Abstract
Osteopontin (OPN) is highly expressed in cancer patients and plays important roles in many stages of tumor progression, such as anti-apoptosis, proliferation, and metastasis. From functional screening of human cDNA library, we isolated OPN as a caspase-8 substrate that regulates cell death during hypoxia/reoxygenation (Hyp/RO). In vitro cleavage assays demonstrate that OPN is cleaved at Asp-135 and Asp-157 by caspase-8. Cellular cleavage of OPN is observed in apoptotic cells exposed to Hyp/RO among various apoptotic stimuli and its cleavage is blocked by zVAD or IETD caspase inhibitor. Further, over-expression of OPN, the form with secretion signal, inhibits Hyp/RO-induced cell death. Caspase cleavage-defective OPN mutant (OPN D135A/D157A) is more efficient to suppress Hyp/RO-induced cell death than wild-type OPN. OPN D135A/D157A sustains AKT activity to increase cell viability through inhibition of caspase-9 during Hyp/RO. In addition, OPN is highly induced in some tumor cells during Hyp/RO, such as HeLa and Huh-7 cells, which is associated with their resistance to Hyp/RO by sustaining AKT activity. Notably, OPN C-terminal cleavage fragment produced by caspase-8 is detected in the nucleus. Plasmid-encoded expression of OPN C-terminal cleavage fragment increases p53 protein level and induces apoptosis of wild-type mouse embryonic fibroblast cells, but not p53−/− mouse embryonic fibroblast cells. These observations suggest that the protective function of OPN during Hyp/RO is inactivated via the proteolytic cleavage by caspase-8 and its cleavage product subsequently induces cell death via p53, postulating caspase-8 as a negative regulator of tumorigenic activity of OPN.
Osteopontin (OPN) is a secreted glycosylated phosphoprotein that is involved in a number of physiological events including bone formation and remodeling (1), immune responses (2, 3), and tumor progression, such as cell proliferation, angiogenesis, metastasis, and anti-apoptosis (4). Especially, OPN is highly up-regulated in cancer patients' plasma, thus it is considered a candidate as a prognostic marker for human cancer diagnosis (4). Multiple cancer-related functions of OPN are mediated by its interaction with integrins or CD44 variants as a cytokine. Generally, secreted OPN acts as an intact protein or fragments cleaved by thrombin; Arg-Gly-Asp (RGD) motif in OPN interacts with integrins (αvβ3, αvβ5) and C-terminal region of OPN binds to CD44 variants, which subsequently activates a PI3K-AKT, NIK, or MEKK1 kinase cascade (4, 5). In addition, alternative isoform of OPN is found in cytosol (6) and OPN is detected as a CD44-ERM complex in the cytosolic side of CD44 (7). Further, OPN also associates with polo-like kinase-1 in the nucleus during cell cycle (8). These observations show diverse roles and subcellular localizations of OPN.
OPN is also highly induced during hypoxia/reoxygenation (Hyp/RO), which is closely related to pathological conditions including myocardial ischemia/reperfusion injury, stroke, inflammation, and solid tumors (9, 10). During Hyp/RO, cell death generally occurs after massive generation of reactive oxygen species (ROS) and caspases activation. Several caspases including caspases-8, -9, and -3 were reported to be activated during reoxygenation, which is required for Hyp/RO-induced cell death (11, 12). Among these caspases, caspase-8 is a well known receptor-proximal caspase. However, accumulating evidence suggests atypical roles of caspase-8 in nonreceptor-mediated cell deaths (13, 14) and NF-κB activation (15). In addition, caspase-8 deficiency is also detected in human cancers (16, 17) and facilitates cellular transfomation (18), showing critical functions of caspase-8 in tumorigenesis and cell death. In the group of hundreds' cellular substrates of various caspases, only a few proteins, such as Bid, p28 Bap31, RIP-1, and plectin, are reported as caspase-8 substrates (19–22).
In this study, we performed genome-wide screening and isolated OPN as a caspase-8 substrate. OPN expression is rapidly increased during Hyp/RO and subsequently cleaved by caspase-8, leading to both inactivation of AKT survival signal and activation of cell death signal via its caspase cleavage fragment in tumor cells.
Results
OPN Is Cleaved by Caspase-8 in Vitro and in Apoptotic Cells During Hyp/RO.
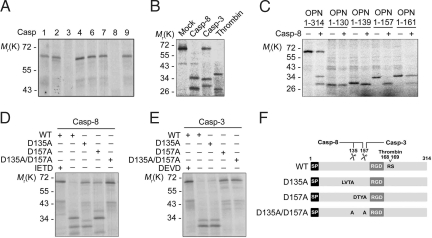
To unearth caspase substrates, we undertook caspase substrate screening using human cDNA library. Small cDNA pools were transcribed and translated in vitro in the presence of [35S]methionine and then incubated with recombinant caspases (23). From this analysis, we isolated OPN as a putative substrate of caspase-8. To characterize the cleavage, in vitro translated OPN was incubated with various recombinant active caspases (caspase-1, -2, -3, -4, -6, -7, -8, or -9) (Fig. 1A). Among these caspases, caspases-3 and -8 were able to cleave OPN, yielding two cleavage products with distinct molecular weights, which are also different from the previously known thrombin cleavage products (Fig. 1B and Fig. S1A).
Fig. 1.
OPN is cleaved at Asp-135 and Asp-157 by caspase-8 in vitro. (A and B) In vitro cleavage assay using caspases. OPN translated in the presence of [35S]methionine was incubated for 60 min with 10 ng of each purified caspase (Casp-1, -2, -3, -4, -6, -7, -8, or -9) (A) or with caspase-3, -8, or thrombin (B). The reaction mixtures were analyzed with autoradiography after SDS/PAGE. (C–E) Mapping of OPN cleavage sites. OPN deletion mutants containing N-terminal region (OPN 1–130, OPN 1–139, OPN 1–157, and OPN 1–161) (C) and OPN mutants replacing Asp with Ala at the indicated position (OPN D135A, OPN D157A, and OPN D135A/D157A) (D and E) were incubated with caspase-8 (C and D) or caspase-3 (E) for in vitro cleavage assay. (F) Respective cleavage sites for caspases-8 and -3 are indicated.
To determine the cleavage sites of OPN, we generated series of OPN-deletion mutants that mimic the fragments cleaved after Asp residue by caspases. We found that all OPN mutants except OPN 1–130 (127DESD130), including OPN 1–139 (136FPTD139), OPN 1–157 (154DTYD157), and OPN 1–161 (158GRGD161), were cleaved by caspase-8 in vitro (Fig. 1C). The OPN cleavage products migrated with same sizes as OPN 1–135 (132LVTD135) and OPN 136–314 (Fig. S1B). Further analysis of OPN mutants replacing Asp-135 (OPN D135A) or Asp-157 (OPN D157A) with Ala showed that these mutants were still partially cleaved by caspase-8 to generate cleavage products of ≈32 kDa and 34 kDa (Fig. 1D). On the contrary, OPN D135A/D157A mutant (OPN DM) was completely resistant to caspase-8. Unlike caspase-8, caspase-3 failed to cleave OPN D157A (Fig. 1E). These results suggest that caspase-8 cleaves OPN after both Asp-135 and Asp-157, and caspase-3 cleaves OPN after Asp-157 (Fig. 1F).
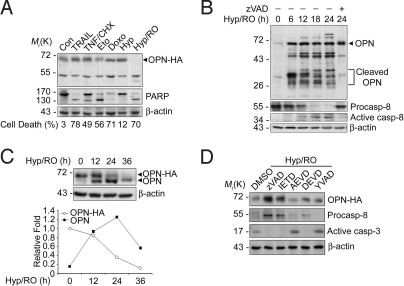
Next, cellular cleavage of OPN was first examined in HeLa cells stably expressing HA-tagged OPN (HeLa/OPN) (Fig. S2A). Exposure to various cell death stimuli induced significant amounts of cell death (Fig. 2A). Although OPN-HA was not significantly degraded in cells exposed to most of apoptotic signals, such as receptor signals (TNF-α and TRAIL) or nonreceptor signals (etoposide and doxorubicin), it was completely disappeared in dying cells after exposure to Hyp/RO (Fig. 2A).
Fig. 2.
OPN is cleaved by caspase-8 in apoptotic cells during Hyp/RO. (A) Cellular cleavage of plasmid-encoded OPN-HA during Hyp/RO. HeLa/OPN stable cells were left untreated (Con) or exposed to 100 ng/mL TRAIL for 6 h, 20 ng/mL TNF-α with 0.5 μg/mL cycloheximide (TNF/CHX) for 12 h, 100 μM etoposide (Eto) for 24 h, 2 μg/mL doxorubicin (Doxo) for 24 h, hypoxia (5% O2) for 12 h (Hyp), or hypoxia for 12 h/reoxygenation for 36 h (Hyp/RO). After determining percentages of cell death as described in Materials and Methods, cell extracts were prepared and subjected to Western blotting analysis using anti-HA, anti-PARP, and anti-β-actin antibodies. (B) Cellular cleavage of genome-encoded OPN during Hyp/RO. HeLa cells were preincubated with 1 μM MG132 and then exposed to Hyp (12 h)/RO for the indicated times in the presence or absence of 25 μM zVAD-fmk. Cell extracts were then analyzed with Western blotting using anti-OPN (LF123), anti-caspase-8, and anti-β-actin antibodies. (C) Quantitative comparison of OPN cleavage during Hyp/RO. HeLa/OPN cells were exposed to Hyp (12 h)/RO for the indicated times and Western blot analysis was performed using anti-OPN antibody (Upper). The signals of genome-encoded OPN and HA-tagged OPN on the Western blot analysis were quantitated by densitometric analysis (Lower). (D) Effects of caspase inhibitors on OPN cleavage. HeLa/OPN cells were pretreated for 3 h with DMSO, zVAD-fmk, IETD-fmk, AEVD-fmk, DEVD-fmk, or YVAD-fmk (25 μM) and then exposed to hypoxia for 12 h. After reoxygenation for another 24 h, cells were analyzed by Western blotting using the indicated antibodies.
We then addressed the cleavage of genome-encoded OPN in HeLa cells during Hyp/RO. Genome-encoded OPN was not easily detected under normoxia condition but was highly accumulated in HeLa cells exposed to Hyp/RO (Fig. 2B and Fig. S2B). This accumulation was contributed at least by the increase of OPN mRNA (Fig. S2C, additional details in SI Text). We found that OPN was cleaved to generate multiple cleavage products in the presence of MG132, a proteasome inhibitor. The cleavage products with molecular mass ranging from 28 kDa to 36 kDa were first detected at 6 h (Fig. 2B). The degradation patterns of plasmid-encoded (OPN-HA) and genome-encoded OPN in HeLa/OPN cells were then compared. Whereas the level of OPN-HA gradually decreased during Hyp/RO, genome-encoded OPN first increased up to 24 h and then decreased at 36 h (Fig. 2C). Consistently, we observed the proteolytic cleavage of PARP and activation of caspase-8 during Hyp/RO (Fig. 2A and B) (11). PARP was used as a marker of apoptosis after caspase-3 activation. Proform of caspase-8 began to be processed for activation as early as at 6 h after reoxygenation (Fig. 2B). The cleavages of OPN and processing of caspase-8 were all inhibited by zVAD-fmk, a pan-caspase inhibitor, or IETD-fmk, a caspase-8 inhibitor (Fig. 2D). These results indicate that caspase-8 cleaves OPN during Hyp/RO.
OPN D135A/D157A Suppresses Hyp/RO-Induced Cell Death.
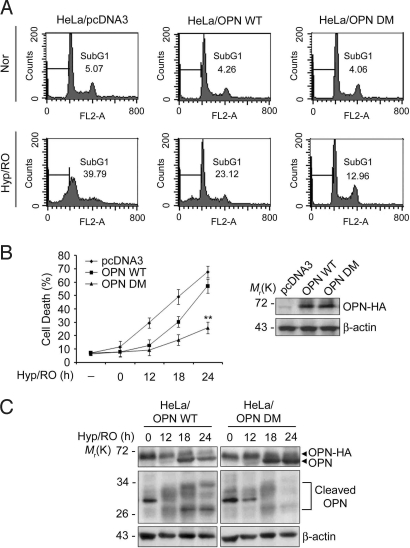
We then extensively investigated the effects of OPN cleavage on cell death during Hyp/RO. From FACS analysis, we found that compared with HeLa/pcDNA3 cells (SubG1 population: 40%), Hyp/RO-induced cell death was weakly inhibited in HeLa/OPN WT stable cells (23%) and strongly inhibited in HeLa/OPN DM stable cells (13%) (Fig. 3A). Similar results were obtained with the transient expression analysis of OPN WT and OPN DM. Plasmid-encoded expression of OPN WT in HeLa cells inhibited Hyp/RO-induced cell death at early time points (12 h and 18 h) but such inhibitory effect was reduced at late time point (24 h). On the contrary, OPN DM was very potent to inhibit Hyp/RO-induced cell death up to 24 h (Fig. 3B). Consistently, Western blot analysis showed that compared with HeLa/OPN WT cells, the cleavage products of OPN were less or not detected at 24 h in HeLa/OPN DM cells during Hyp/RO (Fig. 3C). In SK Hep-1 cells, OPN DM was also resistant to degradation during Hyp/RO (Fig. S3A). These results suggest that the cleavages of OPN at Asp-135 and Asp-157 are associated for efficient incidence of Hyp/RO-induced cell death and the amount of OPN is critical to regulate cell death during Hyp/RO.
Fig. 3.
Cleavage-resistant OPN mutant, OPN D135A/D157A, suppresses Hyp/RO-induced cell death. (A) Suppression of Hyp/RO-induced cell death by OPN WT and OPN DM. HeLa/pcDNA3, HeLa/OPN WT, and HeLa/OPN DM stable cells were exposed to Hyp (12 h)/RO (18 h). Cells were then analyzed by FACS and the population of SubG1 cells (line) is indicated. (B) Cleavage-defective OPN DM suppresses Hyp/RO-induced cell death. HeLa cells were transfected with pcDNA3, pOPN WT, or pOPN DM expression vector and then left untreated or exposed to Hyp (12 h)/RO for the indicated times. Cell death was determined as in Materials and Methods (Left). Values indicate mean values ± SD; (n = 3). **, P < 0.001. Expression level of plasmid-encoded OPN-HA was examined with Western blot analysis using anti-HA antibody (Right). (C) OPN DM is more tolerant to degradation than OPN during Hyp/RO. HeLa/OPN WT and HeLa/OPN DM cells were exposed to Hyp (12 h)/RO for the indicated times in the presence of 1 μM MG132 and cell extracts were analyzed with Western blotting using anti-OPN antibody.
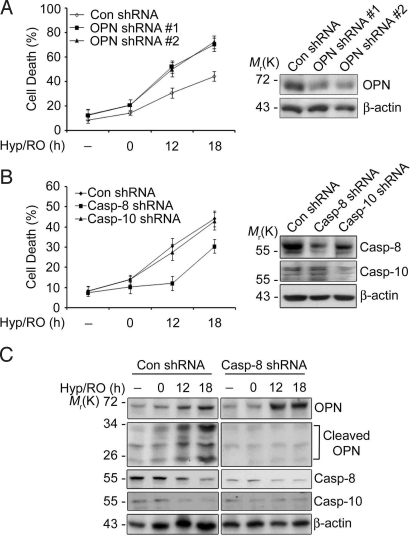
As it exhibits survival activity (4, 24), plasmid-encoded expression of OPN was effective to inhibit cell death induced by Hyp/RO (50% to 30%) but little effective to reduce cell death by TRAIL or TNF-α (Fig. S3B). Conversely, down-regulation of OPN expression using shRNAs sensitized HeLa cells to Hyp/RO-induced cell death (Fig. 4A). Furthermore, reduced expression of caspase-8, but not caspase-10, suppressed Hyp/RO-induced cell death (Fig. 4B) and abolished the generation of OPN cleavage products (Fig. 4C). In addition, the level of OPN was elevated at 12 h and 18 h of Hyp/RO in caspase-8 knockdown cells compared with HeLa control cells. These results further support our proposal that OPN is cleaved by caspase-8 to regulate cell death during Hyp/RO.
Fig. 4.
Reduced expression of caspase-8 increases OPN level and suppresses cell death during Hyp/RO. (A) Knockdown of OPN level sensitizes cells to death during Hyp/RO. HeLa cells were transfected with pSuper (Con), pOPN shRNA #1, or #2 for 36 h and then exposed to Hyp (12 h)/RO for the indicated times. Cell death was assessed as in Materials and Methods (Left). Cell extracts were prepared at 18 h of Hyp/RO and analyzed by Western blotting using anti-OPN antibody (Right). (B) Knockdown of caspase-8 expression desensitizes cells to Hyp/RO. HeLa cells were transfected with pSuper, pcasp-8 shRNA or pcasp-10 shRNA for 36 h and then exposed to Hyp (12 h)/RO for the indicated times. Cell death was analyzed as described in Materials and Methods with mean values ± SD. (A and B, n = 3) (Left). Expression of caspase-8 or -10 was examined by Western blotting (Right). (C) Knockdown of caspase-8 expression inhibits OPN cleavage during Hyp/RO. HeLa cells were transfected with pSuper (Con) or pcasp-8 shRNA for 36 h, exposed to Hyp (12 h)/RO for the indicated times, and analyzed with Western blotting.
Cleavage-Defective OPN Mutant Sustains AKT Activation During Hyp/RO.
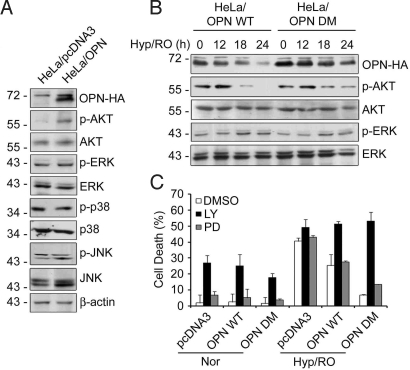
To define the downstream signaling of OPN, we investigated roles of stress kinases during Hyp/RO. Compared with HeLa control cells, we observed the increased levels of phosphorylation of AKT at Ser-473 (pAKT); in contrast, there was no detectable increase in phosphorylation of ERK, JNK, or p38, in HeLa/OPN WT cells (Fig. 5A), consistent with the previous reports (25, 26). We then examined and compared the activation profiles of AKT in HeLa/OPN WT and HeLa/OPN DM cells. During Hyp/RO, the level of pAKT increased up to 12 h and then declined in HeLa/OPN WT cells. However, the level of pAKT remained still high at late time points (18 h and 24 h) in HeLa/OPN DM cells (Fig. 5B). These results indicate that AKT activation may be regulated along with the cleavage of OPN by caspase-8 during Hyp/RO.
Fig. 5.
OPN D135A/D157A sustains AKT activation for cell survival during Hyp/RO. (A) Increase of p-AKT level by OPN. HeLa/pcDNA3 and HeLa/OPN cell extracts were analyzed with Western blotting using the indicated antibodies. (B) Sustained level of pAKT in HeLa/OPN DM cells during Hyp/RO. HeLa/OPN WT and HeLa/OPN DM cells were left untreated or exposed to Hyp/RO for the indicated times. Cell extracts were examined with Western blot analyses. (C) LY294002 potently sensitizes HeLa/OPN DM cells to Hyp/RO. HeLa/pcDNA3, HeLa/OPN WT, and HeLa/OPN DM cells were left untreated (Nor) or exposed to Hyp (12 h)/RO (20 h) in the presence of LY294002 (40 μM) or PD98059 (40 μM). Cell death assay was performed as in Materials and Methods. Bars, mean values ± SD; (n = 3).
Next, we examined whether OPN-dependent activation of AKT regulates Hyp/RO-induced cell death. Although pretreatment with LY294002, an inhibitor of PI3K-AKT, induced certain amounts of cell death in all cell lines tested during normoxia, it abolished anti-apoptotic function of OPN and effectively sensitized HeLa/OPN DM cells to Hyp/RO-induced cell death (Fig. 5C). Thus, HeLa/OPN DM and HeLa/OPN WT cells became as sensitive to Hyp/RO as HeLa control cells after LY294002 treatment. However, PD98059, an inhibitor of MEK1/2 and ERK1/2, did not show any significant effects on Hyp/RO-induced cell death (Fig. 5C). The inhibitory effects of LY294002 and PD98059 on AKT and ERK, respectively, were confirmed by Western blot analysis (Fig. S4). These results indicate that caspase-mediated degradation of OPN and its induction is critical to balance OPN level and to regulate AKT activity for the protection of cells against Hyp/RO.
OPN Induction in Tumor Cells Confers Resistance to Hyp/RO-Induced Cell Death.
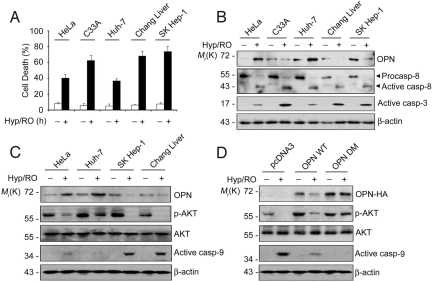
Given that OPN is critical to regulate cell death, we addressed pathophysiological relevance of OPN level in tumorigenesis. We examined sensitivities of various tumor cells to Hyp/RO, such as C33A, Huh-7, Chang liver, SK Hep-1, and HeLa cells. Among these cells, HeLa and Huh-7 cells were more resistant to Hyp/RO-induced cell death than other cells (Fig. 6A). Interestingly, although basal levels of OPN in HeLa and Huh-7 cells were lower than or equivalent to other cells, OPN was highly induced by 3- to 5-fold in HeLa and Huh-7 cells during Hyp/RO (Fig. 6B). Expression and activation of caspase-8 were not that much different in these cells. On the contrary, proteolytic activation of caspase-3 was lower in HeLa and Huh-7 cells than other cells during Hyp/RO (Fig. 6B) and pAKT level was higher in HeLa and Huh-7 cells (Fig. 6C). Consistently, knockdown of OPN expression in HeLa and Huh-7 cells abolished AKT activation during Hyp/RO and sensitized cells to the cell death (Fig. S5 A–C and Fig. 4A). These data suggest that OPN induction in these tumor cells is associated with their resistance to Hyp/RO via AKT activity. With a notion that AKT inhibits the activation of caspase-9 through direct phosphorylation at Ser-196 (27), we also observed reduced activation of caspase-9 in HeLa and Huh-7 cells (Fig. 6C), implying that OPN may regulate caspase-9 via AKT during Hyp/RO.
Fig. 6.
Differential induction of OPN in tumor cells affects viability through regulation of AKT and caspase-9. (A) Different sensitivity of tumor cells to Hyp/RO-induced cell death. Cells were incubated under Hyp (12 h)/RO (18 h) and cell death was examined using EthD staining. Bars, mean values ± SD; (n = 3). (B) Induction of OPN in HeLa and Huh-7 cells during Hyp/RO. Cell extracts prepared from cells exposed to Hyp/RO as in A were analyzed with Western blotting using the indicated antibodies. (C) Sustained level of pAKT in HeLa and Huh-7 cells during Hyp/RO. Cells were left untreated or exposed to Hyp (12 h)/RO (18 h) and Western blot analysis was performed. (D) Expression of OPN DM in HeLa cells increases pAKT level and inhibits caspase-9 activation. HeLa cells were transfected with pcDNA3, OPN WT, or OPN DM expression vector for 24 h and then exposed to Hyp (12 h)/RO (18 h). Cell extracts were analyzed for Western blotting using the indicated antibodies.
In addition, when we examined and compared AKT activities in HeLa/OPN WT and HeLa/OPN DM cells under Hyp/RO, the level of pAKT was higher in HeLa/OPN WT cells than HeLa/pcDNA3 cells and was much higher in HeLa/OPN DM cells than HeLa/OPN WT cells (Fig. 6D). On the contrary, proteolytic activation of caspase-9 was lower in HeLa/OPN WT and much suppressed in HeLa/OPN DM cells than HeLa/pcDNA3 cells. These results suggest that the cleavage of OPN by caspase-8 decreases AKT activity, which may in turn activate caspase-9 for cell death during Hyp/RO.
C-Terminal Caspase Cleavage Fragment of OPN Localizes to the Nucleus and Induces Cell Death via p53.
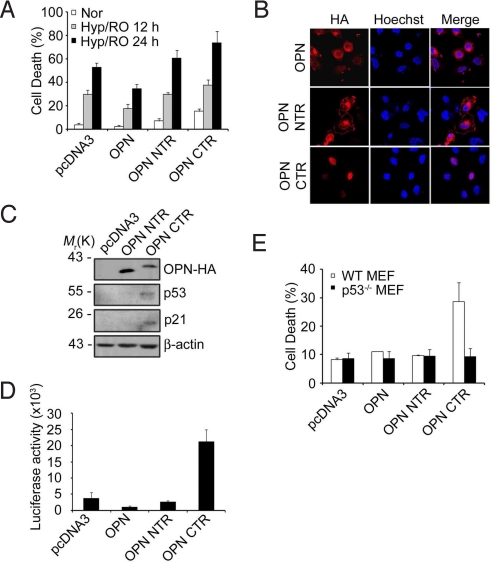
Further, we investigated roles of OPN cleavage products in cell death by using the caspase-8 cleavage products, OPN N-terminal cleavage fragment [OPN NTR (1–135)] and C-terminal cleavage fragment [OPN CTR (136–314)]. OPN CTR fragment, over-expressed in HeLa cells using pcDNA3-HA vector, led to death of the cells after Hyp/RO; OPN NTR was without effect (Fig. 7A). Notably, immunocytochemical analysis showed that OPN CTR is detected in the nucleus (Fig. 7B Bottom) whereas OPN NTR is preferentially found in the plasma membrane (Fig. 7B Middle). The nuclear detection of OPN immunoreactivity was evident in HeLa/OPN WT cells during Hyp/RO (Fig. S6 Upper) but not in HeLa/OPN DM cells (Fig. S6 Lower). These results suggest that OPN CTR may translocate into the nucleus during Hyp/RO.
Fig. 7.
Translocation of OPN C-terminal cleavage fragment (CTR) into the nucleus and induction of p53. (A) Proapoptotic activity of OPN CTR. HeLa cells were transfected with pcDNA3-HA, pOPN-HA, pOPN NTR-HA, or pOPN CTR-HA for 24 h. Cells were then exposed to Hyp (12 h)/RO for the indicated times and cell death rates were determined as in Materials and Methods. Bars, mean values ± SD; (n = 3). (B) OPN CTR is localized in the nucleus. HeLa cells were transfected with pOPN-HA, pOPN NTR-HA, or pOPN CTR-HA for 24 h and then immunostained with anti-HA antibody (red). Nuclei were stained with Hoechst 33258 (blue) and observed under a confocal microscope. (C) OPN CTR increases the level of p53 and p21. HEK293 cells were transiently transfected with pcDNA3-HA, pOPN NTR-HA, or pOPN CTR-HA for 24 h and cell extracts were then immunoblotted with the indicated antibodies. (D) Plasmid-encoded expression of OPN CTR increases the transcriptional activity of p53. HEK293 cells were transiently transfected with pRGC-luc reporter containing synthetic p53-binding sequences, pCMV-β-gal, and the indicated plasmid for 24 h. Luciferase activity was measured and normalized by that of β-galactosidase. Bars, mean values ± SD; (n = 4). (E) OPN CTR-induced cell death is abolished by p53 deficiency. Wild-type MEF and p53−/− MEF were transfected with pcDNA3-HA, pOPN NTR-HA, or pOPN CTR-HA for 24 h and examined for cell death as in Materials and Methods.
Then, we addressed the contribution of OPN CTR to cell death. Interestingly, Western blot analysis showed that plasmid-encoded expression of OPN CTR increases the levels of p53 and p21 protein (Fig. 7C). Luciferase reporter assay also showed that OPN CTR increases the transcriptional activity of p53 (Fig. 7D). Further, cell death assay illustrated that as in HeLa cells, plasmid-encoded expression of OPN CTR induces significant amount of cell death (28%) in wild-type mouse embryonic fibroblast (MEF) cells. However, this proapoptotic activity was not observed in p53−/− MEF cells (Fig. 7E). However, OPN NTR did not show any effects on cell death. Thus, we propose that OPN CTR generated by caspase-8 may gain proapoptotic activity by modulating p53 level in the nucleus.
Discussion
OPN is a multifunctional protein playing roles in cell proliferation, metastasis, and apoptosis. In this study, we found that OPN and its caspase cleavage product exhibit opposite activities to regulate cell death during Hyp/RO. Especially, OPN level is critical to regulate cell death triggered by Hyp/RO among various death signals. Then, there should be a tight balance between cell survival activity generated by OPN and cell death activity triggered by OPN CTR during Hyp/RO. As it is known that AKT activation inhibits p53 (28, 29), we also observed that AKT activation is responsible for the suppression of p53 accumulation during Hyp/RO. Thus, we propose that OPN-induced activation of AKT suppresses p53 accumulation for cell survival. Consequently, OPN cleavage by caspase-8 attenuates AKT-mediated survival signal and at the same time OPN CTR induces the accumulation of p53 in the nucleus to mediate cell death during Hyp/RO.
Upon stimulation of cell surface death receptors, an initiator caspase-8 is activated in death-inducing signaling complex (DISC). However, increasing studies have shown receptor-independent activation of caspase-8 during oxidative stress, UV radiation, or by chemotherapeutic drugs, which may represent either an initiating or a secondary amplifying event in apoptosis (14, 30, 31). We also observed efficient activation of caspase-8 and subsequent OPN cleavage during Hyp/RO. However, we failed to detect OPN cleavage during apoptosis induced by TRAIL or TNF-α. In addition, whereas the reduced expression of caspase-8 attenuates cell death induced by Hyp/RO, TRAIL, or TNF-α, overexpression of OPN or interference with OPN expression using OPN shRNAs exclusively affected cell death induced by Hyp/RO. These data suggest that OPN cleavage by caspase-8 during Hyp/RO is differently regulated from substrate cleavage by caspase-8 activated by DISC formation. Recent studies showed distinct apoptotic complexes containing caspase-8 or caspase-10, and diverse roles of those proximal caspases (32, 33).
We expect that anti-apoptotic OPN functions as a secreted cytokine to activate AKT during Hyp/RO, because we observed that signal sequence-deleted form of OPN (Δsig OPN) (6) failed to both activate AKT or inhibit Hyp/RO-induced cell death (Fig. S7 A–C). OPN substrate of the caspase-8 appears to be exclusively the form with secretion signal, but which may or may not have been secreted. Because OPN has been shown to mainly localize in ER/Golgi of cells along with the secretory pathway, cellular sites for OPN cleavage by caspase-8 during Hyp/RO is not clear at this moment. It might occur at cytosol after cytosolic release of OPN after perturbation of the secretory pathway during cell damage or at some other intracellular sites. Actually, caspase-8 was reported to be localized in the nucleus, mitochondria, and ER as well as in cytosol (13, 34, 35).
OPN is a diagnosis marker as a final product in cancer and a mediator in developing cancer, such as hepatocellular carcinoma, cervical carcinoma, and head and neck carcinoma (4). Consistently, OPN was recently reported to be a systemic tumor instigator via bone marrow activation (36). We also found that several cancer cell lines showing dramatic induction of OPN during Hyp/RO are quite resistant to Hyp/RO-induced cell death. In addition, caspase-8 is also suggested to be closely associated with tumorigenesis: caspase-8 is deleted in lung carcinoma, neuroblastoma, and hepatocellular carcinoma (16, 17) and its deficiency facilitates cellular transformation (18). Thus, we envision that a role of caspase-8 deficiency in tumorigenesis may be in part contributed by its failure to cleave and inactivate OPN to modulate Hyp/RO-induced cell death. It could be important for host organisms to improve survival that caspase-8 inactivates OPN-mediated survival signals to prevent the accumulation of unwanted cancerous cells during Hyp/RO.
In summary, our observations provide evidence that quantitative regulation of OPN through its induction via gene expression and its destruction by caspase-8 during Hyp/RO is an essential check point for determining cell death in some cancer models, shedding insight into OPN as a therapeutic for anti-cancer treatment.
Materials and Methods
Cell Culture and Transfection.
HeLa, Huh-7, C33A, Chang Liver, SK Hep-1, and HEK293 cells were cultured in DMEM (HyClone) supplemented with 10% FBS (HyClone). Transfection was carried out using LipofectAMINE (Invitrogen) or Polyfect reagent (Qiagen) according to the manufacturers' instructions.
Cell Death Assay and Flow Cytometric Analysis.
Cells were exposed to various stimuli and double-stained with Hoechst 33258 (1 μg/mL) and ethidium homodimer (EthD) (Molecular Probes). After which apoptotic cell death was assessed by counting the number of GFP-positive cells showing condensed and fragmented nuclei after under a fluorescence microscope (Olympus). For flow cytometric analysis, cells were collected, fixed with ethanol, and treated with RNase and propidium iodide. Cell death was measured by using a flow cytometer (FACS Calibur; BD Biosciences).
Expression Plasmids and shRNAs.
PCR products of OPN WT and deletion mutants were subcloned into the KpnI site of pcDNA3-HA. OPN D135A and OPN D157A mutants were generated by site-directed mutagenesis. pOPN shRNAs were constructed using forward and reverse 64-nucleotides (#1, 679-ACGAGTCAGCTGGATGACC-697, #2, 805-GTCAGCCGTGAATTCCACA-823) were synthesized, annealed, and cloned into the BglII and HindIII sites of pSuper mammalian expression vector (Oligoengine). pCasp-8 shRNA and pCasp-10 shRNA were generated as described (15, 33).
Stable Cell Lines.
HeLa cells were transfected with pcDNA3-HA, pOPN-HA, or pOPN DM-HA for 24 h and then grown in selection medium containing 1 mg/mL G418 (Invitrogen) for 2 weeks. After further selection, cells were analyzed by Western blotting.
In Vitro Screening for Caspase Substrates and Caspase Cleavage Assays.
Bacterial plasmids expressing caspases were transformed into BL21 (DE3) cells and cell extracts were prepared as described in ref. 23. For in vitro assay, [35S]methionine-labeled OPN was subjected to cleavage reactions with purified caspases as described in ref. 37.
Hypoxia/Reoxygenation.
Hypoxia was achieved by culturing the cells in MEM (Invitrogen) in an air-tight Plexiglass chamber with GasPak Plus (BD Biosciences) (38) or cells were exposed to hypoxia (0.1% O2) by incubating cells at 37 °C in 5% CO2/10% H2/85% N2 anaerobic incubator (Forma Scientific). Cells were then placed under normoxic conditions.
Antibodies.
Anti-PARP (F-2), anti-p53 (Pab 240), anti-p21 (C-19), β-actin (C4) (Santa Cruz Biotechnology), anti-cleaved caspase-3 (#9661), anti-cleaved caspase-9 (#9501), anti-pAKT (#9271), anti-AKT (#9272), anti-pERK (#9101), anti-ERK (#9102), anti-pp38 (#9211), and anti-pJNK (#9251) (Cell Signaling) antibodies were used for Western blot analyses. For genome-encoded OPN detection, polyclonal antibody generated against human recombinant OPN (LF123) was kindly provided by Dr. L.W. Fisher (39).
Immunostaining.
Cells grown on coverslips in 24-well plates were fixed in 4% paraformaldehyde for 5 min, permeablized in 0.1% Triton X-100 for 5 min and blocked with 1% BSA for 2 h. After stained with anti-HA antibody and Hoechst 33258, cells were visualized under a LSM confocal fluorescence microscope (Zeiss).
Luciferase and β-Galactosidase Assays.
Cells were cotransfected with RGC-luc reporter plasmid containing synthetic p53-binding sequences (40) and CMV β-gal plasmids for 24 h. Then luciferase activities were determined using a Luciferase Assay System (Promega) as described in ref. 41.
Supplementary Material
Acknowledgments.
We thank Dr. L.W. Fisher (National Institutes of Health, Bethesda) for providing OPN antibody (LF123) and Dr. H.W. Lee (Yonsei University, Seoul, Korea) for p53−/− MEF. This work was supported by grants from CRI-Acceleration Research, Human Genomics of 21C Frontier Research Program, and Nuclear Research grant (BAERI) funded by the Korea Science and Engineering Foundation. H.-J.K. was supported by the BK21 program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903704106/DCSupplemental.
References
- 1.Alford AI, Hankenson KD. Matricellular proteins: Extracellular modulators of bone development, remodeling, and regeneration. Bone. 2006;38:749–757. doi: 10.1016/j.bone.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Ashkar S, et al. Eta-1 (osteopontin): An early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 3.Shinohara ML, et al. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellahcène A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): Multifunctional proteins in cancer. Nat Rev Cancer. 2008;8:212–226. doi: 10.1038/nrc2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: Role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79–86. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Shinohara ML, Kim HJ, Kim JH, Garcia VA, Cantor H. Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc Natl Acad Sci USA. 2008;105:7235–7239. doi: 10.1073/pnas.0802301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zohar R, et al. Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. J Cell Physiol. 2000;184:118–130. doi: 10.1002/(SICI)1097-4652(200007)184:1<118::AID-JCP13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 8.Junaid A, Moon MC, Harding GE, Zahradka P. Osteopontin localizes to the nucleus of 293 cells and associates with polo-like kinase-1. Am J Physiol Cell Physiol. 2007;292:C919–926. doi: 10.1152/ajpcell.00477.2006. [DOI] [PubMed] [Google Scholar]
- 9.Hwang SM, Wilson PD, Laskin JD, Denhardt DT. Age and development-related changes in osteopontin and nitric oxide synthase mRNA levels in human kidney proximal tubule epithelial cells: Contrasting responses to hypoxia and reoxygenation. J Cell Physiol. 1994;160:61–68. doi: 10.1002/jcp.1041600108. [DOI] [PubMed] [Google Scholar]
- 10.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8:425–437. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim BM, Chung HW. Hypoxia/reoxygenation induces apoptosis through a ROS-mediated caspase-8/Bid/Bax pathway in human lymphocytes. Biochem Biophys Res Commun. 2007;363:745–750. doi: 10.1016/j.bbrc.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Chao W, Shen Y, Li L, Rosenzweig A. Importance of FADD signaling in serum deprivation- and hypoxia-induced cardiomyocyte apoptosis. J Biol Chem. 2002;277:31639–31645. doi: 10.1074/jbc.M204104200. [DOI] [PubMed] [Google Scholar]
- 13.Besnault-Mascard L, et al. Caspase-8 sumoylation is associated with nuclear localization. Oncogene. 2005;24:3268–3273. doi: 10.1038/sj.onc.1208448. [DOI] [PubMed] [Google Scholar]
- 14.von Haefen C, et al. Paclitaxel-induced apoptosis in BJAB cells proceeds via a death receptor-independent, caspases-3/-8-driven mitochondrial amplification loop. Oncogene. 2003;22:2236–2247. doi: 10.1038/sj.onc.1206280. [DOI] [PubMed] [Google Scholar]
- 15.Jun JI, et al. Role of FLASH in caspase-8-mediated activation of NF-kappaB: Dominant-negative function of FLASH mutant in NF-kappaB signaling pathway. Oncogene. 2005;24:688–696. doi: 10.1038/sj.onc.1208186. [DOI] [PubMed] [Google Scholar]
- 16.Teitz T, et al. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med. 2000;6:529–535. doi: 10.1038/75007. [DOI] [PubMed] [Google Scholar]
- 17.Soung YH, et al. Caspase-8 gene is frequently inactivated by the frameshift somatic mutation 1225 1226delTG in hepatocellular carcinomas. Oncogene. 2005;24:141–147. doi: 10.1038/sj.onc.1208244. [DOI] [PubMed] [Google Scholar]
- 18.Krelin Y, et al. Caspase-8 deficiency facilitates cellular transformation in vitro. Cell Death Differ. 2008;15:1350–1355. doi: 10.1038/cdd.2008.88. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 20.Ng FW, et al. p28 Bap31, a Bcl-2/Bcl-XL- and procaspase-8-associated protein in the endoplasmic reticulum. J Cell Biol. 1997;139:327–338. doi: 10.1083/jcb.139.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stegh AH, et al. Identification of the cytolinker plectin as a major early in vivo substrate for caspase 8 during CD95- and tumor necrosis factor receptor-mediated apoptosis. Mol Cell Biol. 2000;20:5665–5679. doi: 10.1128/mcb.20.15.5665-5679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung CW, et al. Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol Dis. 2001;8:162–172. doi: 10.1006/nbdi.2000.0335. [DOI] [PubMed] [Google Scholar]
- 24.Denhardt DT, Noda M, O'Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: Regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin YH, Yang-Yen HF. The osteopontin-CD44 survival signal involves activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. J Biol Chem. 2001;276:46024–46030. doi: 10.1074/jbc.M105132200. [DOI] [PubMed] [Google Scholar]
- 26.Das R, Mahabeleshwar GH, Kundu GC. Osteopontin stimulates cell motility and nuclear factor kappaB-mediated secretion of urokinase type plasminogen activator through phosphatidylinositol 3-kinase/Akt signaling pathways in breast cancer cells. J Biol Chem. 2003;278:28593–28606. doi: 10.1074/jbc.M303445200. [DOI] [PubMed] [Google Scholar]
- 27.Cardone MH, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 28.Ogawara Y, et al. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J Biol Chem. 2002;277:21843–21850. doi: 10.1074/jbc.M109745200. [DOI] [PubMed] [Google Scholar]
- 29.Shankar E, Sivaprasad U, Basu A. Protein kinase C epsilon confers resistance of MCF-7 cells to TRAIL by Akt-dependent activation of Hdm2 and downregulation of p53. Oncogene. 2008;27:3957–3966. doi: 10.1038/onc.2008.39. [DOI] [PubMed] [Google Scholar]
- 30.Boesen-de Cock JG, Tepper AD, de Vries E, van Blitterswijk WJ, Borst J. Common regulation of apoptosis signaling induced by CD95 and the DNA-damaging stimuli etoposide and gamma-radiation downstream from caspase-8 activation. J Biol Chem. 1999;274:14255–14261. doi: 10.1074/jbc.274.20.14255. [DOI] [PubMed] [Google Scholar]
- 31.Huang HL, et al. DNA-damaging reagents induce apoptosis through reactive oxygen species-dependent Fas aggregation. Oncogene. 2003;22:8168–8177. doi: 10.1038/sj.onc.1206979. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 33.Lee HJ, et al. AK2 activates a novel apoptotic pathway through formation of a complex with FADD and caspase-10. Nat Cell Biol. 2007;9:1303–1310. doi: 10.1038/ncb1650. [DOI] [PubMed] [Google Scholar]
- 34.Chandra D, et al. Association of active caspase 8 with the mitochondrial membrane during apoptosis: Potential roles in cleaving BAP31 and caspase 3 and mediating mitochondrion-endoplasmic reticulum cross talk in etoposide-induced cell death. Mol Cell Biol. 2004;24:6592–6607. doi: 10.1128/MCB.24.15.6592-6607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breckenridge DG, Nguyen M, Kuppig S, Reth M, Shore GC. The procaspase-8 isoform, procaspase-8L, recruited to the BAP31 complex at the endoplasmic reticulum. Proc Natl Acad Sci USA. 2002;99:4331–4336. doi: 10.1073/pnas.072088099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAllister SS, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karki P, Dahal GR, Park IS. Both dimerization and interdomain processing are essential for caspase-4 activation. Biochem Biophys Res Commun. 2007;356:1056–1061. doi: 10.1016/j.bbrc.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 38.Cho DH, et al. Suppression of hypoxic cell death by APIP-induced sustained activation of AKT and ERK1/2. Oncogene. 2007;26:2809–2814. doi: 10.1038/sj.onc.1210080. [DOI] [PubMed] [Google Scholar]
- 39.Fisher LW, Stubbs JT, III, Young M. Antisera and cDNA probes to human and certain animal model bone matrix non collagenous proteins. Acta Orthop Scand Suppl. 1995;266:61–65. [PubMed] [Google Scholar]
- 40.Abe Y, et al. Hedgehog signaling overrides p53-mediated tumor suppression by activating Mdm2. Proc Natl Acad Sci USA. 2008;105:4838–4843. doi: 10.1073/pnas.0712216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song S, et al. E2–25K/Hip-2 regulates caspase-12 in ER stress-mediated Aβ neurotoxicity. J Cell Biol. 2008;182:675–684. doi: 10.1083/jcb.200711066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.