Abstract
The objective of this work was to determine whether diagnostic ultrasound and contrast agent could be used to transcranially and nondestructively disrupt the blood-brain barrier (BBB) in mice under ultrasound image guidance, and to quantify that disruption using MRI and MR contrast agent. Each mouse was placed under isoflurane anesthesia and the hair on top of its skull was removed before treatment. A diagnostic ultrasound transducer was placed in a water bag coupled with gel to the mouse skull. Definity (US contrast) and Magnevist (MR contrast) were injected concurrent with the start of a custom ultrasound transmission sequence. The transducer was translated along the rostral-caudal axis to insonify three spatial locations (2 mm apart) along one half of the brain for each sequence. T1-weighted MR images were used to quantify the volume of tissue over which the BBB disruption allowed Magnevist to enter the brain, based upon increases in MR contrast-to-noise ratio (CNR) as compared to the noninsonified portions of the brain. Ultrasonic frequency, pressure, and pulse duration, as well as Definity dose and injection time were varied. Preliminary results suggest that a threshold exists for BBB opening dependent upon both pressure and pulse duration (consistent with reports in the literature performed at lower frequencies). A range of typical diagnostic frequencies (e.g., 5.0-8.0 MHz) generated BBB disruption. Comparable BBB opening was noted with varied delays between Definity injection and insonification (0-2 min) for a range of Definity concentrations (400-2400 μL/kg). The low-pressure, custom sequences (MI≤0.65) had minimal blood cell extravasation as determined by histological evaluation. This study has shown the ability of a diagnostic ultrasound system, in conjunction with Definity, to open the BBB transcranially in a mouse model for molecules approximately 0.5 kDa in size. Opening was achieved at higher frequencies than previously reported and was localized under ultrasound image guidance. A typical, ultrasound imaging mode (PW Doppler) with specific settings (transmit frequency=5.7 MHz, gate size=15 mm, pulse repetition frequency=100 Hz, system power=15%) successfully opened the BBB, which facilitates implementation using the majority of commercially available clinical diagnostic scanners. Localized opening of the BBB may have potential clinical utility for the delivery of diagnostic or therapeutic agents to the brain.
Keywords: Ultrasound, Contrast agent, Blood-brain barrier, Drug delivery, Magnetic resonance imaging
Introduction
The blood-brain barrier (BBB) is a diffusion barrier present in all areas of the brain consisting of endothelial cells, astrocyte end-feet, and pericytes (Ballabh et al, 2004); however, in some brain structures (e.g., circumventricular organs, such as the subfornical organ, area postrema, organum vasculosum of the lamina terminalis), the BBB is incomplete or leaky (McKinley et al, 1998; Price et al, 2008; Hashimoto et al, 1994). BBB endothelial cells have no fenestrations, more extensive tight junctions than the rest of the body, and sparse pinocytic vesicular transport (Ballabh et al, 2004). This specialized endothelial barrier, therefore, limits the passage of substances (including many therapeutic agents) into the brain (Cho et al, 2002).
Several methods, some involving ultrasound, have been investigated to increase the permeability of the BBB to drugs, antibodies, and gene transfer (Sheikov et al, 2004; Kinoshita et al, 2006b; Shimamura et al, 2004). In cell monolayers, mild hyperthermia (41°C) using ultrasound (20 min, 0.4 W/cm2) has been shown to reversibly enhance passive diffusion of hydrophobic drugs and allow them to bypass efflux transporters (Cho et al, 2002). Evidence has also been presented supporting the use of high-intensity focused ultrasound (HIFU) to selectively disrupt the BBB in rats and rabbits; however, this method is often associated with brain tissue damage (Mesiwala et al, 2002; McDannold et al, 2004). Low frequency ultrasound (300 kHz) at low intensities has been shown to open the BBB in humans (Reinhard et al, 2006). An alternative method of localized BBB disruption is to use low-pressure ultrasound (e.g., P- (in situ)=0.4-1.5 MPa), at low diagnostic ultrasound frequencies (0.26-2.04 MHz), in conjunction with microbubble contrast agent (such as Optison or Definity) (McDannold et al, 2007, 2008a). This has been shown to open the BBB to allow molecules, such as gadolinium for MR contrast, imaging fluorophores for molecular imaging, and immunotherapeutics for Alzheimer’s disease, to enter the brain of mice and rabbits (Hynynen et al, 2001; Choi et al, 2007; Raymond et al, 2008); therefore, a dedicated mouse imaging and BBB disruption system will facilitate neurological and genetic evaluation in mice. In addition to basic science research, such a technique would be valuable in preclinical studies in which therapeutics (e.g., chemotherapeutics) could be adminstered focally to the mouse brain.
Several theories exist for possible mechanisms of BBB disruption with ultrasound and contrast agent. Studies where opening occurred with a temperature rise of only 0.025°C suggest that thermal effects are not required in this method of BBB opening (Kinoshita et al, 2006a). Furthermore, this BBB disruption has occurred without the detection of wide-band emissions, the signature for inertial cavitation, and this type of BBB disruption showed no blood cell extravasation (Kinoshita et al, 2006a; McDannold et al, 2006). Consequently, the factors likely to be responsible for such BBB disruption are the oscillation of microbubbles as occurs with stable cavitation, acoustic streaming around the microbubbles, and radiation force on the microbubbles (Datta et al, 2008; Kinoshita et al, 2006a; Sheikov et al, 2004; McDannold et al, 2006). These phenomena may cause mechanical stretching of vessels that leads to opening of tight junctions or the triggering of biochemical reactions to open the BBB (Sheikov et al, 2004). This stretch theory is further supported by work showing that microbubbles can induce a mechanical stretch, activating BKCa channels and leading to rapid hyperpolarization of the cell membrane potential that is in direct contact with the bubbles (Tran et al, 2008). In contrast, Raymond et al (2007) have shown vasoconstriction in the presence of low-intensity ultrasound and microbubbles. They speculate that blood-flow reduction from the vasoconstriction leads to slow leakage along undamaged vessels via transcytosis, while focal disruptions are a result of broken tight junctions. Although the exact mechanism is still under investigation, BBB disruption with ultrasound and contrast agent has been performed with minimal extravasated erythrocytes and neuronal damage (Hynynen et al, 2005; Kinoshita et al, 2006b).
To the best of our knowledge, a diagnostic imaging transducer with contrast has not been previously used for the intentional disruption of the BBB. Hynynen et al. demonstrated BBB disruption with a 1.5 MHz piston transducer with exposure levels near 6.3 MPa using common diagnostic ultrasound pulse lengths (10 μs) and pulse repetition frequencies (PRF, 1 kHz); however, these ultrasound exposures sometimes resulted in vascular and neuronal damage (Hynynen et al, 2003). Typical diagnostic transducers operate at 2-10 MHz, however the highest ultrasound frequency currently reported in the literature for BBB opening is 2.04 MHz in rabbits (McDannold et al, 2008a). Diagnostic transcranial color-coded sonography at 3.5 MHz with microbubble contrast did not result in detectable BBB opening when transmitted through the skull in humans (Schlachetzki et al, 2002). However, if an imaging transducer could be used for BBB disruption, it would have the advantage of providing both image guidance in the brain and therapeutic ultrasound delivery (automatically co-registered) without the need for additional devices. Furthermore, diagnostic scanners are more readily available to biomedical researchers. The primary goal of this work was to investigate the potential use of a diagnostic scanner for focally increasing BBB permeability in animal models using concurrent ultrasound image guidance. In addition to demonstrating the feasibility of using a diagnostic probe with contrast agent for image-guided BBB disruption in mice, secondary goals were to examine the effect of ultrasonic pressure, pulse duration, frequency, and the delay of ultrasound initiation after microbubble injection as well as contrast agent dose. Furthermore, because Definity has not been studied in the context of BBB disruption to the extent of Optison (McDannold et al, 2007), this study also serves to add to the knowledge base concerning one of the most widely used ultrasonic contrast agents in the United States.
Methods
Animal Setup
All animal procedures were approved by the Duke Institutional Animal Care and Use Committee. Thirty-six C57BL/6J mice (20-27 g) were used in this study. Each mouse was anesthetized with isoflurane and the scalp depilated. An IV tail catheter for perflutren lipid microspheres (Definity®, Bristol-Myers Squibb Medical Imaging, N. Billerica, MA, USA) injection and an IP catheter for gadopentetate dimeglumine (Magnevist®, Bayer HealthCare Pharmaceuticals, Inc., Wayne, NJ, USA) injection were put in place. A thin plastic bag containing a 17 mm water path was coupled to the scalp with ultrasound gel. A hemicylindrical plastic shell was placed over the thorax of the mouse to prevent the weight of the water from adversely affecting breathing. A stereotaxic positioning system (Vevo Integrated Rail System, Visualsonics, Toronto, Canada) was used to center the B-mode image in the transverse plane through the eyes.
Ultrasound Application
A Siemens Sonoline™ Antares diagnostic scanner and VF10-5 transducer (Siemens Medical Solutions USA, Inc., Issaquah, WA) were used to insonify the mouse brain approximately 3 mm deep to the dorsal surface of the skull using a transducer focal depth of 2 cm (for both electronic focusing in azimuth and the lens focus in elevation; a water path was used as a standoff to this depth). All acoustic pressure measurements were made with a Sonora SN S4-251 hydrophone with a 0.4-mm spot size membrane (Sonora Medical Systems, Inc., Longmont, CO) in water. The manufacturer quoted precision of the calibration factor for voltage to pressure conversion is ± 1.0 dB. A baseline sequence with 20 ms, 2.7 MPa (peak-to-peak) pulses repeated at 10 Hz for 30 seconds was implemented based on (Choi et al, 2007). Figure 1 shows typical pulses used for this study. Modulation of the ultrasonic sequence as well as the dosage and timing of the Definity injection was performed. Ultrasonic parameters were investigated by varying pulse durations between 0.35 μs and 20 ms, peak-to-peak pressures between 1.1 and 6.2 MPa, and frequencies between 5.0 and 8.0 MHz. Definity doses between 10 and 60 μL (400-2400 μL/kg) and Definity injection times were also briefly examined, ranging from start of insonification to 2 minutes prior to insonification. Table 1 summarizes the parameters investigated and the number of animals evaluated for each sequence.
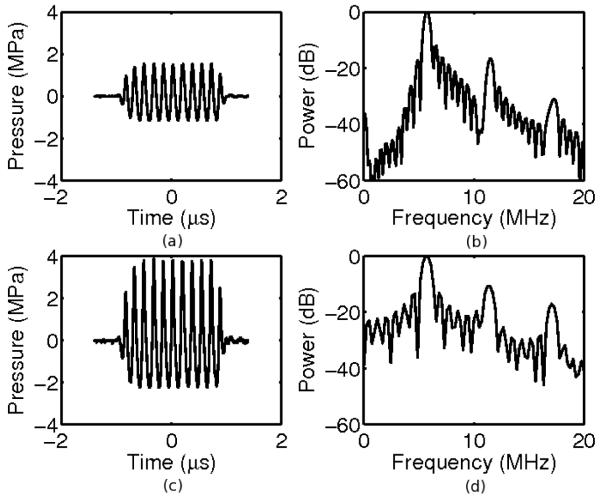
Figure 1.
Example waveforms (a,c) and power spectra (b,d) of pulses with peak-to-peak pressures of 2.7 MPa (a,b) and 6.2 MPa (c,d). At these pressures, the waveforms demonstrate some nonlinearity. The corresponding MI are 0.3 and 0.7, respectively.
Table 1.
This table summarizes the exposure parameters investigated in this study along with the CNR and number of insonifications evaluated for each set of parameters. The number of animals column gives the number of locations (one per sequence on a given animal) evaluated for CNR followed by the number of those animals used in histology in parentheses. Each location was insonified for 30 seconds with a PRF of 10 Hz and an unapodized, F/1.5 configuration except the PW Doppler sequence (*) which used an 100 Hz PRF and an apodized, F/4 configuration. The first 8 sequences are presented in the plots herein, while the others serve as discussion points. The mice in row † were insonified with the most aggressive sequence in an additional location, independent of those used for CNR evaluation, for histology purposes only
| # of Animals | Definity Dosage (μL) | Delay after Definity injection (s) | Frequency (MHz) | Pressure (MPapp) | Pulse Duration (ms) | CNR (mean ± SD) |
|---|---|---|---|---|---|---|
| 4(0) | 30 | 0 | 5.7 | 1.1 | 20 | 0 ± 0 |
| 4(2) | 30 | 0 | 5.7 | 6.2 | 20 | 39 ± 8 |
| 4(1) | 30 | 0 | 5.0 | 2.3 | 20 | 22 ± 12 |
| 4(2) | 30 | 0 | 5.7 | 2.7 | 20 | 36 ± 3 |
| 4(2) | 30 | 0 | 6.7 | 3.8 | 20 | 25 ± 8 |
| 4(1) | 30 | 0 | 8.0 | 5.2 | 20 | 42 ± 13 |
| 4(3) | 30 | 0 | 5.7 | 2.7 | 0.002 | 9 ± 4 |
| 4(1) | 30 | 0 | 5.7 | 2.7 | 0.07 | 24 ± 8 |
| 2(0) | 10 | 0 | 5.7 | 6.2 | 20 | 36 ± 2 |
| 2(0) | 60 | 0 | 5.7 | 6.2 | 20 | 41 ± 11 |
| 2(0) | 30 | 7 | 5.7 | 6.2 | 20 | 24 ± 7 |
| 2(0) | 30 | 60 | 5.7 | 6.2 | 20 | 22 ± 1 |
| 1(0) | 30 | 120 | 5.7 | 6.2 | 20 | 29 |
| 2(0) | 30 | 0 | 6.7 | 2.8 | 20 | 23 ± 1 |
| 2(1) | 30 | 0 | 8 | 2.3 | 20 | 0 ± 0 |
| 1(0) | 30 | 0 | 5.7 | 1.6 | 20 | 24 |
| 1(0) | 30 | 0 | 5.7 | 3.8 | 20 | 39 |
| 1(0) | 30 | 0 | 5.7 | 2.7 | 0.00035 | 0 |
| *5(5) | 30 | 0 | 5.7 | 2.7 | 0.007 | 21 ± 9 |
| †0(7) | 30 | 0 | 5.7 | 6.2 | 20 | - |
BBB Opening Procedure
For opening the BBB, each hemisphere of the brain was treated independently, thus up to two different ultrasound sequences (selected from those shown in Table 1) were tested on each animal - one in each hemisphere of the brain. For a given sequence in a given hemisphere, three different locations were serially insonified 2 mm apart in the rostral-caudal direction (see Figure 2). Using B-mode ultrasound image guidance and the stereotaxic positioning system, the transducer was moved to the first location: 3 mm posterior to the eyes and 1.5 mm to the left of the midline as shown in Figure 2. At the onset of a 30 second ultrasound sequence, Magnevist (6.3 mmol/kg IP) and Definity (30 μL IV) were injected. (We have found that this dose of Magnevist produces a consistent level of enhancement in mice.) After the 30-second sequence was finished, the transducer was then translated such that two more focal spots were insonified 2 and 4 mm posterior to the first spot at 1 and 2 minutes after the Definity injection (only one injection per side), respectively. Prior to administering the second sequence (right side of brain), an IV saline flush was given, and the Definity was allowed to clear over 5 minutes. The half-life for Definity in blood is reported to be only 1.3 minutes (Unger et al, 2004; Def, 2004), which is consistent with qualitative observations in this work. The same procedure was then repeated with a different sequence 1.5 mm to the right of the midline but without reinjection of Magnevist, which clears slowly with a half-life of 1.6 hours (Mag, 2008).
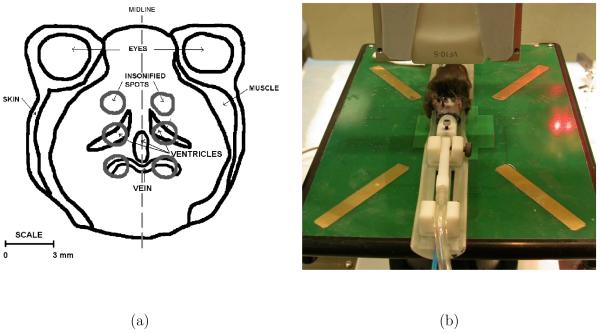
Figure 2.
(a) Anatomical sketch of a coronal slice of the brain with the insonification spots. Only the two most rostral spot positions were analyzed in the MR images. (b) Setup and transducer orientation relative to the mouse. Note: The water bag is not shown here.
Because Magnevist is normally excluded by the BBB, our assay for BBB disruption was to monitor the signal enhancement in MR images. After insonification of all six locations, the animal was placed in a quadrature, 300.5 MHz birdcage coil (M2M Imaging, Cleveland, OH, USA) tunable for mice (20 - 30 grams) and imaged in a 7T MRI system interfaced to a GE EXCITE console. A 3D spoiled gradient recalled echo (SPGR) sequence was used to acquire T1-weighted images approximately 30 minutes after insonification of the first spot. Because Magnevist is normally excluded by the BBB, regions of brain enhancement in the T1-weighted images were interpreted as regions of BBB disruption.
Image Analysis
Image registration between the ultrasound and MR images was performed by aligning a control point defined at the top of the skull directly above the center of the BBB opening from left to right in the MR images and along the beam index used for BBB opening in the ultrasound image. The hyper-intense structures in the ultrasound image, corresponding to bones in this study, were then overlaid onto the corresponding MR images to evaluate the effectiveness of the ultrasonic guidance.
The degree of opening was evaluated by semi-automatic segmentation of the volumes of enhanced brain tissue in the MR images. By inverting the gray-scale values in the MR image, applying a 3-D watershed algorithm (The MathWorks, Inc., Natick, MA), and ignoring any voxels originally below the background level, the contrast-enhanced volumes associated with BBB opening were segmented. The full-width half-maximum (FWHM) contours in each slice of a volume were then used to calculate the mean gray-level as well as the dimensions and total volume for each opened region, or spot, in the brain. If the contralateral region of the brain to the region of interest was not insonified, the unopened BBB level was calculated as the mean gray-level in the opposite hemisphere; otherwise, the mean level in an unopened region of equivalent size and shape a few millimeters lateral and caudal to the opened region was used. The contrast-to-noise ratio (CNR) was calculated as the difference in mean gray-levels of the FWHM-defined volumes for the opened and unopened BBB regions divided by the standard deviation in an empty region of the MR image (no tissue present); therefore, a higher CNR is indicative of more BBB opening (or Magnevist in the brain tissue) and a CNR of 0 indicates no discernible opening.
Histology
The brains of eleven mice were processed for histology. Eight of these mice were insonified with the most aggressive sequence for BBB opening (5.7 MHz, 20-ms pulses, 10 Hz PRF, 6.2 MPapp, 30 μL Definity), sometimes as part of the CNR analysis described previously and sometimes as a single spot spatially offset from those previously imaged by MR. Insonification with less aggressive BBB sequences was also performed in these brains, as described previously. Two other mice were insonified with the sequence common to all of the plots herein (5.7 MHz, 20-ms pulses, 10 Hz PRF, 2.7 MPapp, 30 μL Definity) and another less aggressive sequence. In the eleventh brain, the effect of a commercial, B-mode sequence (5.7 MHz, 0.35-μs pulses, 36 Hz frame rate, 10.0 MPa+/4.6 MPa-, 30 μL Definity, five 30-second periods) on the brain was evaluated, in addition to one spot of the most aggressive BBB opening sequence within the B-mode plane. The number of mice processed for histology for each of the sequences is given in Table 1. The brains of these mice were fixed using transcardiac formalin (10%) perfusion *. Coronal sections of the excised brains were taken at 0.5 mm intervals (at least 17 slices per animal) and stained with hematoxylin-eosin. These sections were examined for evidence of red blood cell extravasation into the brain parenchyma, which has been reported to be the first sign of tissue damage (Burkitt et al, 1996; Hynynen et al, 2005). One author (MP) who was blind to the ultrasound exposure conditions used performed the histology examination. An extravasation was defined as ≥2 red blood cells in the parenchyma adjacent to an intact or partially intact blood vessel.
Results
Confounding effects from the ventricles and other anatomical variations are among the challenges with these experiments. The blood-choroid barrier of the ventricles opened more readily than the blood-brain barrier. As shown in Figure 3, only one of the three insonification spots is visible in brain tissue, but the ventricles are clearly visible. Furthermore, because the ventricles are interconnected, an opening of the blood-choroid barrier in one part of the brain caused enhancement througout the ventricular network. As a result, quantitative measurements are only reported for the most rostral spot (spot 1) for each 3 spot parameter set, because the ventricles were not present within this region (Figures 2 and 3).
Figure 3.
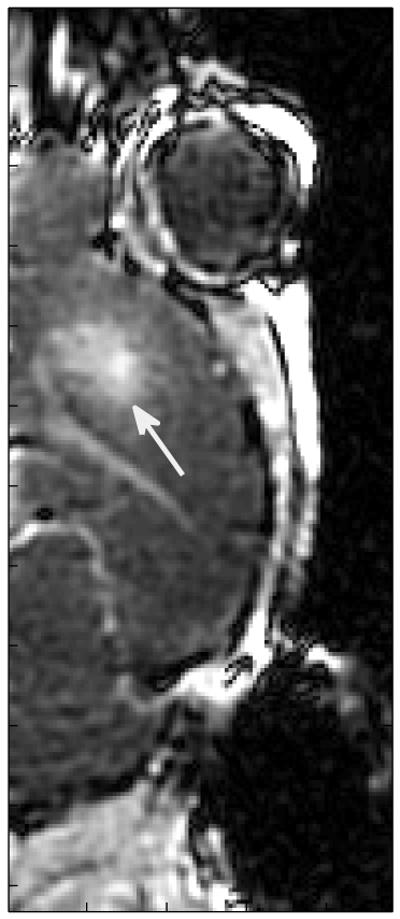
BBB opening with PW Doppler. 5.7-MHz, 7-μs ultrasound pulses repeated at 100 Hz with an apodized F/4 configuration yielding 2.7 MPapp were transmitted for 30 seconds immediately after a 30-μL Definity injection.
The stereotaxic stage in conjunction with ultrasound image guidance prior to injection of Definity made repeatable localization within the brain efficient. The midline between the eyes was easily visible in B-mode images and the stereotaxic positioning system could be moved such that the BBB opening insonification accurately occurred 3 mm caudal and 1.5 mm lateral to this point. As demonstrated in Figure 4, the position of the maximum contrast in the BBB opening was accurate in the medial-lateral and ventral-dorsal axes.
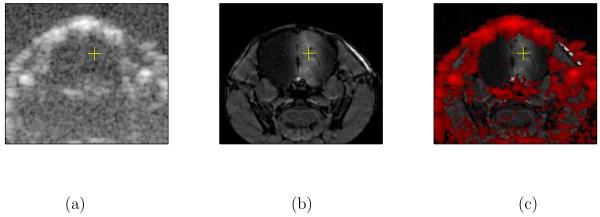
Figure 4.
Images showing a) B-mode ultrasound only (5.7 MHz), b) MR only, and c) structures seen in ultrasound (found by thresholding) overlaid in red on the MR image. The yellow + shows the intended center of the ultrasound focus based on the B-mode image. The white region surrounding the + on the right side of the MR image is indicative of T1 enhancement from Magnevist crossing the BBB. BBB opening was achieved using 5.7-MHz, 20-ms ultrasound pulses repeated at 10 Hz with an F/1.5 configuration, yielding pressures of 6.2 MPapp, in a 30-second insonification immediately after a 30-μL Definity injection.
The general impact of varying the amount of Definity present during insonification was considered in two ways: (1) increasing the dose and (2) changing the time in circulation before insonification. BBB opening with similar CNR was seen with an increasing dose of Definity from 10 to 60 μL (Table 1).
The impact of varying delays between Definity injection and ultrasound initiation were observed over a range of times. For some experimental configurations, it may be hard to have concurrent injection and insonification initiation; therefore, a fast, but reasonable, range of delays between 0 and 2 minutes were considered. Opening occurred in all cases, with slightly higher CNRs observed with no delay (Table 1).
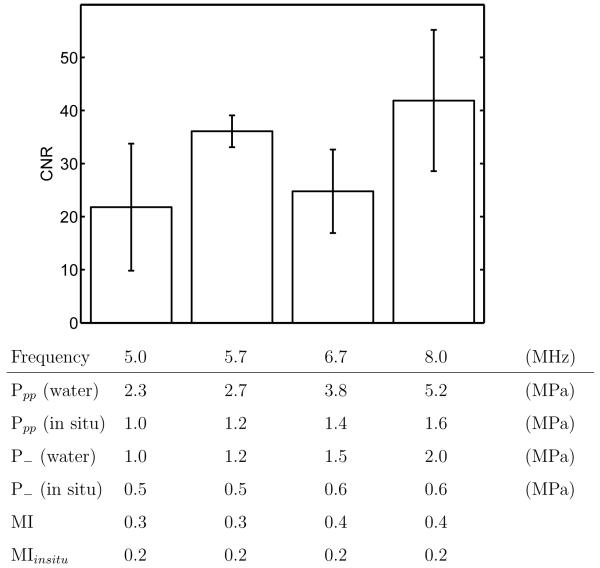
Previous BBB opening studies have looked at frequencies below 2.04 MHz, but the band-widths of diagnostic transducers are usually centered around higher frequencies. Therefore, frequencies of 5.0, 5.7, 6.7 and 8.0 MHz were tested with equal MIin situ (0.2, peak negative in situ pressure over the square root of frequency (McDannold et al, 2008a)). BBB disruption was generated at each of these frequencies with insignificant differences in CNR (p>0.05) between 5.0 and 8.0 MHz, as shown in Figure 5. The acoustic output for the frequencies tested are also shown. The pressures measured in water and derated by the attenuation of the skull and intervening brain tissue (attenuation values reported in (Choi et al, 2007; Duck, 1990)), as well as the MI (peak negative pressure derated by 0.3 dB/cm/MHz over the square root of frequency) (NCRP, 2002) and estimated MIin situ (derated in the same way as the in situ pressure) values are reported. It was noted in preliminary studies that when the MIin situ at 8.0 MHz was lowered to 0.1, no BBB disruption was seen.
Figure 5.
BBB opening for ultrasonic transmission frequencies from 5.0 to 8.0 MHz for the same MIinsitu. 20-ms ultrasound pulses repeated at 10 Hz with an F/1.5 focal configuration were transmitted for 30 seconds immediately after a 30-μL Definity injection. The mean and standard deviation for four animals are indicated at each frequency. Non-derated and derated pressures as well as MI and MIin situ for each frequency are listed.
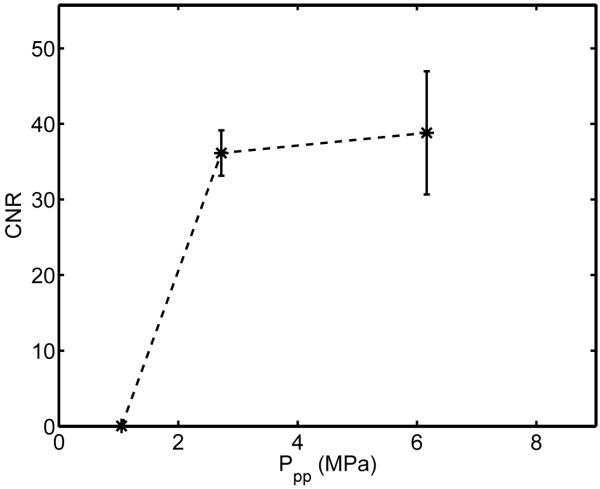
Regardless of the mechanism, most acoustic bioeffects are related to the energy delivered and duration of insonification. Therefore, we evaluated the effects of changing pressure and pulse duration on the degree of BBB opening. While maintaining a constant frequency (5.7 MHz) and changing the pressure, visible opening was shown to require a peak-to-peak pressure exceeding a threshold between 1.1 MPa and 2.7 MPa, as shown in Figure 6. Above 2.7 MPa, the increase in contrast was insignificant (p > .05). A single case from each of two intermediate pressure values (1.6 and 3.8 MPapp) resulted in CNR values (24 and 39) within the appropriate ranges, as predicted by Figure 6.
Figure 6.
Effect of ultrasonic pressures from 1.1 to 6.2 MPapp (non-derated) on BBB opening. 5.7-MHz, 20-ms ultrasound pulses repeated at 10 Hz with an F/1.5 configuration were transmitted for 30 seconds immediately after a 30-μL Definity injection. The mean and standard deviation for four animals is given at each pressure.
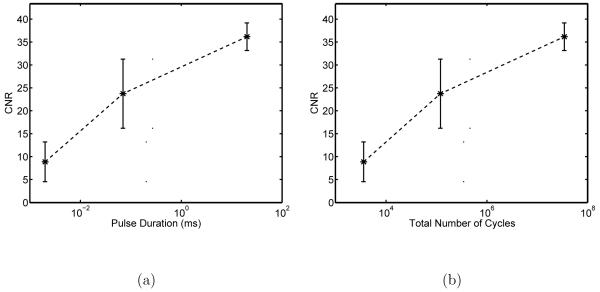
In order to show the feasibility of BBB opening with a clinical scanner, a range of pulse durations corresponding to Color Doppler (0.002 ms) and acoustic radiation force impulse (ARFI, 0.07 ms) imaging were evaluated (see Table 1) and compared with a 20-ms pulse previously shown to open the BBB (Choi et al, 2007), all at a pulse repetition frequency (PRF) of 10 Hz and a total insonification time of 30 seconds. The opening for 2-μs pulses was not always clear without a priori knowledge of the expected location of BBB disruption. However, pulse durations of 70 μs and 20 ms were clearly visible, as evidenced by the CNR values in Figure 7 (semi-log plots in x). For this configuration, the threshold for uniform, well-visible (CNR > 10) opening is a pulse duration between 2 and 70 μs, repeated such that the total number of cycles exceeds ∼ 105. It should also be noted that in a single case where a 2-cycle M-mode pulse (0.35 μs) was used with all other parameters the same (e.g., 2.7 MPapp), no opening was seen.
Figure 7.
a) Effect of pulse durations of 2 μs (Color Doppler), 70 μs (Acoustic Radiation Force Impulse Imaging), and 20 ms on BBB opening. 5.7-MHz ultrasound pulses repeated at 10 Hz with an F/1.5 configuration yielding 2.7 MPapp were transmitted for 30 seconds immediately after a 30-μL Definity injection. The mean and standard deviation for four animals is given at each pulse duration. b) Same data presented as a function of the total number of cycles in the insonification sequence. Note these are semi-log plots in x.
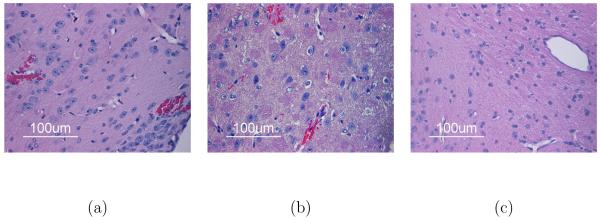
In the preliminary work for this study, standard B-mode insonification (MI=1.3) with Definity present was found to open wide planes of BBB corresponding to the B-mode field of view and to be associated with blood cell extravasation (26 sites in one brain), as evidenced in Figure 8. Therefore, for all other data, no B-mode monitoring after Definity injection was performed in order to avoid both opening and damage from insonification in untargeted areas. The histologic data from mice insonified with the most aggressive, experimental ultrasound regime (MI=0.7, 5.7 MHz transmit frequency, 6.2-MPa peak-to-peak pressure (in water), F/1.5, 20 ms pulse duration, 3.42e7 total cycles, and a 10-Hz PRF) resulted in an average of 4.1±5.5 (mean ± standard deviation) extravasated sites per brain (over 8 entire brains evaluated). Seven or less extravasations were considered minimal. Minimal extravasation was seen in nine of the ten brains insonified by the low-pressure sequences (16 seen in tenth brain). Because not all of the extravasations seen were in sonicated locations, it is not clear whether the small amount of blood cell extravasation was a function of the insonification and contrast agent or the perfusion, fixation, and sectioning methods.
Figure 8.
H & E stained histology of a) blood cell extravasation caused by standard B-mode (MI=1.3, 0.35 μs, 5.7 MHz, 10.0 MPa+, 4.6 MPa- insonifying for five 30-second periods at a 36 Hz frame rate with 30-μL Definity injection) as well as b) extravasated (top) and vessel-enclosed (bottom) blood cells and c) no damage with the most aggressive experimental ultrasound exposure used for this study (MI=0.7, 5.7-MHz transmit frequency, 6.2-MPapp pressure (in water), F/1.5, and 20-ms pulse duration with 30-μL Definity).
Based on the range of pulse durations and pressures that resulted in obvious opening (CNR>10) presented here, it became evident that a pulsed Doppler sequence could be utilized for BBB opening in the mouse. As a proof of concept, the VF10-5 transducer was placed in the standard, clinical, pulsed wave (PW) Doppler mode (B-mode imaging frozen) on the Antares system with a frequency of 5.7 MHz, gate size of 15 mm, PRF of 100 Hz, and a system power of 15%, as indicated on the scanner monitor, for 30 seconds (see Figure 9). These settings resulted in a pulse duration of 7 μs and 1.2×105 total cycles with an apodized F/4 focal configuration. The MI and peak-to-peak pressure of this configuration were equal to one of the standard configurations tested in this study (MI=0.3, 2.7 MPapp). As shown in Figure 3, this sequence easily opened the BBB (CNR = 21±9).
Figure 9.
Example of image guidance and system settings for PW Doppler mode BBB opening.
Discussion
Visualization of the skull, zygomatic arches, and eyes in B-mode images made 3-D localization with the stereotaxic positioning system simple, fast, and repeatable. As evidenced by the registration of B-mode to MR images (Figure 4), the location of peak opening was close to the focal point shown on B-mode. The center of the visible opening was not centered around this focus in the ventral-dorsal direction because the focus of the ultrasound beam was closer to the top of the skull. Furthermore, our studies indicated a change in the depth of the opening (center and dorsal-ventral extent) with anatomical position in the brain (rostral-caudal and left-right). Variable thickness in the skull and confounding effects from the ventricles may explain the variations with position. With a higher attenuation and speed of sound than tissue, variable thicknesses in the skull lead to changes in the pressure delivered in vivo due to increased attenuation and phase aberration (Tanter et al, 1998). Similarly, the fluid in the ventricles will also impact the pressure delivered due to a lower attenuation as compared to tissue (Petkus et al, 2002).
The doses of Definity in this study exceeded the manufacturer’s clinical recommendations (10 μL/kg (Def, 2004)). These clinical recommendations would require injecting 0.2 to 0.27 μL with repeated accuracy. Because it is difficult to administer the clinical doses for the small body weight of a mouse, the doses used in this study were in the range of 400 to 2400 μL/kg (bolus injection). BBB opening was achieved at all studied doses with similar CNRs. These results are consistent with those of another group using focused ultrasound (0.69 MHz in rabbits) with Optison at lower doses (50-250 μL/kg) (McDannold et al, 2008b).
Previous work in mice demonstrated the need for increased pressure (near 3-fold) to observe BBB opening when there was a 15-minute versus a 1-minute delay between contrast agent (Optison) injection and insonification (intact skull, 1.5 MHz, 20-ms pulses at 10 Hz for 30 seconds, 400 μL/kg of Optison) (Choi et al, 2007). Minimal variation in opening for up to a 2 minute delay between injection and the start of insonification was observed in our studies. However, these data do suggest (without statistical significance) that starting the ultrasound insonification at exactly the same time as Definity injection may be optimal. To ensure that the most Definity possible is insonified before it is cleared or degraded by the system, it may be optimal to initiate insonification prior to injection.
A midrange subset of typical diagnostic frequencies were evaluated in this study to show the potential for using diagnostic scanners for BBB opening. A couple of factors, the in situ pressure and the resonance frequency of Definity, could influence the BBB opening observed at a given frequency for a constant pulse duration and insonification time. Of these two factors, the in situ pressure was directly evaluated and had an interesting impact on the BBB opening observed. At 5.7 MHz, there was a significant (p < .05) change in CNR between 1.1 and 2.7 MPapp and an insignificant change between 2.7 and 6.2 MPapp. By assuming linear attenuation and accounting for acoustic loss through the skull (as reported by (Choi et al, 2007) at 1.5 MHz) and brain (Duck, 1990), the in situ pressures shown in Figure 5 result. These pressures are indicative of the estimated increase in attenuation with frequency. Distortions of the beam due to phase aberration effects have also been shown to increase with frequency (Nock et al, 1989) and, therefore, may have further reduced the actual in situ pressure due to defocusing of the beam.
The second factor to consider is the resonance frequency of the Definity microbubbles. The mean bubble diameter of Definity, as described by the manufacturer, is between 1.1 and 3.3 μm (Def, 2004). According to Goertz et al., a lipid encapsulated bubble of those dimensions will have resonance frequencies ranging from about 13 to 3 MHz, respectively. A 2.2 μm diameter bubble (median of Definity diameters) with minimal damping should resonate around 4 to 5 MHz according to simulations (Goertz et al, 2003); however, as bubbles travel through the vasculature, this frequency decreases in vessels of smaller radii (e.g., capillaries) and further decreases near the center (lengthwise) of these smaller vessels (Sassaroli and Hynynen, 2004, 2005).
The combined impact of pressure and frequency on bubble dynamics is included in the mechanical index, which indicates lower frequency insonifications result in an increased likelihood for cavitation (Apfel and Holland, 1991). McDannold et al (2008a) reported recently that the threshold for BBB disruption is constant with a variant of mechanical index, MIin situ. In our data, an MIin situ of 0.1 at 8.0 MHz did not open the BBB, while an MIin situ of 0.2 at 5.0, 5.7, 6.7, and 8.0 MHz did. The threshold herein is lower than McDannold’s threshold of 0.46 (McDannold et al, 2008a); however, this could be due to the much higher dose of contrast agent used in our studies as well as differences in the type of contrast agent, sonication conditions, and other factors.
Possible mechanisms for BBB opening can be hypothesized based on the pulse duration studies. As with ultrasonic pressure, there appears to be a threshold for pulse duration (at a given pulse repetition frequency (PRF) of 10 Hz, and total insonification time of 30 sec) that must be exceeded in order to observe appreciable BBB opening (CNR>10, Figure 7) for the low pressures used in the majority of this study (≤ 6.2 MPapp in water, MI≤0.7). At these low pressures, a pulse length of 2 μs, which is typical for diagnostic Color Doppler pulses, resulted in some opening with a low CNR (9±4). However, when B-mode (0.35-μs) pulses with a high MI (1.3) were used, easily visible opening was seen, but it was associated with more blood cell extravasation. Therefore, longer pulses with lower pressures were found to be preferred for BBB opening with minimal blood cell extravasation. The fact that low pressures are effective is consistent with the hypothesis that inertial cavitation is not necessary for BBB opening (Fowlkes et al, 2008; McDannold et al, 2006).
These pulse duration studies also suggest that acoustic radiation force may be involved in the mechanism for BBB opening. Primary acoustic radiation force is proportional to acoustic temporal-average intensity (Dayton et al, 1997). In this study, a significant (p < 0.05) increase in visible opening was observed between 2-μs (Ispta=1.1 mW/cm2), 70-μs (Ispta=39.5 mW/cm2), and 20-ms (Ispta=11.3 W/cm2) low pressure (2.7 MPapp) pulses at the same PRF and total insonification time, supporting the hypothesis that increased primary radiation force results in more BBB opening (Raymond et al, 2007). Although not monitored herein, these increased pulse durations should provide a longer time period for driving stable cavitation (i.e., bubble resonance without violent rupture) to open the BBB, as described in the literature for thrombolysis (Datta et al, 2008).
Given the data presented here, the total number of cycles deposited at the focus for a given frequency may be a good predictor of the degree of BBB opening (CNR) observed. Two sequences with different pulse lengths and repetition frequencies, but the same total number of cycles, had similar CNRs. Specifically, the PW Doppler sequence with 7-μs pulses at a 100 Hz PRF (Ispta=39.5 mW/cm2) had a CNR of 21±9, and the ARFI pulse length sequence (70-μs pulses, 10 Hz, Ispta=39.5 mW/cm2) had a CNR of 24±8. The difference in CNR between these two sequences, with the same total number of cycles (1.2e5) and pressure at the focus (2.7 MPapp), was insignificant (p>0.05).
Transcranial opening of the BBB with a diagnostic system was proven feasible in mice herein; however, significant barriers exist for extending this to humans. The mouse skull is very thin resulting in minimal acoustic loss due to attenuation (18% of the pressure amplitude at 1.525 MHz (Choi et al, 2007)) and phase aberration. Increased attenuation and defocusing due to thicker skulls in larger animals would require more acoustic output from the transducer to achieve the necessary in situ pressures. Although diagnostic systems may be capable of the necessary output, it may lead to excessive skull heating unless aberration correction or other techniques are employed (Clement et al, 2005; Aubry et al, 2003). However, there could be intra-operative situations in which a whole or partial craniotomy has already been performed and similar methods to those presented herein could be applied directly to the brain, but with the use of a more clinically relevant Definity dosage. A post-operative situation in which an acoustically transparent window has been implanted might also be feasible.
Conclusion
The results of this study demonstrate the feasibility of BBB opening in mice with a commercial, diagnostic system and ultrasound contrast agent. Ultrasound at a frequency capable of imaging relevant anatomical landmarks in the mouse skull was successfully utilized both to image the mouse brain and to open the BBB in the presence of ultrasound contrast agent. Longer duration pulses (≥ 2 μs over a 30-second insonification time, at PRFs of 10-100Hz, for a total number of cycles from ∼ 104 to 108) with low pressure amplitudes (1.6 to 6.2 MPapp, MI≤0.7) were found to allow MR contrast agent to enter the brain with minimal blood cell extravasation. Using these ranges as a guide, standard system settings with a low MI (e.g., PW Doppler, 15% power, maximum gate size) can be chosen to open the BBB without significant damage (≤7 extravasations/brain for the majority of cases). The results of this study can be used to gauge the potential of other custom sequences or existing diagnostic regimes for studies to locally deliver drugs or other therapeutic agents through the BBB in mice.
Acknowledgments
This work was supported by NIH grants 2R01 EB-002132 and 1R01 CA-114075, as well as NSF Graduate Research Fellowship 2003014921. All work was performed at the Duke Center for In Vivo Microscopy, an NIH/NCRR National Biomedical Technology Resource Center (P41 RR005959) and an NCI Small Animal Imaging Resource Program (U24 CA092656). The authors would also like to thank Siemens Medical Solutions USA, Inc. Ultrasound Division for their technical assistance.
Footnotes
One brain was fixed by immersion in formalin. Although this may have resulted in a higher number of extravasations counted, it did not change the conclusions drawn.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Package insert. Bristol-Myers Squibb Medical Imaging; North Billerica, MA: Sep, 2004. Definity® Vial for (Perflutren Lipid Microsphere) Injectable Suspension. [Google Scholar]
- Package insert. Berlex Imaging; Montville, New Jersey: 2008. Magnevist® (brand of gadopentetate dimeglumine) Injection. [Google Scholar]
- Apfel R, Holland C. Gauging the likelihood of cavitation from short-pulse, low-duty cycle diagnostic ultrasound. Ultrasound Med. Biol. 1991;17:179–185. doi: 10.1016/0301-5629(91)90125-g. [DOI] [PubMed] [Google Scholar]
- Aubry J, Tanter M, Pernot M, Thomas J, Fink M. Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans. J. Acoust. Soc. Am. 2003;113(1):84–93. doi: 10.1121/1.1529663. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview; structure, regulation, and clinical implications. Neurobiology of Disease. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Burkitt H, Stevens A, Lowe J, Young B. Wheater’s Basic Histopathology: A Colour Atlas and Text. 3rd Edition Churchill Livingstone; New York: 1996. [Google Scholar]
- Cho CW, Liu Y, Cobb W, Henthorn T, Lillehei K, Christians U, Ng KY. Ultrasoundinduced mild hyperthermia as a novel approach to increase drug uptake in brain microvessel endothelial cells. Pharmaceutical Research. 2002;19(8):1123–1129. doi: 10.1023/a:1019837923906. [DOI] [PubMed] [Google Scholar]
- Choi J, Pernot M, Small S, Konofagou E. Noninvasive, transcranial and localized opening of the blood-brain barrier using focused ultrasound in mice. Ultrasound Med. Biol. 2007;33(1):95–104. doi: 10.1016/j.ultrasmedbio.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Clement G, White P, King R, McDannold N, Hynynen K. A magnetic resonance imagingcompatible, large-scale array for trans-skull ultrasound surgery and therapy. J Ultrasound Med. 2005;24:1117–1125. doi: 10.7863/jum.2005.24.8.1117. [DOI] [PubMed] [Google Scholar]
- Datta S, Coussios CC, Ammi A, Mast D, De Courten-Myers G, Holland C. Ultrasound-enhanced thrombolysis using Definity® as a cavitation nucleation agent. Ultrasound Med. Biol. 2008;34(9):1421–1433. doi: 10.1016/j.ultrasmedbio.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayton P, Morgan K, Klibanov A, Brandenburger G, Nightingale K, Ferrara K. A preliminary evaluation of the effects of primary and secondary radiation forces on acoustic contrast agents. IEEE Trans. Ultrason., Ferroelec., Freq. Contr. 1997;44(6):1264–1277. [Google Scholar]
- Duck F. Physical Properties of Tissue. Harcourt Brace Jovanovich; San Diego: 1990. [Google Scholar]
- Fowlkes J, Abramowicz J, Church C, Holland C, Miller D, O’Brien W, Sanghvi N, Stratmeyer M, Zachary J. American institute of ultrasound in medicine consensus report on potential bioeffects of diagnostic ultrsound: Executive summary. Journal of Ultrasound in Medicine. 2008;27:503–515. doi: 10.7863/jum.2008.27.4.503. [DOI] [PubMed] [Google Scholar]
- Goertz D, Frinlink M, Bouakaz A, Chin C, de Jong N, van der Steen A. The effect of bubble size on nonlinear scattering from microbubbles at high frequencies; 2003 IEEE Ultrasonics Symposium; 2003.pp. 1503–1506. [Google Scholar]
- Hashimoto M, Ueno T, Iriki M. What roles does the organum vasculosum laminae terminalis play in fever in rabbits? Pflügers Arch Eur J Physiol. 1994;429:50–57. doi: 10.1007/BF02584029. [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Martin H, Jolesz F, Vykhodtseva N. The threshold for brain damage in rabbits induced by bursts of utlrasound in the presence of an ultrasound contrast agent (Optison®) Ultrasound Med. Biol. 2003;29(3):473–481. doi: 10.1016/s0301-5629(02)00741-x. [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Sheikov N, Jolesz F, Vykhodtseva N. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage. 2005;24:12–20. doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Hynynen K, McDannold N, Vykhodtseva N, Jolesz F. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, McDannold N, Jolesz F, Hynynen K. Noninvasive localized delivery of herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proceedings of the National Academy of Sciences. 2006a;103(31):11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, McDannold N, Jolesz F, Hynynen K. Targeted delivery of antibodies through the blood-brain barrier in MRI-guided focused ultrasound. Biochemical and Biophysical Research Communications. 2006b;340:1085–1090. doi: 10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood-brain barrier with focused ultrasound: association with cavitation activity. Phys. Med. Biol. 2006;51:793–807. doi: 10.1088/0031-9155/51/4/003. [DOI] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Use of ultrasound pulses combined with Definity for targeted blood-brain barrier disruption: A feasibility study. Ultrasound in Med. & Biol. 2007;33(4):584–590. doi: 10.1016/j.ultrasmedbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Blood-brain barrier disruption induced by focused ultrasound and circulating preformed microbubbles appears to be characterized by the mechanical index. Ultrasound Med. Biol. 2008a;34(5):834–840. doi: 10.1016/j.ultrasmedbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Hynynen K. Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood-brain barrier disruption. Ultrasound Med. Biol. 2008b;34(6):930–937. doi: 10.1016/j.ultrasmedbio.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. MRI investigation of the threshold for thermally induced blood-brain barrier disruption and brain tissue damage in the rabbit brain. Magnetic Resonance in Medicine. 2004;51:913–923. doi: 10.1002/mrm.20060. [DOI] [PubMed] [Google Scholar]
- McKinley M, Allen A, Burns P, Colvill L, Oldfield B. Interaction of circulating hormones with the brain: The roles of the subfornical organ and the organum vasculosum of the lamina terminalis. Clinical and Experimental Pharmacology and Physiology. 1998;25(S1):S61–S67. doi: 10.1111/j.1440-1681.1998.tb02303.x. [DOI] [PubMed] [Google Scholar]
- Mesiwala A, Farrel L, Wenzel H, Silbergeld D, Crum L, Winn H, Mourad P. High-intensity focused ultrasound selectively disrupts the blood-brain barrier in vivo. Ultrasound Med. Biol. 2002;28:389–400. doi: 10.1016/s0301-5629(01)00521-x. [DOI] [PubMed] [Google Scholar]
- NCRP . Exposure Criteria for Medical Diagnostic Ultrasound: II. Criteria Based on All Known Mechanisms. National Council on Radiation Protection and Measurements; Bethesda, MD: 2002. [Google Scholar]
- Nock L, Trahey G, Smith S. Phase aberration correction in medical ultrasound using speckle brightness as a quality factor. J. Acoust. Soc. Am. 1989;85(5):1819–1833. doi: 10.1121/1.397889. [DOI] [PubMed] [Google Scholar]
- Petkus V, Ragauskas A, Jurkonis R. Investigation of intracranial media using ultrasoic monitoring model. Ultrasonics. 2002;40:829–833. doi: 10.1016/s0041-624x(02)00216-0. [DOI] [PubMed] [Google Scholar]
- Price C, Hoyda T, Ferguson A. The area postrema: A brain monitor and integrator of systemic autonomic state. The Neuroscientist. 2008;14(2):182–194. doi: 10.1177/1073858407311100. [DOI] [PubMed] [Google Scholar]
- Raymond S, Skoch J, Hynynen K, Bacskai B. Multiphoton imaging of ultrasound/Optison mediated cerebrovascular effects in vivo. Journal of Cerebral Blood Flow & Metabolism. 2007;27:393–403. doi: 10.1038/sj.jcbfm.9600336. [DOI] [PubMed] [Google Scholar]
- Raymond S, Treat L, Dewey J, McDannold N, Hynynen K, Bacskai B. Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer’s disease mouse models. PLoS One. 2008;3(5):1–7. doi: 10.1371/journal.pone.0002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard M, Hetzel A, Kruger S, Kretzer S, Talazko J, Ziyeh S, Weber J, Els T. Blood-brain barrier disruption by low-frequency ultrasound. Stroke. 2006;37:1546–1548. doi: 10.1161/01.STR.0000221813.27519.0b. [DOI] [PubMed] [Google Scholar]
- Sassaroli E, Hynynen K. Forced linear oscillations of microbubbles in blood capillaries. J. Acoust. Soc. Am. 2004;115(6):3235–3243. doi: 10.1121/1.1738456. [DOI] [PubMed] [Google Scholar]
- Sassaroli E, Hynynen K. Resonance frequency of microbubbles in small blood vessels: a numerical study. Phys. Med. Biol. 2005;50:5293–5305. doi: 10.1088/0031-9155/50/22/006. [DOI] [PubMed] [Google Scholar]
- Schlachetzki F, Holscher T, Koch H, Draganski B, May A, Schuierer G, Bogdahn U. Observation on the integrity of the blood-brain barrier after microbubble destruction by diagnostic transcranial color-coded sonography. J Ultrasound Med. 2002;21:419–429. doi: 10.7863/jum.2002.21.4.419. [DOI] [PubMed] [Google Scholar]
- Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound in Med. & Biol. 2004;30(7):979–989. doi: 10.1016/j.ultrasmedbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Shimamura M, Sato N, Taniyama Y, Yamamoto S, Endoh M, Kurinami H, Aoki M, Ogihara T, Kaneda Y, Morishita R. Development of efficient plasmid DNA transfer into adult rat central nervous system using microbubble-enhanced ultrasound. Gene Therapy. 2004;11:1532–1539. doi: 10.1038/sj.gt.3302323. [DOI] [PubMed] [Google Scholar]
- Tanter M, Thomas J, Fink M. Focusing and steering through absorbing and aberrating layers: Application to ultrasonic propagation through the skull. Journal of the Acoustical Society of America. 1998;103(5):2403–2410. doi: 10.1121/1.422759. [DOI] [PubMed] [Google Scholar]
- Tran T, Le Guennec J, Bougnoux P, Tranquart F, Bouakaz A. Characterization of cell membrane response to ultrasound activated microbubbles. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 2008;55(1):44–49. doi: 10.1109/TUFFC.2008.615. [DOI] [PubMed] [Google Scholar]
- Unger E, Porter T, Culp W, Labell R, Matsunaga T, Zutshi R. Therapeutic applications of lipid-coated microbubbles. Advanced Drug Delivery Reviews. 2004;56(9):1291–1314. doi: 10.1016/j.addr.2003.12.006. [DOI] [PubMed] [Google Scholar]