Abstract
Breast cancer resistance protein (BCRP, ABCG2) is expressed in the hepatic canalicular membrane and mediates biliary excretion of xenobiotics including sulfate and glucuronide metabolites of some compounds. Hepatic Bcrp expression is sex-dependent, with higher expression in male mice. The hypothesis that sex-dependent Bcrp expression influences the hepatobiliary disposition of phase II metabolites was tested in the present study using acetaminophen (APAP) and the generated APAP glucuronide (AG) and sulfate (AS) metabolites in single-pass in situ perfused livers from male and female wild-type and Abcg–/– (Bcrp-deficient) mice. Pharmacokinetic modeling was used to estimate parameters governing the hepatobiliary disposition of APAP, AG, and AS. In wild-type mice, the biliary excretion rate constant was 2.5- and 7-fold higher in males than in females for AS and AG, respectively, reflecting male-predominant Bcrp expression. Sex-dependent differences in AG biliary excretion were not observed in Bcrp-deficient mice, and AS biliary excretion was negligible. Interestingly, sex-dependent basolateral excretion of AG (higher in males) and AS (higher in females) was noted in wild-type mice with a similar trend in Bcrp-deficient mouse livers, reflecting an increased rate constant for AG formation in male and AS formation in female mouse livers. In addition, the rate constant for AS basolateral excretion was increased significantly in female mouse livers compared with that in male mouse livers. It is interesting to note that multidrug resistance-associated protein 4 was higher in female than in male mouse livers. In conclusion, sex-dependent differences in conjugation and transporter expression result in profound differences in the hepatobiliary disposition of AG and AS in male and female mouse livers.
Breast cancer resistance protein (BCRP, ABCG2) belongs to the ATP-binding cassette (ABC) transporter G family and was first cloned in 1998 from mitoxantrone-resistant cell lines (Doyle et al., 1998). BCRP is highly expressed in the canalicular membrane of the hepatocyte as well as in the intestinal epithelia, blood-brain barrier, and placenta (Robey et al., 2009). BCRP has wide substrate specificity, including organic anions, cations, and zwitterions. BCRP mediates the transport of sulfated hormone metabolites, SN-38, mitoxantrone, topotecan, doxorubicin, flavopiridol, nitrofurantoin, various flavonoids, and the chlorophyll metabolite pheophorbide A (Robey et al., 2001; Jonker et al., 2002, 2005; Nakagawa et al., 2002; Bates et al., 2004; Imai et al., 2004; Merino et al., 2005a).
Male-predominant expression of Bcrp has been reported in mouse liver (Merino et al., 2005b; Tanaka et al., 2005). Bcrp is influenced by sex hormones; estradiol suppresses and testosterone induces Bcrp expression (Tanaka et al., 2005). Sex-dependent differences in Bcrp expression have been shown to influence the pharmacokinetics of drugs (Merino et al., 2005b). For example, the area under the plasma concentration-time curve of nitrofurantoin in female wild-type mice was ∼2-fold higher than that in male mice after oral administration; the cumulative biliary excretion of nitrofurantoin was 9-fold higher in male than in female mice. In contrast, no significant sex differences in plasma concentrations or the hepatobiliary excretion of nitrofurantoin were observed in Bcrp-deficient mice, indicating that hepatic Bcrp protein contributes to sex differences in wild-type mice (Merino et al., 2005b).
The role of various transport proteins in the biliary excretion of phase II metabolites has been examined using several compounds including acetaminophen, 4-methylumbelliferone, and harmol, which are metabolized primarily to glucuronide and sulfate conjugates in mice (Zamek-Gliszczynski et al., 2006b). In situ single-pass liver perfusion in male mice demonstrated that Bcrp plays a predominant role in the biliary excretion of sulfate conjugates, whereas the biliary excretion of glucuronide conjugates was mediated by both Bcrp and, to a lesser extent, multidrug resistance-associated protein (Mrp) 2. Glucuronide and sulfate conjugates of acetaminophen (AG and AS, respectively) are transported from the hepatocytes into sinusoidal blood by basolateral transport proteins; Mrp3 mediates basolateral excretion of AG whereas Mrp3, Mrp4, and other transport proteins may be involved in the basolateral excretion of AS in mice (Xiong et al., 2002; Manautou et al., 2005; Zamek-Gliszczynski et al., 2006a). In the liver, mRNA levels of Mrp4 were higher in female than in male C57BL/6 mice, but Mrp2 and Mrp3 mRNA levels did not differ based on gender (Maher et al., 2005). Sex-dependent differences in the disposition of some phase II conjugates might be anticipated, considering the important role of transport proteins in the biliary and basolateral excretion of phase II conjugates. In addition, these sex-dependent differences in hepatobiliary disposition may be of potential clinical relevance, as some sulfate conjugates are important from a physiologic (e.g., dehydroepiandrosterone sulfate and estrone sulfate) or pharmacologic (e.g., minoxidil sulfate) standpoint.
The purpose of this study was to examine the influence of Bcrp and sex on the hepatobiliary disposition of phase II metabolites, using APAP as a model substrate. In situ single-pass liver perfusion in male and female wild-type and Bcrp-deficient mice was used to examine the hepatobiliary disposition of AG and AS. Compartmental pharmacokinetic modeling was performed to recover relevant parameters governing hepatobiliary disposition of AG and AS. In light of the physiologic and pharmacologic roles that some conjugates play in vivo, it is important to investigate the potential for significant sex-dependent differences in hepatobiliary disposition of sulfate and glucuronide metabolites.
Materials and Methods
Materials. APAP, AG, AS, cimetidine, taurocholate, and Krebs-Henseleit buffer packets were purchased from Sigma-Aldrich (St. Louis, MO). All other reagents were of analytical grade and were available commercially.
Mice. Male and female C57BL/6 (wild-type) and Abcg2–/– (Bcrp-deficient) (created by Deltagen Inc., San Mateo, CA) mice (23–30 g) were a gift from Eli Lilly and Company (Indianapolis, IN). Further information regarding background and breeding of these mice has been detailed previously (Nezasa et al., 2006). All animal procedures were approved by the institutional animal care and use committee at the University of North Carolina at Chapel Hill.
In Situ Single-Pass Liver Perfusion. Single-pass mouse liver perfusion studies were performed as described previously (Zamek-Gliszczynski et al., 2006b). The abdominal cavity of each mouse was opened to expose the intestines, liver, and gallbladder. The common bile duct above the duodenum was ligated, and the gallbladder was cannulated with PE-10 tubing (BD Biosciences, Franklin Lakes, NJ). A loose suture was placed around the inferior vena cava below the liver. The portal vein was cannulated with a 20-gauge catheter (B. Braun Medical, Inc., Bethlehem, PA), and the liver was perfused with oxygenated drug-free Krebs-Henseleit buffer containing 5 μM taurocholate at a flow rate of 5 ml/min. The inferior vena cava below the liver was severed immediately by an incision below the loose suture. Thereafter, the inferior vena cava above the liver was cannulated with a 20-gauge catheter. The inferior vena cava below the liver was ligated to direct all perfusate outflow to the inferior vena cava above the liver. After a 15-min preperfusion period to equilibrate liver temperature and stabilize bile flow, the liver was perfused with buffer containing 500 nM APAP for 40 min. Liver perfusion was continued for an additional 30 min after APAP infusion with drug-free buffer. Perfusate outflow was collected at designated time points throughout the 70-min experiment. Bile was collected in toto at 10-min intervals. At the end of the perfusion, livers were isolated and snap-frozen.
Analytical Methods. Perfusate, bile, and liver homogenate samples were diluted 1:5, 1:250, and 1:10, respectively, to obtain a final sample aliquot of 500 μl. Samples were protein-precipitated by the addition of 390 μl of ice-cold methanol containing the internal standard cimetidine. After removal of protein by centrifugation at 3000g for 15 min at 4°C, the supernatants were analyzed by liquid chromatography with detection by tandem mass spectrometry (API 4000 triple quadrupole spectrometer with TurboIonSpray interface; Applied Biosystems Foster City, CA). AS, AG, and the internal standard (cimetidine) were eluted from an Aquasil C18 column (2.1 × 50 mm, dp = 5 μm; Thermo Fisher Scientific, Waltham, MA) using a mobile phase gradient (A, 0.1% formic acid in water and B, 0.1% formic acid in methanol; 0–0.75 min hold at 5% B, 0.75–2.5 min linear gradient to 70% B, 2.5–3.5 min hold at 70% B, 3.5–3.6 min linear gradient to 5% B, and 3.6–4 min hold at 5% B; flow rate, 0.75 ml/min; 0.8–4 min directed to mass spectrometer) and were detected in negative ion mode using multiple reaction monitoring: AS, 230 → 150 m/z; AG, 326 → 150 m/z; and cimetidine, 251 → 157 m/z. Concentrations of APAP and the internal standard (cimetidine) were quantified using the chromatographic conditions detailed above but were detected in positive ion mode using multiple reaction monitoring: acetaminophen, 152 → 110 m/z; and cimetidine, 253 → 117 m/z. Care was taken to confirm that no in-source fragmentation of the conjugates back to APAP occurred. Standard curves of each analyte (0.5–1000 nM) were prepared in the appropriate matrices; inter- and intraday coefficients of variation were <15%.
Western Blot Analysis. Mouse livers, perfused with phosphate-buffered saline solution, were removed promptly and frozen. Livers were homogenized with 3 volumes of Tris-HCl solution (pH 7.4) containing complete protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). The homogenates were centrifuged at 1500g for 10 min, and the supernatant was ultracentrifuged at 100,000g for 30 min. The resulting pellet was suspended in Tris-HCl solution (pH 7.4) containing complete protease inhibitor cocktail, 1% SDS, and 1 mM EDTA. The protein concentration of the solution was determined with a BCA protein assay reagent kit (Pierce Biotechnology, Rockford, IL). Bcrp expression was determined using the anti-BCRP primary monoclonal antibody (BXP-53; Alexis Biochemicals, Carlsbad, CA) at a 1:1000 dilution. Anti-MRP4 (M4I-10; Alexis Biochemicals) was used at a 1:1000 dilution to determine the expression of Mrp4. Protein loading was normalized to β-actin, which was detected using a β-actin antibody at a dilution of 1:5000 (C4; Millipore Bioscience Research Reagents, Temecula, CA). Immunoreactive protein bands were detected by chemiluminescence using a VersaDoc imaging system (Bio-Rad, Hercules, CA), and densitometry analysis was performed using the Quantity One software package (version 4.1; Bio-Rad).
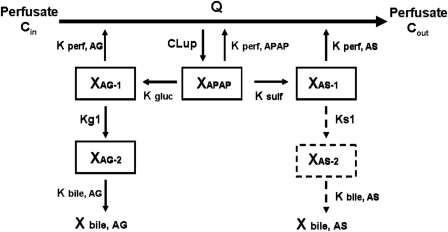
Pharmacokinetic Modeling. A compartmental modeling approach was used to describe the hepatobiliary disposition of APAP and metabolites in perfused livers from male and female wild-type and Bcrp-deficient mice and to recover estimates of key pharmacokinetic parameters. AG and AS outflow perfusate and biliary excretion rates were calculated as the product of flow (perfusate or bile) and outflow perfusate or biliary concentrations, respectively, during each collection period and then were normalized to liver weight. Several structurally distinct models were evaluated; standard model selection criteria including residual distribution and Akaike's information criterion were used to identify the optimal structure. The compartmental model that best described the data is shown in Fig. 1. Differential equations derived from the model scheme in Fig. 1 were as follows (initial conditions were denoted with a superscript zero):
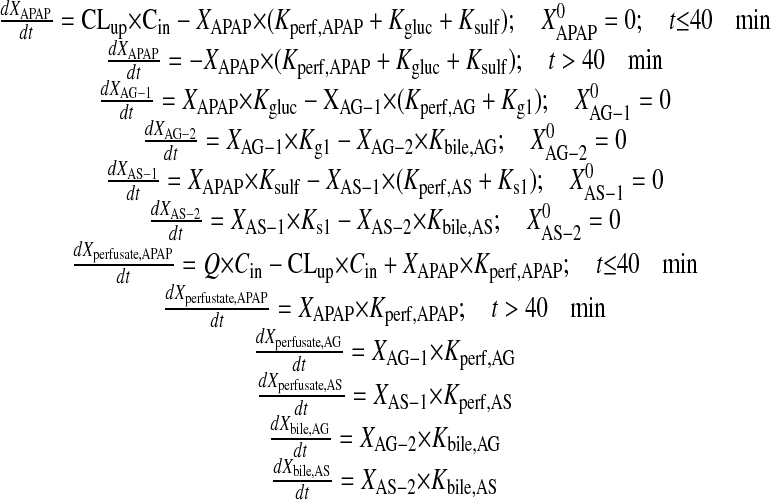 |
Fig. 1.
Model scheme depicting the hepatic disposition of APAP, AG, and AS in the single-pass perfused mouse liver. Q, flow rate; Cin, concentration of APAP in inflow perfusate; Cout, concentration of APAP in outflow perfusate; XAPAP, amount of APAP in liver compartment; XAG-1 and XAG-2, amount of AG in liver compartments 1 and 2, respectively; XAS-1 and XAS-2, amount of AS in liver compartments 1 and 2, respectively; CLup, uptake clearance into the liver; Kperf, APAP, first-order rate constant for basolateral excretion of APAP; Kperf, AG, first-order rate constant for basolateral excretion of AG; Kperf, AS, first-order rate constant for basolateral excretion of AS; Kgluc and Ksulf, first-order rate constants for formation of AG and AS, respectively; Kg1, first-order rate constant for movement of AG from hepatic compartment 1 to 2; Ks1, first-order rate constant for movement of AS from hepatic compartment 1 to 2; and Kbile, AG and Kbile, AS, first-order rate constants for biliary excretion of AG and AS, respectively. AS biliary excretion was lower than the limit of quantitation in Bcrp-deficient mouse livers.
The final model included compartments representing hepatocellular spaces for APAP, AG, and AS. To describe the prolonged biliary excretion of AG and AS after APAP washout relative to basolateral excretion of AG and AS, an intervening compartment between the site of conjugate formation and the bile compartment was included in the model. This intervening compartment was required to describe disposition of AG in all livers and of AS in wild-type but not in Bcrp-deficient mouse livers (AS biliary concentrations measured in Bcrp-deficient mouse livers were below the limit of quantitation of the assay). Differential equations describing substrate flux in each compartment were fit simultaneously to the perfusate and biliary excretion rate data by nonlinear least-squares regression (WinNonlin Pro version 4.2; Pharsight, Mountain View, CA) with a weighting scheme of 1/(Y predicted). The precision of parameter estimates was maximized with stepwise nonlinear least-squares regression. AG perfusate and biliary excretion rate data from male and female wild-type and Bcrp-deficient mouse livers were used to estimate Kperf, AG, Kg1, and Kbile, AG, whereas CLup, Kperf, APAP, Kgluc, and Ksulf were held constant at the estimated values. Likewise, AS perfusate and biliary excretion rate data were used to estimate Kperf, AS, Ks1, and Kbile, AS, whereas CLup, Kperf, APAP, Kgluc, and Ksulf were held constant at the estimated values.
Data Analysis. All data are expressed as the mean ± SD unless otherwise indicated. The effects of Bcrp expression (wild-type or Bcrp-deficient) and sex (male or female) were assessed using a two-way analysis of variance. Data shown in Table 1 and Figs. 3 and 4 were analyzed further with a Bonferroni post hoc test, but no further analysis was performed for the pharmacokinetic parameter estimates shown in Table 2. An unpaired Student's t test was used to determine the statistical significance of differences in AS biliary excretion from male and female mouse livers because AS biliary excretion was negligible in Bcrp-deficient mice. Differences were considered to be statistically significant when p < 0.05.
TABLE 1.
Mouse body weights, liver weights normalized to body weight, APAP extraction ratio, and total recovery at 70 min in in situ single-pass perfused livers from male and female wild-type and Bcrp-deficient mice
Livers were perfused with Krebs-Henseleit buffer containing 500 nM APAP for 40 min followed by an additional 30-min infusion with drug-free buffer. Data are the mean ± S.D. (n = 3–4/group).
|
Wild-Type
|
Bcrp-Deficient
|
|||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Body weight (g) | 28.4 ± 0.8* | 25.8 ± 1.8* | 25.9 ± 1.7 | 24.5 ± 1.7 |
| Liver weight/body weight (%) | 4.7 ± 0.2 | 4.8 ± 0.5 | 4.3 ± 0.2 | 4.4 ± 0.7 |
| Extraction ratio (%) | 35.4 ± 4.8 | 29.6 ± 7.1 | 30.8 ± 6.1 | 24.8 ± 1.2 |
| Total recovery (% of dose) | 98.4 ± 5.2 | 91.7 ± 8.5 | 93.6 ± 3.7 | 98.2 ± 4.8 |
Groups denoted with an asterisk were significantly different, p < 0.05.
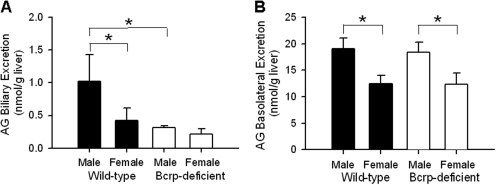
Fig. 3.
Cumulative biliary excretion and basolateral excretion of AG (A and B, respectively; nanomoles per gram of liver) in male and female wild-type and Bcrp-deficient perfused mouse livers through 70 min as described in the legend to Fig. 2 (n = 3–4/group; *, groups denoted with an asterisk were significantly different, p < 0.05).
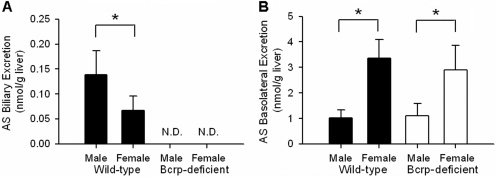
Fig. 4.
Cumulative biliary excretion and basolateral excretion of AS (A and B, respectively; nanomoles per gram of liver) in male and female wild-type and Bcrp-deficient perfused mouse livers through 70 min as described in the legend to Fig. 2 (n = 3–4/group; *, groups denoted with an asterisk were significantly different, p < 0.05). N.D., not detected.
TABLE 2.
Mean pharmacokinetic parameter estimates governing APAP, AG, and AS disposition in in situ single-pass perfused livers from wild-type and Bcrp-deficient male and female mice
n = 3–4/group.
|
Mean Estimates (CV %)
|
||
|---|---|---|
| Parameter | Wild-Type | Brcp-Deficient |
| CLup (ml/min) | ||
| Male | 5.63 (12.1) | 5.60 (13.2) |
| Female | 5.79 (4.9) | 5.55 (8.8) |
| Kperf, APAP (min-1) | ||
| Male | 0.468 (16.5) | 0.588 (44.7) |
| Female | 0.525 (31.1) | 0.564 (36.3) |
| Kgluc (min-1)* | ||
| Male | 0.183 (36.9) | 0.199 (17.3) |
| Female | 0.121 (16.5) | 0.119 (8.2) |
| Ksulf (min-1)* | ||
| Male | 0.007 (13.1) | 0.009 (50.6) |
| Female | 0.026 (8.8) | 0.018 (44.6) |
| Kperf, AG (min-1) | ||
| Male | 0.168 (45.4) | 0.247 (22.5) |
| Female | 0.217 (49.3) | 0.214 (77.3) |
| Kg1 (min-1) | ||
| Male | 0.044 (89.3) | 0.104 (36.7) |
| Female | 0.093 (49.4) | 0.092 (55.3) |
| Kbile, AG (min-1)*, ** | ||
| Male | 0.014 (45.4) | 0.001 (66.4) |
| Female | 0.002 (68.1) | 0.001 (72.1) |
| Kperf, AS (min-1)* | ||
| Male | 0.379 (38.6) | 0.271 (11.7) |
| Female | 0.663 (17.6) | 0.634 (9.6) |
| Ks1 (min-1)* | ||
| Male | 0.048 (25.3) | N.A. |
| Female | 0.017 (39.5) | N.A. |
| Kbile, AS (min-1)* | ||
| Male | 0.107 (8.0) | N.A. |
| Female | 0.043 (72.6) | N.A. |
CV, coefficient of variation; N.A., not available.
p < 0.05, male vs. female.
p < 0.05, wild-type vs. Bcrp-deficient mice.
Results
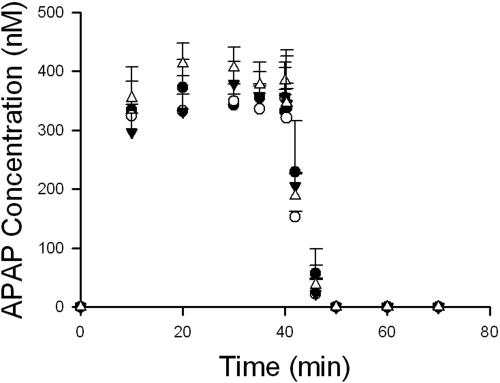
Statistically significant differences were observed in body weights among male and female wild-type mice, but mouse liver weights normalized for body weight were comparable between wild-type and Bcrp-deficient male and female mice (Table 1). Outflow perfusate concentrations of APAP reached a plateau within 20 min of continuous perfusion (Fig. 2). The hepatic extraction ratios of APAP, calculated from inflow and outflow APAP perfusate concentrations between 30 and 40 min of perfusion, ranged from 24.8 to 35.4% among mouse groups (Table 1). Because APAP, AG, and AS concentrations in liver homogenates after the 30-min washout phase were negligible, total recovery (% of dose) was calculated by adding recovered APAP, AG, and AS in outflow perfusate and bile; in all cases, total recovery was comparable among all mouse groups (Table 1).
Fig. 2.
APAP concentrations (nanomolar) in outflow perfusate from male wild-type (•) and Bcrp-deficient (○) and female wild-type (▾) and Bcrp-deficient (▵) mouse liver perfusions. Livers were perfused with Krebs-Henseleit buffer containing 500 nM APAP for 40 min followed by an additional 30-min infusion with drug-free buffer. (n = 3–4/group).
Biliary excretion of AG in livers from male wild-type mice was ∼2.5-fold higher than that in female wild-type mouse livers, but AG biliary excretion between male and female Bcrp-deficient mice was comparable (Fig. 3A). Relative to the corresponding wild-type mouse livers, biliary excretion of AG was decreased ∼73 and ∼54% in male and female Bcrp-deficient mouse livers, respectively. Cumulative AG excretion in outflow perfusate from male wild-type and Bcrp-deficient livers was ∼1.6-fold higher than that from female wild-type and Bcrp-deficient livers (Fig. 3B).
Biliary excretion of AS in livers from male wild-type mice was ∼2.2-fold higher than that from female wild-type livers (Fig. 4A). In Bcrp-deficient mice, AS biliary recovery was negligible. AS basolateral excretion in female wild-type and Bcrp-deficient mouse livers was ∼3.1- and ∼2.4-fold higher, respectively, than that in livers from their male counterparts (Fig. 4B).
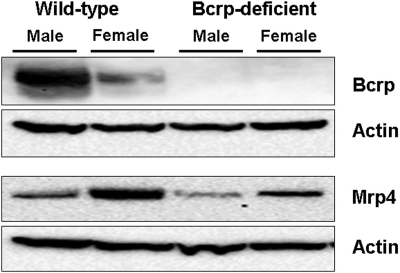
Immunoblot analysis was performed with crude membrane fractions of liver homogenates from male and female wild-type and Bcrp-deficient mice to compare the levels of Bcrp and Mrp4 protein (Fig. 5). As reported previously (Tanaka et al., 2005), densitometry analysis revealed that hepatic Bcrp protein, normalized to the β-actin signal, was 3-fold higher in male wild-type mice than in female wild-type mice. Interestingly, there was a trend toward increased hepatic Mrp4 protein in females; Mrp4 protein, normalized to the β-actin signal, in female wild-type and Bcrp-deficient mouse livers was 3.5- and 1.6-fold higher than that in male mouse livers from wild-type and Bcrp-deficient mice, respectively.
Fig. 5.
Representative immunoblot of Bcrp, Mrp4, and actin in crude membrane fractions from male and female wild-type and Bcrp-deficient mouse liver homogenates from three independent experiments (30 μg protein/well).
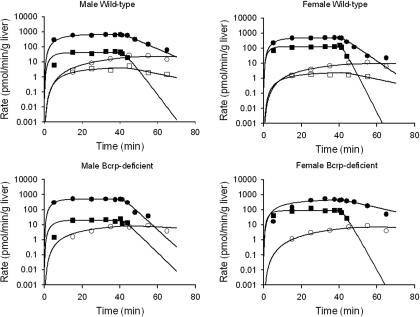
The model scheme presented in Fig. 1 best described the current experimental data. Representative fits of the model to APAP, AG, and AS biliary and basolateral excretion rate versus time data are shown in Fig. 6. Pharmacokinetic parameter estimates obtained by resolving the differential equations representing biliary and basolateral excretion rate versus time data for APAP, AG, and AS are presented in Table 2. The uptake clearance (CLup) was comparable with the perfusate flow rate (Q; 5 ml/min). The rate constant for APAP basolateral excretion (Kperf, APAP) was comparable among male and female wild-type and Bcrp-deficient mouse livers. Sex-dependent differences in metabolite formation rate constants were observed; the rate constant for AG formation (Kgluc) was ∼2-fold higher in male mouse livers, and the rate constant for AS formation (Ksulf) was ∼2 to 4-fold higher in female mouse livers. The basolateral excretion rate constant of AS (Kperf, AS), but not that of AG (Kperf, AG), differed based on sex; the AS basolateral excretion rate constant was ∼2-fold higher in female versus male mouse livers. The AG biliary excretion rate constant (Kbile, AG) was higher in male than in female wild-type mouse livers (0.014 versus 0.002 min–1) but was similar between male and female Bcrp-deficient mouse livers. The rate constant for movement of AS from hepatic compartment 1 to 2 (Ks1) was higher in male than in female wild-type mouse livers. Likewise, the rate constant for AS biliary excretion (Kbile, AS) was higher in male versus female wild-type mouse livers (0.107 versus 0.043 min–1).
Fig. 6.
Representative outflow perfusate rate (picomoles per minute per gram of liver) of AG (•) and AS (▪) and biliary excretion rate (picomoles per minute per gram of liver) of AG (○) and AS (□) in male and female wild-type and Bcrp-deficient mouse livers. Livers were perfused with Krebs-Henseleit buffer containing 500 nM APAP for 40 min followed by an additional 30-min infusion with drug-free buffer. Curves represent the best fit of the model scheme depicted in Fig. 1 to the data.
Discussion
The current studies support the hypothesis that sex-dependent hepatic Bcrp expression influences the hepatobiliary disposition of sulfate and glucuronide conjugates of the model substrate APAP during in situ single-pass mouse liver perfusion. Male-predominant Bcrp protein expression in wild-type mouse livers is consistent with ∼2.5-fold higher biliary excretion of AG and AS in male than in female wild-type mouse livers. Sex-dependent differences in biliary excretion of AG were not observed in Bcrp-deficient mice. Pharmacokinetic modeling revealed that the rate constants governing AG and AS biliary excretion were significantly higher in male than in female wild-type mouse livers. AS biliary excretion was negligible and AG biliary excretion rate constants were markedly reduced in Bcrp-deficient mice, consistent with recent findings demonstrating an important role of Bcrp in AG and AS biliary excretion in male mice (Zamek-Gliszczynski et al., 2006b). Both Bcrp and Mrp2 seem to be involved in AS biliary excretion in rats (Zamek-Gliszczynski et al., 2005); however, AS biliary excretion was not decreased in Mrp2-deficient mice (Zamek-Gliszczynski et al., 2006b). This apparent discrepancy in the mechanisms of AS biliary excretion may be due to species-specific differences in the affinity of multiple hepatic efflux proteins for AS.
It is interesting to note that sex differences were observed for basolateral excretion of AG and AS, with more extensive basolateral excretion of AG in male and more extensive AS basolateral excretion in female mouse livers (Figs. 3B and 4B). One possible explanation for these findings is that the rate constant governing AG formation (Kgluc) was greater in male mouse livers, whereas the rate constant for AS formation (Ksulf) was greater in female mouse livers, suggesting sex-dependent differences in formation of AG and AS. Although the primary isoforms of UDP-glucuronosyltransferases (Ugt) and sulfotransferases (Sult) responsible for AG and AS formation in mice remain to be elucidated, previously published data demonstrate male-predominant mRNA expression of Ugt1a5 and female-predominant mRNA expression of Sult1a1 and Sult2a1/2 in mouse livers (Alnouti and Klaassen, 2006; Buckley and Klaassen, 2007). In human liver microsomes, protein expression of UGT1A6, a major isoform responsible for AG formation, was 50% higher in males than in females (Court et al., 2001).
Sex-dependent basolateral excretion of AG and AS also may be due to differences in the expression of transport protein(s) responsible for AG and AS basolateral excretion. As reported previously, Mrp3 plays a significant role in basolateral excretion of AG, whereas both Mrp3 and Mrp4, as well as other unidentified mechanisms, may be involved in AS basolateral excretion (Xiong et al., 2002; Manautou et al., 2005; Zamek-Gliszczynski et al., 2006a). Previous studies showed moderate expression of Mrp3 mRNA in mouse livers, but no differences in Mrp3 mRNA were noted between male and female mouse livers (Maher et al., 2005). In the current study, pharmacokinetic modeling revealed that the rate constants for AG basolateral excretion between male and female mouse livers were not different. Thus, greater AG basolateral excretion in male versus female mouse livers appears to be due primarily to more extensive glucuronidation in male mouse livers. In contrast, the rate constant governing AS basolateral excretion (Kperf, AS) was significantly higher in female versus male mouse livers; this finding cannot be explained by increased AS formation in female mice. It is interesting to note that Western blot analysis demonstrated greater Mrp4 protein in crude membrane fractions from female than from male mouse livers, consistent with previous findings that hepatic Mrp4 mRNA was increased in female compared with male wild-type mice (Maher et al., 2005). This sex-dependent increase in Mrp4 protein levels, coupled with more extensive sulfation in female mouse livers, may explain, in part, the higher AS basolateral excretion in female mouse livers because Mrp4 has been implicated in the basolateral excretion of AS (Lickteig et al., 2007).
The pharmacokinetic model included an intervening compartment between the sites of conjugate formation and excretion into bile. This additional compartment was required to describe the prolonged biliary excretion of AG and AS during the washout phase (Fig. 1). Movement of AG and AS from the site of conjugate formation to the site of biliary excretion was best described as a unidirectional rather than bidirectional process, based on markedly lower coefficients of variation associated with this model fit. Likewise, pharmacokinetic modeling of the in vivo disposition of APAP and AG in rats has suggested the existence of a discrete cytosolic compartment before AG biliary excretion (Studenberg and Brouwer, 1993). In a study of hepatic intracellular distribution of several compounds, the presence of a cytosolic link between metabolite formation and subsequent biliary excretion also was proposed (Levine and Singer, 1972). Kipp et al. (2001) demonstrated that newly synthesized canalicular transport proteins (e.g., P-glycoprotein) may be directly trafficked from the Golgi apparatus to the apical membrane, or they may be stored transiently in intracellular compartments before apical delivery to the membrane (e.g., bile salt export pump); taurocholate or dibutyryl cAMP can stimulate trafficking of bile salt export pump and P-glycoprotein. Phosphatidylinositol 3-kinase is involved in the vesicular trafficking of ABC transporters (Misra et al., 1999). Of note, the phosphatidylinositol 3-kinase inhibitor LY294002 translocated Bcrp1 from the plasma membrane to the cytoplasmic compartment in side population cells from mice (Mogi et al., 2003). AG and AS may bind or associate with subcellularly localized ABC transport proteins in mouse liver before biliary excretion, as described by intrahepatic compartments in the current pharmacokinetic model.
In summary, the present study demonstrated sex-dependent hepatobiliary disposition of glucuronide and sulfate conjugates of APAP in mice due to differences in hepatic transport protein expression and conjugation. In light of the extensive use of the mouse as a model species to examine hepatobiliary drug disposition and drug-induced liver injury, such sex-dependent differences in transport proteins and phase II conjugation may explain different outcomes between male and female mice during preclinical drug development.
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM41935]; and Eli Lilly and Company.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.109.026815.
ABBREVIATIONS: BCRP/Bcrp, breast cancer resistance protein; ABC, ATP-binding cassette; SN-38, ethyl-10-hydroxy-camptothecin; AG, acetaminophen glucuronide; AS, acetaminophen sulfate; MRP/Mrp, multidrug resistance-associated protein; APAP, acetaminophen; UGT/Ugt, UDP-glucuronosyltransferases; Sult, sulfotransferase; LY294002, 2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride.
References
- Alnouti Y and Klaassen CD (2006) Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicol Sci 93 242–255. [DOI] [PubMed] [Google Scholar]
- Bates SE, Medina-Pérez WY, Kohlhagen G, Antony S, Nadjem T, Robey RW, and Pommier Y (2004) ABCG2 mediates differential resistance to SN-38 (7-ethyl-10-hydroxycamptothecin) and homocamptothecins. J Pharmacol Exp Ther 310 836–842. [DOI] [PubMed] [Google Scholar]
- Buckley DB and Klaassen CD (2007) Tissue- and gender-specific mRNA expression of UDP-glucuronosyltransferases (UGTs) in mice. Drug Metab Dispos 35 121–127. [DOI] [PubMed] [Google Scholar]
- Court MH, Duan SX, von Moltke LL, Greenblatt DJ, Patten CJ, Miners JO, and Mackenzie PI (2001) Interindividual variability in acetaminophen glucuronidation by human liver microsomes: identification of relevant acetaminophen UDP-glucuronosyltransferase isoforms. J Pharmacol Exp Ther 299 998–1006. [PubMed] [Google Scholar]
- Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, and Ross DD (1998) A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA 95 15665–15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Tsukahara S, Asada S, and Sugimoto Y (2004) Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Cancer Res 64 4346–4352. [DOI] [PubMed] [Google Scholar]
- Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, Rosing H, et al. (2002) The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci USA 99 15649–15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker JW, Merino G, Musters S, van Herwaarden AE, Bolscher E, Wagenaar E, Mesman E, Dale TC, and Schinkel AH (2005) The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat Med 11 127–129. [DOI] [PubMed] [Google Scholar]
- Kipp H, Pichetshote N, and Arias IM (2001) Transporters on demand: intrahepatic pools of canalicular ATP binding cassette transporters in rat liver. J Biol Chem 276 7218–7224. [DOI] [PubMed] [Google Scholar]
- Levine WG and Singer RW (1972) Hepatic intracellular distribution of foreign compounds in relation to their biliary excretion. J Pharmacol Exp Ther 183 411–419. [PubMed] [Google Scholar]
- Lickteig AJ, Fisher CD, Augustine LM, Aleksunes LM, Besselsen DG, Slitt AL, Manautou JE, and Cherrington NJ (2007) Efflux transporter expression and acetaminophen metabolite excretion are altered in rodent models of nonalcoholic fatty liver disease. Drug Metab Dispos 35 1970–1978. [DOI] [PubMed] [Google Scholar]
- Maher JM, Slitt AL, Cherrington NJ, Cheng X, and Klaassen CD (2005) Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (Mrp) family in mice. Drug Metab Dispos 33 947–955. [DOI] [PubMed] [Google Scholar]
- Manautou JE, de Waart DR, Kunne C, Zelcer N, Goedken M, Borst P, and Elferink RO (2005) Altered disposition of acetaminophen in mice with a disruption of the Mrp3 gene. Hepatology 42 1091–1098. [DOI] [PubMed] [Google Scholar]
- Merino G, Jonker JW, Wagenaar E, Pulido MM, Molina AJ, Alvarez AI, and Schinkel AH (2005a) Transport of anthelmintic benzimidazole drugs by breast cancer resistance protein (BCRP/ABCG2). Drug Metab Dispos 33 614–618. [DOI] [PubMed] [Google Scholar]
- Merino G, van Herwaarden AE, Wagenaar E, Jonker JW, and Schinkel AH (2005b) Sex-dependent expression and activity of the ATP-binding cassette transporter breast cancer resistance protein (BCRP/ABCG2) in liver. Mol Pharmacol 67 1765–1771. [DOI] [PubMed] [Google Scholar]
- Misra S, Ujházy P, Varticovski L, and Arias IM (1999) Phosphoinositide 3-kinase lipid products regulate ATP-dependent transport by sister of P-glycoprotein and multidrug resistance associated protein 2 in bile canalicular membrane vesicles. Proc Natl Acad Sci U S A 96 5814–5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Yang J, Lambert JF, Colvin GA, Shiojima I, Skurk C, Summer R, Fine A, Quesenberry PJ, and Walsh K (2003) Akt signaling regulates side population cell phenotype via Bcrp1 translocation. J Biol Chem 278 39068–39075. [DOI] [PubMed] [Google Scholar]
- Nakagawa R, Hara Y, Arakawa H, Nishimura S, and Komatani H (2002) ABCG2 confers resistance to indolocarbazole compounds by ATP-dependent transport. Biochem Biophys Res Commun 299 669–675. [DOI] [PubMed] [Google Scholar]
- Nezasa K, Tian X, Zamek-Gliszczynski MJ, Patel NJ, Raub TJ, and Brouwer KLR (2006) Altered hepatobiliary disposition of 5 (and 6)-carboxy-2′,7′-dichlorofluorescein in Abcg2 (Bcrp1) and Abcc2 (Mrp2) knockout mice. Drug Metab Dispos 34 718–723. [DOI] [PubMed] [Google Scholar]
- Robey RW, Medina-Pérez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, Senderowicz AM, Ross DD, and Bates SE (2001) Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res 7 145–152. [PubMed] [Google Scholar]
- Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, and Bates SE (2009) ABCG2: a perspective. Adv Drug Deliv Rev 61 3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studenberg SD and Brouwer KLR (1993) Hepatic disposition of acetaminophen and metabolites. Pharmacokinetic modeling, protein binding and subcellular distribution. Biochem Pharmacol 46 739–746. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Slitt AL, Leazer TM, Maher JM, and Klaassen CD (2005) Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem Biophys Res Commun 326 181–187. [DOI] [PubMed] [Google Scholar]
- Xiong H, Suzuki H, Sugiyama Y, Meier PJ, Pollack GM, and Brouwer KLR (2002) Mechanisms of impaired biliary excretion of acetaminophen glucuronide after acute phenobarbital treatment or phenobarbital pretreatment. Drug Metab Dispos 30 962–969. [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Hoffmaster KA, Tian X, Zhao R, Polli JW, Humphreys JE, Webster LO, Bridges AS, Kalvass JC, and Brouwer KLR (2005) Multiple mechanisms are involved in the biliary excretion of acetaminophen sulfate in the rat: role of Mrp2 and Bcrp1. Drug Metab Dispos 33 1158–1165. [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Nezasa K, Tian X, Bridges AS, Lee K, Belinsky MG, Kruh GD, and Brouwer KLR (2006a) Evaluation of the role of multidrug resistance-associated protein (Mrp) 3 and Mrp4 in hepatic basolateral excretion of sulfate and glucuronide metabolites of acetaminophen, 4-methylumbelliferone, and harmol in Abcc3–/– and Abcc4–/– mice. J Pharmacol Exp Ther 319 1485–1491. [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Nezasa K, Tian X, Kalvass JC, Patel NJ, Raub TJ, and Brouwer KLR (2006b) The important role of Bcrp (Abcg2) in the biliary excretion of sulfate and glucuronide metabolites of acetaminophen, 4-methylumbelliferone, and harmol in mice. Mol Pharmacol 70 2127–2133. [DOI] [PubMed] [Google Scholar]