Abstract
The melibiose permease of Escherichia coli (MelB) catalyzes the coupled stoichiometric symport of a galactoside with a cation (either Na+, Li+, or H+), using free energy from the downhill translocation of one cosubstrate to catalyze the accumulation of the other. Here, we present a 3D structure model of MelB threaded through a crystal structure of the lactose permease of E. coli (LacY), manually adjusted, and energetically minimized. The model contains 442 consecutive residues (≈94% of the polypeptide), including all 12 transmembrane helices and connecting loops, with no steric clashes and superimposes well with the template structure. The electrostatic surface potential calculated from the model is typical for a membrane protein and exhibits a characteristic ring of positive charges around the periphery of the cytoplasmic side. The 3D model indicates that MelB consists of two pseudosymmetrical 6-helix bundles lining an internal hydrophilic cavity, which faces the cytoplasmic side of the membrane. Both sugar and cation binding sites are proposed to lie within the internal cavity. The model is consistent with numerous previous mutational, biochemical/biophysical characterizations as well as low-resolution structural data. Thus, an alternating access mechanism with sequential binding is discussed. The proposed overall fold of MelB is different from the available crystal structures of other Na+-coupled transporters, suggesting a distinctive fold for Na+ symporters.
Keywords: bioenergetics, ligand binding, MelB, protein threading, sugar/cation symporter
Melibiose permease of Escherichia coli (MelB), encoded by the melB gene in the mel operon (1), is a well-studied representative of the glycoside-pentoside-hexuronide/cation symporter family of membrane transporters (2). MelB utilizes free energy released from the energetically downhill movement of a cation (Na+, Li+, or H+), in response to an electrochemical cation gradient, to drive the uphill stoichiometric accumulation of a galactopyranoside (3–5). The type of the cotransported cation depends on the stereostructure of the transported sugar (6). α-Galactopyranosides (melibiose, raffinose, and p-nitrophenyl-α-galactoside) are cotransported with Na+, H+, or Li+, whereas β-galactopyranosides (lactose, methyl-1-β-d-galactopyranoside, and p-nitrophenyl-β-galactoside) are cotransported with Na+ or Li+ but not H+ (6). In the absence of an electrochemical cation gradient, MelB catalyzes the reverse reaction, using free energy from the downhill translocation of the sugar to drive the stoichiometric transport of the coupled cation in either direction across the membrane (5, 7, 8). Similar features of transport exist in the lactose permease of E. coli (LacY) (9, 10), the best-studied representative of the major facilitator superfamily (MFS) of membrane transporters (2). Both MelB and LacY transport d-galactopyranosides, with similar substrate specificity; however, sugar transport in LacY is coupled solely with H+ (9, 10).
MelB consists of 473 aa with ≈65% apolar/hydrophobic residues (1, 11), and the Met-1 residue does not appear by N-terminal sequencing (12). The single polypeptide is responsible for the Na+, H+, or Li+/sugar symport (11). The membrane topology of MelB has been determined by hydropathy and phoA-fusion analyses (11, 13) as well as by limited proteolysis (14).Similar to LacY (10), MelB exhibits 12 transmembrane helices connected by hydrophilic loops with both N- and C-termini located at the cytoplasmic side of the membrane. A signature of “6 helices-middle loop-6 helices” is observed in both permeases. A projection map of MelB obtained by cryo-EM of 2D crystals at a resolution of 8 Å (15) confirms the total number of transmembrane helices as well as the feature of 2-helix bundles lining a central cavity. Furthermore, a 3D EM map at a resolution of 10 Å reveals that MelB exhibits a heart shape and that the crystal structure of LacY can be docked within the EM map (16).
Five X-ray 3D crystal structures of LacY have been solved for the wild-type protein (17) and a conformationally restricted mutant (18, 19). All crystal structures show that LacY is organized into two 6-helix bundles related by 2-fold pseudosymmetry, which are separated by a large hydrophilic cavity open to the cytoplasmic side, representing an inward-facing conformation. The side chains important for binding both sugar and H+ lie at the apex of the central cavity in the middle of the protein. Similar overall folds have been observed for two other members of MFS, namely, the glycerol-3-phosphate transporter (GlpT) (20) and the postulated multidrug efflux pump (EmrD) (21) of E. coli. Here, we report a 3D structure model of MelB obtained by threading analysis using LacY crystal structure as the template. The model suggests that MelB shares a similar overall fold with MFS members and exhibits a distinctive fold for Na+ symporter superfamilies.
Results and Discussion
Structure Prediction.
The full-length sequence of MelB was subjected to threading analysis using several server-based tools as described in Materials and Methods. Hit lists of potential templates were obtained from the FUGUE (22), LOOPP (23), and Phyre (24) programs [supporting information (SI) Table S1]. The crystal structure of LacY was consistently selected as a top candidate by all three programs. Among other templates, two other MFS permeases (GlpT and EmrD) and three non-MFS proteins [LeuTAa (Na+/alanine transporter, PDB ID 2a65), Amt-1 (an ammonium transporter, PDB ID 2b2f), and CLC (an H+/Cl− antiporter, PDB ID 1ots)] were also recognized with varying scores.
All 3D threading models of MelB were converted to membrane topological representations (Fig. S1). The topological profiles of MelB derived from LeuTAa and Amt-1 structures (Fig. S1 D and E) clearly disagree with the well-established topology of MelB (Fig. 1). The conversion of the CLC-based model was rather difficult because of the large number of irregular and broken helices. Therefore, these three models are excluded from the analysis. On the other hand, all topological profiles based on the MFS templates (Fig. S1 A–C) are similar to the experimentally determined topology of MelB (11, 13, 14), where the characteristic signature of 6 helices-middle loop-6 helices is present. LacY shares similar substrate specificity and overall transport mechanism with MelB; hence, the LacY-based model was chosen for further systematic evaluation.
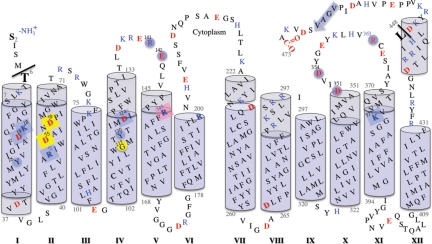
Fig. 1.
Membrane topology of MelB. The topology model of MelB (11) is matched to the threading 3D structure (see the text). Transmembrane helices are presented as blue cylinders; the light-blue color indicates extramembrane segments. Helices are numbered with Roman numerals. Charged residues are shown in red (negative) and blue (positive). Eight cavity-exposed charged residues from transmembrane regions are marked as filled square boxes. Residues known to participate in the sugar binding are highlighted with a pink background. Residues expected to be involved in cation binding are highlighted with a yellow background (see the text). Residues important for substrate(s) binding and/or coupling are highlighted with a blue background. The Met-1 residue is removed, as suggested by N-terminal sequencing (12). The threaded region is between residues Thr-6 and Leu-448, as indicated by black lines.
Careful examination of the threading model shows that the register of the residues in helix X needed to be modified by half a turn so that both hydrophilic and hydrophobic faces of the helix orient properly. Therefore, manual adjustments, followed by energy minimization, were performed as described in Materials and Methods.
Alignment of 3D Structures.
The primary sequence alignment between MelB and LacY is relatively poor with ≈37% sequence similarity and ≈15% identity; thus, MelB was separated from the MFS (2). However, it is remarkable that a secondary structure-weighted alignment yields an excellent match between the predicted secondary structure of MelB and the crystal structure of LacY, as observed by the LOOPP program (Fig. S2). Subsequently, functionally important residues align well between the two permeases as discussed below. The poor outcome of alignments based only on primary sequence may be attributable to the presence of four longer extramembrane loops on both ends of helices V and XI in MelB (Figs. S2 and S5).
The backbone model (FUGUE program) and the all-atom model (LOOPP program) suggest that MelB adopts an overall fold resembling that of LacY (Fig. 2A). The all-atom model of MelB includes ≈94% of the residues (positions 6–448) covering all 12 transmembrane helices and connecting loops with acceptable stereochemistry and no steric clashes. A Ramachandran plot shows that 80% of the residues concentrate in the α-helical region and only 1% of the total residues are located in the stereochemically disallowed area. These outliers are mainly located in the flexible extramembrane loops. Furthermore, superposition of the main chain atoms of the MelB model and LacY structure, restricting the 3D alignment to the corresponding transmembrane helices (Fig. 2A), yields a rmsd of only 0.5 Å. These results indicate that MelB can adopt an inward-facing conformation similar to that of LacY. In addition, threading models based on GlpT and EmrD exhibit arrangement of helices similar to that of LacY but with different overall conformations. It is also noteworthy that pair-wise superpositions of LacY-based threading models of four MelB orthologues from Salmonella typhimurium, Citrobacter freundii, Klebsiella pneumoniae, and Vibrio shilonii yield rmsd values of less than 1.5 Å. Moreover, evolutionary conservation analyses were performed using the ConSurf program (SI Text). When the evolutionary conservation patterns are mapped on the MelB model (Fig. S3A) and LacY structure (Fig. S3B), the most conserved residues are located at the interhelix interfaces within transmembrane regions, whereas the most variable residues are located in the periphery (Fig. S3).
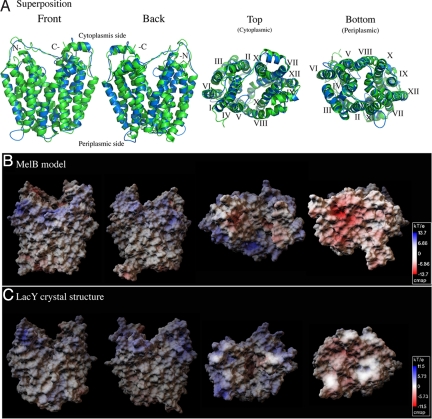
Fig. 2.
Comparison between the threading model of MelB and the crystal structure of LacY. (A) Superposition. The main chain coordinates of MelB (residues 6–448, blue), obtained from the LOOPP program, are superimposed on the LacY crystal structure (green, PDB ID 1pv6). The alignment was restricted to the corresponding transmembrane helices. (B and C) Surface electrostatic potential maps of the MelB threading model and the crystal structure of LacY were calculated using Adaptive Poisson-Boltzmann Solver (APBS) software (53). The scale indicates color-coded values of the electrostatic potentials (kT/e). A positive electrostatic potential is noticeable around the cytoplasmic opening of the central cavity in both permeases. Front and back, viewing parallel to the membrane; top, viewing from the cytoplasmic side; bottom, viewing from the periplasmic side.
Overall Architecture.
MelB, like LacY (Fig. 2 A and C), is heart shaped when viewed parallel to the membrane and oval shaped when viewed from the cytoplasmic side (Fig. 2 A and B). The overall shape of the MelB model is consistent with previous structural results based on 2D crystallographic projections and 3D EM maps (15, 16). The calculated electrostatic surface potential of the MelB model reveals a hydrophobic transmembrane region and charged/polar extramembrane loops (Fig. 2B). In both permeases, it is interesting that a positively charged belt, like “blue lips,” is clearly observed around the periphery of the cytoplasmic opening, with negative charges concentrated at both the top and bottom surfaces of the proteins (Fig. 2 B and C). The asymmetrical surface charge distribution in both permeases supports the positive inside rule (25), which is important for controlling membrane topology of the protein, and extends the rule to the tertiary structure level. Moreover, the blue lips at the cytoplasmic opening are expected to facilitate interactions with the phosphate oxygens of phospholipids and might also imply a preferential association with anionic phospholipids at the inner leaflet of the membrane. Additionally, the negatively charged surfaces at the top and bottom sides may contribute to the local cation concentrations.
Domain Structure.
MelB is organized in 2-helix bundles connected with a central loop and separated by an internal cavity facing the cytoplasmic side. Both N- and C-termini are located at the cytoplasmic side of the protein (Fig. 2A). The N- and C-helix bundles are related by a 2-fold pseudosymmetry. Within each domain, there is another 2-fold inverted pseudosymmetry. The primary sequence alignment between the N- and C-terminal halves shows significant homology of 57.8% (17.5% identity), with a nearly perfect match in the secondary structure (Fig. S4). A pseudosymmetry between the two 6-helix bundles is evident in the crystal structures of LacY. However, the homology between the two halves is ≈57.4%, with only ≈14.2% identity (Fig. S4). Thus, the threading structure is consistent with the notion that MelB, like other MFS members, contains two internal tandem repeats from the same genetic origin (2). The pseudosymmetry between the two domains is not observed in the low-resolution 2D projection map (15). Possible interpretations would be the presence of several irregular or tilted helices and/or a tilt in the pseudosymmetrical axis relative to the membrane plane.
Conserved Hydrophobic Interactions.
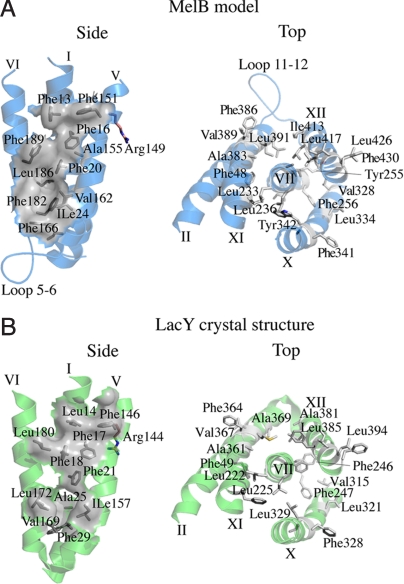
Both the N- and C-helices bundles contain conserved hydrophobic patches. Three Phe residues in positions 17 and 21 (helix I), as well as position 146 (IV) in LacY, contribute to a hydrophobic patch, which seems to be involved in stabilizing the N-terminal domain (Fig. 3B). The corresponding positions in MelB are all Phe residues in positions 16, 20 (helix I), and 151 (helix IV), respectively (Fig. 3A). In addition, this characteristic packing seems to play a role in the optimal orientation of a conserved Arg residue, Arg-149 in MelB (26) and Arg-144 in LacY, which is important for sugar binding (9) (Fig. 3). Furthermore, residues Phe-49 (helix II); Leu-222, Leu-225, Phe-246, and Phe-247 (helix VII); Val-315 (helix X); and Leu-385 (helix XII) of LacY contribute to the packing around helix VII in the C-terminal helices bundle (Fig. 3B). In MelB, residues Phe-48 (helix II); Leu-233, Leu-236, Tyr255, and Phe-256 (helix VII); Val-328 (helix X); and Leu-417 (helix XII) occupy similar positions (Fig. 3A). The well-conserved helical-packing environments support the predicted overall arrangement of helices in the model.
Fig. 3.
Conserved helical packing. Hydrophobic patches (gray) between helices I, V, and VI in the N-terminal domain and around helix VII in the C-terminal domain of the MelB threading model (A, blue) and LacY crystal structure (B, green).
Characteristic Cytoplasmic Loops of MelB.
The presence of four distinguishable loops is observed in the 3D model of MelB (Fig. S5). These loops are significantly longer than the corresponding regions in LacY. The length and location of these loops are highly conserved across all MelB orthologues. Loops 4–5/5–6 and 10–11/11–12 are located on both sides of the corresponding helices V and XI. It is remarkable that more than two-thirds of the cytoplasmic loops 4–5 and 10–11 contain charged/polar residues, (Fig. 1) most of which are highly conserved (Fig. S3). Loop 4–5 contains two residues, Arg-141 and Glu-142, that are essential for sugar/cation translocation (26, 27). In the model, Arg-141 is in close proximity to the important residue Asp-120 of helix IV. In loop 10–11, residues Asp-351, Asp-354, and Arg-363 are the only three residues important for sugar transport (28). These hydrophilic loops could be conformationally flexible, and the model may only suggest one possible orientation. Nevertheless, it is highly likely that rearrangements of loops 4–5 and 10–11 may play important role(s) in ligand recognition and/or conformational switches between functional states during the turnover.
Central Hydrophilic Cavity with Asymmetrical Charge Distribution.
Helices I, II, IV, and V (N-terminal domain) as well as helices VII, VIII, X, and XI (C-terminal domain) line the central cavity in the MelB model (Fig. 2A). The cavity is closed to the periplasmic side by the helical contacts between helices V/VIII, I/VII, and II/XI and is open to the cytoplasmic side. Based on the model, it is possible that interactions between or involving loops 4–5 and 10–11 might cover the cytoplasmic opening of the cavity. Remarkably, all helices in the inner layer (I, IV, VII, and X) tilt and have kinks near the cavity as observed in LacY (17–19), which may suggest unsynchronized movements of these helices.
Unlike LacY, a clear asymmetrical distribution of charges is observed within the cavity of MelB. Seven of eight charged residues exposed to the cavity are from the N-terminal helices (Figs. 1 and 4), and the only C-terminal–charged residue (Lys-377, helix XI) is in close proximity to the N-terminal helices bundle. In LacY, charged residues involved in sugar and H+ bindings are mainly located at the N- and C-terminal helices of the cavity, respectively. Residues Asp-240 (helix VII); Glu-269 (helix VIII); Arg-302 (helix IX); and Lys-319, His-322, and Glu-325 (helix X) in LacY form the charged/H-bond network responsible for the H+ binding and translocation (Fig. 4B) (29). The equivalent positions in MelB are mainly occupied with polar residues with no charged side chains.
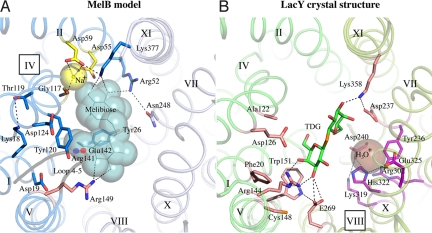
Fig. 4.
The central cavity in the MelB model containing putative binding sites for Na+ and sugar. Identical views from the cytoplasmic side down the central cavity of MelB (A, blue) and LacY (B, green). The N- and C-terminal helices of MelB and LacY are shown in blue/light-blue and green/light-green colors, respectively. Residues important for cosubstrate binding are shown as sticks. The large spheres reflect postulated positions of the ligands. Dotted lines show possible interactions. (A) In the threading model of MelB, a melibiose molecule and a Na+ ion are manually docked in putative binding sites. The modeling of melibiose (line, cyan) was guided by the X-ray coordinates of the sugar substrate in LacY, as shown in B. The Na+ and possible interacting residues (Asp-55, Asp-59, and Gly-117) are colored yellow. The functionally important loop 4–5 is shown in gray, with 2 important positions (Arg-141 and Glu-142) highlighted. Residues colored in blue are important for substrate(s) binding and/or coupling (see the text). The Arg-149 is important for sugar binding (26) and is colored pink. In LacY (B, PDB 1pv7), residues that are essential or important for the binding of sugar and H3O+ are shown in pink and magenta, respectively. A ß-D-galactopyranosyl 1-thio-ß-D-galactopyranoside (TDG) molecule is shown in green, and manually docked H3O+ is shown as an enlarged brown sphere.
In addition, apolar/hydrophobic residues dominate the periplasmic half of the MelB molecule; even the periplasmic external loops are not as charged as the cytoplasmic loops (Fig. 1). In the central cavity, the majority of the charged residues are located in the middle and inner surfaces. Therefore, hydrophobic interactions at the periplasmic side and the charged environment at the cytoplasmic side and inner surface of the cavity may stabilize an inward-facing conformation, which is predicted to be the most populated conformer of MelB, similar to what is established for LacY (17, 30, 31).
Putative Na+ Binding Site.
Among eight charged residues in the cytoplasmic cavity, four Asp residues in positions 19 (helix I), 55 and 59 (helix II), and 124 (helix IV) have been identified to contribute to the Na+-dependent sugar binding and transport in MelB (32–37). Cys or Asn mutations at either Asp-55 or Asp-59 abolish completely and specifically the Na+-dependent increase in both sugar affinity and stimulation of transport (35, 37). Unlike the D59C mutant that uncouples the cations (Na+, Li+, or H+) from sugar translocation, the D55C mutation retains only the H+-coupled melibiose transport (37). It is implied by attenuated total reflection Fourier transform infrared spectroscopy studies that some carboxyl groups may directly interact with the Na+ ion in MelB (38). Moreover, it was concluded that Trp-54, adjacent to Asp-55, is the major contributor to the Na+ binding-induced Trp fluorescence changes (39). Based on these observations, one Na+ ion was manually docked with no steric clashes between the carboxyl groups of the conserved Asp-55 and Asp-59 (helix II) (Fig. 4A). The backbone carbonyl oxygen of the conserved Gly-117 (helix IV) is also in close proximity to the docked Na+ ion. It is notable that the available crystal structures of Na+-coupled symporters (40–43) reveal a common Na+ binding motif, wherein the Na+ ions are buried between unwound regions of two helices, which is different from the Na+ binding site suggested for MelB. On the other hand, Asp-19 (helix I) and Asp-124 (helix IV) are not in close proximity to the proposed Na+ binding pocket in the model. Cys replacements at these two positions affect both sugar- and Na+ binding affinities. It is likely that these residues contribute to the charged/polar interactions around helix IV through the pairs Lys-18/Thr-119 (helices I/IV) Asp-124/Arg-141 (helix IV/loops 4–5), Arg-52/Asn-248 (helices II/VII), Arg-52/Asp-55 (helix II) (44), and Asp-59/Lys-377 (helices II/XI). Consistently, second-site mutation analyses suggest close proximities between the pairs Lys-18/Met-123 (1 helical turn below Thr-119) (44), Arg-52/Asn-248 (44), and Lys-377/Asp-59 (45). It is likely that the interhelix- and/or interdomain-charged/polar networks play important roles in optimizing the cation binding site and sugar recognition as well as the coupling between sugar and H+ translocation.
Residues Involved in Sugar Binding.
Although both MelB and LacY share similar substrate specificities, there is no detectable match of residues important for sugar binding in the traditional alignment. In LacY, the salt bridge between Arg-144 (helix V) and Glu-126 (helix IV) is essential for sugar binding and selectivity (9, 10). In MelB, Arg-149 (helix V) is important for sugar recognition (26), and Asp-124 and Tyr-120 (helix IV) are also suggested to be involved in both sugar and/or cation coupling (36). It is remarkable that the two pairs Arg-144/Glu-126 and Arg-149/Asp-124, which are strictly conserved among all LacY and MelB orthologues, respectively, align well at the tertiary structure level (Fig. S2 and Fig. 4). Trp-151 of LacY stacks hydrophobically against the galactopyranosyl ring of the sugar and plays a role in sugar recognition (46). In MelB, there is no Trp residue at the corresponding position, and Tyr-120 (36) is at a position corresponding to Ala-122 of LacY, which abuts the nongalactosyl moiety of d-galactosides (10). Phe-20 (helix I) of LacY is in close proximity to the sugar binding pocket (17, 47), and the side chain of the corresponding residue Asp-19 of MelB could be within a salt-bridge distance with Arg-149. Arg-52, on the same face of helix II as Asp-55 and Asp-59, is critical for H+-coupled melibiose transport but is not essential for Na+-coupled melibiose transport (44).
One molecule of melibiose was manually docked in the internal cavity of the MelB model (Fig. 4A), guided by the coordinates of ß-d-galactopyranosyl 1-thio-ß-d-galactopyranoside in the LacY crystal structure (Fig. 4B) (18). The docked melibiose exhibits no steric clashes within the proposed binding pocket and is surrounded to within 4 Å by potential hydrogen-bonding partners: Arg-149 (helix V), Arg-52 (helix II), and Lys-377 (helix XI). It is noteworthy that the K377C mutant shows no sugar transport (45).
Although it is not clear whether Arg-149 in MelB confers sugar specificity similar to the essential residue Arg-144 in LacY, it is striking that the two residues occupy almost identical positions (Fig. 3 A and B and Fig. 4). Based on the model, the aromatic residues Tyr-26 (helix I) and Tyr-120 (helix IV) may interact hydrophobically with the galactopyranosyl rings. Moreover, the sugar seems to be in close proximity to loop 4–5, which is consistent with a previous photoaffinity labeling study (48). The cooperative binding of the cosubstrates has been established for MelB (26, 35, 39, 49). It is interesting that both binding sites appear to be in close proximity in the model. It is also interesting that precedence has been shown in LeuTAa (40), wherein the substrate participates in the Na+ coordination.
In the MelB model, helix IV is in the middle of the N-terminal helices bundle with a kink at the center (Figs. 2A and 4A). Both cation and sugar binding sites are near the hinge on the adjacent faces of the helix, which implies a physical role in the coupling between the two cosubstrates (39). The charged/H-bond network observed between helices IV, I, and V, loops 4–5, as well as in helices II and XI could account for the cooperative binding of the cation and sugar in MelB (35, 36).
Postulated Mechanism.
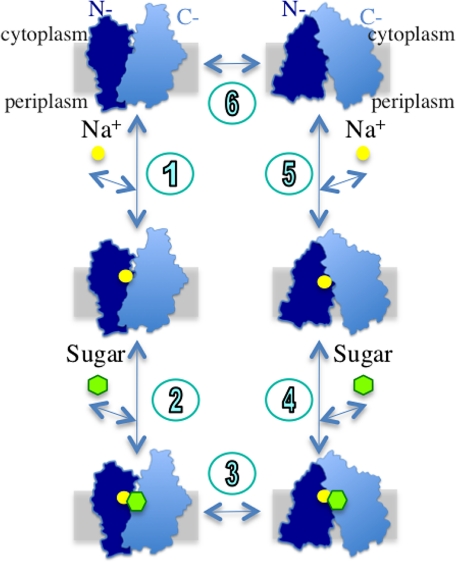
Combined with other studies (27, 50), the alternating access mechanism can be postulated for the galactoside/Na+ symport in MelB (i.e., both cation and sugar binding sites are reciprocally exposed to either side of the membrane during turnover). The symport mechanism for the efflux mode can be explained by a simplified 6-step scheme similar to that proposed for LacY (10) (Fig. 5). In the reversible and sequential binding model, the Na+ ion binds initially (step 1) and the sugar then binds at the inner surface (step 2). Binding of the cosubstrates in the inward-facing conformation causes a transition to the outward-facing conformation (step 3). The sugar releases initially (step 4), and the Na+ ion then releases at the outer surface (step 5). The empty carrier returns to the inward-facing conformation (step 6). The reverse reaction can be applied to the influx mode. Thus, it is likely that the turnover occurs when both cosubstrate binding sites are concurrently occupied (step 3) or unoccupied (step 6).
Fig. 5.
A kinetic scheme of the efflux mode of galactoside/Na+ symport for MelB. A cross section of the membrane is shown as a gray rectangle.
Conclusion
Several lines of evidence, including bioinformatics, previous functional studies, and biochemical/biophysical characterizations, are consistent with the overall fold of MelB suggested from the threading model. Two pseudosymmetrical 6-helix bundles surround an internal cavity that faces the cytoplasm and contains the binding sites for both cosubstrates. As suggested from the model, the overall fold of the Na+-coupled symporter MelB is different from the available structures of other Na+-coupled transporters, including LeuTAa, the sodium/galactose symporter (41), the Na+/aspartate symporter (43), and the Na+/H+ antiporter (51), but is similar to MFS members. Therefore, the MelB model may represent a unique fold for Na+-coupled permeases. Although exact side-chain orientations/interactions in the model await further evaluation, the sugar binding pocket of MelB seems to be similar to that of LacY; however, the cation binding site is different between the two permeases.
Materials and Methods
The detailed methodology is provided in SI Text. In summary, using the full-length primary sequence of MelB as the sole input, three threading programs [FUGUE, (22); LOOPP, (23); and Phyre, (24)] were used for the structure prediction. The model was then manually adjusted and energetically minimized. The CCP4 package (52) was used for superposition of the structures and evaluation of the stereochemistry. The electrostatic surfaces were calculated using Adaptive Poisson-Boltzmann Solver (APBS) software (53). The evolutionary conservation analysis was performed using the Web-based server ConSurf (54).
Supplementary Material
Acknowledgments.
The authors thank Gérard Leblanc and H. Ronald Kaback for their critical comments and insightful suggestions and Arathi Krishnakumar and Richa Chandra for their critical reading of the manuscript. This work is supported by Award number R21HL087895 from the National Heart, Lung, and Blood Institute (to L.G.) and Center for Membrane Protein Research, the Texas Tech University Health Sciences Center. The coordinates of the MelB model are available in SI Appendix.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905516106/DCSupplemental.
References
- 1.Yazyu H, et al. Nucleotide sequence of the melB gene and characteristics of deduced amino acid sequence of the melibiose carrier in Escherichia coli. J Biol Chem. 1984;259:4320–4326. [PubMed] [Google Scholar]
- 2.Saier MH., Jr Families of transmembrane sugar transport proteins. Mol Microbiol. 2000;35:699–710. doi: 10.1046/j.1365-2958.2000.01759.x. [DOI] [PubMed] [Google Scholar]
- 3.Tokuda H, Kaback HR. Sodium-dependent methyl 1-thio-β-d-galactopyranoside transport in membrane vesicles isolated from Salmonella typhimurium. Biochemistry. 1977;16:2130–2136. doi: 10.1021/bi00629a013. [DOI] [PubMed] [Google Scholar]
- 4.Tsuchiya T, Raven J, Wilson TH. Co-transport of Na+ and methul-beta-d-thiogalactopyranoside mediated by the melibiose transport system of Escherichia coli. Biochem Biophys Res Commun. 1977;76:26–31. doi: 10.1016/0006-291x(77)91663-1. [DOI] [PubMed] [Google Scholar]
- 5.Bassilana M, Pourcher T, Leblanc G. Facilitated diffusion properties of melibiose permease in Escherichia coli membrane vesicles. Release of co-substrates is rate limiting for permease cycling. J Biol Chem. 1987;262:16865–16870. [PubMed] [Google Scholar]
- 6.Wilson DM, Wilson TH. Cation specificity for sugar substrates of the melibiose carrier in Escherichia coli. Biochim Biophys Acta. 1987;904:191–200. doi: 10.1016/0005-2736(87)90368-3. [DOI] [PubMed] [Google Scholar]
- 7.Tsuchiya T, Wilson TH. Cation-sugar cotransport in the melibiose transport system of Escherichia coli. Membr Biochem. 1978;2:63–79. doi: 10.3109/09687687809063858. [DOI] [PubMed] [Google Scholar]
- 8.Bassilana M, Pourcher T, Leblanc G. Melibiose permease of Escherichia coli. Characteristics of co-substrates release during facilitated diffusion reactions. J Biol Chem. 1988;263:9663–9667. [PubMed] [Google Scholar]
- 9.Kaback HR, Sahin-Toth M, Weinglass AB. The kamikaze approach to membrane transport. Nat Rev Mol Cell Biol. 2001;2:610–620. doi: 10.1038/35085077. [DOI] [PubMed] [Google Scholar]
- 10.Guan L, Kaback HR. Lessons from lactose permease. Annu Rev Biophys Biomol Struct. 2006;35:67–91. doi: 10.1146/annurev.biophys.35.040405.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pourcher T, Bibi E, Kaback HR, Leblanc G. Membrane topology of the melibiose permease of Escherichia coli studied by melB-phoA fusion analysis. Biochemistry. 1996;35:4161–4168. doi: 10.1021/bi9527496. [DOI] [PubMed] [Google Scholar]
- 12.Pourcher T, Leclercq S, Brandolin G, Leblanc G. Melibiose permease of Escherichia coli: Large scale purification and evidence that H+, Na+, and Li+ sugar symport is catalyzed by a single polypeptide. Biochemistry. 1995;34:4412–4420. doi: 10.1021/bi00013a033. [DOI] [PubMed] [Google Scholar]
- 13.Botfield MC, Naguchi K, Tsuchiya T, Wilson TH. Membrane topology of the melibiose carrier of Escherichia coli. J Biol Chem. 1992;267:1818–1822. [PubMed] [Google Scholar]
- 14.Gwizdek C, Leblanc G, Bassilana M. Proteolytic mapping and substrate protection of the Escherichia coli melibiose permease. Biochemistry. 1997;36:8522–8529. doi: 10.1021/bi970312n. [DOI] [PubMed] [Google Scholar]
- 15.Hacksell I, et al. Projection structure at 8 AÅ resolution of the melibiose permease, an Na-sugar co-transporter from Escherichia coli. EMBO J. 2002;21:3569–3574. doi: 10.1093/emboj/cdf378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purhonen P, Lundback AK, Lemonnier R, Leblanc G, Hebert H. Three-dimensional structure of the sugar symporter melibiose permease from cryo-electron microscopy. J Struct Biol. 2005;152:76–83. doi: 10.1016/j.jsb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Guan L, Mirza O, Verner G, Iwata S, Kaback HR. Structural determination of wild-type lactose permease. Proc Natl Acad Sci USA. 2007;104:15294–15298. doi: 10.1073/pnas.0707688104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abramson J, et al. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 19.Mirza O, Guan L, Verner G, Iwata S, Kaback HR. Structural evidence for induced fit and a mechanism for sugar/H+ symport in LacY. EMBO J. 2006;25:1177–1183. doi: 10.1038/sj.emboj.7601028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301:616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 21.Yin Y, He X, Szewczyk P, Nguyen T, Chang G. Structure of the multidrug transporter EmrD from Escherichia coli. Science. 2006;312:741–744. doi: 10.1126/science.1125629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi J, Blundell TL, Mizuguchi K. FUGUE: Sequence-structure homology recognition using environment-specific substitution tables and structure-dependent gap penalties. J Mol Biol. 2001;310:243–257. doi: 10.1006/jmbi.2001.4762. [DOI] [PubMed] [Google Scholar]
- 23.Teodorescu O, Galor T, Pillardy J, Elber R. Enriching the sequence substitution matrix by structural information. Proteins. 2004;54:41–48. doi: 10.1002/prot.10474. [DOI] [PubMed] [Google Scholar]
- 24.Kelley LA, Sternberg MJ. Protein structure prediction on the Web: A case study using the Phyre server. Nat Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 25.von Heijne G. Control of topology and mode of assembly of a polytopic membrane protein by positively charged residue. Nature. 1989;341:456–458. doi: 10.1038/341456a0. [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Dayem M, Basquin C, Pourcher T, Cordat E, Leblanc G. Cytoplasmic loop connecting helices IV and V of the melibiose permease from Escherichia coli is involved in the process of Na+-coupled sugar translocation. J Biol Chem. 2003;278:1518–1524. doi: 10.1074/jbc.M210053200. [DOI] [PubMed] [Google Scholar]
- 27.Meyer-Lipp K, et al. The inner interhelix loop 4–5 of the melibiose permease from Escherichia coli takes part in conformational changes after sugar binding. J Biol Chem. 2006;281:25882–25892. doi: 10.1074/jbc.M601259200. [DOI] [PubMed] [Google Scholar]
- 28.Ding PZ. Loop X/XI, the largest cytoplasmic loop in the membrane-bound melibiose carrier of Escherichia coli, is a functional re-entrant loop. Biochim Biophys Acta. 2004;1660:106–117. doi: 10.1016/j.bbamem.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Kaback HR. Structure and mechanism of the lactose permease. C R Biol. 2005;328:557–567. doi: 10.1016/j.crvi.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Smirnova I, et al. Sugar binding induces an outward facing conformation of LacY. Proc Natl Acad Sci USA. 2007;104:16504–16509. doi: 10.1073/pnas.0708258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaback HR, et al. Site-directed alkylation and the alternating access model for LacY. Proc Natl Acad Sci USA. 2007;104:491–494. doi: 10.1073/pnas.0609968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuzaki S, Weissborn AC, Tamai E, Tsuchiya T, Wilson TH. Melibiose carrier of Escherichia coli: Use of cysteine mutagenesis to identify the amino acids on the hydrophilic face of transmembrane helix 2. Biochim Biophys Acta. 1999;1420:63–72. doi: 10.1016/s0005-2736(99)00087-5. [DOI] [PubMed] [Google Scholar]
- 33.Pourcher T, Deckert M, Bassilana M, Leblanc G. Melibiose permease of Escherichia coli: Mutation of aspartic acid 55 in putative helix II abolishes activation of sugar binding by Na+ ions. Biochem Biophys Res Commun. 1991;178:1176–1181. doi: 10.1016/0006-291x(91)91016-6. [DOI] [PubMed] [Google Scholar]
- 34.Hama H, Wilson TH. Replacement of alanine 58 by asparagine enables the melibiose carrier of Klebsiella pneumoniae to couple sugar transport to Na+ J Biol Chem. 1994;269:1063–1067. [PubMed] [Google Scholar]
- 35.Pourcher T, Zani ML, Leblanc G. Mutagenesis of acidic residues in putative membrane-spanning segments of the melibiose permease of Escherichia coli. I. Effect on Na(+)-dependent transport and binding properties. J Biol Chem. 1993;268:3209–3215. [PubMed] [Google Scholar]
- 36.Zani ML, Pourcher T, Leblanc G. Mutation of polar and charged residues in the hydrophobic NH2-terminal domains of the melibiose permease of Escherichia coli. J Biol Chem. 1994;269:24883–24889. [PubMed] [Google Scholar]
- 37.Zani ML, Pourcher T, Leblanc G. Mutagenesis of acidic residues in putative membrane-spanning segments of the melibiose permease of Escherichia coli. II. Effect on cationic selectivity and coupling properties. J Biol Chem. 1993;268:3216–3221. [PubMed] [Google Scholar]
- 38.Leon X, Lemonnier R, Leblanc G, Padros E. Changes in secondary structures and acidic side chains of melibiose permease upon cosubstrates binding. Biophys J. 2006;91:4440–4449. doi: 10.1529/biophysj.106.090241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cordat E, Leblanc G, Mus-Veteau I. Evidence for a role of helix IV in connecting cation- and sugar-binding sites of Escherichia coli melibiose permease. Biochemistry. 2000;39:4493–4499. doi: 10.1021/bi991852i. [DOI] [PubMed] [Google Scholar]
- 40.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 41.Faham S, et al. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science. 2008;321:810–814. doi: 10.1126/science.1160406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weyand S, et al. Structure and molecular mechanism of a nucleobase-cation-symport-1 family transporter. Science. 2008;322:709–713. doi: 10.1126/science.1164440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boudker O, Ryan RM, Yernool D, Shimamoto K, Gouaux E. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature. 2007;445:387–393. doi: 10.1038/nature05455. [DOI] [PubMed] [Google Scholar]
- 44.Franco PJ, Wilson TH. Arg-52 in the melibiose carrier of Escherichia coli is important for cation-coupled sugar transport and participates in an intrahelical salt bridge. J Bacteriol. 1999;181:6377–6386. doi: 10.1128/jb.181.20.6377-6386.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franco PJ, Jena AB, Wilson TH. Physiological evidence for an interaction between helices II and XI in the melibiose carrier of Escherichia coli. Biochim Biophys Acta. 2001;1510:231–242. doi: 10.1016/s0005-2736(00)00353-9. [DOI] [PubMed] [Google Scholar]
- 46.Guan L, Hu Y, Kaback HR. Aromatic stacking in the sugar binding site of the lactose permease. Biochemistry. 2003;42:1377–1382. doi: 10.1021/bi027152m. [DOI] [PubMed] [Google Scholar]
- 47.Kasho VN, Smirnova IN, Kaback HR. Sequence alignment and homology threading reveals prokaryotic and eukaryotic proteins similar to lactose permease. J Mol Biol. 2006;358:1060–1070. doi: 10.1016/j.jmb.2006.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ambroise Y, Leblanc G, Rousseau B. Active-site-directed photolabeling of the melibiose permease of Escherichia coli. Biochemistry. 2000;39:1338–1345. doi: 10.1021/bi9916224. [DOI] [PubMed] [Google Scholar]
- 49.Mus-Veteau I, Leblanc G. Melibiose permease of Escherichia coli: Structural organization of cosubstrate binding sites as deduced from tryptophan fluorescence analyses. Biochemistry. 1996;35:12053–12060. doi: 10.1021/bi961372g. [DOI] [PubMed] [Google Scholar]
- 50.Meyer-Lipp K, Ganea C, Pourcher T, Leblanc G, Fendler K. Sugar binding induced charge translocation in the melibiose permease from Escherichia coli. Biochemistry. 2004;43:12606–12613. doi: 10.1021/bi0489053. [DOI] [PubMed] [Google Scholar]
- 51.Hunte C, et al. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature. 2005;435:1197–1202. doi: 10.1038/nature03692. [DOI] [PubMed] [Google Scholar]
- 52.Collaborative Computational Project N. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 53.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc Natl Acad Sci USA. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Landau M, et al. ConSurf 2005: The projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res. 2005;33:W299–W302. doi: 10.1093/nar/gki370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.