, M., Valenta, T., Silhankova, M., Korinek, V. and Jindra, M. (2006). Crosstalk between a nuclear receptor and β-catenin signaling decides cell fates in the C. elegans somatic gonad. Dev. Cell 11, 203-211. [DOI] [PubMed] [Google Scholar]
- Bae, Y. K., Qin, H., Knobel, K. M., Hu, J., Rosenbaum, J. L. and Barr, M. M. (2006). General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development 133, 3859-3870. [DOI] [PubMed] [Google Scholar]
- Baird, S. E., Fitch, D. H. A., Kassem, I. A. A. and Emmons, S. W. (1991). Pattern formation in the nematode epidermis: determination of the arrangement of peripheral sense organs in the C. elegans male tail. Development 113, 515-526. [DOI] [PubMed] [Google Scholar]
- Betschinger, J. and Knoblich, J. A. (2004). Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr. Biol. 14, R674-R685. [DOI] [PubMed] [Google Scholar]
- Botrugno, O. A., Fayard, E., Annicotte, J. S., Haby, C., Brennan, T., Wendling, O., Tanaka, T., Kodama, T., Thomas, W., Auwerx, J. et al. (2004). Synergy between LRH-1 and beta-catenin induces G(1) cyclin-mediated cell proliferation. Mol. Cell 15, 499-509. [DOI] [PubMed] [Google Scholar]
- Brenner, S. (1974). The genetics of C. elegans. Genetics 77, 71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z., Eastburn, D. J. and Han, M. (2004). The Caenorhabditis elegans nuclear receptor gene nhr-25 regulates epidermal cell development. Mol. Cell. Biol. 24, 7345-7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, K. L. and Emmons, S. W. (1994). HOM-C/Hox genes and four interacting loci determine the morphogenetic properties of single cells in the nematode male tail. Development 120, 2579-2593. [DOI] [PubMed] [Google Scholar]
- Fujii, T., Nakao, F., Shibata, Y., Shioi, G., Kodama, E., Fujisawa, H. and Takagi, S. (2002). Caenorhabditis elegans plexinA, PLX-1, interacts with transmembrane semaphorins and regulates epidermal morphogenesis. Development 129, 2053-2063. [DOI] [PubMed] [Google Scholar]
- Ginzburg, V. E., Roy, P. J. and Culotti, J. G. (2002). Semaphorin 1a and semaphorin 1b are required for correct epidermal positioning and adhesion during morphogenesis in C. elegans. Development 129, 2065-2078. [DOI] [PubMed] [Google Scholar]
- Gissendanner, C. R. and Sluder, A. E. (2000). nhr-25, the Caenorhabditis elegans ortholog of ftz-f1, is required for epidermal and somatic gonad development. Dev. Biol. 221, 259-272. [DOI] [PubMed] [Google Scholar]
- Gönzy, P. (2008). Mechanisms of asymmetric cell division: flies and worms pave the way. Nat. Rev. Mol. Cell Biol. 9, 355-366. [DOI] [PubMed] [Google Scholar]
- Gummow, B. M., Winnay, J. N. and Hammer, G. D. (2003). Convergence of Wnt signaling and steroidogenic factor-1 (SF-1) on transcription of the rat inhibin α gene. J. Biol. Chem. 278, 26572-26579. [DOI] [PubMed] [Google Scholar]
- Hedgecock, E. M., Culotti, J. G., Thomson, J. N. and Perkins, L. A. (1985). Axonal guidance mutants of Caenorhabditis elegans identified by filling sensory neurons with fluorescein dyes. Dev. Biol. 111, 158-170. [DOI] [PubMed] [Google Scholar]
- Herman, M. A. (2001). C. elegans POP-1/TCF functions in a canonical Wnt pathway that controls cell migration and in a noncanonical Wnt pathway that controls cell polarity. Development 128, 581-590. [DOI] [PubMed] [Google Scholar]
- Herman, M. A. (2002). Control of cell polarity by noncanonical Wnt signaling in C. elegans. Cell Dev. Biol. 13, 233-241. [DOI] [PubMed] [Google Scholar]
- Herman, M. A. and Horvitz, R. (1994). The Caenorhabditis elegans gene lin-44 controls the polarity of asymmetric cell divisions. Development 120, 1035-1047. [DOI] [PubMed] [Google Scholar]
- Herman, M. A. and Wu, M. (2004). Noncanonical Wnt signaling pathways in C. elegans converge on POP-1/TCF and control cell polarity. Front. Biosci 9, 1530-1539. [DOI] [PubMed] [Google Scholar]
- Hodgkin, J., Brenner, S. and Horvitz, B. (1979). Nondisjunction mutants of the nematode C. elegans. Genetics 91, 67-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain, A. and Saunders, G. F. (2003). Synergistic cooperation between the β-catenin signaling pathway and steroidogenic factor 1 in the activation of the Mullerian inhibiting substance type II receptor. J. Biol. Chem. 278, 26511-26516. [DOI] [PubMed] [Google Scholar]
- Hunter, C. P., Harris, J. M., Maloof, J. N. and Kenyon, C. (1999). Hox gene expression in a single Caenorhabditis elegans cell is regulated by a caudal homolog and intercellular signals that inhibit Wnt signaling. Development 126, 805-814. [DOI] [PubMed] [Google Scholar]
- Ji, Y. J., Nam, S., Jin, Y. H., Cha, E. J., Lee, K. S., Choi, K. Y., Song, H. O., Lee, J., Bae, S. C. and Ahnn, J. (2004). RNT-1, the C. elegans homologue of mammalian RUNX transcription factors, regulates body size and male tail development. Dev. Biol. 274, 402-412. [DOI] [PubMed] [Google Scholar]
- Jordan, B. K., Shen, J. H. C., Olaso, R., Ingraham, H. A. and Vilain, E. (2003). Wnt4 overexpression disrupts normal testicular vasculature and inhibits testosterone synthesisby repressing SF-1/b-catenin synergy. Proc. Natl. Acad. Sci. USA 100, 10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagoshima, H., Sawa, H., Mitani, S., Burglin, T. R., Shigesada, K., Kohara, Y. (2005). The C. elegans RUNX transcription factor RNT-1/MAB-2 is required for asymmetrical cell division of the T blast cell. Dev. Biol. 287, 262-273. [DOI] [PubMed] [Google Scholar]
- Kagoshima, H., Shigesada, K. and Kohara, Y. (2007a). RUNX regulates stem cell proliferation and differentiation: insights from studies of C. elegans. J. Cell. Biochem. 100, 1119-1130. [DOI] [PubMed] [Google Scholar]
- Kagoshima, H., Nimmo, R., Saad, N., Tanaka, J., Miwa, Y., Mitani, S., Kohara, Y. and Woollard, A. (2007b). The C. elegans CBFβ homologue BRO-1 interacts with the Runx factor, RNT-1, to promote stem cell proliferation and self-renewal. Development 134, 3905-3915. [DOI] [PubMed] [Google Scholar]
- Kaiser, U. B., Halvorson, L. M. and Chen, M. T. (2000). Sp1, steroidogenic factor 1 (SF-1) and early growth response protein 1 (Egr-1) binding sites form a tripartite gonadotropin-releasing hormone response element in the rat luteinizing hormone-β gene promoter: an integral role for SF-1. Mol. Endocrinol. 14, 1235-1245. [DOI] [PubMed] [Google Scholar]
- Kastner, P., Mark, M. and Chambon, P. (1995). Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell 83, 859-869. [DOI] [PubMed] [Google Scholar]
- Kenyon, C. (1986). A gene involved in the development of the posterior body region of C. elegans. Cell 46, 477-487. [DOI] [PubMed] [Google Scholar]
- Kidd, A. R., 3rd, Miskowski, J. A., Siegfried, K. R., Sawa, H. and Kimble, J. (2005). A β-catenin identified by functional rather than sequence criteria and its role in Wnt/MAPK signaling. Cell 121, 761-772. [DOI] [PubMed] [Google Scholar]
- Kim, K. and Li, C. (2004). Expression and regulation of an FMRFamide-related neuropeptide gene family in Caenorhabditis elegans. J. Comp. Neurol. 475, 540-550. [DOI] [PubMed] [Google Scholar]
- Kimble, J. and Crittenden, S. L. (2007). Controls of germaline stem cells, entry to meiosis, and sperm/oocyte decision in Caenorhabditis elegans. Annu. Rev. Cell Dev. Biol. 23, 405-433. [DOI] [PubMed] [Google Scholar]
- Korswagen, H. C., Coudreuse, D. Y. M., Betist, M. C., van de Water, S., Zivkovic, D. and Clevers, H. C. (2002). The axin-like protein PRY-1 is a negative regulator of a canonical Wnt pathway in C. elegans. Genes Dev. 15, 1291-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lints, R., Jia, L., Kim, K., Li, C. and Emmons, S. W. (2004). Axial patterning of C. elegans male sensilla identities by selector genes. Dev. Biol. 269, 137-151. [DOI] [PubMed] [Google Scholar]
- Liu, Z. and Simpson, E. R. (1997). Steroidogenic factor 1 (SF-1) and SP1 are required for regulation of bovine CYP11A gene expression in bovine luteal cells and adrenal Y1 cells. Mol. Endocrinol. 11, 127-137. [DOI] [PubMed] [Google Scholar]
- Maglich, J. M., Sluder, A., Guan, X., Shi, Y., McKee, D. D., Carrick, K., Kamdar, K., Willson, T. M. and Moore, J. T. (2001). Comparison of complete nuclear receptors sets from the human, Caenorhabditis elegans and Drosophila genomes. Genome Biol. 2, 29.1-29.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloof, J. N., Whangbo, J., Harris, J. M., Jongeward, G. D. and Kenyon, C. (1999). A Wnt signaling pathway controls Hox gene expression and neuroblast migration in C. elegans. Development 126, 37-49. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf, D. J., Thummel, C., Beato, M., Herrlich, P., Schütz, G., Umesono, K., Blumberg, B., Kastner, P., Mark, M., Chambon, P. et al. (1995). The nuclear receptor superfamily: the second decade. Cell 83, 835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello, C. C., Kramer, J. M., Stinchcomb, D. and Ambros, V. (1991). Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10, 3959-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto, K. and Sawa, H. (2007a). Cortical β-catenin and APC regulate asymmetric nuclear β-catenin localization during asymmetric cell division in C. elegans. Dev. Cell 12, 287-299. [DOI] [PubMed] [Google Scholar]
- Mizumoto, K. and Sawa, H. (2007b). Two βs or not two βs: regulation of asymmetric divisions by β-catenin. Trends Cell Biol. 17, 465-473. [DOI] [PubMed] [Google Scholar]
- Mizusaki, H., Kawabe, K., Mukai, T., Ariyoshi, E., Kasahara, M., Yoshioka, H., Swain, A. and Morohashi, K. (2003). Dax-1 (dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1) gene transcription is regulated by Wnt4 in the female developing gonad. Mol. Endocrinol. 17, 507-519. [DOI] [PubMed] [Google Scholar]
- Mohler, W. A., Simske, J. S., Williams-Masson, E. M., Hardin, J. D. and White, J. G. (1998). Dynamics and ultrastructure of developmental cell fusions in the Caenorhabditis elegans hypodermis. Curr. Biol. 8, 1087-1090. [DOI] [PubMed] [Google Scholar]
- Mulholland, D. J., Dedhar, S., Coetzee, G. A. and Nelson, C. C. (2005). Interaction of nuclear receptors with Wnt/beta-catenin/Tcf signaling: Wnt you like to know? Endocr. Rev. 26, 898-915. [DOI] [PubMed] [Google Scholar]
- Nimmo, R., Antebi, A. and Woollard, A. (2005). mab-2 encodes RNT-1, a C. elegans Runx homologue essential for controlling cell proliferation in a stem cell-like developmental lineage. Development 132, 5043-5054. [DOI] [PubMed] [Google Scholar]
- Nukazuka, A., Fujisawa, H., Inada, T., Oda, Y. and Takagi, S. (2008). Semaphorin controls epidermal morphogenesis by stimulating mRNA translation via eIF2α in Caenorhabditis elegans. Genes Dev. 22, 1025-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parakh, T. N., Hernandez, J. A., Grammer, J. C., Weck, J., Hunzicker-Dunn, M., Zeleznik, A. J. and Nilson, J. H. (2006). Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires beta-catenin. Proc. Natl. Acad. Sci. USA 103, 12435-12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett, C. L., Breen, K. T. and Ayer, D. E. (2007). A C. elegans Myc-like network cooperates with semaphorin and Wnt signaling pathways to control cell migration. Dev. Biol. 310, 226-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roegiers, F. and Jan, Y. N. (2004). Asymmetric cell division. Curr. Opin. Cell Biol. 16, 195-205. [DOI] [PubMed] [Google Scholar]
- Salisbury, T. B., Binder, A. K., Grammer, J. C. and Nilson, J. H. (2007). Maximal activity of the Luteinizing hormone β-subnunit gene requires β-catenin. Mol. Endocrinol. 21, 963-971. [DOI] [PubMed] [Google Scholar]
- Salser, S. J. and Kenyon, C. (1996). A C. elegans Hox gene switches on, off, on and off again to regulate proliferation, differentiation and morphogenesis. Development 122, 1651-1661. [DOI] [PubMed] [Google Scholar]
- Sawa, H., Kouike, H. and Okano, H. (2000). Components of the SWI/SNF complex are required for asymmetric cell division in C. elegans. Mol. Cell 6, 617-624. [DOI] [PubMed] [Google Scholar]
- Siegfried, K. R. and Kimble, J. (2002). POP-1 controls axis formation during early gonadogenesis in C. elegans. Development 129, 443-453. [DOI] [PubMed] [Google Scholar]
- Siegfried, K. R., Kidd, A. R., 3rd, Chesney, M. A. and Kimble, J. (2004). The sys-1 and sys-3 genes cooperate with Wnt signaling to establish the proximal-distal axis of the Caenorhabditis elegans gonad. Genetics 166, 171-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhankova, M., Jindra, M. and Asahina, M. (2005). Nuclear receptor NHR-25 is required for cell-shape dynamics during epidermal differentiation in Caenorhabditis elegans. J. Cell Sci. 118, 223-232. [DOI] [PubMed] [Google Scholar]
- Sluder, A. E., Mathews, S. W., Hough, D., Yin, V. P. and Maina, C. V. (1999). The nuclear receptor superfamily has undergone extensive proliferation and diversification in nematodes. Genome Res. 9, 103-120. [PubMed] [Google Scholar]
- Sternberg, P. W. and Horvitz, H. R. (1988). lin-17 mutations of Caenorhabditis elegans disrupt certain asymmetric cell divisions. Dev. Biol. 130, 67-73. [DOI] [PubMed] [Google Scholar]
- Sugawara, T., Saito, M. and Fujimoto, S. (2000). Sp1 and SF-1 interact and cooperate in the regulation of human steroidogenic acute regulatory protein gene expression. Endocrinology 141, 2895-2903. [DOI] [PubMed] [Google Scholar]
- Sulston, J. E. and Horvitz, H. (1977). Post-embryonic cell lineages of the nematode, C. elegans. Dev. Biol. 56, 110-156. [DOI] [PubMed] [Google Scholar]
- Sulston, J. E., Schierenberg, E., White, J. G. and Thompson, J. N. (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64-119. [DOI] [PubMed] [Google Scholar]
- Sze, J. Y., Victor, M., Loer, C., Shi, Y. and Ruvkun, G. (2000). Food and metabolic signaling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature 403, 560-564. [DOI] [PubMed] [Google Scholar]
- Terns, R. M., Kroll-Conner, P., Zhu, J., Chung, S. and Rothman, J. H. (1997). A deficiency screen for zygotic loci required for for establishment and patterning of the epidermis in Caenorhabditis elegans. Genetics 146, 185-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons, L., Court, D. L. and Fire, A. (2001). Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263, 103-112. [DOI] [PubMed] [Google Scholar]
- Timmons, L., Tabara, H., Mello, C. C. and Fire, A. Z. (2003). Inducible systemic RNA silencing in Caenorhabditis elegans. Mol. Biol. Cell 14, 2972-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, D., Zhang, Y., Huang, X., Sun, Y. and Zhang, H. (2007). The C. elegans CBF-β homolog, BRO-1, regulates the proliferation, differentiation and specification of the stem cell-like seam cell lineages. Dev. Biol. 309, 259-272. [DOI] [PubMed] [Google Scholar]
- Wu, M. and Herman, M. A. (2007). Asymmetric localizations of LIN-17/Fz and MIG-5/Dsh are involved in the asymmetric B cell division in C. elegans. Dev. Biol. 303, 650-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn, T. R., Macmorris, M. A., Dong, W., Day, R. and Hutton, J. C. (2001). IDA-1, a Caenorhabditis elegans homolog of the diabetic autoantigens IA-2 and phogrin, is expressed in peptidergic neurons in the worm. J. Comp. Neurol. 429, 127-143. [DOI] [PubMed] [Google Scholar]
- Zhao, X., Yang, Y., Fitch, D. H. A. and Herman, M. A. (2002). TLP-1 is an asymmetric cell fate determinant that responds to Wnt signals and controls male tail tip morphogenesis in C. elegans. Development 129, 1497-1508. [DOI] [PubMed] [Google Scholar]