Abstract
The capacity of the collateral circulation to lessen injury in occlusive vascular disease depends on the density and caliber of native (pre-existing) collaterals, as well as their ability to outwardly remodel in ischemia. Native collateral conductance varies widely among healthy individuals, yet little is known about what specifies collateral formation. Chloride intracellular channel-4 protein (CLIC4) is required for endothelial cell hollowing, a process necessary for vessel formation during embryogenesis and ischemia. Whether CLIC4 has other physiological roles in vascular biology is uncertain. We studied collateral formation and remodeling in mice deficient in CLIC1 and CLIC4. Vascular responses to femoral artery ligation were similar in Clic1-/- and wild-type mice. In contrast, immediately after ligation perfusion dropped more in Clic4-/- than wild-type mice, suggesting fewer pre-existing collaterals—a finding confirmed by angiography, greater ischemia and worse recovery of perfusion; however, collateral remodeling was unaffected. Likewise, native cerebral collateral density in Clic4-/- (but not Clic1-/-) mice was reduced, resulting in severe infarctions. This was associated with impaired perinatal formation and stabilization of nascent collaterals. Clic4 hemizygous mice had intermediate deficits in the above parameters, suggesting a gene-dose effect. Ischemia augmented CLIC1 and CLIC4 expression similarly in wild-type mice. However, CLIC1 increased 3-fold more in Clic4-/- mice, suggesting compensation. Despite greater ischemia in Clic4-/- mice, HIF-1α, VEGF and angiopoietin-2 increased less compared to wild-type, suggesting CLIC4 exerts influences upstream of HIF-1α—VEGF signaling. Hence, CLIC4 represents the second gene that, along with VEGF shown by us previously, specifies native collateral formation.
Keywords: arteriogenesis, intracellular chloride channels, cerebral circulation, angiogenesis, vascular development
Introduction
Ischemic vascular disease of the heart, brain and peripheral limbs are leading causes of morbidity and death. In dependent tissues, angiogenesis can only improve distribution of the remaining arterial inflow, whereas collateral remodeling (“arteriogenesis”) is capable of restoring the original resting flow1,2. Thus, the density and diameter of native (pre-existing) collaterals in healthy tissues and their capacity to remodel in ischemia are major determinants of the severity of tissue injury in obstructive vascular disease2-4. Evidence suggests that native collateral conductance varies widely in healthy individuals5,6. This presumably extends from the influence of as yet unknown genetic and environmental factors.
Identification of such factors requires an understanding of how these unique inter-tree artery-to-artery connections are formed. However, while the mechanisms that direct collateral remodeling are receiving considerable attention2-4 no studies have examined when or how the native collateral circulation forms, although formation has been suggested to occur embryonically7. We recently reported that collaterals are present at birth and mature/stabilize rapidly thereafter, and that VEGF is important in both processes8. VEGF is critical in vascular development, wherein VEGF isoform gradients, in concert with hemodynamic and other factors, expand and remodel the primary embryonic plexus into the mature network present at birth9,10, through new vessel sprouting, intussusception, branching morphogenesis, pruning and remodeling of existing vessels, and subsequent mural cell recruitment11,12. Variation in these processes may underlie VEGF's involvement in the wide variation in collateral conductance among healthy individuals8.
Undoubtedly, molecules in addition to VEGF are involved in collateral formation. Chloride intracellular channel-4 (CLIC4) belongs to a newly described family of proteins with actions distinct from traditional cell membrane chloride channels that include formation of anion channels in intracellular organelles and involvement in membrane trafficking13, apoptosis14 and cell differentiation15. CLIC4 is highly expressed in endothelial cells of renal16 and retinal17 blood vessels, as well as in formation of new capillary sprouts in embryoid bodies and tumors16. Interestingly, Bohman and co-workers have implicated CLIC4 in VEGF-induced tubulogenesis in mammalian endothelial cells16. A similar process—epithelial cell hollowing and formation of the alimentary canal in C. elegans—is mediated by a CLIC homolog denoted exc-418. Moreover, Ulmasov et al. recently demonstrated that Clic4-/- mice show defective angiogenesis both in a Matrigel plug assay and during oxygen-induced retinal neo-angiogenesis, that endothelial cells from Clic4-/- mice show impaired tubologenesis in culture, that intracellular endothelial vacuoles along the tubulogenic pathway acidify, and that vacuolar acidification is defection in Clic4-/- endothelial cells. These studies identify important functions of CLIC proteins in vessel formation, ie, endothelial cell hollowing and tubulogenesis.
Presently, we examined involvement of CLIC4, as well as CLIC1 which is expressed widely19,20, in determining collateral density and diameter in healthy tissue and in collateral remodeling in hindlimb ischemia. Given the above evidence for CLIC4's role in VEGF-mediated vessel formation, together with findings that VEGF contributes to collateral formation in the embryo, maturation in the neonate, and remodeling in ischemia8, we hypothesized that CLIC proteins contribute to all three processes.
Materials and Methods
Procedures were conducted blindly. Clic4-/- were on CD-1 background17; Clic1-/- mice were constructed similarly (manuscript in preparation). Both lack an apparent phenotype (Ref 17, see Results). Wild-type littermates served as controls. The right femoral artery was ligated distal to the lateral caudal femoral and superficial epigastric arteries (latter also ligated) and proximal to the genu, followed by transection. Histology, angiography and arteriography were done after pressure-perfused maximal dilation and fixation8,21,22. Postmortem arterial micro-angiography was done with lead-based latex with a viscosity sufficient to minimize capillary transit on separate groups after right femoral ligation 7 days earlier and left femoral ligation on day-7. Data (x±SEM) were tested by ANOVA, Dunn-Bonferonni, Student's t-tests or Mann Whitney U tests. Additional details are in Supplement Material (http://circres.ahajournals.org).
Results
Clic4-/- mice have reduced hindlimb perfusion immediately after femoral artery ligation and reduced recovery of perfusion
Mice with CLIC1 and CLIC4 disruption (Clic1-/-, Clic4-/-) have minimal apparent phenotype in unstressed laboratory conditions, except that Clic4-/- have less dense retinal vasculature and lower body weight17 [herein, Clic4-/-, 29±2g; CD-1 wild-type, 32±2g; Clic1-/-, 30±2g]. Immediately (“acutely”) after ligation, perfusion in the plantar foot (Figure 1a,c), which correlates with overall hindlimb blood flow8,21,22 dropped to similar values in Clic1-/- and wild-type mice. However, perfusion decreased more in Clic4-/-. Lower conductance of the native collateral circulation results in lower plantar perfusion immediately after ligation8,22. Thus, these data suggest that Clic4-/- mice (but not Clic1-/-) have fewer and/or narrower native hindlimb collaterals than wild-type. Over 10-day recovery following femoral ligation, plantar perfusion in Clic4-/- mice increased in parallel with wild-type, suggesting similar collateral remodeling in both strains.
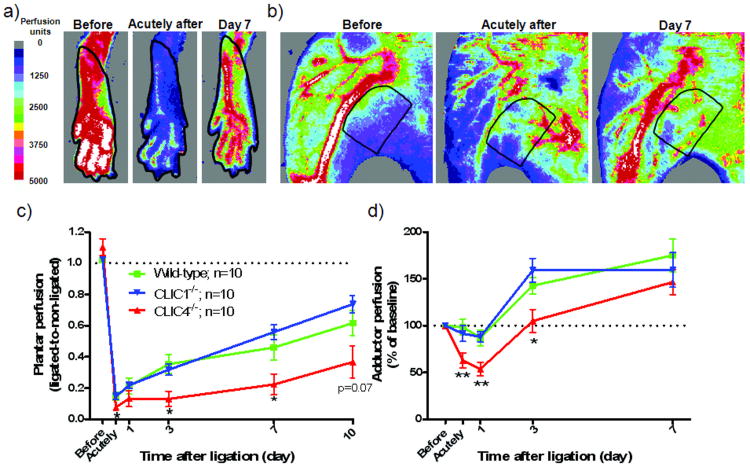
Figure 1.
Clic4-/- mice have lower perfusion immediately after femoral artery ligation and reduced recovery of perfusion. Doppler images (a,b) showing regions of interest (outlined area) for quantification (c,d) of perfusion of the plantar foot (index of hindlimb perfusion) and adductor thigh (index of combined collateral and adductor perfusion). Plantar and adductor perfusion dropped more in Clic4-/- immediately after ligation, suggesting lower collateral conductance, but recovered at a similar rate relative to wild-type, suggesting similar collateral remodeling. *, ** p<0.05, 0.01 vs. WT. In this and in subsequent figures, values are means ± SEM for n number of mice per group, time-point or bar.
We also measured perfusion in the adductor region (ie, the “collateral zone”) that contains collaterals supplying the lower leg8,21,22 (Figure 1b,d). Collaterals are presumed to have little or no net flow in the absence of obstruction. Thus, flow in small arterioles and capillaries supplied by the distal branches of the saphenous and lateral caudal femoral artery trees, which are also present in this region, determine the perfusion signal before femoral artery ligation. Immediately after ligation, measured perfusion reflects the combined effects of a drop in flow in arterioles and capillaries of the saphenous artery tree, together with an increase in flow in collaterals interconnecting it with the lateral caudal femoral artery tree. The increase in perfusion over subsequent days reflects primarily collateral enlargement (remodeling), since no angiogenesis occurs in this region in the ligation model used herein2,8,22. Adductor perfusion showed a pattern similar to plantar: Baseline perfusion before ligation was comparable among the 3 groups. Perfusion after ligation followed comparable patterns in Clic1-/- and wild-type, but decreased more in Clic4-/- mice immediately after ligation and thereafter followed a parallel recovery. These plantar and adductor patterns suggest that germ-line deletion of CLIC4 (but not CLIC1) results in fewer and/or narrower native collaterals, which, however, undergo comparable remodeling (confirmed below).
To test functional significance, scores were obtained for hindlimb use (index of muscle function) and appearance (index of ischemia)8,21,22. In agreement with the perfusion data, use and appearance scores were similar in wild-type and Clic1-/- mice but significantly greater/worse in Clic4-/- (Figure 2a,b).
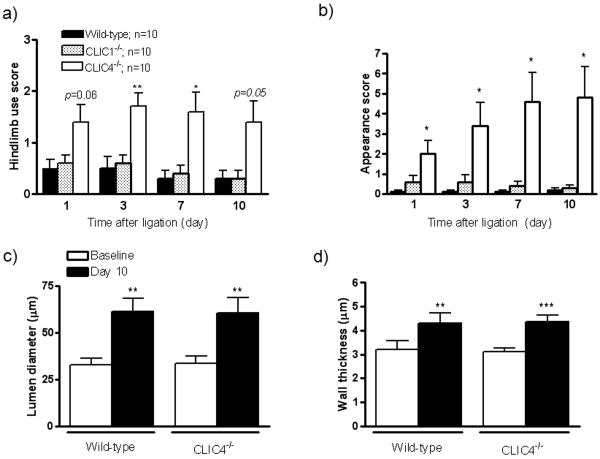
Figure 2.
Clic4-/- mice experience greater functional impairments but normal collateral remodeling after ligation. Use score (a) and appearance score (b). c,d) Collateral lumen diameter and medial wall thickness before (baseline) and 10 days after ligation. Diameters and wall thicknesses of the single collateral in the anterior and posterior gracilis muscles did not differ significantly and were thus averaged. *, **, *** p<0.05, 0.01, 0.001 vs. wild-type or baseline.
Collateral remodeling is unaffected in Clic4-/- mice
Lower conductance of the native collateral circulation in Clic4-/- mice could reflect reduced collateral number and/or lumen diameter. We thus measured diameter of collaterals in the anterior and posterior gracilis muscles by histomorphometry after maximal dilation and fixation. After ligation, flow in these superficial collaterals is a primary contributor to Doppler perfusion measured in the adductor collateral zone (Figure 1b,d). Wall thickness (intima-to-outer limit of media) was also determined to confirm lumen measurements21, since it varies in proportion to diameter according to Laplace's equation. Baseline diameter and wall thickness did not differ in the non-ligated contralateral limbs of wild-type and Clic4-/- mice (Figure 2c,d). This suggests that the lower perfusion in Clic4-/- mice acutely after ligation (Figures 1c,d) is not due to narrower native collaterals but, instead, reflects fewer collaterals (see below). After ligation, gracilis collaterals in Clic4-/- and wild-type mice underwent similar lumen enlargement and medial thickening (Figure 2c,d). This indicates that arteriogenesis is not impaired in Clic4-/-, which is consistent with the parallel increases in plantar and adductor perfusion in wild-type and Clic4-/- mice (Figure 1c,d).
Clic4-/- mice have reduced density of native collaterals in the hindlimb
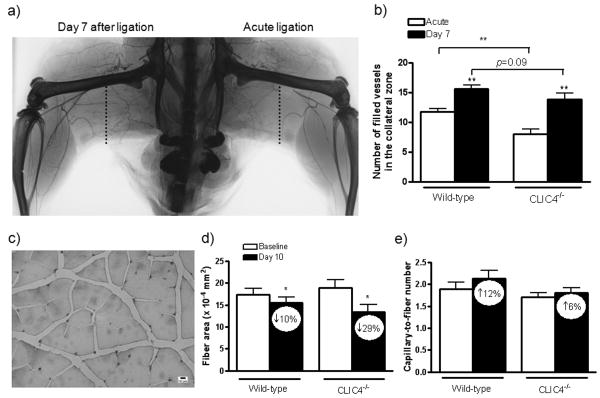
Micro-angiography was obtained in separate groups to determine whether Clic4-/- mice have fewer pre-existing collaterals. Microfil®, with a viscosity adjusted to impede capillary transit (8:1 latex-to-diluent), was infused (aorta) after acute ligation of the left femoral artery in mice that received right femoral ligation 7 days earlier. We then counted the number of arterial vessels crossing a line drawn through the center of the thigh collateral zone in x-ray angiograms (Figure 3a)22. Detection of both native and remodeled vessels (≥ 25 micron diameter resolution8,21,22) were reduced in Clic4-/- mice (Figure 3b). This agrees with the lower perfusion in the plantar and adductor regions immediately after femoral ligation in Clic4-/- (Figure 1c,d). Arteries in the calf of the acutely ligated limb had reduced filling, compared to the 7-day ligated limb, because upstream collaterals had not undergone remodeling (Fig 3a)22. These data suggest that absence of CLIC4 results in reduced density of native collaterals in skeletal muscle.
Figure 3.
Clic4-/- mice have fewer hindlimb collaterals. X-ray arteriogram (a) and averaged data (b) showing arterial vessels crossing the collateral zone (dotted line) of the posterior thigh after acute ligation (native collaterals) and 7 days after ligation (remodeled collaterals). c) Lectin staining for capillaries in non-ischemic (baseline) WT gastrocnemius muscle. d) Clic4-/- tended toward greater atrophy measured at day-10 after ligation. e) Capillary-to-muscle fiber number ratio tended toward less increase in Clic4-/-. Values within bars are percentage change from non-ligated limb (baseline). *,**,*** p<0.05, 0.01 vs. white bar or as bracketed; n=4 per bar (panel b) and n=10 (panels d,e).
Ischemia-induced angiogenesis in Clic4-/- mice
Although baseline collateral conductance and their subsequent enlargement primarily determine limb perfusion after ligation, reduced baseline capillary density and ischemic angiogenesis after ligation could also contribute, respectively, to the lower plantar and adductor perfusion immediately after ligation and subsequent recovery in Clic4-/- mice. The prior observation that CLIC4 deficiency results in reduced angiogenesis in vitro16,18 and in Matrigel plug and retinal neo-angiogenesis models in vivo17 would support this hypothesis. To address this possibility, we measured capillarity:muscle fiber ratio and fiber area/size in the gastrocnemius muscle of the left (non-ligated) and right leg ligated 10 days earlier (Figure 3c-e). The gastrocnemius experiences ischemic angiogenesis and atrophy after femoral ligation8,21,22. Muscle fiber size and the ratio of capillary-to-muscle fiber number before ligation (non-ligated limb; “baseline”) were similar in wild-type and Clic4-/- mice (Figure 3d,e). This strengthens the suggestion from the preceding analyses that lower pre-existing collateral number in Clic4-/- mice, rather than baseline capillary-to-fiber ratio (capillary density), accounts for the larger drop in perfusion immediately after ligation. Clic4-/- mice had greater atrophy and a smaller increase in capillary-to-fiber ratio in the ligated limb (Figure 3d,e), although the latter were not significant. Greater atrophy and less angiogenesis is consistent with the greater ischemia and use-impairment scores in Clic4-/- (Figure 2a,b) and may contribute, along with reduced collateral density, to the lower recovery of plantar perfusion in Clic4-/- mice (Figure 1c). The absence of a significant increase in capillary-to-fiber ratio in these CD-1 wild-type mice may reflect their smaller increase in VEGF induced by ischemia, compared to other strains such as C57BL/68, or the need to make measurements later than 10 days (eg, at 21 days8).
CLIC1 expression is upregulated during ischemia in Clic4-/- mice
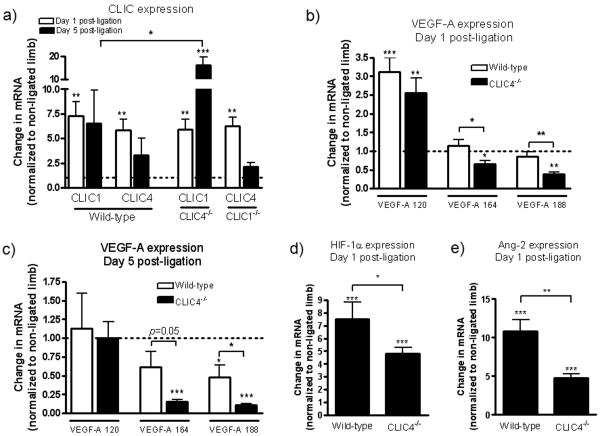
Clic1-/- and Clic4-/- mice have no apparent phenotype. In addition, CLIC4 is expressed in ECs17,18, while CLIC1 is expressed ubiqitously20. Thus, these proteins may provide functional redundancy or compensation. To examine this possibility, we compared expression of CLIC1 and CLIC4 in the calf muscle. CLIC1 and CLIC4 mRNA in the non-ligated limb (baseline) was comparable among wild-type, Clic4-/- and Clic1-/- (data not shown). CLIC1 and CLIC4 increased similarly (6-7 fold) one day after ligation in all three strains (Figure 4a). Five days after ligation expression of CLIC1 increased ∼15 fold in Clic4-/- mice. These data suggest that CLIC1 may provide partial compensation for deficient CLIC4 expression, and lessen the deficits we observed in Clic4-/- mice. However, CLIC1 appears to have little contribution when CLIC4 is intact, since CLIC4 expression in Clic1-/- mice was comparable to wild-type, in agreement with our failure to find deficiencies in Clic1-/- mice (Figures 1,2a,b).
Figure 4.
Expression (qRT-PCR) of CLIC1, CLIC4, VEGF-A isoforms, Hif-1α and angiopoietin-2 in calf muscle after femoral artery ligation. Data are fold-change from baseline (dotted line, non-ligated leg). *,**,*** p<0.05, 0.01, 0.001 vs. baseline or as bracketed; n=6 per bar.
HIF-1α, VEGF-A and angiopoietin-2 expression during ischemia are reduced in Clic4-/- mice
Besides its role in angiogenesis, VEGF is an important determinant of collateral formation in the embryo and neonate, and collateral remodeling in adult occlusive disease8. To determine if VEGF-A is affected by CLIC4 deletion, we examined levels of mRNA encoding VEGF-120, -164, and -188 in the calf. Basal expression in the non-ligated limb was similar in Clic4-/- and wild-type mice (data not shown). One day after ligation, VEGF-120 increased similarly in the ligated limb of wild-type and Clic4-/- mice and returned to control by 5 days, whereas the other two isoforms were reduced at both times in Clic4-/- (Figure 4b,c). These observations are consistent with the known importance of soluble VEGF-120 in ischemia23. More importantly, they suggest that CLIC4 may positively regulate high-molecular weight VEGF isoforms when their levels are augmented, eg, in ischemia and during embryonic growth. During the latter period, impaired CLIC4 expression could thus disturb VEGF gradients, resulting in the impaired collateral formation we observed.
Among several possible mechanisms for lower induction of VEGF in Clic4-/- mice, Hif-1α expression could be reduced. Indeed, increased Hif-1α in the ischemic calf of wild-type mice was reduced in Clic4-/- mice (Figure 4d). Moreover, Hif-1α and VEGF were lower in Clic4-/- mice despite their greater ischemia (Figures 1, 2a,b). Similarly, upregulation of angiopoietin-2 (like VEGF-A, regulated by HIF-1α) was reduced in Clic4-/- mice (Figure 4e). Thus, CLIC4 impacts upstream regulation of Hif-1α and its downstream targets, VEGF and angiopoietin-2.
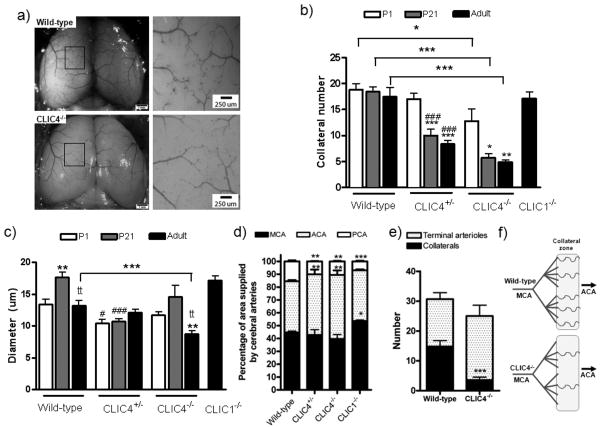
Collateral density and diameter in the cerebral circulation are reduced in Clic4-/-mice
The greater drop in perfusion immediately after ligation (Figure 1c,d), plus fewer arteries in the collateral zone detected by angiography in the acute and chronically ligated leg (Figure 3b), suggest that Clic4-/- mice have reduced numbers of pre-existing collaterals. To test this with higher-resolution methods and determine if it extends to other tissues, we examined the collateral circulation that interconnects the anterior cerebral artery (ACA) and middle cerebral artery (MCA) trees (Figure 5a). Cerebral cortical collaterals are confined to the pial surface and can thus be imaged in ∼2-dimension with high fidelity22. In wild-type mice, collateral number remained unchanged from postnatal day-1 (P1) through adulthood (12-16 weeks), whereas lumen diameter underwent modest restructuring over this interval (Figure 5b,c). Native collateral density and diameter were unaffected in adult Clic1-/- mice, in agreement with hindlimb data (Fig 1, 2a,b); thus Clic1-/- were not examined at postnatal time points. In contrast, Clic4-/- were born with a lower collateral density that further declined by P21 to that present in adults wherein diameter was also smaller. These data indicate that CLIC4 positively regulates pial collateral formation in the embryo and is important for stabilization of nascent collateral number and size after birth. Similar results were obtained for VEGF-hypomorphic mice8.
Figure 5.
Clic4-/- and Clic4-/+ mice have fewer collaterals of smaller diameter between the middle cerebral artery (MCA) and anterior cerebral artery (ACA) trees. a) Polyurethane-filled arteriograms of the pial circulation. b,c) Collateral number and diameter at P1 (postnatal day-1), P21 and adult (12-16 weeks of age). d) Percent of cortical surface area overlain by the MCA, ACA and posterior cerebral artery (PCA) trees. e) Number of terminal arterioles and collaterals. f) Cartoon illustrating data in panel 5e. *,**,*** p<0.05, 0.01, 0.001 vs. P1 or wild-type as bracketed (b,c,d) or wild-type collateral number (e); tt p<0.01 vs P21 (c), #, ## p<0.05, 0.01 vs wild-type (b,c); n=4-10 per bar.
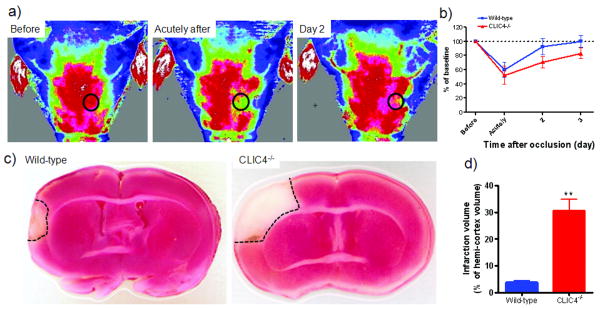
Clic4-/- mice suffer larger infarctions after middle cerebral artery (MCA) occlusion
To determine if the lower density and diameter of pial collaterals in adult Clic4-/- mice is functionally significant, infarct volume was assessed three days after MCA occlusion. We used our modified laser Doppler imager21 to assess the success of the occlusion surgery by non-invasively monitoring trans-cranial perfusion (Figure 6a,b). The signal derives from perfusion of the skin, bone and upper cortex. While the former two are not supplied by the cerebral arteries and thus could lessen detection of differences after occlusion, a similar ∼40% decrease in perfusion after occlusion in mice was obtained with a Doppler probe positioned over a sealed cranial window made in a similar location as our ROI24. Infarct volume was increased ∼4-fold in Clic4-/- mice (Figure 6c,d). Similar findings were obtained in VEGF hypomorphic mice8. Larger infarct volume in Clic4-/- is not due to an increase cortical territory supplied by the MCA. Area of the MCA tree was comparable in Clic4-/- and wild-type mice, whereas ACA area was increased and PCA area was decreased (Figure 5d). Cerebral artery territories were also different in Clic1-/- mice (see Discussion).
Figure 6.
Clic4-/- mice have larger cerebral infarctions after middle cerebral artery occlusion. a) Non-invasive, trans-cranial laser Doppler perfusion imaging to verify occlusion and recovery of perfusion (perfusion units as in figure 1). b) Perfusion averaged in the circled region of interest in panel a. c) Coronal slices 3 days after occlusion showing infarct area (dashed lines). d) Infarct volume. ** p<0.01; n=7-8 per time-point or bar.
Since MCA tree size trended smaller in Clic4+/- and Clic4-/- (Fig 5d), this could be accompanied by fewer distal branches and a corresponding fewer number of collaterals. However, Clic4-/- have ∼21% fewer distal-most branches abutting the collateral zone, yet ∼75% fewer collaterals (this results in a commensurate increase in “terminal arterioles”, ie, distal-most branches that do not give rise to a collateral) (Figure 5e,f). Hence, CLIC4's role in collaterogenesis is not simply secondary to its influence on tree structure. This is consistent with our other studies showing no relationship between MCA tree size and collateral abundance.32
Clic4+/-mice display collateral deficits intermediate to Clic4-/- mice
To investigate whether a gene dosage effect could be detected, we compared Clic4 heterozygotes to homozygous wild-type and Clic4-/-. Immediately after femoral artery ligation, perfusion in Clic4+/- mice declined to values in the plantar as low as—and in the adductor intermediate to—CLIC4-/- mice (Online Figure Ia,b). In the pial circulation, the number of nascent collaterals present at birth in Clic4+/- were comparable to wild-type mice, although diameter was smaller (Figure 5b,c). By 3-weeks postnatal (when the adult collateral density is established8), loss of pial collaterals was intermediate compared to Clic4-/- mice (Figure 5b). These and the hindlimb adductor data provide evidenced for a gene dose-effect of CLIC4 expression on postnatal maturation and thus collateral anatomy in the adult.
Discussion
Recently, we reported that collaterals form in the murine pial circulation between e13.5-e18.5 and undergo maturation and stabilization over the first several weeks after birth; moreover, these processes are strongly impaired in the BALB/c strain, compared to C57BL/625. Thus, “collaterogenesis” in the cerebral circulation occurs during the embryonic and early postnatal period, and is susceptible to genetic background. In the present study, CLIC4 disruption resulted in greater drop in hindlimb perfusion immediately after femoral artery ligation. Clic4-/- mice also showed less recovery of perfusion, in association with worse ischemia, use impairment and muscle atrophy—yet normal collateral remodeling. These data suggest Clic4-/- mice have fewer native collaterals. This was confirmed by arteriography, where we detected 40% fewer vessels crossing the collateral zone of the non-ligated leg in Clic4-/-, and a more dramatic 70% fewer native collaterals in the pial circulation that was associated with 4-fold larger infarctions after MCA occlusion. These findings demonstrate that CLIC4 is a major determinant of native collateral density in healthy skeletal muscle and brain. Because of technical limitations, we do not know if our findings in newborn brain predict the situation in newborn hindlimb.
We did not measure arterial pressure, because like other groups, perfusion ratio between the two hindlimbs was measured to minimize the effect of any potential difference. Moreover, CLIC4 is expressed predominantly in endothelial cells, with little or no signal in vascular smooth muscle cells or cardiomyocytes17, J Edwards, pers comm.. In addition, collateral wall thickness did not differ between wildtype and Clic4-/- (Figure 2d), arguing against the existence of a difference in pressure.
We also found that CLIC4 expression increased in ischemic muscle, plus evidence suggesting CLIC4 promotes HIF-1α—VEGF signaling, based on reduced Hif-1α and VEGF expression in Clic4-/- mice despite their greater ischemia. Given VEGF's role as a positive regulator of density and diameter of native collaterals8, these findings provide a potential hypothesis for why CLIC4 deficiency leads to impaired collaterogenesis: reduced or absent CLIC4 results in lower VEGF expression during conditions when VEGF expression is induced rather than constitutive (eg, during perinatal development and ischemia). Interestingly, although VEGF regulates both collaterogenesis and collateral remodeling8, no deficiency in remodeling was observed in Clic4-/- mice after femoral ligation. One possibility, in addition to potential compensation by other factors, is that the increase in VEGF around enlarging collaterals8 is not diminished enough in CLIC4-/- mice to impair remodeling.
Significant variation in collateral conductance in healthy tissues and collateral remodeling in ischemic disease exists among humans and species2,5,26-29. We previously reported that BALB/c mice have markedly fewer native collaterals in the hindlimb (as did Helisch et al30), brain and intestine, less remodeling in ischemia, and less induction of VEGF in association with a putative polymorphism in the Vegfa gene22. Herein, CLIC4 reduction resulted in fewer native collaterals in skeletal muscle and cerebral cortex, with a suggested dose-dependency in the latter. We also found a similar gene dose-effect of VEGF on native collateral density8. Thus, CLIC4 and VEGF are the first proteins that regulate native collateral formation. We speculate that polymorphisms in CLIC4, VEGF and other genes contribute to variation in native collateral density.
Our present and previous8 findings demonstrate that the cerebral collateral density present at P21 in CD-1, C57BL/6, BALB/c and VEGF hypermorphic mice, is the same density present in adults. However, VEGF hypomorphic mice8—and in the present study—CLIC4 deficient mice, are born with fewer collaterals than wild-type mice, and a significant percentage are lost by the third postnatal week. Approximately 40 and 60 percent of the collaterals present at birth were lost by P21 in Clic4+/- and Clic4-/- mice, respectively. Thus, in addition to formation of collaterals during embryogenesis, VEGF and CLIC4 are also involved in early post-natal stabilization of nascent collaterals when the adult collateral density is established. It is possible that factors regulating the density or branching of the primary embryonic plexus and/or mechanisms regulating branching morphogenesis and hemodynamic remodeling of it into the mature artery trees, participate in collateral formation. Pathways induced by VEGF play critical roles in each of these embryonic processes9-12. Interestingly, CLIC4 has been shown to mediate endothelial cell tubulation16,18, angiogenesis17 and in establishing the density and branch patterning of the developing retinal circulation17. Thus, VEGF and CLIC4 share actions on endothelial cells that may underlie their common effects on collaterogenesis.
Although how VEGF and CLIC4 interact are important unanswered questions, our results offer some possible starting points. In wild-type mice, femoral ligation increased CLIC4 and CLIC1 expression, together with expected increases in VEGF and Hif-1α. Interestingly, expression of the high (164, 188) but not low (120) molecular weight VEGF isoforms was reduced in Clic4-/- mice, suggesting CLIC4 may differentially regulate VEGF isoform expression. We recently found that collateral growth is associated with induction of the high molecular weight isoforms—a pattern opposite to that occurring in ischemic angiogenesis8. Induction of Hif-1α and angiopoietin-2 after ligation were also lower in Clic4-/- mice despite their greater ischemia; both VEGF and angiopoietin-2 are well known to be regulated by Hif-1α. These findings suggest that CLIC4 levels may impact transcriptional events upstream of HIF-1α—VEGF—angiopoietin-2 signaling. Although additional studies will be required to investigate these speculations and the mechanism for CLIC4 (and CLIC1) increase in ischemic calf, CLIC4 has been reported to undergo nuclear translocation and bind transcription factors20, and when induced by TGF-β1 to cause conversion of fibroblasts to myofibroblasts31. In addition, 6 CGTG sequences that comprise the core sequence of the Hif-1α binding site are present in 2Kb of sequence 5′ to the CLIC4 start site, with two of these being ACGTG and one of them (-1201) having 3′ flanking G-C repeats (Transcriptional Regulatory Element Database, http://rulai.cshl.edu/ TRED/).
Unlike CLIC4, we found no evidence that CLIC1 is required for collaterogenesis. However, Clic1-/- mice had a larger MCA tree and smaller ACA tree, compared to wild-type (Figure 5d). This suggests that CLIC1 has a role in pre- or post-natal growth of these trees. The MCA tree was smaller in CLIC4+/- and trended smaller yet in Clic4-/- mice, while the ACA trees were proportionately larger and PCA trees smaller. Although the smaller MCA trees in Clic4+/- and Clic4-/- mice mirror the successively smaller collateral number interconnecting them to the ACA trees, a comparison of 19 mouse strains found no correlation between collateral number and size of any of the cerebral artery trees32. This indicates that the number of collaterals that form is not simply a result of tree size (which is specified in the embryo25). Native collateral number in skeletal muscle was also reduced in Clic4-/- mice (Figures 1 and 3B), but unlike in the pia, diameter was unaffected, at least in the gracilis muscles (Figure 2c). Our previous studies found that differences in collateral density in pial, hindlimb and intestinal circulations, arising from strain differences or targeted mutation of VEGF, agree qualitatively; however differences in the pial circulation are much larger than hindlimb and intestine beds22. Likewise, in the present study, deficits in native collaterals in Clic4+/- mice (Figure 5) appeared greater in the pia than in the hindlimb (Online Figure I). Thus, we did not examine Clic4 hemizygotes for differences in recovery of perfusion, etc (Figs 1-3) after femoral ligation.
Native pial collateral density and diameter and response to hindlimb ligation were unaltered in Clic1-/- mice, suggesting that CLIC1 is not required for collaterogenesis or collateral remodeling. However, both CLIC4 and CLIC1 increased 7-fold in ischemic calf of wild-type mice, and CLIC1 increased double this in Clic4-/- mice. This suggests that CLIC1 could compensate for deficient CLIC4 during collateral formation, stabilization and remodeling. Thus, greater deficits in the former processes—and a frank deficit in collateral remodeling—might be observed when expression of both genes is reduced. The CLIC family of proteins consists of multiple highly homologous members20. CLIC1 and CLIC4 are expressed in virtually all tissues20, whereas CLIC2 is found in fetal liver and skeletal muscle20, and CLIC3 is expressed in placental and fetal membranes33. Thus, it is possible that both CLIC1 and CLIC4 are involved in collateral remodeling and are able to compensate for the loss of the other, or that both do not participate in this process. Additional approaches, eg, double knockout (if viable), cell-specific or conditional knockout mice, will be required to address the possibility of compensation.
In conclusion, our findings suggest that CLIC4 contributes to formation and stabilization of collaterals during vascular development and thus native collateral density in adults. These effects may be due in part to its influence on HIF-1α--VEGF signaling which is critical in formation of the general (ie, arterio-venous)11,12 and collateral8 circulations. Identification of how these and other factors interact to regulate collaterogenesis is needed to begin to understand the basis for the wide variance in collateral capacity in healthy humans and determine if therapeutic strategies can be devised to induce formation of new collaterals in individuals that have, or are prone to, occlusive vascular disease.
Acknowledgments
We thank Kirk McNaughton and Carolyn Suitt for histology, and Nicarter Gordon for maintaining mice and measuring capillarity.
Sources of Funding: Support: NIH-HL62584 (JEF), NIH-DK060551 (JCE), and F32-HL080847 (DC).
Footnotes
Disclosures: None
References
- 1.Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49:507–521. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 2.Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries. Microcirculation. 2003;10:83–97. doi: 10.1038/sj.mn.7800173. [DOI] [PubMed] [Google Scholar]
- 3.Sherman JA, Hall A, Malenka DJ, De Muinck ED, Simons M. Humoral and cellular factors responsible for coronary collateral formation. Am J Cardiol. 2006;98:1194–1197. doi: 10.1016/j.amjcard.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 4.Schaper W. Collateral circulation: past and present. Basic Res Cardiol. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wustmann K, Zbinden S, Windecker S, Meier B, Seiler C. Is there functional collateral flow during vascular occlusion in angiographically normal coronary arteries? Circulation. 2003;107:2213–2220. doi: 10.1161/01.CIR.0000066321.03474.DA. [DOI] [PubMed] [Google Scholar]
- 6.Meier P, Gloekler S, Zbinden R, Beckh S, de Marchi SF, Zbinden S, Wustmann K, Billinger M, Vogel R, Cook S, Wenaweser P, Togni M, Windecker S, Meier B, Seiler C. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116:975–983. doi: 10.1161/CIRCULATIONAHA.107.703959. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez B. Development of Collateral Arteries. In: Schaper W, Schaper J, editors. Arteriogenesis. Kluwer Academic; Boston: 2004. [Google Scholar]
- 8.Clayton JA, Chalothorn D, Faber JE. Vascular Endothelial Growth Factor-A Specifies Formation of Native Collaterals and Regulates Collateral Growth in Ischemia. Circ Res. 2008;103:1027–1036. doi: 10.1161/CIRCRESAHA.108.181115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:268426–268498. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kearney JB, Kappas NC, Ellerstrom C, DiPaola FW, Bautch VL. The VEGF receptor flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood. 2004;103:4527–4535. doi: 10.1182/blood-2003-07-2315. [DOI] [PubMed] [Google Scholar]
- 11.Risau RW, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 12.Flamme I, Frolich T, Risau W. Molecular mechanisms of vasculogenesis and embryonic angiogenesis. J Cell Physiol. 1997;173:206–210. doi: 10.1002/(SICI)1097-4652(199711)173:2<206::AID-JCP22>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 13.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 14.Fernández-Salas E, Sagar M, Cheng C, Yuspa SH, Weinberg WC. p53 and tumor necrosis factor alpha regulate the expression of a mitochondrial chloride channel protein. J Biol Chem. 1999;274:36488–36497. doi: 10.1074/jbc.274.51.36488. [DOI] [PubMed] [Google Scholar]
- 15.Suh KS, Mutoh M, Gerdes M, Yuspa SH. CLIC4, an intracellular chloride channel protein, is a novel molecular target for cancer therapy. J Investig Dermatol Symp Proc. 2005;10:105–109. doi: 10.1111/j.1087-0024.2005.200402.x. [DOI] [PubMed] [Google Scholar]
- 16.Bohman S, Matsumoto T, Suh K, Dimberg A, Jakobsson L, Yuspa S, Claesson-Welsh L. Proteomic analysis of vascular endothelial growth factor-induced endothelial cell differentiation reveals a role for chloride intracellular channel 4 (CLIC4) in tubular morphogenesis. J Biol Chem. 2005;280:42397–42404. doi: 10.1074/jbc.M506724200. [DOI] [PubMed] [Google Scholar]
- 17.Ulmasov B, Bruno J, Gordon N, Hartnett ME, Edwards JC. CLIC4 functions in angiogenesis by supporting acidification of vacuoles along the intracellular tubulogenic pathway. Am J Path. 2009;174:1084–1096. doi: 10.2353/ajpath.2009.080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry KL, Bulow HE, Hall DH, Hobert O. A C. elegans CLIC-like protein required for intracellular tube formation and maintenance. Science. 2003;302:2134–2137. doi: 10.1126/science.1087667. [DOI] [PubMed] [Google Scholar]
- 19.Ashley RH. Challenging accepted ion channel biology: p64 and the CLIC family of putative intracellular anion channel proteins. Mol Membr Biol. 2003;20:1–11. doi: 10.1080/09687680210042746. [DOI] [PubMed] [Google Scholar]
- 20.Suh KS, Malik M, Shukla A, Yuspa SH. CLIC4, skin homeostasis and cutaneous cancer: surprising connections. Mol Carcinog. 2007;46:599–604. doi: 10.1002/mc.20324. [DOI] [PubMed] [Google Scholar]
- 21.Chalothorn D, Zhang H, Clayton JA, Thomas SA, Faber JE. Catecholamines augment collateral vessel growth and angiogenesis in hindlimb ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H947–H959. doi: 10.1152/ajpheart.00952.2004. [DOI] [PubMed] [Google Scholar]
- 22.Chalothorn D, Clayton JA, Zhang H, Pomp D, Faber JE. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics. 2007;30:179–191. doi: 10.1152/physiolgenomics.00047.2007. [DOI] [PubMed] [Google Scholar]
- 23.Hattori K, Heissig B, Wu Y, Dias S, Tejada R, Ferris B, Hicklin DJ, Zhu Z, Bohlen P, Witte L, Hendrikx J, Hackett NR, Crystal RG, Moore MA, Werb Z, Lyden D, Rafii S. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1 (+) stem cells from bone-marrow microenvironment. Nat Med. 2002;8:841–849. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitagawa K, Yagita Y, Sasaki T, Sugiura S, Omura-Matsuoka E, Mabuchi T, Matsushita K, Hori M. Chronic mild reduction of cerebral perfusion pressure induces ischemic tolerance in focal cerebral ischemia. Stroke. 2005;36:2270–2274. doi: 10.1161/01.STR.0000181075.77897.0e. [DOI] [PubMed] [Google Scholar]
- 25.Chalothorn D, Faber JE. Differences in collateral formation in the embryo are associated with genetic variation in patterning and maturation of the cerebral cortical circulation. Atheroscler Thromb Vasc Biol. 2009 June;:P250. in press. [Google Scholar]
- 26.Chittenden TW, Sherman JA, Xiong F, Hall AE, Lanahan AA, Taylor JM, Duan H, Pearlman JD, Moore JH, Schwartz SM, Simons M. Transcriptional profiling in coronary artery disease: indications for novel markers of coronary collateralization. Circulation. 2006;114:1811–1820. doi: 10.1161/CIRCULATIONAHA.106.628396. [DOI] [PubMed] [Google Scholar]
- 27.Schirmer SH, Fledderus JO, Bot PT, Moerland PD, Hoefer IE, Baan J, Jr, Henriques JP, van der Schaaf RJ, Vis MM, Horrevoets AJ, Piek JJ, van Royen N. Interferon-beta signaling is enhanced in patients with insufficient coronary collateral artery development and inhibits arteriogenesis in mice. Circ Res. 2008;102:1286–1294. doi: 10.1161/CIRCRESAHA.108.171827. [DOI] [PubMed] [Google Scholar]
- 28.Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, Schaper W. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34:775–7787. doi: 10.1006/jmcc.2002.2013. [DOI] [PubMed] [Google Scholar]
- 29.Fukino K, Sata M, Seko Y, Hirata Y, Nagai R. Genetic background influences therapeutic effectiveness of VEGF. Biochem Biophys Res Commun. 2003;310:143–147. doi: 10.1016/j.bbrc.2003.08.134. [DOI] [PubMed] [Google Scholar]
- 30.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. 2006;26:520–526. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- 31.Rønnov-Jessen L, Villadsen R, Edwards JC, Petersen OW. Differential expression of a chloride intracellular channel gene, CLIC4, in transforming growth factor-beta1-mediated conversion of fibroblasts to myofibroblasts. Am J Pathol. 2002;161:471–480. doi: 10.1016/s0002-9440(10)64203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Keum S, Marchuk D, Sealock R, Faber JE. Analysis of 19 mouse strains demonstrates that genetic variations in pial collateral dimensions are the major determinants of severity of infarct volume after middle cerebral artery occlusion (MCAO) Cardiovasc Revasc Med J. 2009;10(3) in press. [Google Scholar]
- 33.Money TT, King RG, Wong MH, Stevenson JL, Kalionis B, Erwich JJ, Huisman MA, Timmer A, Hiden U, Desoye G, Gude NM. Expression and cellular localization of chloride intracellular channel 3 in human placenta and fetal membranes. Placenta. 2007;28:429–436. doi: 10.1016/j.placenta.2006.08.002. [DOI] [PubMed] [Google Scholar]