Abstract
Petiole explants were obtained from in vitro grown diploid (2x = 22) Echinacea purpurea plantlets. Shoots were regenerated by culturing the explants on MS basal medium containing 0.3 mg/L benzyladenine (BA), 0.01 mg/L naphthaleneacetic acid (NAA) and four concentrations (30, 60, 120, and 240 mg/L) of colchicine for 30 days, or 120 mg/L of colchicine for various durations (7, 14, 21, and 28 days). The regenerated shoots were induced to root on MS basal medium with 0.01 mg/L NAA, and then the root-tips of the regenerated shoots were sampled for count of chromosome number. It was found that a treatment duration of >7 days was necessary for induction of tetraploid (4x = 44) shoots, and treatment with 120 mg/L colchicine for 28 days was the most efficient for induction of tetraploids, yielding 23.5% of tetraploids among all the regenerated shoots. Chimeras were observed in almost all the treatments. However, the ratio of tetraploid to diploid cells in a chimeric plant was usually low. In comparison with diploid plants, tetraploid plants in vitro had larger stomata and thicker roots with more root branches, and had prominently shorter inflorescence stalk when mature.
1. Introduction
Echinacea purpurae L., commonly known as purple coneflower, is one of the most popular herbs with a long history of medicinal use for a wide variety of illnesses, such as syphilis, septic wounds, snakebites, blood poisoning, common cold, influenza, and other upper respiratory tract infections [1–3]. Nowadays, the chemistry of Echinacea species has been well documented, and several groups of components, including alkamides, caffeic acid derivatives, and polysaccharides are considered important for activity [4]. In the recent years, global demand for products of purple coneflower has increased steadily due to the frequent occurring of threatening pandemic diseases caused by viruses. Under this background, biotechnological researches on mass propagation and genetic improvement of this crop have been conducted [5–10].
Polyploids, although frequently encounter low seed-setting rates or complete sterility, are usually superior to diploids with respect to genetic adaptability and tolerant to environmental stress [11]. For medicinal plants of those the functional compounds are accumulated in the vegetative parts such as purple coneflower, polyploids may be more valuable because they may exhibit increased biomass or content of effective compounds [12]. Induction of polyploidy is thus one of the strategies for crop improvement. Out of many applicable methods, the application of colchicine to double the chromosome numbers has been adopted successfully in many plant species [13–19]. Purple coneflower has been confirmed to be a diploid with a chromosome number of 2x = 22 in somatic cell [20]. However, chromosome doubling has not yet been achieved in purple coneflower.
After successful regeneration of haploid plants through anther culture in purple coneflower for the first time [8], we focused on doubling the chromosome number of diploid as well as haploid plants. In this paper, details of regeneration of the tetraploid plants by treating diploid petiole explants with colchicine are reported.
2. Materials and Methods
2.1. Plant Source
Seeds of purple coneflower were purchased in a supermarket produced by the Company of Plantation Products (Norton, MA, USA), and plants were grown at the Garden of Chinese Medicinal Plants on the campus of South China Agricultural University. Seeds were collected from these seed-grown plants and used for the present studies.
2.2. Establishment of Aseptic Seedlings and Preparation of Petiole Explants
Seeds were surface sterilized by immersing in 70% ethanol for 1 minutes and soaking in a 0.1% mercuric chloride solution for 10 minutes followed by 1% sodium hypochlorite solution containing one drop of Tween 20 per 50 mL for 10 minutes. Surface-sterilized seeds were then rinsed three times in sterile deionized water and inoculated to a medium comprised of half-strength MS salts, 1% sucrose and 500 mg/L lactalbumin hydrolysis and the medium was solidified with 0.2% Phytagel prior to autoclaving. After 14 days in dim light for germination, the resulted seedlings were transferred to a medium containing full-strength MS salt, 1% sucrose, and gelled with 0.2% Phytagel for further growth. Petioles of about two-month old seedlings were cut into 7–10 mm long segments and used as explants.
2.3. Preparation of Media
Each bottle was filled with 40 mL medium and covered with an air-tightly polycarbonate screwed cap. Shoot regeneration medium for culture of petiole explants comprised of MS salts, 3% sucrose, 0.3 mg/L BA, 0.01 mg/L NAA, and rooting medium of the regenerated shoots comprised of MS salts, 3% sucrose, 0.01 mg/L NAA. All the media used were adjusted to a pH value of 5.8 ~ 6.0 with 1 N NaOH or 1 N HCl solution, and gelled with 0.6% agar prior to autoclaving at pressure of 1.4 kg cm−2 for 20 minutes. When colchicine was used, it was dissolved in distilled water to a concentration of 5 mg/mL, filtered sterilized and then added to warm (about 70°C ) autoclaved media.
2.4. Induction of Chromosome Doubling
Petiole explants were precultured on shoot regeneration medium for one week to heal the cutting wound and initiate cell division, and then transferred to shoot regeneration medium with different concentrations (0, 30, 60, 120, 240, mg/L) of colchicine for 30 days or with 120 mg/L colchicine for different durations (7, 14, 21, 28 days). Eight explants were cultured in one bottle.
2.5. Shoot Regeneration and Rooting of the Regenerated Shoots
After the colchicine treatment, the treated explants were transferred to the shoot regeneration medium and cultured for 40 days. The regenerated shoots were cut from the mother tissues and cultured to the rooting medium for the initiation of roots and further growth of the intact regenerated plantlets.
2.6. Maintenance of Cultures
Except seed germination culture which was kept under dim light, all the other cultures were kept in lighted conditions with a 12-hour photoperiod under cool-white light (about 50 μmol m−2s−1), and all the cultures were kept in a room with temperature of 25–27°C.
2.7. Observation of Chromosomes and Determination of Ploidy Level
Fifty one regenerated plants were randomly selected and from each plant all actively growing root tips of 5–10 mm in length were excised. These root tips were treated with 0.1% colchicine water solution at room temperature for 4 hours, washed with tap water and transferred to Carnoy's solution for fixing at least 24 hours at room temperature. The fixed root tips were then hydrolyzed in 1 N HCl for 10–15 minutes at 65°C. After hydrolysis, root tips were rinsed with tap water for 10 minutes and cut into shorter root tips of ~1.5 mm in length. These prepared root tips were then placed on slide glass, stained with one drop of carbol fuchsin solution for 1-2 minutes, squashed under cover glass and observed for chromosome numbers under a microscope (Leica DLMB2) through a 60x object lens, and photos were taken with the associated apparatus [8]. A plant with all the root tip cells showing 22 chromosomes was determined as diploid, with some cells showing 22 and the other cells showing 44 chromosomes was determined as chimera, and with all the cells showing 44 chromosomes was determined as tetraploid. For confirmation of the nonchimeric condition of the tetraploid plants that had been determined by chromosome counting of the root tip cells, shoot tips were sampled from five plants of which all the root tip cells had 44 chromosomes and prepared by the same method shown above and the chromosomes of the shoot meristem cells were counted.
2.8. Stomata Analysis
For stomata analysis, a few pieces of epidermal layer were torn from the abaxial side of relatively mature leaves (leaf no. 4 or 5 from the top of the shoot) of tetraploid, diploid and chimeric plants. These epidermal layers were then mounted on slide glass with one drop of distilled water and a piece of cover glass for measuring stomata sizes under the above mentioned microscope with an associated computer.
2.9. Data Analysis
All the experiments were arranged in Randomized Complete Block Design (RCBD) and every experiment reported here was repeated at least four times with a minimum of four replicates. Statistical analysis was carried out using the student Newman-Kuells means separation test of SAS (SAS Institute, Cary, NC, 1995). Significance of differences among means was determined by Duncan's multiple range testes at P ≤ .05.
3. Results
3.1. Influence of Concentration of Colchicine on Shoot Regeneration
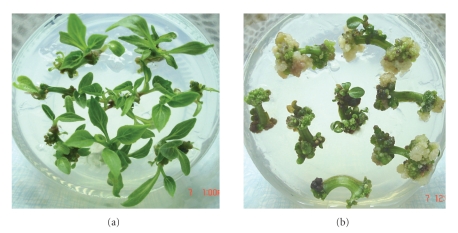
Shoot regeneration from petiole explants generally took place after one month of culture on regeneration medium, and the regenerated shoots could continue grow on the same medium for another 10 days without declining in vitality (Figure 1(a)). Colchicine added in the medium significantly inhibited shoot regeneration; the higher was concentration of colchicine, the lower regeneration rate (Table 1). Colchicine also delayed the regeneration progress and induced obviously more callus on the cutting surface of the explants (Figure 1(b)).
Figure 1.
Cultures of petiole explants on regeneration medium containing 0.3 mg/L BA and 0.01 mg/L NAA. (a), For 40 days without subculture; (b), For 40 days after being cultured on a medium containing 0.3 mg/L BA, 0.01 mg/L NAA and 120 mg/L colchicine for 30 days.
Table 1.
Comparison of the effect of colchicine concentration on shoot regeneration from petiole explants. Data were collected after 30 days on regeneration medium containing colchicine and 40 days on colchicine-free regeneration medium except for the control, data for which were collected after 40 days of culture without transfer to new medium.
| Colchicine concentration (mg/L) | % Shoot regeneration | No. shoots per bottle |
|---|---|---|
| 0 (control) | 97.5 a* | 16.2 a |
| 30 | 67.5 b | 6.2 b |
| 60 | 50.0 c | 5.0 c |
| 120 | 20.0 d | 2.0 c |
| 240 | 7.5 e | 0.8 d |
*Data in the same column followed by different letters are significantly different by Duncan’t test at ≤5% level.
3.2. Effect of Colchicine Concentration on Chromosome Doubling
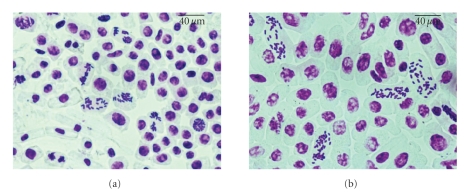
Explants were treated with various concentrations of colchicine for 30 days. Effect of colchicine on chromosome doubling was evaluated by counting the chromosome numbers in the root tip cells of the plants regenerated from the colchicine treated petioles. Figure 2 shows root-tip cells and chromosomes of diploid and tetraploid plantlets regenerated after colchicine treatment. Chromosome counting of shoot meristem cells confirmed further the nonchimeric status of these tetraploid plants.
Figure 2.
Chromosomes in root tip cells of (a), Diploid (2x = 22) and (b), Tetraploid (4x = 44) plantlets. Bar = 40 μm.
The effect of colchicine concentration on doubling chromosome number was summarized in Table 2. It is indicated that 30 mg/L was not very effective because no tetraploid plants but only a low portion (11.8%) of chimera could be induced, with 11 out of 429 (2.6%) cells had doubled chromosome numbers. 120 mg/L colchicine in the medium exhibited the best doubling effect, among 51 plantlets examined, chromosomes in 11 plantlets were confirmed to have been completely doubled. From these 11 plantlets, 19 root tips were sampled, and all the 389 cells subjected to chromosome counting had 4x chromosomes. Colchicine at concentrations of 60 and 240 mg/L was also effective, being able to induce complete chromosome doubling in 5.9–7.8% of the examined plantlets, but was not as effective as at concentration of 120 mg/L.
Table 2.
Comparison of the effect of colchicine concentration on chromosome doubling of regenerated plants.
| Colchicine concentration (mg/L) | No. plants sampled | No. root tips sampled | No. cells observed | No. cells with 2x chromosome | No. cells with 4x chromosome | No. each kind of plants on the base of chromosome counting* | ||
| 2x plants | 4x plants | Chimeras | ||||||
| 30 | 51 | 89 | 3653 | 3642 | 11 | 45 | 0 | 6 (11/429/9) |
| 60 | 51 | 86 | 3690 | 3515 | 175 | 42 | 3 (154/154/6) | 6 (21/653/11) |
| 120 | 51 | 83 | 3542 | 3132 | 410 | 37 | 11 (389/389/19) | 4 (21/360/7) |
| 240 | 51 | 90 | 3418 | 3251 | 167 | 45 | 4 (154/154/7) | 2 (13/114/4) |
*Data in the parenthesis are no. of 4x cells/ no. of cells observed/ No. of root tips sampled.
Data in Table 2 also indicate that the ratio of 4x cells to 2x cells in a chimeric plant was generally low, with the highest tetraploid ratio in a root tip sample of 21.51% and the highest average of chimeric plants in a treatment (30 or 60 mg/L colchicine) was 11.8%. Although the possibility exists theoretically, there have not been observed that all cells of one root-tip sample in a plant were 4x and other root-tip samples showing chimeric or all the cells being 2x.
3.3. Effect of Treatment Duration of Colchicine on Chromosome Doubling
In the above-described experiments, 120 mg/L colchicine treatment induced chromosome doubling with the highest efficiency. In this experiment, explants were inoculated on media supplemented with 120 mg/L colchicine for various durations. The data in Table 3 showed that longer duration of colchicine treatment inhibited the regeneration efficiency.
Table 3.
Comparison of the effect of duration of 120 mg/L colchicine treatment on shoot regeneration from petiole explants. Data were collected 40 days after transfer of the colchicine treated explants to colchicine-free regeneration medium.
| Treatment duration (days) | % shoot regeneration | No. shoots per bottle |
|---|---|---|
| 7 | 72.5 a* | 7.0 a |
| 14 | 60.0 b | 5.2 b |
| 21 | 47.0 c | 3.8 c |
| 28 | 17.5 d | 2.2 d |
*Data in the same column followed by different letters are significantly different by Duncan’t test at ≤5% level.
Roots were sampled from plantlets growing from the regenerated shoots and chromosome number of the root-tip cells was counted. Result of chromosome counting is summarized in Table 4. The highest percentage (23.5%) of tetraploid induction occurred on the regeneration medium treated with 120 mg/L colchicine for 28 days. However, 14- and 21-day treated explants also generated a substantial number of tetraploids (11.8% and 19.6%, respectively) whereas no tetraploids were observed in 7-day treatment.
Table 4.
Comparison of the effect of colchicine treatment duration on chromosome doubling of regenerated plants.
| Treatment duration (days) | No. plants sampled | No. root tip sampled | No. cells observed | No. cells with 2x chromosomes | No. cells with 4x chromosomes | No. each kind of plants on the base of chromosome counting* | ||
| 2x plants | 4x plants | Chimera | ||||||
| 7 | 51 | 88 | 3915 | 3903 | 12 | 45 | 0 | 6 (12/902/14) |
| 14 | 51 | 89 | 3746 | 3463 | 283 | 37 | 6 (248/248/10) | 8 (35/762/13) |
| 21 | 51 | 88 | 3237 | 2823 | 414 | 35 | 10 (391/391/15) | 6 (23/371/10) |
| 28 | 51 | 87 | 3350 | 2781 | 569 | 35 | 12 (546/546/20) | 4 (23/311/8) |
*Data in the parenthesis are no. of 4x cells/ no. of cells observed/No. of root tips sampled.
3.4. Morphological Difference among Diploid, Tetraploid and Chimeric Plantlets
Sizes of stomata on leaves varied largely even among those of the same leaf, but statistically significant mean size difference could still be detected between diploid and tetraploid (Table 5). The obviously bigger mean size of tetraploid than diploid stomata suggests bigger cells in tetraploid plants than in diploid plants.
Table 5.
Comparison of stomata size between diploid and tetraploid plantlets.
| Ploidy level of plant | Stomata length (μm) | Stomata width (μm) |
|---|---|---|
| Diploid | 104.519 b* | 90.741 b |
| Tetraploid | 144.810 a | 111.758 a |
*Data in the same column followed by different letters are significantly different by Duncan’t test at 5% level.
There were no noticeable morphological differences in shape of the stomata among the three kinds of plants, however, on a piece of leaf epidermal layer of a chimeric plant, some areas were situated with more number of larger size stomata which are very likely tetraploid and some areas were situated with more number of smaller size stomata which are very likely diploid.
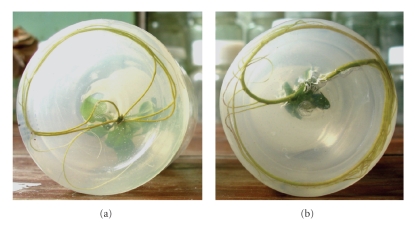
Morphological difference in root system between in vitro diploid and tetraploid plantlets was significant (Figure 3). Diploid plantlets generally initiated more roots from the base of the shoots, and these roots were thinner and had fewer branches in comparison with those of the tetraploid plantlets. After transfer to pots, the tetraploid plants produced relatively broader leaves but shorter petioles than diploid plants, and the morphology of chimeric plants with higher diploid to tetraploid cell ratios were more similar to diploid plants than those with lower diploid to tetraploid cell ratios.
Figure 3.
Roots of regenerated plantlets in culture bottles. (a) roots of diploid plantlets; (b) roots of tetraploid plantlets.
In pots, all the three kinds of plants developed normal looking inflorescences with a clear difference in the length of the inflorescence stalk: the inflorescence stalk of the tetraploid plant being the shortest and diploid the longest, with chimeric in the middle (Figure 4). Seeds were collected afterward from diploid and chimeric plants, but so far from tetraploid plants no seeds could been collected.
Figure 4.
Mature diploid (left), chimeric (middle), and tetraploid (right) plants with inflorescence.
4. Discussion
In preliminary experiments of the present study, various colchicine concentrations (from 100 to 1000 mg/L) associated with short duration (one to five days) treatments, as methods of in vitro chromosome doubling frequently reported [21–23], were found not effective. No tetraploid but only chimeric plants were produced in these experiments (data not shown). On the bases of the preliminary experimental results, we tried to treat the plant material with colchicine for longer duration.
In comparison with short duration of high colchicine concentration treatments, long duration treatments of colchicine were much less attempted. Chakraborti et al. treated mulberry with 1000 mg/L colchicine for one day and 28 days and obtained equally good results [24]. However, Portela de Carvalho et al. treated annatto with 25, 250 and 1250 μM (about 10, 100 and 500 mg/L resp.,) colchicine for 15 and 30 days obtained only one tetraploid shoots in the treatment of 25 μM colchicine for 15 days [25]. In the present study, we found that colchicine at 120 mg/L was suitable for induction of chromosome doubling in purple coneflower, and a certain long duration (longer than seven days) of colchicine treatment was necessary for obtaining completely doubled chromosome tetraploid plantlets from diploid explants, with longer duration the better among the four durations tested.
Chimeric plants were frequently produced in the experiments. However the production of chimeras was of much less value in agricultural production in comparison with tetraploid plants. This is not only because the ratio of 4x cells in the chimeras was generally low, but also the ratio might not stable due to the possible difference in the time required for completing a cell cycle between these two types of cells [26]. Although evidence has not been found, it was very likely that tetraploid cells required longer time to complete a cell cycle and resulted in lower and lower ratio to the diploid cells during the growth of the chimeric plants. Beside the data presented in this paper, the high production rate of chimeras in regenerated plants from colchicine treated materials have already been reported in many cases, such as in mulberry [24] and in Miscanthus sinensis [17]. The accumulated information suggests that the production of chimeric plants is in most of the cases a by-product of the production of tetraploids induced chemically, by colchicine or by oryzalin, which is almost an alternative of colchicine for polyploidization [17, 27]. Comparatively, it is easier to obtain chimeras than tetraploid plants from diploid materials. It is therefore important to develop new methods for increasing the efficiency of tetraploid induction.
Although there have been many methods for clarifying ploidy level of plants [23–25, 28, 29], all these methods require certain and even sophisticated techniques. Simple methods for early identification of ploidy level in regenerated plants have important application value, especially when a large amount of regenerated plants are to be identified. In a previous report on regeneration of haploid from anther cultures of purple coneflower, we found that the haploid plantlets have evidently thinner roots than those of diploid ones. In the present study, obvious difference in root morphology between diploid and tetraploid were also observed. The finding of the difference in root morphology between plants of different ploidy levels can serve as a convenient and reliable method for identifying plants of certain ploidy level from the others in purple coneflower, and may probably be applicable to other plant species as well.
The regenerated tetraploid plants which have been transferred to pots several months ago could develop normal looking inflorescences. Relevant researches making use of these tetraploid plants, such as crossing with diploid plants and regenerating plants from anther culture of tetraploid plants for breeding new varieties are under way. Because purple coneflower plants are commonly harvested after three or four years of growth when the accumulation of medicinally functional compounds reaches a high level, full details on growth and accumulation of the medicinal compounds of these tetraploid plants will be reported later in comparison with the original diploid plants.
Acknowledgments
This research was funded by a Grant (no. 2008B090500250) from a special program for Enterprise, University and Research Institute Cooperation of Guangdong Province and the Ministry of Education of China.
Abbreviations
- BA:
6-benzyladenine
- MS:
Murashige and Skoog. (1962)
- NAA:
Naphthaleneacetic acid
References
- 1.Hobbs C. Echinacea. A literature review. Herbal Gram. 1994;30(supplement):33–47. [Google Scholar]
- 2.Melchart D, Walther E, Linde K, Brandmaier R, Lersch C. Echinacea root extracts for the prevention of upper respiratory tract infections: a double-blind, placebo-controlled randomized trial. Archives of Family Medicine. 1998;7(6):541–545. doi: 10.1001/archfami.7.6.541. [DOI] [PubMed] [Google Scholar]
- 3.Melchart D, Linde K, Fischer P, Kaesmayr J. The Cochrane Library. Oxford, UK: Update Software; 2004. Echinacea for preventing and treating the common cold (Cochrane review) [DOI] [PubMed] [Google Scholar]
- 4.Barnes J, Anderson LA, Gibbons S, Phillipson JD. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea(L.) Moench): a review of their chemistry, pharmacology and clinical properties. Journal of Pharmacy and Pharmacology. 2005;57(8):929–954. doi: 10.1211/0022357056127. [DOI] [PubMed] [Google Scholar]
- 5.Mechanda SM, Baum BR, Johnson DA, Arnason JT. Direct shoot regeneration from leaf segments of mature plants of Echinacea purpurea (L.) Moench. In Vitro Cellular and Developmental Biology—Plant. 2003;39(5):505–509. [Google Scholar]
- 6.Zobayed SMA, Saxena PK. In vitro regeneration of Echinacea purpurea L.: enhancement of somatic embryogenesis by indolebutyric acid and dark pre-incubation. In Vitro Cellular and Developmental Biology—Plant. 2003;39(6):605–612. [Google Scholar]
- 7.Sauve RJ, Mmbaga MT, Zhou S. In vitro regeneration of the Tennessee coneflower (Echinacea tennesseensis) In Vitro Cellular and Developmental Biology—Plant. 2004;40(3):325–328. [Google Scholar]
- 8.Zhao F-C, Nilanthi D, Yang Y-S, Wu H. Anther culture and haploid plant regeneration in purple coneflower (Echinacea purpureaL.) Plant Cell, Tissue and Organ Culture. 2006;86(1):55–62. [Google Scholar]
- 9.Abbasi BH, Saxena PK, Murch SJ, Liu C-Z. Echinacea biotechnology: challenges and opportunities. In Vitro Cellular and Developmental Biology—Plant. 2007;43(6):481–492. [Google Scholar]
- 10.Wu C-H, Murthy HN, Hahn E-J, Paek K-Y. Large-scale cultivation of adventitious roots of Echinacea purpurea in airlift bioreactors for the production of chichoric acid, chlorogenic acid and caftaric acid. Biotechnology Letters. 2007;29(8):1179–1182. doi: 10.1007/s10529-007-9399-1. [DOI] [PubMed] [Google Scholar]
- 11.Lewis WH. Ploidy: Biological Relevance. New York, NY, USA: Plenum; 1980. [Google Scholar]
- 12.Gao SL, Zhu DN, Cai ZH, Xu DR. Autotetraploid plants from colchicine-treated bud culture of Salvia miltiorrhiza Bge. Plant Cell, Tissue and Organ Culture. 1996;47(1):73–77. [Google Scholar]
- 13.Luckett DJ. Colchicine mutagenesis is associated with substantial heritable variation in cotton. Euphytica. 1989;42(1-2):177–182. [Google Scholar]
- 14.Ishizaka H, Uematsu J. Amphidiploids between Cyclamen persicum Mill. and C. hederifolium Aiton induced through colchicine treatment of ovules in vitro and plants. Breeding Science. 1994;44(2):161–166. [Google Scholar]
- 15.Takamura T, Miyajima I. Colchicine induced tetraploids in yellow-flowered cyclamens and their characteristics. Scientia Horticulturae. 1996;65(4):305–312. [Google Scholar]
- 16.Pinheiro AA, Pozzobon MT, Do Valle CB, Penteado MIO, Carneiro VTC. Duplication of the chromosome number of diploid Brachiaria brizantha plants using colchicine. Plant Cell Reports. 2000;19(3):274–278. doi: 10.1007/s002990050011. [DOI] [PubMed] [Google Scholar]
- 17.Petersen KK, Hagberg P, Kristiansen K. Colchicine and oryzalin mediated chromosome doubling in different genotypes of Miscanthus sinensis. Plant Cell, Tissue and Organ Culture. 2003;73(2):137–146. [Google Scholar]
- 18.Liu G, Li Z, Bao M. Colchicine-induced chromosome doubling in Platanus acerifolia and its effect on plant morphology. Euphytica. 2007;157(1-2):145–154. [Google Scholar]
- 19.Clarindo WR, Roberto de Carvalho C, Araujo FS, Santiago de Abreu I, Otoni WC. Recovering polyploidy papaya in vitro regenerants as screened by flow cytometry. Plant Cell, Tissue and Organ Culture. 2008;92:207–214. [Google Scholar]
- 20.McGregor RL. The taxonomy of the genus Echinacea (Compositae) University of Kansas Science Bulletin. 1968;48(4):113–142. [Google Scholar]
- 21.Song P, Kang W, Peffley EB. Chromosome doubling of Allium fistulosum x A. cepa interspecific F1 hybrids through colchicine treatment of regenerating callus. Euphytica. 1997;93(3):257–262. [Google Scholar]
- 22.Roy AT, Leggett G, Koutoulis A. In vitro tetraploid induction and generation of tetraploids from mixoploids in hop (Humulus lupulus L.) Plant Cell Reports. 2001;20(6):489–495. [Google Scholar]
- 23.Thao NTP, Ureshino K, Miyajima I, Ozaki Y, Okubo H. Induction of tetraploids in ornamental Alocasia through colchicine and oryzalin treatments. Plant Cell, Tissue and Organ Culture. 2003;72(1):19–25. [Google Scholar]
- 24.Chakraborti SP, Vijayan K, Roy BN, Quari SMH. In vitro induction of tetraploid in mulberry. Plant Cell Reports. 1998;17:799–803. doi: 10.1007/s002990050486. [DOI] [PubMed] [Google Scholar]
- 25.de Carvalho JFRP, de Carvalho CR, Otoni WC. In vitro induction of polyploidy in annatto (Bixa orellana) Plant Cell, Tissue and Organ Culture. 2005;80(1):69–75. [Google Scholar]
- 26.Mergen F, Lester DT. Colchicine induced polyploidy in Abies. Forest Science. 1971;7:314–319. [Google Scholar]
- 27.Eeckhaut TGR, Werbrouck SPO, Leus LWH, Van Bockstaele EJ, Debergh PC. Chemically induced polyploidization in Spathiphyllum wallisii Regel through somatic embryogenesis. Plant Cell, Tissue and Organ Culture. 2004;78(3):241–246. [Google Scholar]
- 28.Borrino EM, Powell W. Stomata guard cell length as an indicator of ploidy in microspore-derived plants of barley. Genome. 1988;30:158–160. [Google Scholar]
- 29.Gu XF, Yang AF, Meng H, Zhang JR. In vitro induction of tetraploid plants from diploid Zizyphus jujuba Mill. cv. Zhanhua. Plant Cell Reports. 2005;24(11):671–676. doi: 10.1007/s00299-005-0017-1. [DOI] [PubMed] [Google Scholar]