Summary
Normal self-renewal of follicle stem cells (FSCs) in the Drosophila ovary requires Hedgehog (Hh) signaling. Excess Hh signaling, induced by loss of patched (ptc), causes cell-autonomous duplication of FSCs. We have used a genetic screen to identify Mastermind (Mam), the Notch pathway transcriptional co-activator, as a rare dose-dependent modifier of aberrant FSC expansion induced by excess Hh. Complete loss of Mam activity severely compromises the persistence of both normal and ptc mutant FSCs, but does not affect the maintenance of ovarian germline stem cells. Thus, Mam, like Hh, is a crucial stem cell factor that acts selectively on FSCs in the ovary. Surprisingly, other Notch pathway components, including Notch itself, are not similarly required for FSC maintenance. Furthermore, excess Notch pathway activity alone accelerates FSC loss and cannot ameliorate the more severe defects of mam mutant FSCs. This suggests an unconventional role for Mam in FSCs that is independent of Notch signaling. Loss of Mam reduces the expression of a Hh pathway reporter in FSCs but not in wing discs, suggesting that Mam might enhance Hh signaling specifically in stem cells of the Drosophila ovary.
Keywords: Hedgehog, Notch, Mastermind, Drosophila, Ovary, Stem cells
Introduction
Drosophila ovaries provide an excellent model system for investigating the signaling pathways and internal circuitry that regulate stem cell function. The Drosophila female has a pair of ovaries, each composed of 14-18 ovarioles. The ovariole is an egg assembly unit, with a germarium at the anterior, where the follicle stem cells (FSCs; formerly known as ovarian somatic stem cells or SSCs) and germline stem cells (GSCs) are located (Fig. 1A). Differentiated cell types, known as terminal filament cells, and cap cells at the anterior tip of the germarium are important niche components, with the cap cells being in direct contact with each of the two to three GSCs (Kirilly and Xie, 2007). The cystoblast daughter of a GSC undergoes four mitotic divisions with incomplete cytokinesis to produce a cluster of 16 cystocytes. The germline cysts differentiate to form one oocyte and 15 nurse cells as they come into contact with somatic cell progeny of the FSCs in region 2b (see Fig. 1A). These somatic cells form a single-layered epithelium of follicle cells around each germline cyst to form an egg chamber, which grows and develops further as it passes posteriorly through the ovariole to become a mature egg.
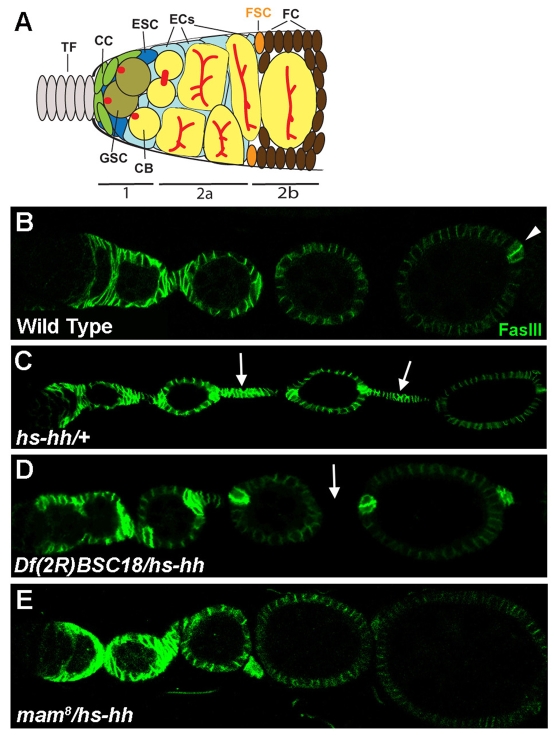
Fig. 1.
Suppression of hs-hh somatic cell overproliferation. (A) Schematic of the Drosophila germarium (posterior to the right). Germline stem cells (GSCs, olive colored) give rise to cystoblasts, which divide to form cystocyte clusters (yellow). Cap cells (green) at the base of the terminal filament (TF, gray) and escort stem cells (ESCs, dark blue) directly contact GSCs. Escort cells (ECs, light blue) contact the follicle stem cells (FSCs, orange) at the region 2a/2b border. FSC progeny (brown cells posterior to the FSCs) are follicle cells that surround cystocytes and the stalk cells (not shown) that separate egg chambers. (B-E) FasIII staining (green) of ovarioles after multiple heat shocks and a 3-day chase period. The germarium is to the left (anterior) with increasingly more developed egg chambers to the right in every image. (B) FasIII is expressed in the germarium of wild-type ovarioles beginning with the immediate progeny of the FSC and in all follicle cells until approximately stage four of egg chamber development when FasIII is expressed predominantly in a pair of polar cells at each pole of an egg chamber (arrowhead). (C) Arrows indicate excessive FSC progeny between egg chambers with high levels of FasIII in a heterozygous hs-hh ovariole. (D,E) In Df(2R)BSC18/hs-hh ovarioles (D) and mam8/hs-hh ovarioles (E) most egg chambers are separated by stalks of normal length that do not stain with FasIII (arrow).
FSCs reside at the border of regions 2a and 2b, where they are likely to contact the basement membrane underlying the germarial sheath, transient escort cells and, perhaps, passing germline cysts (Fig. 1A) (Margolis and Spradling, 1995; Nystul and Spradling, 2007). Escort cells derive from escort stem cells (ESCs) adjacent to the GSCs, and associate closely with germline cells before undergoing apoptosis at the 2a/2b border. Some FSC contacts are thought to be important because loss of the homotypic adhesion molecule E-cadherin, or of the heterodimeric integrins βPS, αPS1 and αPS2 from FSCs leads to their rapid disappearance (Song and Xie, 2002; O'Reilly et al., 2008). However, at least two of the extracellular factors that contribute to FSC function, Hedgehog (Hh) and Wingless, are expressed most prominently in the terminal filament and cap cells, which are located quite far from the FSCs (Forbes et al., 1996a; Forbes et al., 1996b; Kirilly and Xie, 2007).
FSCs actively self-renew, with little evidence of a lengthy quiescence, simultaneously producing non-stem cell daughters that generally divide an estimated seven to nine times before adopting position-specific cell fates within the follicle cell epithelium of maturing egg chambers (Margolis and Spradling, 1995). A few FSC derivatives arrest much earlier to form polar cells and adjacent stalk cells, which separate egg chambers (Margolis and Spradling, 1995; Tworoger et al., 1999).
The Hh pathway appears to be an especially important regulator of FSCs. Although loss of Hh, Wnt or BMP pathway activity results in accelerated FSC loss (Kirilly and Xie, 2007), only excess activity of the Hh pathway drives cell-autonomous FSC duplication (Zhang and Kalderon, 2001). Hh acts by binding to Patched (Ptc), a transmembrane protein that normally acts to restrict the activity of Smoothened (Smo), a seven-transmembrane domain protein (Hooper and Scott, 2005). Binding of Hh to Ptc activates Smo, leading eventually to the activation of the transctription factor Cubitus interruptus (Ci). Loss of Ptc activity results in maximal activation of the intracellular Hh pathway even in the absence of Hh. Both ectopic, ubiquitous Hh expression and loss of Ptc activity in FSC lineages lead to the accumulation of an excess of FSC derivatives (Forbes et al., 1996a; Forbes et al., 1996b; Zhang and Kalderon, 2001). In the latter case, this was shown to involve the cell autonomous duplication of ptc mutant FSCs, which increases the total number of FSCs in a germarium (Zhang and Kalderon, 2001). By contrast, inactivation of smo, which blocks Hh signal transduction cell autonomously, accelerates loss of the FSC lineage (Zhang and Kalderon, 2001).
Here, we used a genetic modifier screen designed to isolate factors that either collaborate with, or are regulated by, the Hh pathway to promote FSC maintenance. We found that the transcriptional co-activator Mastermind (Mam) is essential for the expansion of ptc mutant FSCs, and for the maintenance of both normal and ptc mutant FSCs. Remarkably, the regulation of FSCs by Mam is not through its known role in the Notch signaling pathway. Mam is required for the elevated expression of the Hh pathway reporter ptc-lacZ that is seen in the FSC and its immediate progeny, but has no clear effect on Hh signaling in wing discs. This suggests that Mam may be a tissue-specific co-activator for the Hh pathway in ovarian follicle stem cells.
MATERIALS AND METHODS
Fly strains
Fly stocks are described on FlyBase: Flies with alleles on an FRT42D chromosome [sha (control), mam8, mamIL115 (also known as mam10), ptcS2 and ptcS2 mam8] were mated to hsp70-flp; FRT42D Ubi-GFP nls flies for negative marking. ptc-lacZ was added to the third chromosome (also for UbiGFP FRT40A) when needed. Flies with alleles on an FRT40A chromosome [NM (Nuclear Myc, control), smo2, smoD16 and Su(H)Δ47 P[l(2)35Bg+] (Morel and Schweisguth, 2000)] were mated to hsp70-flp; Ubi-GFP FRT40A flies for negative marking. Flies with alleles on an FRT101 chromosome [ywv (control) and N55e11] were mated to hsp70-flp hsp70-GFP FRT101 flies for negative marking. Flies with alleles on an FRT82B chromosome [NM (Nuclear Myc, control) and nctR46] were mated to hsp70-flp; FRT82B tub-lacZ flies for negative marking. Positive marking strains [E22C FRT42D act-GAL4 (control), E22C FRT42D ptcS2 act-GAL4, E22C FRT42D ptcS2 mam8 act-GAL4, FRT42D ptc S2, FRT42D sha, FRT42D mam8 and FRT42D ptcS2 mam8] were mated to hsp70-flp UAS-GFP tub-GAL4; FRT42D tub-GAL80/Cyo flies for positive marking. UAS-MamWT, UAS-MamN, UAS-Nintra, UAS-Su(H)VP16, ptc-lacZ and act>CD2>GAL4 (> indicates FRT) were added to the third chromosomes when appropriate for positive marking. For weaker expression of UAS-Nintra, UAS-Su(H)VP16, UAS-MamN or UAS-MamWT, flies with these third chromosome transgenes together with the appropriate FRT42D-linked alleles without E22C-GAL4 and act-GAL4 were crossed to hsp70-flp UAS-GFP tub-GAL4; FRT42D tub-GAL80 tub-lacZ/Cyo flies.
hs-hh screen
hsp70-hh (hhhs.PI) flies were crossed to second and third chromosome deficiency stocks (from the Bloomington Stock Center). The resulting transheterozygous adult flies were heat shocked twice a day for three days (1 hour at 37°C), dissected 3 or 6 days later, and stained with FasIII and Hoechst DNA stain.
Clonal analysis and stem cell counts
Adult flies of the appropriate genotype were heat shocked twice (approximately 8 hours apart) for 1 hour at 37°C. FRT101 flies were given an additional 1-hour heat shock at 37°C four hours prior to dissection to induce hsp70-GFP expression.
For positive marking, flies were incubated at 29°C for at least two days prior to dissection in order to increase the expression of UAS-GFP. Low-level UAS-MamWT expressing flies (Fig. 2J) were also marked in this way, following incubation at 18°C for 12 days immediately after clone induction.
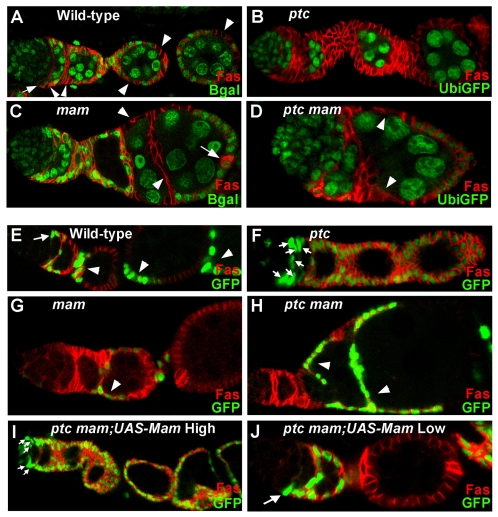
Fig. 2.
Essential and dose-dependent Mam functions in FSCs. (A-D) Ovarioles with FSC clones marked by the absence of (green) nuclear Ubi-GFP or tub-lacZ gene product (`Bgal') and stained with FasIII (red). Clones were induced in adults and evaluated 14 days after induction. (A) A typical control (wild-type) FSC clone includes a FSC (arrow) just anterior to FasIII-staining cells and numerous FSC derivatives (arrowheads). (B) An `all marked' ptcS2 mutant ovariole. All FSCs and FSC progeny are mutant as indicated by lack of GFP expression. All germline cells retain GFP expression in this ovariole. (C) A mam8 mutant FSC clone has lost the mutant FSC, as no somatic cells in the germarium lack tub-lacZ expression, but retained FSC derivatives (arrowheads). Several mam mutant cells lie between adjacent fused egg chambers. Arrow marks normal FasIII staining in wild-type polar cells at the posterior of the egg chamber. (D) ptcS2 mam8 FSC derivatives (arrowheads) lie between the germarium and successive fused egg chambers. (E-J) Ovarioles containing FSC clones positively marked with GFP (green) and stained with FasIII (red). (E) Wild-type FSC clone with a FSC (arrow) and its derivatives (arrowheads). (F) An `all marked' ptcS2 mutant ovariole, with multiple ptc mutant FSCs (arrows). (G) A mam8 mutant FSC clone includes FSC derivatives (arrowhead) but no FSC. (H) A ptcS2 mam8 mutant FSC clone has lost the FSC but derivatives (arrowheads) separate fused egg chambers. (I) High-level expression of UAS-Mam in a ptcS2 mam8 FSC clone produces multiple FSCs (arrows) giving rise to an `all marked' ovariole. (J) Low-level UAS-Mam expression in ptcS2 mam8 FSC clones results in a single mutant FSC (arrow) in ovarioles that retain a morphology that is close to normal.
At least 50 ovarioles were evaluated for stem cell counts and in most cases over 100 ovarioles were counted. In only one case were fewer than 50 ovarioles evaluated for stem cell clones because of insufficient flies (a 14-day adult UAS-Nintra). Significance of differences was calculated by χ2 tests.
For wing disc clones, larvae were heat shocked during first instar for 1 hour at 37°C and dissected two days later.
Immunohistochemistry
Ovaries and wing discs were dissected in phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde in PBS for 20 minutes at room temperature. The tissue was blocked in 1% bovine serum albumin (BSA) for 1 hour and stained with the appropriate primary antibodies: anti-Fasciclin III and anti-Engrailed [University of Iowa Developmental Studies Hybridoma Bank (DSHB), under the auspices of the NICHD] at 1:250 and 1:5, respectively; anti-β-Galactosidase (Cappel) at 1:2000; and anti-GFP (for FRT101 experiments, A6455, Molecular Probes) at 1:2000. Secondary antibodies were Alexa-488, Alexa-594 or Alexa-647 from Molecular Probes, used at 1:1000. DNA was stained with Hoechst 33258 (Molecular Probes) at 1 μg/ml (DNA not shown but used for stem cell counts).
RESULTS
A screen for dominant suppressors of Hh-induced overproliferation identifies Mastermind
To identify molecules that collaborate with Hh or respond to Hh in regulating follicle stem cell behavior, we screened for dominant genetic modifiers of the ovarian follicle cell over-proliferation that is induced by ectopic Hh expression. We tested 157 heterozygous second and third chromosome deficiencies (covering most of these autosomes) in flies carrying a transgene (hs-hh) with heat-shock-inducible hh activity (Forbes et al., 1996a). Young adult females were heat-shocked twice daily for three days followed by a three day `chase' to allow the progeny of excess FSCs induced by ectopic Hh expression to proliferate and exit the germarium. These progeny accumulate between egg chambers and, unlike normal differentiated stalk cells, they maintain high levels of Fasciclin III (FasIII; Fas3 - FlyBase; Fig. 1B,C). Only two deficiencies suppressed the `hs-hh' phenotype, judged by a strong reduction in the number of inappropriate cells between egg chambers.
Df(2R)BSC18 is a relatively small deficiency, deleting polytene segments between 50D1 and 50D2-7 and was the strongest suppressor (Fig. 1D). One interesting gene located within this deficiency is mastermind (mam). Mam is an effector of the Notch signal transduction pathway, where it associates with the DNA-binding protein Suppressor of Hairless [Su(H)] and the cleaved intracellular domain of Notch (Nintra) to form a transcriptional activator complex (Bray, 2006; Schweisguth, 2004). We therefore examined the interaction between mam and hh. Two point mutations in mam (mam8 and mamIL115) dominantly suppressed the hs-hh phenotype to almost the same degree as did the original deficiency (Fig. 1E), indicating that mam is the relevant modifier gene within Df(2R)BSC18. The dominant interaction of Mam is especially notable in light of our observation that an essential component of Hh signaling, Smo, does not dominantly suppress the hs-hh phenotype (data not shown).
Mastermind is required for normal FSC maintenance
Although excessive Hh signaling in FSCs is known to increase the number of FSCs (Zhang and Kalderon, 2001), consequent overproliferation in the FSC lineage might, in principle, be suppressed either by preventing Hh-induced FSC duplication or by reducing the proliferation of FSC progeny. We therefore examined whether the FSCs themselves were affected by changes in Mam activity. We began by looking at the consequences of inactivating Mam under conditions of normal Hh signaling.
FSC maintenance can be measured by examining the persistence of marked FSC lineages of defined genotype that are generated by heat-shock-induced FRT-mediated mitotic recombination (Xu and Rubin, 1993) in young adults. We marked FSC lineages by loss of either tubulin-lacZ (tub-lacZ) or UbiGFP, and examined ovaries 7 and 14 days after heat-shock. FSC daughters proliferate, differentiate and finally exit the ovariole within 5-6 days at 25°C (Margolis and Spradling, 1995). Hence, all marked clones examined 7 or more days after heat shock must derive from recombination events induced in FSCs (`FSC clones'). A normal, single, persistent FSC clone extends from the FSC through proliferating progeny to differentiated stalk and follicle cells, generally occupying about a third of the somatic cells throughout an ovariole (Fig. 2A). The FSC can be recognized by its position as the most anterior cell in the lineage and by the criterion that it does not stain significantly with FasIII, in contrast to its immediate descendants (Zhang and Kalderon, 2001).
To measure the effects of mam (and other) mutations on FSC maintenance, we counted the number of ovarioles with persistent FSC clones at various times after clone induction. We compared this to the number of control (wild-type) marked FSC clones that were induced under identical conditions and strictly in parallel. Because both experimental and control flies employ the same FRT and hs-FLP transgenes, we assumed that the proportion of ovarioles in which a marked FSC clone is initially induced is the same. Hence, any reduction in the percentage of ovarioles with mutant FSC clones relative to control clones at 7 days or thereafter reflected a selective loss of FSC clones that could be attributed to their mutant genotype. mam8 mutant FSC clones were present at a much lower frequency than control clones at 7 days (30% versus 73%) and 14 days (8% versus 57%) after clone induction (Table 1). These measurements include only marked clones that stretched all the way back to the FSC. However, for mam (and other mutations that impair FSC maintenance), we also saw an increased frequency of ovarioles in which a marked FSC was no longer present but several marked descendants were still evident, providing a more direct visual confirmation of FSC loss (Fig. 2C).
Table 1.
Persistence of negatively marked FSC clones
|
Percentage of ovarioles with marked FSC clones
|
||||
|---|---|---|---|---|
|
7 Days
|
14 Days
|
|||
| Genotype | Total | AM | Total | AM |
| FRT42D control | 73 | 2 | 57 | 4 |
| mam8 | 30 | 0 | 8 | 4 |
| ptcS2 | 70 | 7 | 55 | 22 |
| ptcS2 mam8 | 33 | 0 | 7 | 0 |
| FRT40A control | 67 | 0 | 36 | 4 |
| Su(H)delta47 | 56 | 0 | 42 | 5 |
| smo2 | 34 | 0 | 8 | 0 |
| smoD16 | 25 | 0 | 6 | 0 |
| FRT101 control | 82 | 3 | 56 | 3 |
| N55e11 | 79 | 4 | 52 | 0 |
| FRT82B control | 53 | 1 | 43 | 8 |
| nctR46 | 57 | 0 | 42 | 0 |
AM, percentage of ovarioles with `all marked' FSC derivatives.
The numbers of mam, ptc mam, and smo FSC clones at 14 days are significantly different from control (P<1 × 10−7), whereas the numbers of ptc, Su(H), N and nct FSC clones are not (P>0.3).
By contrast, the recovery of germline stem cell (GSC) clones, measured in an analogous fashion in the same ovarioles that were used to count FSC clones, was not affected by mam inactivation (39% mam versus 44% control at 14 days for clones induced in adults). These data suggest that Mam is essential for the normal maintenance of ovarian FSCs but not GSCs.
Ovarioles that included mam mutant follicle cells exhibited frequent egg chamber fusions and ectopic FasIII expression beyond stage 4 (Fig. 2C). These phenotypes are characteristic of loss of Notch signaling (Assa-Kunik et al., 2007; Lopez-Schier and St Johnston, 2001; Torres et al., 2003), implying that Mam is an essential co-activator for Notch signaling in the FSC lineage.
Positive FSC marking confirms loss of Mam-deficient FSCs
The observed disappearance of negatively marked mam mutant FSC lineages over time is most likely to reflect an irreversible loss of the FSC itself. However, because an isolated, quiescent, negatively marked FSC might be missed, we also positively marked mam mutant FSC lineages in order to see the FSC more easily. We used the well-established MARCM (mosaic analysis with a repressible cell marker) system for producing positively marked lineages of defined genotype (Lee and Luo, 2001). We found that the addition of actin-GAL4 (act-GAL4) alone or together with E22C-GAL4 was necessary to supplement the commonly used tubulin-GAL4 (tub-GAL4) driver in order to increase the GFP signal strength sufficiently for reliable positive marking of FSC clones (Fig. 2E).
Positive marking confirmed that mam mutant FSC lineages were rapidly lost (see Table 2). Furthermore, several ovarioles included positively marked mam mutant FSC derivatives but clearly lacked a mutant FSC, indicating relatively recent FSC loss (Fig. 2G). Most importantly, in ovarioles with no positively marked mam mutant FSC derivatives, we could be certain that no temporarily quiescent FSC remained. Thus, FSC longevity is clearly greatly reduced by the loss of Mam function.
Table 2.
Persistence of positively marked FSC clones
|
Percentage of ovarioles with positively marked FSC clones at 14 days
|
|||
|---|---|---|---|
| Genotype* | Total | AM | Normalized total |
| FRT42D control | 44 | 4 | 100 |
| mam | 7 | 0 | 16 |
| ptc | 58 | 24 | 132 |
| ptc mam | 9 | 1 | 20 |
| UAS-MamWT | 38 | 4 | 86 |
| mam;UAS-MamWT | 18 | 1 | 41 |
| ptc;UAS-MamWT | 56 | 14 | 127 |
| ptc mam;UAS-MamWT | 49 | 24 | 111 |
| UAS-MamN | 34 | 2 | 77 |
| mam;UAS-MamN | 10†,‡ | 0 | 23 |
| ptc;UAS-MamN | 49 | 18 | 111 |
| ptc mam;UAS-MamN | 19†,§ | 1 | 43 |
AM, percentage of ovarioles with `all marked' FSC derivatives.
UAS-Mam transgenes were expressed using tub-GAL4 and actin-GAL4.
Significantly different from control (P<1 × 10−5).
Not significantly different from mam (P=0.36).
Difference from ptc mam over two such experiments marginally significant (P=0.046).
To confirm that FSC defects associated with the mam mutant chromosome were actually due to the loss of Mam, we ectopically expressed a UAS-Mam transgene in mam mutant FSCs. Expression of UAS-Mam reduced mam mutant FSC loss either partially (increasing FSC survival from 7% to 18% over 14 days; control 44%; Table 2) or more completely (from 11% to 29%; control 33%; Table 3), depending on the GAL4 transgenes used to drive expression. We also observed selective FSC loss for an independently derived allele, mamIL115 (6% FSC persistence at 14 days versus 46% for control). Thus, the inactivation of Mam clearly leads to premature, selective loss of FSCs.
Table 3.
Rescue of mam FSC phenotype by UAS-Mam transgene
|
Percentage of ovarioles with marked FSC clones at 14 days
|
|||
|---|---|---|---|
| Genotype* | Total | AM | Normalized total |
| FRT42D control | 33 | 10 | 100 |
| ptc | 41 | 21 | 124 |
| mam | 11 | 1 | 33 |
| ptc mam | 10 | 1.5 | 30 |
| UAS-MamWT | 44 | 17 | 133 |
| ptc;UAS-MamWT | 53 | 23 | 161 |
| mam;UAS-MamWT | 29† | 9 | 88 |
| ptc mam;UAS-MamWT | 38† | 17 | 115 |
| ptc mam;UAS-MamN | 11‡ | 2 | 33 |
AM, percentage of ovarioles with `all marked' FSC derivatives.
UAS-Mam transgenes were expressed using tub-GAL4.
Not significantly different from control (P>0.4).
Significantly different from control (P=3.5 × 10−5) but not significantly different from ptc mam (P=0.952).
Hedgehog-driven FSC duplication requires normal Mam activity
Our initial suppressor screen indicated that reduced Mam function might impair the induction of excess FSCs caused by increased Hh signaling activity. Excess Hh signaling is elicited in ptc mutant FSC clones, leading to the duplication and enhanced longevity of ptc mutant FSCs and the accumulation of many FasIII-positive progeny between egg chambers (Zhang and Kalderon, 2001) (Fig. 2B). By contrast, ptc mam double mutant FSC clones were lost as rapidly as mam mutant clones (Table 1), and produced the characteristic mam mutant phenotype of fused egg chambers (Fig. 2D).
Individual FSCs are lost from ovarioles with an estimated half-life of roughly two weeks, but are quickly replaced by descendants of another FSC in the ovariole (Margolis and Spradling, 1995; Nystul and Spradling, 2007; Song and Xie, 2002; Zhang and Kalderon, 2001). This leads to the gradual homogenization of ovarioles that initially contained FSCs of different control genotypes. However, this process is much faster for ptc mutant FSCs, which readily take over an entire ovariole (Zhang and Kalderon, 2001). For example, 22% of ovarioles contained only ptc mutant FSC derivatives (`all marked', Fig. 2B) 14 days after clone induction, compared with 4% for control FSC clones (Table 1, `AM' column). Loss of mam fully suppressed this property of ptc mutant FSCs, such that no ovarioles contained only ptc mam FSC derivatives at 7 or 14 days after clone induction (Table 1).
We then used positive marking to examine FSC duplications directly in ptc and ptc mam mutant FSC clones. It was previously shown that ptc mutant FSCs had generally duplicated by 5 days after clone induction (Zhang and Kalderon, 2001). At later times, we saw that a single ovariole accumulates progressively more ptc mutant FSCs over time (defined by their anterior position, positive marking and lack of FasIII expression), sometimes producing as many as ten marked FSCs by 14 days after clone induction (Fig. 2F). By contrast, in ovarioles containing positively marked ptc mam double mutant FSC clones, we found two or more marked FSCs at a frequency even lower than that observed for controls (Table 2). Thus, loss of mam entirely prevented the progressive, cell-autonomous duplication of FSCs that is normally elicited by excessive Hh pathway activity.
Next, we tested whether the levels of Mam affected FSC duplication elicited by loss of ptc, as had been suggested by our initial screen. To do this, we exploited the temperature sensitivity of GAL4-induced gene expression in Drosophila. When expressed at 25°C, a UAS-Mam transgene fully rescued the persistence of ptc mam mutant FSC clones (Table 2), confirming that loss of mam is responsible for the FSC loss seen in ptc mam mutant FSC clones. In addition, we saw the characteristic ptc mutant phenotypes of FSC duplications and the accumulation of large numbers of cells between egg chambers (Fig. 2I; Table 2). When we expressed Mam from the same UAS-Mam transgene but at a lower temperature (18°C for 12 days), the consequently lower levels of Mam supported modest hypertrophy of ptc mam somatic cells in most ovarioles, although other ovarioles displayed an almost normal morphology and, importantly, contained only a single follicle stem cell (10 out of 12 `normal' ovarioles contained one FSC; Fig. 2J). From this result and our original screen, we conclude that reduced levels of Mam (as in mam+/- heterozygotes) cannot support the inappropriate duplication of FSCs that is induced by excessive Hh pathway activity.
Mam requirement in FSCs does not involve Notch signaling
Since Mam is known primarily as a transcriptional co-activator in the Notch signaling pathway, we investigated other Notch pathway components. We found that FSC clones homozygous for a null allele of Su(H) persist roughly as well as control clones over a 14-day period (42% versus 36%; Table 1), and much better than FSC clones lacking Smo (8% and 6% for two alleles of smo versus 36% control; Table 1, Fig. 3A), which is on the same chromosome arm as Su(H). Thus, FSC clones lacking Su(H) are clearly maintained much better than FSC clones lacking mam or smo activity. Su(H) has two roles in the Notch pathway: it acts as a repressor of Notch target genes in the absence of Notch activation and as an activator when ligands activate Notch (Schweisguth, 2004). Thus, the persistence of Su(H) mutant FSCs suggests that Notch signaling has no instructive role in FSC function, but leaves open the possibility that FSC function requires the de-repression of Notch target genes.
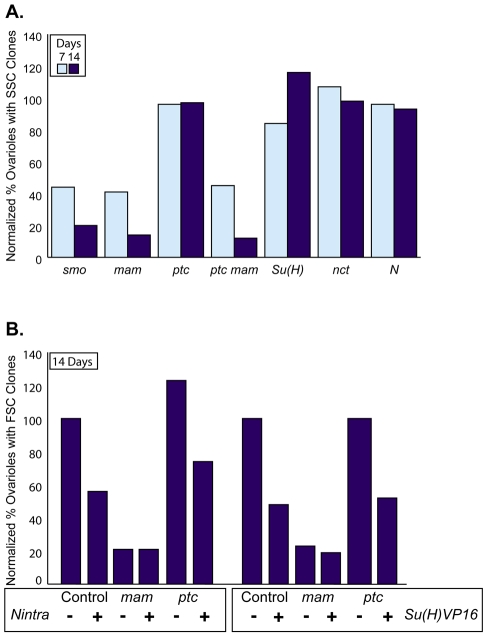
Fig. 3.
Mam function in FSCs is independent of the Notch pathway. (A,B) The percentage of ovarioles with negatively marked FSC clones of the indicated mutant genotypes was divided by the equivalent percentage for control (wild-type) clones for the same chromosome arm to give the plotted normalized values. (A) Clones were counted after 7 (light blue bars) and 14 days (dark blue bars). An average is presented for the two alleles of smo shown in Table 1. (B) Data from Table 4 for control, mam mutant and ptc mam mutant FSC clones with or without UAS-Nintra or UAS-Su(H)VP16 expressed using tub-GAL4. Clones were counted 14 days (dark blue bars) after clone induction.
Nicastrin (Nct) promotes the cleavage of Notch into Nintra, which is required for transcriptional activation and de-repression of target genes (Chung and Struhl, 2001; Lopez-Schier and St Johnston, 2002; Schweisguth, 2004). Loss of nct did not impair FSC clone persistence (42% versus 43% control at 14 days; Table 1). We also tested Notch itself. As expected, egg chamber fusions were present and FasIII staining was increased cell autonomously in ovarioles with follicle cell clones homozygous for a null Notch mutant allele (data not shown). However, Notch mutant FSC clones were maintained almost as well as control clones (52% versus 56% at 14 days; Table 1, Fig. 3A). These data show that Notch pathway activity is not required within FSCs for their maintenance. Hence, the clear requirement for Mam in FSCs cannot be explained by its well-established role in the Notch signaling pathway.
We also examined the consequences of expressing a dominant-negative truncated form of Mam (MamN), which binds well to Su(H)/Nintra complexes but lacks one of two transcriptional activation domains (Helms et al., 1999). MamN expression did not significantly impair FSC persistence (34% versus 44% for control clones after 14 days; Table 2), but did induce the characteristic Notch pathway phenotypes of ectopic FasIII expression and egg chamber fusions (data not shown). Expression of MamN also failed to reduce the duplication of ptc mutant FSCs, the homogenization of ovarioles with ptc mutant cells or the accumulation of ptc mutant FSC progeny (Table 2; data not shown), all of which are sensitive to the dose of Mam. Thus, expression of MamN, at levels sufficient to inhibit Notch pathway activity, does not affect FSC behavior, even under sensitized conditions.
The absence of a strong dominant-negative activity of MamN in FSCs suggests that the truncated MamN protein either functions like normal Mam in FSCs or competes poorly with wild-type Mam for a limiting effector. Expression of UAS-MamN, in contrast to UAS-MamWT, did not rescue FSC loss due to either mam or ptc mam mutations (Tables 2, 3). We infer that MamN interacts poorly with its critical partner in FSCs, confirming that the relevant partner is not a Su(H)/Nintra complex.
Elevated Notch signaling results in FSC loss
To characterize the role of Notch signaling on FSC regulation further, we examined the consequences of excess Notch pathway activity. For this purpose, we used Su(H) fused to the VP16 transactivation domain. Su(H)-VP16 activates Notch target genes constitutively even in the absence of Notch or Mam (Kidd et al., 1998). We also used a constitutively active form of Notch, Nintra, which still requires the presence of Mam and Su(H) in order to induce Notch target genes (Bray, 2006). Strong expression of either Su(H)-VP16 or Nintra in FSC clones caused a very large number of FasIII-positive cells to accumulate both around the germarium and between egg chambers (Fig. 4A,B). The cells expressing Su(H)-VP16 or Nintra appeared to have altered adhesion properties, as they always clustered together and were generally not incorporated into the normal structure of germaria or egg chambers. The abundance of FSC progeny might suggest that FSC numbers or proliferation is enhanced. However, FSCs expressing either Su(H)-VP16 or Nintra were in fact lost more rapidly than control FSCs over time (Table 4). This was also observed when lower levels of Su(H)-VP16 or Nintra were expressed using the tub-GAL4 driver alone (Table 4, Fig. 3B), confirming that FSC maintenance is impaired by abnormally high levels of Notch pathway activity.
Fig. 4.
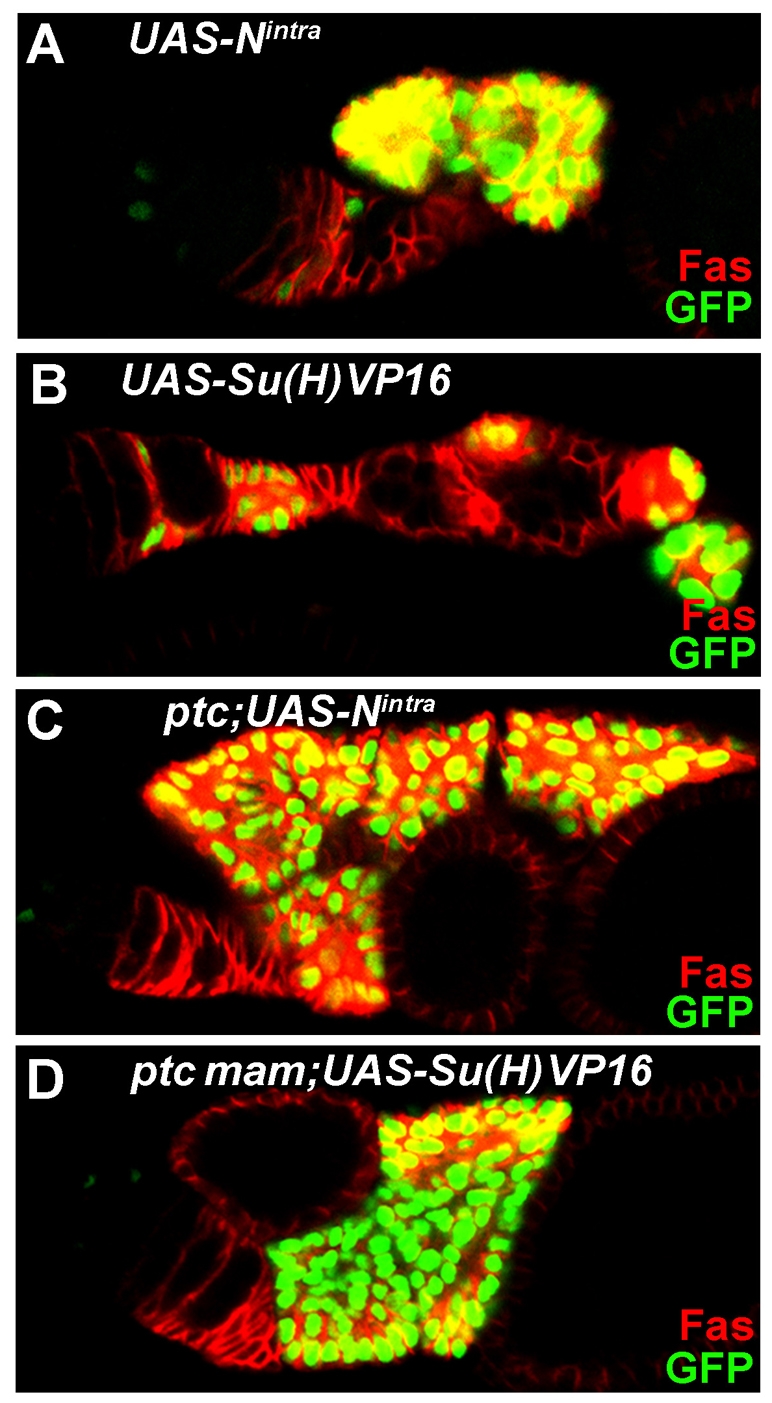
Excessive Notch pathway activity leads to transient accumulation of FSC derivatives. (A-D) Ovarioles with positively marked (green nuclei) FSC clones expressing UAS-Nintra or UAS-Su(H)VP16 and stained with FasIII (red). Both Nintra (A) and Su(H)VP16 (B) cause FSC loss and ectopic accumulation of FasIII-expressing FSC derivatives that generally separate from the germarium without being incorporated into egg chambers. Loss of Ptc (C) or Ptc and Mam (D) from FSC clones exacerbates the accumulation of FSC derivatives due to Nintra or Su(H)VP16, even though FSCs are still frequently lost.
Table 4.
Ectopic Notch pathway activity depletes FSCs and does not rescue mam mutant FSCs
|
Percentage of ovarioles with marked FSC clones
|
|||||
|---|---|---|---|---|---|
|
7 Days
|
14 Days
|
||||
| Genotype* | Total | AM | Total | AM | Normalized total (14 days) |
| Control | 61 | 7 | 41 | 14 | 100 |
| UAS-Nintra (strong) | 23 | 1 | 17 | 0 | 41 |
| Control | 54 | 6 | 59 | 16 | 100 |
| UAS-Su(H)VP16 (strong) | 57 | 4 | 27 | 1 | 46 |
| Control | 51 | 2 | 39 | 10 | 100 |
| UAS-Nintra | 26 | 1 | 22 | 0 | 56 |
| mam | 26 | 0 | 8 | 0 | 21 |
| mam;UAS-Nintra | 18 | 0 | 8 | 0 | 21 |
| ptc | 57 | 16 | 48 | 24 | 123 |
| ptc;UAS-Nintra | 35 | 1 | 29 | 2 | 74 |
| ptc mam | 22 | 0 | 13 | 0 | 33 |
| ptc mam;UAS-Nintra | 23 | 0 | 14 | 0 | 36 |
| Control | 57 | 2 | 48 | 13 | 100 |
| UAS-Su(H)VP16 | 33 | 0 | 23 | 0 | 48 |
| mam | 25 | 0 | 11 | 0 | 23 |
| mam;UAS-Su(H)VP16 | 15 | 0 | 9 | 2 | 19 |
| ptc | 61 | 11 | 48 | 21 | 100 |
| ptc;UAS-Su(H)VP16 | 34 | 0 | 25 | 0 | 52 |
| ptc mam | 19 | 0 | 12 | 0 | 25 |
| ptc mam;UAS-Su(H)VP16 | 18 | 0 | NC | ||
AM, percentage ovarioles with `all marked' FSC derivatives.
NC, not counted due to poor morphology from degeneration.
UAS transgenes were driven by tub-GAL4 only, except where `strong' denotes addition of E22C-GAL4 and Act-GAL4 drivers.
We used synthetic activation of the Notch pathway effector Su(H) to provide one more test of whether Mam function in FSCs involves the Notch pathway at all. Su(H)-VP16 should restore Notch target gene induction in FSCs that lack mam function and therefore ought to rescue mam mutant FSC loss if Mam acts through the Notch signaling pathway in FSCs. Expression of Nintra provides an important control because it cannot stimulate the Notch pathway in the absence of Mam. These rescue assays are not ideal because Nintra and Su(H)-VP16 do not necessarily mimic normal levels of Notch signaling in FSCs and because they reduce FSC function. The assays are nevertheless feasible because FSC loss induced by the expression of Su(H)-VP16 or Nintra is significantly lower than that induced by the loss of mam (Table 4, Fig. 3B). As expected, expression of Nintra had no effect on the maintenance of mam mutant FSCs (Table 4, Fig. 3B). Su(H)-VP16 also failed to rescue the loss of mam (or ptc mam) mutant FSCs (Table 4, Fig. 3B), confirming our earlier deduction that Mam has a crucial function in FSCs that is entirely distinct from its role in the Notch pathway.
As expected, Su(H)-VP16 (but not Nintra) rescued the fused egg chamber phenotype characteristic of ovaries with mam mutant clones (data not shown). Also, even though inactivation of Mam increased FSC loss in clones expressing Su(H)-VP16, it did not suppress the characteristic transient outgrowths of those clones (data not shown), underlining the conclusion that these outgrowths reflect the effects of excess Notch signaling on FSC progeny rather than on FSCs themselves.
Because excess Hh pathway activity increases the number of FSCs and excess Notch pathway activity impairs FSC maintenance, we tested the effects of activating both pathways simultaneously. We found that the ability of ptc mutations to induce FSC duplications and to take over whole ovarioles was fully suppressed by excess Notch activity (Table 4). In addition, loss of ptc did not substantially suppress the loss of FSC clones caused by the expression of Su(H)-VP16 or Nintra (Table 4, Fig. 3B). Even though ptc mutations did not enhance FSC survival in any of these situations, loss of ptc strongly enhanced the accumulation of FSC progeny in response to Nintra or Su(H)VP16, especially in cells that also lacked mam function (Fig. 4C,D). Thus, although excess Hh and Notch signaling have opposing effects on FSC function that collectively lead to FSC loss, they have additive or synergistic effects in FSC progeny that can lead to massive, transient hypertrophy.
Loss of mam reduces Hh pathway reporter expression selectively in FSCs
As Mam exhibits a dosage-sensitive interaction with Hh signaling and acts outside of the Notch pathway in FSCs, it is possible that Mam either acts to enhance Hh signaling or collaborates with the products of crucial Hh target genes to promote stem cell behavior. A key test of these hypotheses is whether Mam activity affects the induction of Hh target genes.
We first tested the idea that Mam might generally facilitate Hh signaling by looking at wing discs in which crucial, dose-sensitive Hh target genes are well defined. In the wing disc, Hh signals along the anteroposterior (AP) border, a band of anterior compartment cells adjacent to Hh-secreting cells of the posterior compartment. At the highest levels of Hh signaling, Engrailed (En) expression is activated, whereas other target genes, including ptc, are activated at lower levels of Hh signaling (see Fig. S1 in the supplementary material) (Hooper and Scott, 2005). Anterior ptc mutant clones induce strong ectopic, cell-autonomous expression of both En and ptc, monitored here by a ptc-lacZ reporter. We found that anterior ptc mam double mutant clones also induced strong ptc-lacZ and En expression, and that mam mutant clones at the AP border did not reduce either endogenous En or ptc-lacZ expression (see Fig. S1 in the supplementary material). Thus, we saw no evidence for a general role of Mam in Hh signaling.
In FSCs, the only known Hh target gene is ptc. Various ptc-lacZ reporter genes, including the one with 12 kb of ptc regulatory sequence used here (Zhou et al., 2006), are expressed in the FSC lineage, with highest expression in the germarium and lower expression in egg chambers up to stage 6 (Zhang and Kalderon, 2000). It is surprising that ptc-lacZ expression extends so far beyond the only strong source of Hh expression in terminal filament and cap cells, because ptc-lacZ expression in wing discs is strictly dependent on Hh signaling and extends for fewer than ten small cell diameters. We therefore first examined ptc-lacZ expression carefully in wild-type ovaries and in cells with smo or ptc mutations.
In the majority of the wild-type germaria we have examined, we saw an elevated level of expression of ptc-lacZ in the FSC (Fig. 5A, arrow) and sometimes in the adjacent FSC progeny (Fig. 5A, line). The levels of ptc-lacZ decline towards the posterior of the germarium to reach the distinctly lower levels observed in budded egg chambers. This suggests a gradient of Hh signaling that is highest in the FSC. In budded egg chambers and towards the posterior end of the germarium, ptc-lacZ levels were generally unaltered in FSC derivatives lacking smo (Fig. 5B). By contrast, ptc-lacZ expression was clearly reduced in most smo mutant FSCs and in their most recent progeny (six out of nine cases; Fig. 5B). Thus, elevated ptc-lacZ expression in the FSC and its immediate descendants is dependent on Hh signaling.
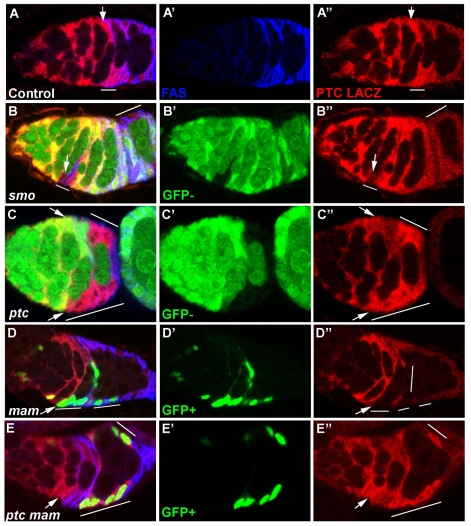
Fig. 5.
Elevated Hh pathway activity in FSCs depends on Mam. (A-A″) Control germarium stained with FasIII (A′, blue) and β-gal (A″, red) to show ptc-lacZ expression (posterior to the right). FSCs are recognized as being immediately anterior to follicle cells with strong FasIII staining. Higher levels of ptc-lacZ are seen in the FSC (arrow) or the FSC and its immediate progeny (line) than in more posterior FSC derivatives. (B,C) Mutant clones are marked by loss of GFP (green). (B-B″) Reduced levels of ptc-lacZ (B″, red) are seen in a smo2 mutant FSC and its immediate progeny (line, arrow marks FSC). Lower ptc-lacZ levels at th posterior of the germarium and in budded egg chambers are not altered in smo mutant cells (B, line). (C-C″) Loss of ptc (lines, bottom arrow indicates a FSC within a ptc mutant clone) results in higher levels of ptc-lacZ (red, C″) than seen in a wild-type FSC (top arrow). (D,E) Mutant clones are marked by GFP (green) expression. (D-D″) Reduced levels of ptc-lacZ (red, D″) are seen in a mam mutant FSC and its recent progeny (lines, arrow marks the FSC). (E-E″) ptc mam double mutant clones (lines) show similar levels of ptc-lacZ (red, E″) expression to the wild-type FSC (arrow) within the same germarium.
The lower levels of ptc-lacZ from stage 1 onwards that are independent of smo are influenced by Ci expression (Sun and Deng, 2007) and are therefore likely to result from basal, ligand-independent pathway activity. Hence, high ptc-lacZ expression reflects selectively strong Hh signaling in the FSC but probably underestimates the gradient of Hh activity in the germarium because of β-galactosidase perdurance and a significant baseline of Hh-independent pathway activity. Expression of ptc-lacZ in ptc mutant cells was slightly stronger than in wild-type FSCs and extended throughout the germarium into budded egg chambers, consistent with the expectation of maximal, Hh-independent pathway activation (Fig. 5C).
In germaria containing mam mutant FSC derivatives, 20 out of 28 mam clones showed reduced levels of ptc-lacZ relative to wild-type neighboring follicle cells (Fig. 5D). In 17 of these clones, an FSC was among the mam mutant cells, with eleven showing clearly reduced levels of ptc-lacZ. In the remaining clones, no FSC was present, suggesting recent loss of the FSC from the lineage. Thus, loss of Mam activity substantially reduces Hh-dependent induction of ptc-lacZ in FSCs, implying that Mam normally enhances Hh signaling in follicle stem cells. Expression of ptc-lacZ was also marginally lower in ptc mam mutant clones than in ptc mutant clones, but similar to the levels observed in wild-type FSCs (Fig. 5E).
DISCUSSION
Mastermind acts as a specific stem cell factor in FSCs
Understanding the molecular circuitry and varied behaviors of stem cells will require the detailed study of many model systems. Drosophila follicle stem cells (FSCs) provide a paradigm that includes the basic attributes of visualizing a defined stem cell and its environment, coupled with the potential to manipulate stem cell genotypes extensively and measure stem cell function. Nevertheless, only a limited number of factors have so far been defined as being essential to FSC function. Among these are the Hh, Wnt and BMP signaling pathways, adhesion molecules, a chromatin-remodeling factor and a histone ubiquitin protease (Buszczak et al., 2009; Kirilly and Xie, 2007; O'Reilly et al., 2008). Here, we define Mastermind as an essential FSC factor. Mam is not generally required for cell proliferation or survival in follicle cells or other Drosophila tissues. Mam is also not required for GSC function, assayed under exactly the same conditions and in the same animals that reveal its role in FSCs. However, in the absence of Mam, FSCs are lost as rapidly from adult ovaries as FSCs that cannot transduce a Hh signal.
Our experiments show that Mam function is required cell-autonomously within the FSC lineage and we assume that this reflects a function in the FSC itself. We saw no evidence of increased apoptosis of mam mutant FSCs (data not shown) or of prolonged quiescence of such cells (positive-marking studies), suggesting that mam FSCs are prematurely lost from their characteristic position in the germarium, taking on the fate of non-stem FSC daughter cells. Whether loss of Mam primarily affects a fundamental stem-daughter cell decision or adhesive properties contributing to niche retention is, as for most other FSC factors, unknown.
Mam function in FSC is independent of Notch signaling
Mam has long been considered to be a dedicated co-activator in the Notch signaling pathway, because genetic analyses in Drosophila and other model organisms generally show a congruence between Notch and Mam loss-of-function phenotypes (Bray, 2006; McElhinny et al., 2008). Biochemically, Mam binds to a composite surface contributed by the cleaved intracellular domain of activated Notch and the DNA-binding protein Su(H), and provides an essential transcriptional activation function that includes the recruitment of CREB-Binding Protein (CBP) (Fryer et al., 2002; Wallberg et al., 2002). In ovaries, we observed characteristic Notch mutant phenotypes in response to mam mutations, showing that Mam does indeed act as an essential co-activator for Notch signaling in the follicle cell lineage. However, that function of Mam cannot account for its role in FSCs because FSC function is not impaired by null mutations affecting Notch and Su(H), the direct binding partners of Mam. That assertion is also consistent with our finding that expression of a dominant-negative Mam derivative inhibited the Notch-dependent behaviors of FSC derivatives without impairing FSC maintenance. To determine whether loss of Notch signaling contributed even partially to the mam mutant FSC phenotype, we sought to ameliorate the mam phenotype by activating Notch signaling in a Mam-independent manner. We found that a synthetic Su(H)-VP16 activator could not rescue mam or ptc mam mutant FSC loss. Furthermore, increased activity of the Notch pathway by itself [using Su(H)-VP16 or Nintra] caused moderate FSC loss. Thus, the essential activity of Mam in FSCs appears to be entirely independent of the well-known role of Mam as a co-activator for Notch signaling.
Notch signaling in the FSC lineage
The finding that FSC maintenance is not markedly impaired by the elimination of Notch signaling is notable in itself, because it contrasts with a requirement for each of the three other pathways (Hh, BMP and Wnt) that have been investigated to date. Notch signaling is important in the germarium for the earliest known decision of FSC progeny to adopt polar and stalk cell fates, and for the specification and maintenance of cap cells, which are themselves essential for maintaining GSCs (Assa-Kunik et al., 2007; Song et al., 2007; Ward et al., 2006).
The loss of FSCs in response to increased Notch activity might also be informative, although the heightened Notch activity induced by Nintra or Su(H)-VP16 is likely to be beyond physiological levels. The FSC loss induced by Nintra cannot be explained by titration of Mam away from other essential partners, because Su(H)-VP16 induces a similar phenotype but cannot bind to Mam in the absence of activated Notch.
The Notch ligand Delta is known to be expressed in terminal filament, cap, follicle and germline cells, and a clear increase in Delta signaling from the germline to overlying follicle cells at stage 6 triggers a switch from mitosis to follicle cell endocycles (Deng et al., 2001; Lopez-Schier and St Johnston, 2001). Interestingly, that switch is still imposed on ptc mutant follicle cells that contact the germline, and is accompanied by Notch-dependent inhibition of ci expression and Hh pathway activity, which is mediated by the transcription factors Hindsight (Pebbled - FlyBase) and Tramtrack (Sun and Deng, 2007). FSC loss induced by Notch hyperactivity is also seen for ptc mutant cells and might conceivably involve an analogous mechanism, although Hindsight expression is not normally observed prior to stage 6. Moreover, it is possible that FSCs normally evade Notch-induced repression of Hh signaling by minimizing contact with the germline, while non-stem cell daughters embrace passing germline cysts.
Does Mam have a direct role in FSC Hh signaling?
There are currently very few reports of Notch-independent roles of Mam proteins (McElhinny et al., 2008). In two cases, the mammalian Mam homolog MAML1 was shown to bind and collaborate with DNA-binding proteins (p53 and MEF2C) other than those of the Su(H) family. In the third case, MAML1 was shown to bind β-catenin and to contribute to TCF-dependent induction of Wnt target genes. It seems likely from these examples, and from the established role of Mam in recruiting Mediator and histone acetyltransferase complexes (Fryer et al., 2002; Fryer et al., 2004), that the essential action of Mam in stem cells is as a transcriptional co-activator.
Mam function in FSCs has a notable dosage-sensitive interaction with the Hh signaling pathway. Mam was first identified in this context because a heterozygous mam mutation strongly suppressed ovarian somatic cell overproliferation induced by excess Hh signaling. This suppression was partially reproduced by controlling the level of Mam expression from a UAS-Mam transgene and was shown under those circumstances to suppress the duplication of FSCs normally induced by excessive Hh pathway activity. Our initial genetic screen suggests that dose-dependent suppressors of Hh-induced FSC expansion are rare. Complete loss of mam was fully epistatic over ptc mutations with regard to FSC duplication and FSC maintenance.
Several mechanisms might theoretically account for the observed interactions of Mam with the Hh pathway. However, we also saw that loss of Mam inhibited expression of the Hh pathway reporter ptc-lacZ in FSCs, focusing attention on the idea that Mam might act as a co-activator in the Hh pathway. Some further observations are relevant to this hypothesis.
First, we found no evidence of Mam affecting Hh signaling output in wing discs. Thus, any effect of Mam on FSC Hh signaling is tissue specific. Very little is known of the mechanisms underlying tissue-specific responses to Hh signaling, but tissue-specific interactions of Ci with other transcription factors and co-activators are likely conduits. Second, loss of Mam limited the induction of ptc-lacZ in ptc mutant FSC clones, but only to levels seen in normal FSCs. Thus, if Mam does indeed act in FSCs to potentiate Hh signaling, it could only be crucial for target genes induced by strong Hh pathway activity, for which ptc-lacZ is an insufficient marker. There is a precedent for exactly this situation in wing discs. There, loss of Fu kinase activity in ptc mutant clones completely eliminates the expression of Engrailed (which responds only to strong pathway activity) and substantially alters the resulting wing phenotype without reducing ptc-lacZ expression (Ohlmeyer and Kalderon, 1998) (C.V., unpublished).
In summary, epistasis of mam over ptc and the specific requirement for Mam in FSCs, which experience higher Hh signaling than their progeny, are consistent with a role for Mam as a co-activator of crucial FSC target genes induced only by strong Hh pathway activity. However, it is also possible that Mam contributes to FSC function independently of the Hh pathway, affecting ptc-lacZ expression in FSCs only indirectly. Further investigation would benefit greatly from the identification of crucial FSC Hh target genes and detailed examination of the chromatin localization of Mam, Ci and other transcription factors in the FSC lineage.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/13/2177/DC1
Supplementary Material
We thank the Bloomington Drosophila Stock Center at Indiana University, N. Baker, D. Harrison, P. Ingham, K. Irvine, F. Schweisguth, M. Wehrli and especially B. Yedvobnick for reagents; S. Marks, Z. Wang, Christina Atiya and Q. Zhou for helpful discussions; and S. Shang for help with statistics. This work was supported by an NIH grant (GM41815) to D.K. Deposited in PMC for release after 12 months.
References
- Assa-Kunik, E., Torres, I. L., Schejter, E. D., Johnston, D. S. and Shilo, B. Z. (2007). Drosophila follicle cells are patterned by multiple levels of Notch signaling and antagonism between the Notch and JAK/STAT pathways. Development 134, 1161-1169. [DOI] [PubMed] [Google Scholar]
- Bray, S. J. (2006). Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7, 678-689. [DOI] [PubMed] [Google Scholar]
- Buszczak, M., Paterno, S. and Spradling, A. C. (2009). Drosophila stem cells share a common requirement for the histone H2B ubiquitin protease scrawny. Science 323, 248-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, H. M. and Struhl, G. (2001). Nicastrin is required for Presenilin-mediated transmembrane cleavage in Drosophila. Nat. Cell Biol. 3, 1129-1132. [DOI] [PubMed] [Google Scholar]
- Deng, W. M., Althauser, C. and Ruohola-Baker, H. (2001). Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells. Development 128, 4737-4746. [DOI] [PubMed] [Google Scholar]
- Forbes, A. J., Lin, H., Ingham, P. W. and Spradling, A. C. (1996a). hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development 122, 1125-1135. [DOI] [PubMed] [Google Scholar]
- Forbes, A. J., Spradling, A. C., Ingham, P. W. and Lin, H. (1996b). The role of segment polarity genes during early oogenesis in Drosophila. Development 122, 3283-3294. [DOI] [PubMed] [Google Scholar]
- Fryer, C. J., Lamar, E., Turbachova, I., Kintner, C. and Jones, K. A. (2002). Mastermind mediates chromatin-specific transcription and turnover of the Notch enhancer complex. Genes Dev. 16, 1397-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer, C. J., White, J. B. and Jones, K. A. (2004). Mastermind recruits CycC:CDK8 to phosphorylate the Notch ICD and coordinate activation with turnover. Mol. Cell 16, 509-520. [DOI] [PubMed] [Google Scholar]
- Helms, W., Lee, H., Ammerman, M., Parks, A. L., Muskavitch, M. A. and Yedvobnick, B. (1999). Engineered truncations in the Drosophila mastermind protein disrupt Notch pathway function. Dev. Biol. 215, 358-374. [DOI] [PubMed] [Google Scholar]
- Hooper, J. E. and Scott, M. P. (2005). Communicating with Hedgehogs. Nat. Rev. Mol. Cell Biol. 6, 306-317. [DOI] [PubMed] [Google Scholar]
- Kidd, S., Lieber, T. and Young, M. W. (1998). Ligand-induced cleavage and regulation of nuclear entry of Notch in Drosophila melanogaster embryos. Genes Dev. 12, 3728-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilly, D. and Xie, T. (2007). The Drosophila ovary: an active stem cell community. Cell Res. 17, 15-25. [DOI] [PubMed] [Google Scholar]
- Lee, T. and Luo, L. (2001). Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 24, 251-254. [DOI] [PubMed] [Google Scholar]
- Lopez-Schier, H. and St Johnston, D. (2001). Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev. 15, 1393-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Schier, H. and St Johnston, D. (2002). Drosophila nicastrin is essential for the intramembranous cleavage of notch. Dev. Cell 2, 79-89. [DOI] [PubMed] [Google Scholar]
- Margolis, J. and Spradling, A. (1995). Identification and behavior of epithelial stem cells in the Drosophila ovary. Development 121, 3797-3807. [DOI] [PubMed] [Google Scholar]
- McElhinny, A. S., Li, J. L. and Wu, L. (2008). Mastermind-like transcriptional co-activators: emerging roles in regulating cross talk among multiple signaling pathways. Oncogene 27, 5138-5147. [DOI] [PubMed] [Google Scholar]
- Morel, V. and Schweisguth, F. (2000). Repression by suppressor of hairless and activation by Notch are required to define a single row of single-minded expressing cells in the Drosophila embryo. Genes Dev. 14, 377-388. [PMC free article] [PubMed] [Google Scholar]
- Nystul, T. and Spradling, A. (2007). An epithelial niche in the Drosophila ovary undergoes long-range stem cell replacement. Cell Stem Cell 1, 277-285. [DOI] [PubMed] [Google Scholar]
- Ohlmeyer, J. T. and Kalderon, D. (1998). Hedgehog stimulates maturation of Cubitus interruptus into a labile transcriptional activator. Nature 396, 749-753. [DOI] [PubMed] [Google Scholar]
- O'Reilly, A. M., Lee, H. H. and Simon, M. A. (2008). Integrins control the positioning and proliferation of follicle stem cells in the Drosophila ovary. J. Cell Biol. 182, 801-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweisguth, F. (2004). Notch signaling activity. Curr. Biol. 14, R129-R138. [PubMed] [Google Scholar]
- Song, X. and Xie, T. (2002). DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc. Natl. Acad. Sci. USA 99, 14813-14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, X., Call, G. B., Kirilly, D. and Xie, T. (2007). Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development 134, 1071-1080. [DOI] [PubMed] [Google Scholar]
- Sun, J. and Deng, W. M. (2007). Hindsight mediates the role of notch in suppressing hedgehog signaling and cell proliferation. Dev. Cell 12, 431-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, I. L., Lopez-Schier, H. and St Johnston, D. (2003). A Notch/Delta-dependent relay mechanism establishes anterior-posterior polarity in Drosophila. Dev. Cell 5, 547-558. [DOI] [PubMed] [Google Scholar]
- Tworoger, M., Larkin, M. K., Bryant, Z. and Ruohola-Baker, H. (1999). Mosaic analysis in the Drosophila ovary reveals a common hedgehog-inducible precursor stage for stalk and polar cells. Genetics 151, 739-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallberg, A. E., Pedersen, K., Lendahl, U. and Roeder, R. G. (2002). p300 and PCAF act cooperatively to mediate transcriptional activation from chromatin templates by notch intracellular domains in vitro. Mol. Cell. Biol. 22, 7812-7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, E. J., Shcherbata, H. R., Reynolds, S. H., Fischer, K. A., Hatfield, S. D. and Ruohola-Baker, H. (2006). Stem cells signal to the niche through the Notch pathway in the Drosophila ovary. Curr. Biol. 16, 2352-2358. [DOI] [PubMed] [Google Scholar]
- Xu, T. and Rubin, G. M. (1993). Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223-1237. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. and Kalderon, D. (2000). Regulation of cell proliferation and patterning in Drosophila oogenesis by Hedgehog signaling. Development 127, 2165-2176. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. and Kalderon, D. (2001). Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature 410, 599-604. [DOI] [PubMed] [Google Scholar]
- Zhou, Q., Apionishev, S. and Kalderon, D. (2006). The contributions of protein kinase A and smoothened phosphorylation to hedgehog signal transduction in Drosophila melanogaster. Genetics 173, 2049-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.