Abstract
OBJECTIVES
To examine the effect of a multi-component intervention on pain and function following orthopedic surgery.
DESIGN
Controlled prospective propensity score matched clinical trial.
SETTING
New York City acute rehabilitation hospital.
PARTICIPANTS
249 patients admitted to rehabilitation following hip fracture repair (N=51) hip (N=64) or knee (N=134) arthroplasty.
INTERVENTION
Pain assessment at rest and with physical therapy (PT) by staff using numeric rating scales (1 to 5). Physician protocols for standing analgesia and pre-emptive analgesia prior to PT were implemented on the intervention unit. Control unit patients received usual care.
MAIN OUTCOME MEASURES
Pain, analgesic prescribing, gait speed, transfer time, and percent of PT sessions completed during admission. Pain and difficulty walking at 6, 12, 18, and 24 weeks following discharge.
RESULTS
In multivariable analyses compared to controls, intervention patients were significantly more likely to report no or mild pain at rest (66% versus 49%, P=.004) and with PT (52% versus 38%, P=.02) on average for the first 7 days of rehabilitation; had faster 8 foot walk times on days four (9.3 seconds versus 13.2 seconds, P=.02) and seven (6.9 versus 9.2 seconds, P=.02); received more analgesia (8.0 milligrams of morphine sulfate equivalents/day, P<0.001); were more likely to receive standing analgesia (98% versus 48%, P<.001); and had significantly shorter lengths of stay (10.1 versus 11.3 days, P=.005). At 6 months compared to controls, intervention patients were less likely to report moderate/severe pain with walking (15% versus 4%, P=.02), that pain did not interfere with walking (7% versus 18%, P=.004), and were less likely to be taking analgesics (35% versus 51%, P=.03).
CONCLUSION
The intervention improved post-operative pain, reduced chronic pain, and improved function.
Keywords: pain, function, orthopaedic surgery
INTRODUCTION
Uncontrolled pain is a major impediment to post-operative functional recovery and is a persistent problem in the United States.1-3 Older adults who undergo lower extremity orthopedic surgery (e.g., hip and knee arthroplasty, hip fracture repair) experience intense post-operative pain and are at risk for sub-optimal analgesic therapy.4-6 Higher pain levels following lower extremity orthopedic surgery have been associated with increased lengths of stay, increased complications, delays in ambulation, impaired functional recovery, and increased suffering.4 5 7-9 Given the paucity of data with respect to the effective treatment of pain in the geriatric patient and the increasing number of elders undergoing surgery10 we performed a controlled prospective propensity score matched case control clinical trial to examine the effect of a multi-component inter-disciplinary intervention on the treatment of pain in older adults following lower extremity orthopedic surgery. We hypothesized that this generalizable intervention would decrease pain, enhance rehabilitation, and improve post-discharge function compared to usual care.
METHODS
This controlled clinical trial enrolled all eligible and consenting patients over age 50 years admitted to the acute rehabilitation service of a large New York hospital following lower extremity orthopedic surgery (hip fracture repair (HFX), unilateral total knee replacement (TKR), unilateral total hip replacement (THR)). The intervention - a standardized pain management protocol including daily comprehensive pain assessments by nursing and physical therapy (PT) staff, a standardized analgesic protocol including guidelines for treating opioid side-effects for physicians, and daily feedback of pain scores to all clinical staff– was implemented on one acute rehabilitation unit (intervention) while two additional units served as controls. Subjects were interviewed daily about their pain, had physical performance testing conducted on days 4 and 7, and were followed by telephone every 6 weeks for 24 weeks following discharge to assess pain and walking ability. The study was approved by the Mount Sinai School of Medicine Institutional Review Board.
Study Design
This study used a prospective individual matching procedure employing propensity score methods modeled on other successful clinical trials evaluating comprehensive hospital unit based interventions.1 11 12 We enrolled all eligible subjects admitted to our intervention and control units and at the time of analysis matched intervention to control patients by propensity scores.13
Setting and Patients
We prospectively screened all admissions to the acute rehabilitation service on a daily basis Monday through Friday from March 2004 through August 2006. Patients were eligible for inclusion if they were over age 50 and were admitted within seven days of: a) hip fracture repair; b) unilateral total hip arthroplasty, or c) unilateral total knee arthroplasty. We excluded non-English speaking patients, patients not cleared to fully bear weight, patients who scored less than 17 out of 22 on the telephone mini-mental state exam,14 patients with cancer-related fractures, patients with a history of substance abuse, patients with a known adverse reaction to opioids, and patients taking adjuvant agents for neuropathic pain (e.g, gabapentin, tricyclic antidepressants). Enrolled patients were not informed as to whether they were on an intervention or control unit. Study enrollment details are in Figure 1.
Figure 1.
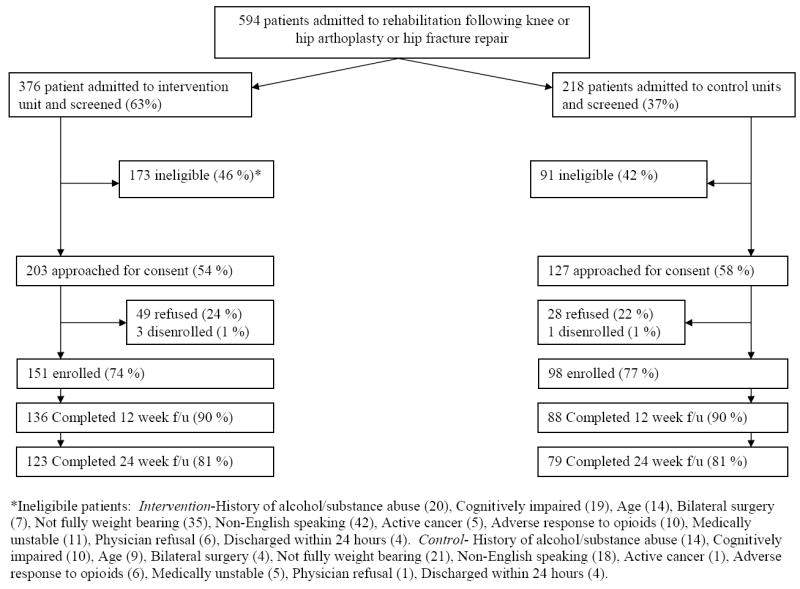
Details of Subject Enrollment
The acute rehabilitation service consisted of 3 self-contained units with therapeutic gyms located on the same floor as patient rooms. Orthopedic patients who met Medicare criteria for acute rehabilitation were admitted to any of the three units based solely upon bed availability. Two of the units contained dedicated beds for brain injury and spinal cord injury with the remaining beds available for orthopedic patients. We chose to place the intervention on the larger unit and the two smaller units served as controls. The units were staffed with equivalent ratios of physicians, physical and occupational therapists, physical therapy assistants, certified occupational therapy assistants, registered nurses, nurses’ aides, and support associates. All team members (including physicians) were unit based and all units followed the same clinical protocols with the exception of pain management. Therapeutic rehabilitation regimens for the three conditions (HFX, TKR, THR) were identical across the 3 units as were nursing protocols (available from the authors on request).
Usual Care
Patients admitted to the control units received the standard nursing pain assessment as mandated by the Joint Commission on Accreditation of Healthcare Organizations.15 Patients were assessed for pain at rest, worst pain, and pain relief on every nursing shift using 5 point numeric rating scales. Analgesia was prescribed based upon the treating physicians’ preference.
Intervention
The intervention was based upon published guidelines, 16-18 studies,5 19 20 and our prior research.1 The intervention consisted of a pain protocol for standing, pre-emptive, rescue, and titrated analgesia with training and feedback to clinical staff as supporting systems.
Analgesic Protocol
Patients admitted to the intervention unit were placed on a standard analgesic protocol detailed in Appendix 1. The protocol was available only to intervention unit physicians and was contained within their set of admitting orders. The protocol called for standing analgesia based upon patients’ self-reported pain levels, as needed analgesia for breakthrough pain (i.e., intermittent pain flares that occur despite regularly scheduled analgesia), and pre-emptive analgesia administered one hour before PT. Analgesia was titrated as in Appendix 1. Protocols for tapering of opioids to prevent withdrawal symptoms (Appendix 1) and for treating opioid-induced side effects were also provided (Appendix 2). All medications were prescribed by the treating physiatrist.
Appendix 1.
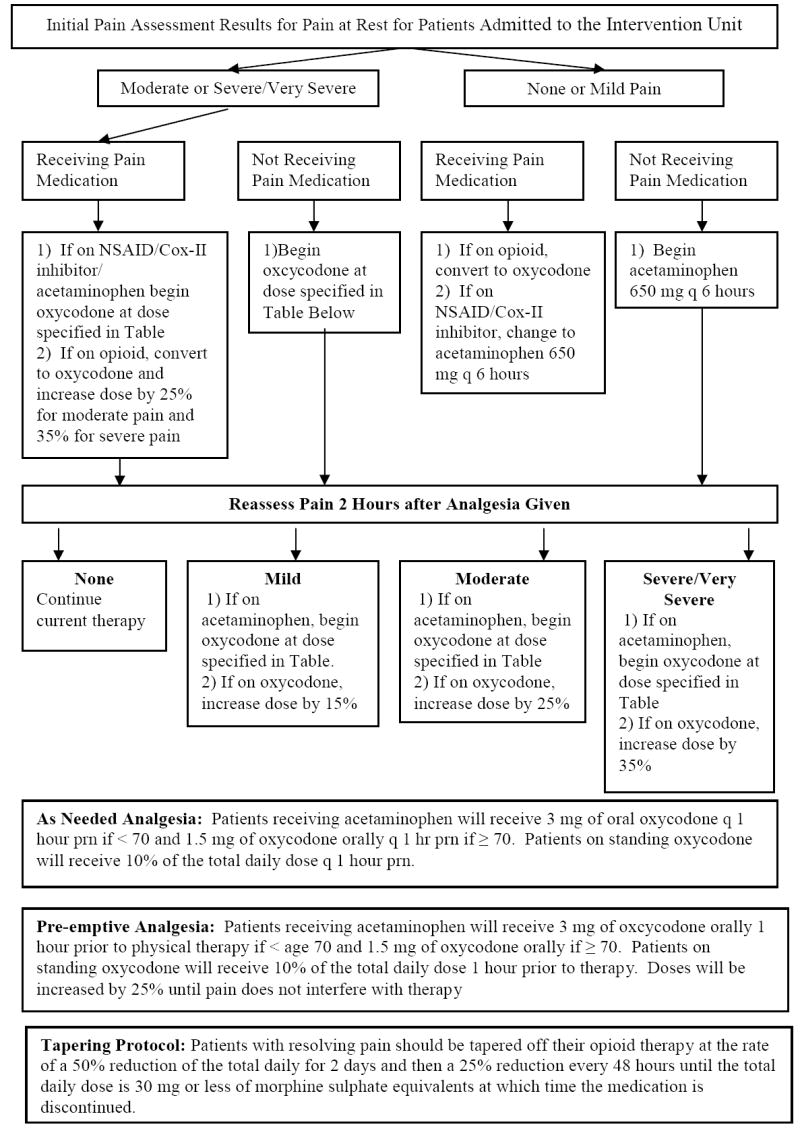
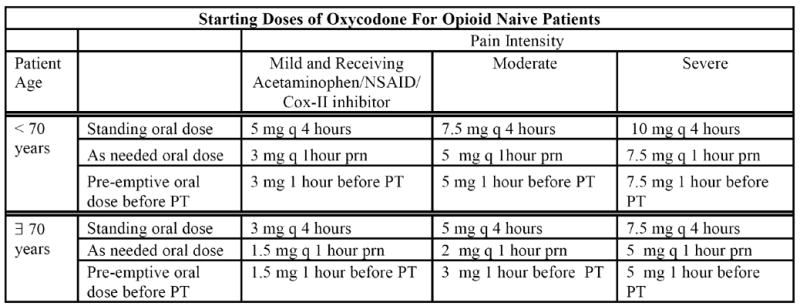
Analgesic Prescribing Protocol for Intervention Unit
Appendix 2.
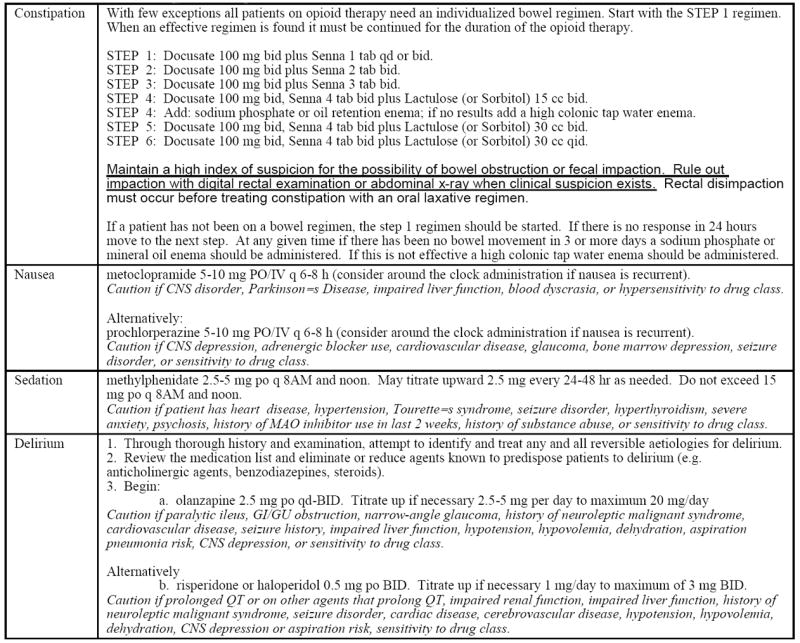
Protocols for Management of Opioid Related Side Effects
Staff Training and Pain Assessment
Additional support was provided to clinical staff through education, enhanced pain assessment, and audit and feedback interventions developed in a prior study.1 At baseline, all intervention unit staff received education on pain management and additional training was provided at regular intervals for newly hired staff. 1 Four additional questions (pain with transfer, pain with ambulation, and the degree to which pain interfered with transferring and walking) were added to the hospital’s existing nursing pain assessment to capture the more dynamic nature of pain present in this patient population. Physical therapists queried patients about their pain at the start and conclusion of therapy, the worst pain experienced during therapy, and the degree to which pain interfered with therapy. Pain ratings were charted with vital signs as in the usual care group. Additionally a daily report detailing patients who reported severe pain at rest or pain that interfered moderately/completely with transfer or PT over the prior 24 hours was provided to the intervention interdisciplinary team on morning rounds.
Data Collection and Measures
All patients, intervention and control, were asked to rate their pain at rest, with transfer, and with physical therapy on 5 point numeric rating scales (1-none to 5-very severe) at admission and daily throughout their admission by clinical research interviewers. Additionally, clinical interviewers performed daily assessments of opioid side effects.1
Patient characteristics were obtained from interviews and medical record review. Comorbid conditions were measured using the RAND comorbidity score.21 Functional status prior to surgery, at admission to rehabilitation, and at discharge was measured using the motor subscales of the Functional Independence Measure (FIM). Cognitive status was assessed by the telephone version of the Mini-mental State Examination, depression by the 15 item Geriatric Depression Scale, and overall health related quality of life by self-report using excellent, very good, good, fair, or poor. 14 22 23
Physical performance was assessed prior to the patient’s first PT session on rehabilitation days four and seven by a research physical therapist who was not a member of the rehabilitation staff and who was blinded to the patients’ intervention status using two measures - the timed 8 foot walk and single and repetitive standing from chair. 24-26 These measures have both been shown to be reliable and valid measures of lower extremity performance following HFX and TKR/THR.24-26 Performance on these tasks was scored as described in prior published studies.25 26 Finally, we collected data on the number of missed or shortened physical therapy sessions.8
Patients were interviewed by telephone by a clinical interviewer blinded to the patients’ intervention status following discharge every 6 weeks for 24 weeks. Patients were queried as to their pain at rest and with walking (1-none to 5-very severe), and the degree to which pain interfered with walking (1-none to 4-completely).
Analyses
Matching
Within each type of surgery, (HFX, TKR, THR) we computed a propensity score for each intervention and control subject.13 27 Propensity scores were determined by regressing whether patients received the intervention on variables of patient age, gender, ethnicity, medical insurance, modified RAND comorbidity score, GDS score, self-perceived health related quality of life score, admission rest pain, admission FIM score, overall FIM score prior to surgery, and 8 foot walk and sit to stand times at study entry. As more intervention than control patients were enrolled given the larger size of the intervention unit, we employed one to many matching without replacement.28 Each patient on the control unit was matched to one or more patients on the intervention unit whose logit of their propensity score was within ±0.2 standard deviations of the logit of the control patient’s score. All analyses of the intervention’s effect included only matched subjects.
Main Analyses
Pain scores used in the analyses were obtained from the clinical research interviewers and data were weighted to account for the one-to-many propensity matching. We employed multivariable logistic regression and multivariable ordered logistic regression for categorical outcomes, generalized linear models for continuous outcomes (GLM), and generalized estimating equations (GEE) for longitudinal outcomes to examine the association between the independent variables and our outcomes of interest. Covariates were selected based on prior studies.8 21 29 30
Confirmatory Analyses
To supplement to the propensity score analyses, we also performed multivariable modeling as described above using all subjects, not just those matched by propensity score.
All analyses were performed using STATA 9.2.31
RESULTS
Eighty eight of 98 patients on the control unit (90%) were matched to 129 of 150 patients (85%) on the intervention units (Table 1). There were no significant differences observed between the two matched groups. Specifically, baseline pain scores and performance measures were not significantly different between the two groups. All results presented below reflect the propensity score matched normalized weighted data.
Table 1.
Characteristics for Propensity Score Matched and Unmatched Control and Intervention Patients
| Propensity Score Matched Patients | Unmatched Patients | ||||
|---|---|---|---|---|---|
| Control (N=88) Weighted Value | Intervention (N=129) Weighted Value | P | Control (N=10) | Intervention (N=21) | |
| Women | 74.4 % | 76.7 % | .72 | 10 (100 %) | 9 (43 %) |
|
| |||||
| Mean age in years (sd) | 70.9 (8.3) | 70.9 (9.9) | .95 | 76 (10.6) | 71 (11.5) |
|
| |||||
| Race | .71 | ||||
| White | 58.6 % | 63.2 % | 9 (90 %) | 7 (33 %) | |
| African American | 21.8 % | 23.0 % | 0 | 9 (43 %) | |
| Hispanic | 14.9 % | 9.2 % | 0 | 1 (5 %) | |
| Other | 4.6 % | 4.6 % | 1 (10 %) | 4 (19%) | |
|
| |||||
| Insurance | .56 | ||||
| Medicare | 81.4 % | 76.7 % | 10 (100%) | 13 (62%) | |
| Medicaid | 5.8 % | 4.7 % | 0 | 1 (5 %) | |
| Other | 12.8 % | 18.6 % | 0 | 7 (33 %) | |
|
| |||||
| Surgery | .99 | ||||
| Hip fracture repair | 20.9 % | 20.9 % | 8 (80 %) | 4 (19 %) | |
| Total hip replacement | 24.4 % | 24.4 % | 1 (10 %) | 5 (24 %) | |
| Total knee replacement | 54.7 % | 54.7 % | 1 (10 %) | 12 (57) | |
|
| |||||
| Pre-hospital residence | .55 | ||||
| Home/apartment | 88.8 % | 86.5 % | 10 (100) | 20 (94 %) | |
| Nursing home | 1.2 % | 3.5 % | 0 | 1 (5 %) | |
|
| |||||
| Self-perceived health related quality of life | .83 | ||||
| Excellent | 14.9 % | 14.1 % | 3 (30) | 3 (14) | |
| Very Good | 24.1 % | 31.8 % | 3 (30) | 5 (24) | |
| Good | 39.1 % | 35.3 % | 2 (20) | 5 (24) | |
| Fair | 14.9 % | 14.1 % | 1 (10) | 8 (38) | |
| Poor | 6.9 % | 4.7 % | 1(10) | 0 | |
|
| |||||
| Geriatric depression score (sd) | 2.9 (2.8) | 2.6 (2.5) | .38 | 3.8 (4.0) | 3.2 (3.4) |
|
| |||||
| Rand comorbidity index (sd) | 1.7 (1.1) | 1.6 (1.1) | .45 | 1.7 (1.2) | 2.1 (1.7) |
|
| |||||
| Fim total score prior to surgery (sd) | 85.0 (7.3) | 85.4 (5.8) | .66 | 86.1 (9.7) | 82.1 (11.8) |
|
| |||||
| Fim locomotion score prior to surgery (sd) | 11.3 (2.6) | 11.7 (2.0) | .18 | 12.9 (1.9) | 10.8 (2.9) |
|
| |||||
| Fim locomotion score on admission to rehabilitation (sd) | 4.0 (2.5) | 4.4 (2.9) | .44 | 2.3 (1.1) | 3.4 (2.5) |
|
| |||||
| Pain at rest on admission | .57 | ||||
| None | 12.8 % | 17.4 % | 1 (10 %) | 2 (9.5 %) | |
| Mild | 31.4 % | 32.6 % | 4 (40 %) | 7 (33 %) | |
| Moderate | 27.9 % | 25.6 % | 1 (10 %) | 6 (28.6 %) | |
| Severe-very severe | 27.9 % | 24.4% | 4 (40 %) | 6 (28.6 %) | |
|
| |||||
| Pain with transfer from bed to chair on admission | .38 | ||||
| None | 1.2 % | 5.9 % | 0 | 0 | |
| Mild | 25.9 % | 17.6 % | 3 (30 %) | 5 (23.8 %) | |
| Moderate | 29.4 % | 30.6 % | 3 (30 %) | 5 (23.8 %) | |
| Severe-very severe | 43.8 % | 45.8 % | 4 (40 %) | 11 (52.3 %) | |
|
| |||||
| Pain with walking on admission | .87 | ||||
| None | 8.3 % | 7.3 % | 0 | 1 (4.8 %) | |
| Mild | 27.4 % | 23.2 % | 1 (10 %) | 6 (28.6 %) | |
| Moderate | 27.4 % | 32.9 % | 5 (50 %) | 4 (19 %) | |
| Severe-very severe | 36.9 % | 36.7 % | 4 (40 %) | 10 (47.6 %) | |
|
| |||||
| Sit to stand time | .95 | ||||
| Unable to perform task | 12.9 % | 11.6 % | 4 (40 %) | 5 (22.7 %) | |
| > 56 seconds | 15.3 % | 15.1 % | 3 (30 %) | 10 (45.5 %) | |
| 36-55.9 seconds | 23.5 % | 20.9 % | 0 | 5 (22.7 %) | |
| 26-35.9 seconds | 23.5 % | 29.1 % | 2 (20 %) | 1 (4.5 %) | |
| < 26 seconds | 24.7 % | 23.3 % | 1 (10 %) | 1 (4.5 %) | |
|
| |||||
| Average time for 8 foot walk | .97 | ||||
| Unable to perform task | 12.6 % | 10.6 % | 4 (40 %) | 3 (13.6 %) | |
| > 19 seconds | 16.1 % | 16.5 % | 3 (30 %) | 9 (40.9 %) | |
| 12.0 to 18.9 seconds | 21.8 % | 21.2 % | 0 | 5 (22.7 %) | |
| 7.5 to 11.9 seconds | 31.0 % | 29.4 % | 2 (20 %) | 3 (13.6 %) | |
| <7.5 seconds | 18.4 % | 22.4 % | 1 (10 %) | 2 (9.1 %) | |
Figure 2 displays rest pain and pain with PT scores through hospital day 7. Intervention patients reported significantly less pain at rest and with PT than usual care patients. In multivariable analyses compared to controls, intervention patients on average were significantly more likely to report no or mild pain at rest (66% (95% CI 64.8% to 66.9%) versus 51% (50.0% to 52.9%); parameter estimate = .63; P=.004) and with PT (52% (50.8% to 52.8%) versus 38% (36.8% to 39.1%); parameter estimate = .47; P=.02) for the first 7 days of rehabilitation. Compared to control patients, intervention patients were significantly less likely to report moderate to very severe pain at discharge (21% versus 37%; odds ratio = .42; 95% CI .24 to .74; P=.003) and during their last PT session prior to discharge (37% versus 56%; odds ratio = .47; 95% CI .29 to .77; P=.002).
Figure 2.
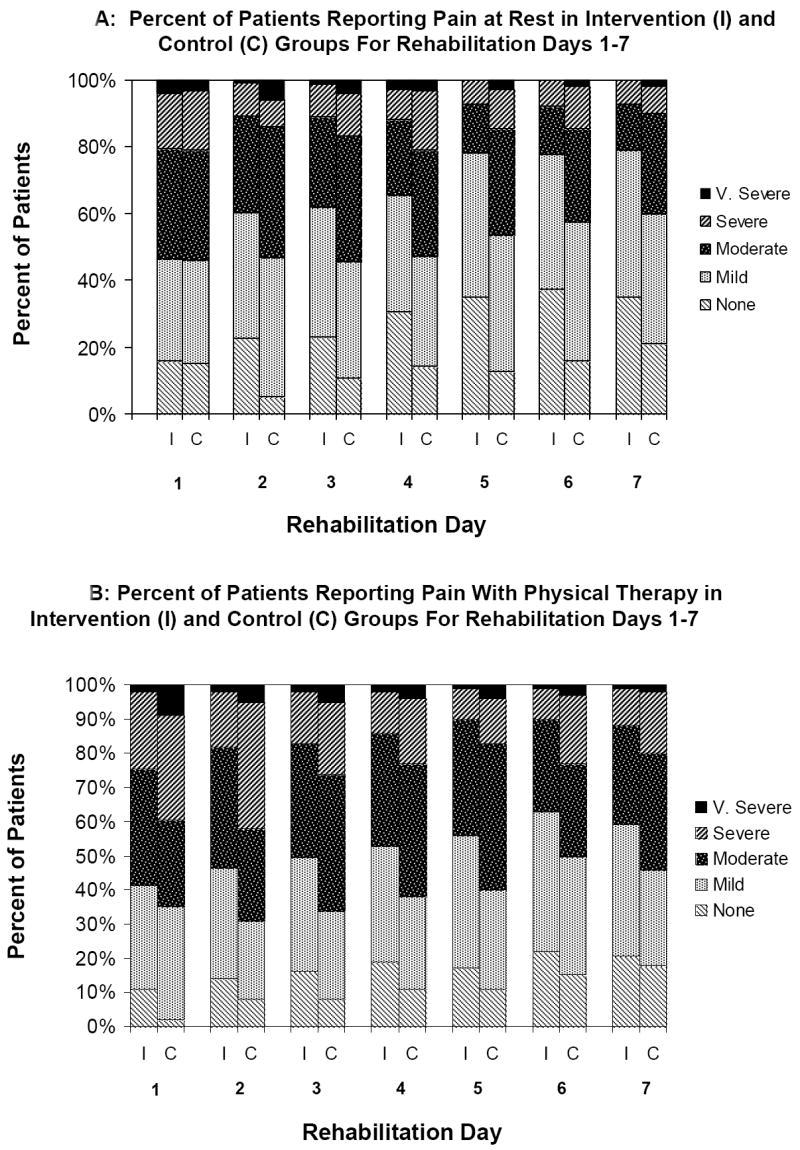
Pain at rest (Figure 2A) and pain with PT (Figure 2B) for intervention and control patients for intervention and control patients from rehabilitation day 1 to rehabilitation day 7. Overall adjusted mean pain at rest scores from admission through day 7 were 2.2 (95% CI 2.14 to 2.30) for intervention patients and 2.6 (2.46 to 2.63) for control patients (parameter estimate = -.33; 95% CI -.52 to -14; P<.001). Overall adjusted mean pain scores during PT for rehabilitation days 1-7 were 2.6 (2.48 to 2.65) for intervention patients and 2.8 (2.72 to 2.91) for control patients (parameter estimate = -.20; -.34 to -01; P=.04). All values reflect propensity score matched normalized weighted data.
Table 2 presents the multi-variable adjusted in-hospital performance outcomes for the two groups. Compared to controls, patients in the intervention arm had significantly faster 8 foot walk times at rehabilitation day 4 (9.3 seconds (95% CI 8.13 to 10.50) versus 13.2 seconds (11.98 to 14.44)) and day 7 (6.9 seconds (6.17 to 7.64) versus 9.2 seconds (8.31 to 10.18)). Intervention patients were significantly less likely to have a PT session missed or shortened (81% versus 73% of controls, odds ratio = 0.08; 95% CI .004 to.17; P=.04). Intervention patients had significantly shorter mean length of stay as compared to control patients (10.1 days (9.6 to 10.5) versus 11.3 days (11.0 to 11.7)).
Table 2.
Multivariable Adjusted Performance Outcomes For Propensity Score Matched Subjects*
| Control (N=88) | Intervention (N=129) | Parameter estimate (95% CI) or Odds Ratio (95% CI) | P | |
|---|---|---|---|---|
| 8 Foot Walk Performance | ||||
|
| ||||
| 8 ft walk performance on day 4 | 3.03 (1.78, 5.19) | <.001 | ||
| Unable to complete task | 13.8% | 5.8% | ||
| Over 13 seconds to complete task | 25.3% | 12.8% | ||
| 7.5 to 13 seconds to complete task | 26.4% | 22.1% | ||
| 5.6 to 7.4 seconds to complete task | 21.8% | 20.9% | ||
| 5.5 seconds or less to complete task | 12.6% | 38.4% | ||
|
| ||||
| Mean 8 ft walk time (sec) on day 4 for those able to complete the task (sd) | 13.2 (7.0) | 9.3 (4.9) | -.28 (-.51, -.04) | .02 |
|
| ||||
| 8 ft walk performance on day 7† | 2.77 (1.45, 5.28) | .002 | ||
| Unable to complete task | 11.1 | 10.4 | ||
| Over 13 seconds to complete task | 31.7 | 11.9 | ||
| 7.5 to 13 seconds to complete task | 28.6 | 17.9 | ||
| 5.6 to 7.4 seconds to complete task | 15.9 | 28.4 | ||
| 5.5 seconds or less to complete task | 12.7 | 31.3 | ||
|
| ||||
| Mean 8 ft walk time (sec) on day 7 for those able to complete the task (sd)† | 9.2 (3.7) | 6.9 (2.9) | -.21 (-.39, -.03) | .02 |
|
| ||||
| Sit to Stand Performance | ||||
|
| ||||
| Sit to stand performance on day 4 | 1.87 (1.08, 3.2) | .03 | ||
| Unable to complete task | 16.1% | 9.3% | ||
| Over 40 seconds to complete task | 23% | 15.1% | ||
| 28 to 40 seconds to complete task | 17.2% | 19.8% | ||
| 19-27 seconds to complete task | 28.7% | 26.7% | ||
| 18 seconds or less to complete task | 14.9% | 29.1% | ||
|
| ||||
| Sit to stand time (sec) on day 4 for those able to complete the task (sd) | 34.7 (13.0) | 32.7 (13.4) | -.08 (-.25, .08) | .34 |
|
| ||||
| Sit to stand performance on day 7† | 1.08 (.56, 2.1) | .81 | ||
| Unable to complete task | 13.5% | 15% | ||
| Over 32 seconds to complete task | 23.1% | 21.7% | ||
| 23 to 31 seconds to complete task | 26.9% | 21.7% | ||
| 16 to 22 seconds to complete task | 34.6% | 30% | ||
| 15 seconds or less to complete task | 1.9% | 11.7% | ||
|
| ||||
| Sit to stand time (sec) on day 7 for those able to complete the task (sd)† | 26.0 (10.0) | 27.8 (14.5) | -.02 (-.22, .18) | .85 |
|
| ||||
| Mean length of stay in days (sd) | 11.3 (1.8) | 10.1 (2.2) | -1.52 (-2.57, -.46) | .005 |
Variables included in the multivariable models: FIM locomotion score at admission to rehabilitation, modified RAND comorbidity score, age, sex, type of surgery (hip fracture repair, hip replacement, knee replacement), race/ethnicity, and GDS score
Sample size on day 7 was 168 (64 control and 104 intervention patients) due to discharges
Intervention patients received 8.0 milligrams (95% CI for difference 1.8 to 14.2) more oral morphine sulfate equivalents per day (23.6 milligrams/day versus 15.6 milligrams/day, parameter estimate = 6.48; P<0.001 respectively) and were significantly more likely to receive regularly scheduled opioid analgesia (98% versus 48%, odds ratio = 295.18; 95% CI 34.12 to 2553.44; P<.001 respectively) than control patients. For the 52% of control patients who did not receive any standing analgesia, 21% were ordered an “as needed opioid”, 77% were ordered an “as needed” combination product (acetaminophen with codeine, acetaminophen with oxycodone); and 3% were ordered “as needed” acetaminophen. Intervention patients were significantly more likely to have concurrent laxatives prescribed while receiving opioids (92% versus 83%, odds ratio=2.54, 95% CI 1.04 to 6.20 P=.03) than control patients. There were no significant differences between the two groups with respect to opioid side effects of constipation (e.g., 3 or more days without a bowel movement) (32% of intervention patients versus 25% of controls, P=.06), delirium (4% of intervention versus 7% of controls, P=.30), nausea (16% of intervention patients versus 17% of controls, P=.31), somnolence (33% of intervention patients versus 36% of controls, P=.55), or thought clarity (6% of intervention patients versus 6% of controls, P=.96).
At 6 months, intervention patients were less likely to report moderate to very severe pain with walking (4% versus 15% of controls, odds ratio =.16; 95% CI .05 to.56; P=.02), that pain interfered with walking (7% versus 18% of controls, odds ratio =.16; 95% CI .05 to.56; P=.004), and were less likely to be taking analgesics (35% versus 51% of controls; odds ratio = 0.50; 95% CI .26, .94; P=.03) as compared to control patients. Figure 3 presents pain and interference with walking for intervention and control patients for the six months following discharge.
Figure 3.
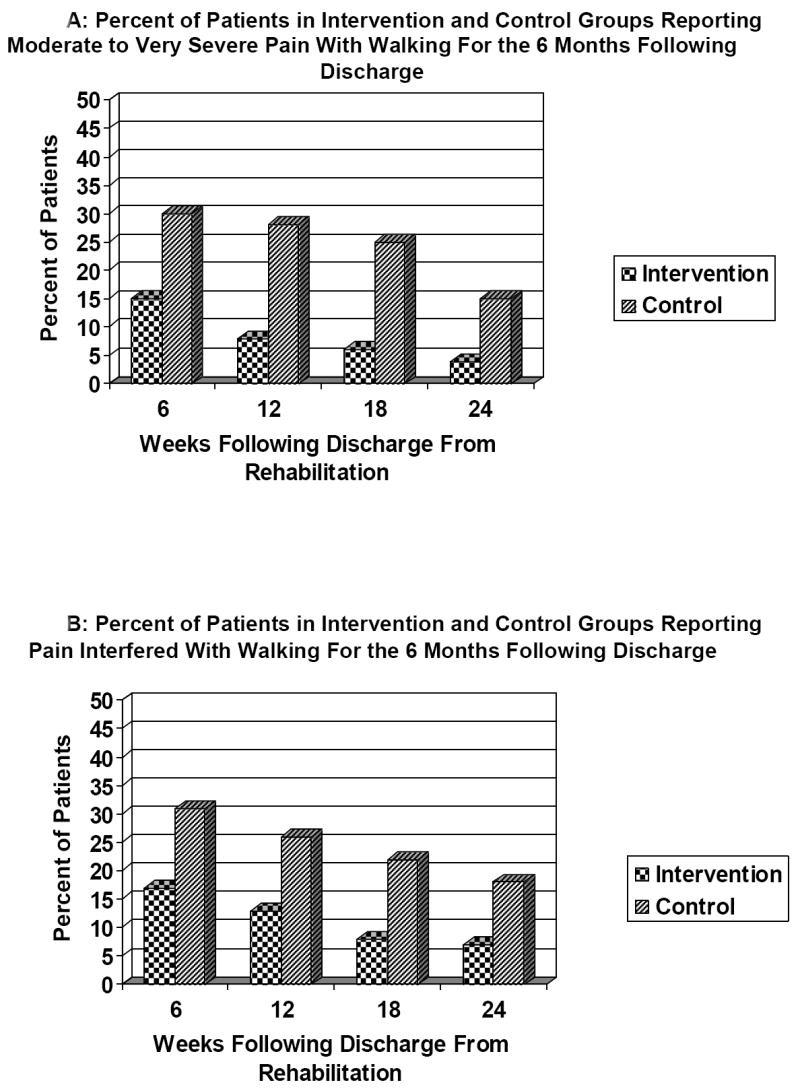
Percent of patients reporting moderate to very severe pain with ambulation (Figure 3A) and percent of patients reporting pain interfered with ambulation (Figure 3B) for intervention and control patients for the 24 weeks following hospital discharge. Overall adjusted mean pain with ambulation scores over the 24 weeks were 1.50 (95% CI 1.47 to 1.53) for intervention patients and 1.77 (1.73 to 1.81) for control patients (parameter estimate = -.27; 95 CI -.46, -.05; P=.01). Overall adjusted mean interference scores over the 24 weeks were 1.18 (1.16 to 1.20) for intervention patients and 1.48 (1.45 to 1.51) for control patients (parameter estimate =-.25; 95 CI -.41, -.10; P=.001). All values reflect propensity score matched normalized weighted data.
Results from multivariable analyses that included all subjects were qualitatively similar to those of the propensity score matched analyses across all outcome measures (i.e., the parameter estimates were contained within the 95% confidence intervals of the estimates of the primary propensity score analyses).
DISCUSSION
This study of a generalizable interdisciplinary pain management program is one of the first rigorous clinical trials to demonstrate significant reductions in post-operative pain, reduction in chronic pain, and improved lower extremity mobility both acutely and 6 months following discharge. This study makes several valuable contributions to the evidence base for pain treatment. First, it is one of the only controlled clinical trials published to date that describes generalizable interventions that routinely identify and scale pain, result in appropriate analgesic medication prescribing, and are associated with reduced pain severity. Second, it is the largest study to date to show that improved pain control results in enhanced rehabilitation for older adults following surgery and shorter lengths of stay. Finally, this study is the first to our knowledge that demonstrates that reducing acute post-operative pain results in a lower prevalence of chronic pain and improved walking at 6 months following discharge.
Strengths and Limitations
There are several limitations to this study that should be noted. This was not a randomized trial and it is possible that an unmeasured confounder may have accounted for the results observed. As others have noted, it is impractical to randomize patients on admission to unit based interventions in settings where fiscal pressures require that patients be assigned to the first available open bed.12 Although patient assignment was based upon the random availability of an open bed and was out of our control, it is possible that measured and unmeasured confounders were not randomly distributed across intervention and control units. To account for this possibility, our study employed a prospective individual matching procedure to achieve a balanced allocation of subjects11 and used newly developed propensity score methods to ensure even more stringent balancing between intervention and control groups.32 As shown in Table 1, subjects in the two arms were well matched and there were no significant difference in observable characteristics between the two groups.
It is also possible that cross-contamination could have occurred with providers caring for patients on more than on floor and applying the intervention or some variant to the control group as a result of weekend or holiday coverage. We believe that such contamination is unlikely. It was extremely rare for nursing or physician staff to “float” from intervention to control units – even during times of cross-coverage - during the study period. Even if such cross-contamination had occurred the resulting bias would favor the null hypothesis and as such, our results would reflect a more conservative estimate of the true intervention effect.
Finally, our study was limited to the sub-set of older adults admitted to acute rehabilitation. It is possible that the long-term pain and functional outcomes that we observed might not generalize to other settings or patient populations. Nevertheless, our intervention was designed so that it could be easily incorporated into standard rehabilitation protocols for lower-extremity orthopedic surgery in other settings (peri-operatively in the hospital, sub-acute rehabilitation). Although empirical testing of our intervention in these other settings is warranted, we are cautiously optimistic that similarly positive results will be obtained.
Relationship to Other Studies
Pain Severity and Analgesic Prescribing
After more than 2 decades of panels, symposia, and editorials calling for remedial action,15 33-36 inadequate treatment of pain remains a serious problem in the United States and world-wide.1 37 38 A recent systematic review of institutional interventions to improve the management of pain in hospitalized adults did not identify a single generalizable intervention that successfully and consistently improved patients’ pain severity.2 We believe our intervention was effective because it targeted the entire interdisciplinary team rather than nursing staff alone,2 employed interventions that have been previously shown to be effective in improving pain assessment,1 and unlike other studies, targeted physicians by giving them guidance regarding both opioid prescribing and side effect management. Although this study was conducted in the rehabilitation setting, the intervention could be easily implemented in the immediate postoperative period and in sub-acute rehabilitation. Future studies are needed to confirm our finding in these additional settings.
Rehabilitation
Prior observational studies have found that post-operative pain in older adults undergoing lower extremity orthopedic surgery is associated with an increased the risk of delirium,7 39 40 longer hospital and rehabilitation length of stay, 8 41 higher probability of a PT session being missed or shortened,8 delays in ambulation post-operatively,8 impaired functional recovery,8 41 and greater pain at 6 months 42 Data from controlled studies examining the effect of improving postoperative pain on functional outcomes in older adults are sparse. The few small studies that have been performed suggest that improved pain management is associated with reduced length of stay, earlier mobilization, and improved range of motion in patients undergoing knee arthroplasty.5 43 44 Our study both confirms and extends these preliminary reports by enrolling patients who underwent hip and knee arthroplasty and hip fracture repair, being of adequate size to adjust for confounding variables, and using validated performance measures of lower extremity function. Patients in the intervention arm not only had better pain control, but were noted to have increased gait speed, faster transfer times, were significantly more likely to complete their regularly scheduled PT sessions, and had shorter lengths of stay.
Chronic Pain and Six Month Function
Despite the prevalence (over one million surgeries annually in the United States) and reported success rates of knee and hip arthroplasty and hip fracture repair, a substantial number of patients undergoing these procedures report chronic pain at six months.45 46 The prevalence of chronic pain has been reported to be as high as 28.1% following hip arthroplasty, 18.4% following knee arthroplasty, and 26% following hip fracture repair.8 46 These rates are comparable to those observed in the control arm of this study.
Why chronic pain syndromes develop following surgery is not well understood. It is hypothesized that chronic pain results from the interaction of prolonged peripheral and central nervous system sensitization that subsequently leads to the development of permanent aberrant excitatory synaptic connections.46 Observational data suggest that enhanced post-operative pain management is associated with improved functional outcomes at six months in older adults.8 Our study confirms and amplifies these findings by demonstrating that patients exposed to the intervention reported less pain, improved function, and less analgesic requirements 6 months after receiving our analgesic protocol. We postulate that our intervention, by providing effective analgesia in the immediate post-operative period, prevented the development of central sensitization and contributed to the reduction of chronic pain and improved 6 month function observed in our intervention group (Figure 3). Studies are needed to confirm these results and explore the underlying pathophysiology for them in both animal models and additional clinical trials.
CONCLUSIONS
The inability of health care providers and the health care system to address the problem of untreated pain has been well documented. This failure is partly a reflection of the emphasis in medicine on diagnosis and treatment of causative factors rather than on symptomatic treatment. The common belief that acute pain is merely a symptom, will resolve as healing occurs, and is not harmful in itself relegates the relief of acute pain to a minor level of priority in the minds of many doctors and nurses.47 The absence of data linking untreated pain to adverse clinical and functional outcomes has further reinforced these beliefs. This study of an interdisciplinary unit based intervention to improve the treatment of pain revealed important and novel findings. It is the largest and most rigorously designed study to date to identify an effective systematic intervention to reduce post-operative pain in older adults. Second, it supplements existing observational data by providing direct evidence that reducing post-operative pain improves functional outcomes in older adults and reduces rehabilitation length of stay. Finally, and perhaps most intriguingly, it suggests that aggressive pain management in the post-operative setting may reduce the development of chronic post-operative pain. Additional research focused on replicating these results in other patient populations and settings and on the underlying biological mechanisms that underlie these clinical findings is required.
Acknowledgments
The authors would like to acknowledge the invaluable assistance of Susan Calise and Saima Siddiqui.
Funding sources: This study was supported by grant # R01AG022108 from the National Institute on Aging. Drs. Morrison and Siu are recipients of Mid-career investigator awards in patient oriented research from the National Institute on Aging (K24 AG022345 and K24 AG000918 respectively). Dr. Cintron was supported by a Diversity Supplement from the National Institute on Aging (R01AG022108-S1).
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions R. Sean Morrison – study concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of manuscript
Steven Flanagan - study concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of manuscript
Daniel Fischberg – study concept and design, analysis and interpretation of data, and preparation of manuscript
Alexie Cintron – acquisition of subjects and data, analysis and interpretation of data, and preparation of manuscript
Albert Siu – study concept and design, analysis and interpretation of data, and preparation of manuscript
Sponsors’ Role: The funding agencies had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
References
- 1.Morrison RS, Meier DE, Fischberg D, et al. Improving the management of pain in hospitalized adults. Arch Intern Med. 2006;166(9):1033–9. doi: 10.1001/archinte.166.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldberg GR, Morrison RS. Pain management in hospitalized cancer patients. J Clin Oncol. 2007;25:1792–801. doi: 10.1200/JCO.2006.07.9038. [DOI] [PubMed] [Google Scholar]
- 3.Max MB. How to move pain and symptom research from the margin to the mainstream. J Pain. 2003;4:355–360. doi: 10.1016/s1526-5900(03)00719-3. [DOI] [PubMed] [Google Scholar]
- 4.Munin MC, Kwoh CK, Glynn N, et al. Predicting discharge outcome after elective hip and knee arthroplasty. Am J Phys Med Rehabil. 1995;74:294–301. doi: 10.1097/00002060-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Cheville A, Chen A, Oster G, et al. A randomized trial of controlled-release oxycodone during inpatient rehabilitation following unilateral total knee arthroplasty. J Bone Joint Surg Am. 2001;83-A:572–576. doi: 10.2106/00004623-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Morrison RS, Siu AL. A comparison of pain and its treatment in advanced dementia and cognitively intact patients with hip fracture. J Pain Symptom Manage. 2000;19:240–248. doi: 10.1016/s0885-3924(00)00113-5. [DOI] [PubMed] [Google Scholar]
- 7.Morrison RS, Magaziner J, Gilbert M, et al. Relationship between pain and opioid analgesics on the development of delirium following hip fracture. J Gerontol A Biol Sci Med Sci. 2003;58:76–81. doi: 10.1093/gerona/58.1.m76. [DOI] [PubMed] [Google Scholar]
- 8.Morrison RS, Magaziner J, McLaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain. 2003;103:303–11. doi: 10.1016/S0304-3959(02)00458-X. [DOI] [PubMed] [Google Scholar]
- 9.Kroll MA, Otis JC, Sculco TP, et al. The relationship of stride characteristics to pain before and after total knee arthroplasty. Clin Orthop. 1989:191–195. [PubMed] [Google Scholar]
- 10.Jonasson OL, Kwakwa F. Aging of America: Implications For the Surgical Workforce. In: Rosenthal RA, Zenilman ME, et al., editors. Principles and Practice of Geriatric Surgery. New York: Springer; 2001. pp. 105–110. [Google Scholar]
- 11.Makuch RW, Zhang Z, Charpentier PA, et al. Prospective individual matching: covariate balance and power in a comparative study. Stat Med. 1998;17(13):1517–26. doi: 10.1002/(sici)1097-0258(19980715)17:13<1517::aid-sim859>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. see comments. [DOI] [PubMed] [Google Scholar]
- 13.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 14.Roccaforte WH, Burke WJ, Bayer BL, Wengel SP. Validation of a telephone version of the mini-mental state examination. J Am Geriatr Soc. 1992;40:697–702. doi: 10.1111/j.1532-5415.1992.tb01962.x. [DOI] [PubMed] [Google Scholar]
- 15.Joint Commission on Accreditation of Healthcare Organizations. Joint Commission on Accreditation of Healthcare Organizations Pain Standards. 2004 doi: 10.1016/s1089-9472(03)00184-9. [DOI] [PubMed] [Google Scholar]
- 16.American Pain Society Quality of Care Committee. Quality improvement guidelines for the treatment of acute pain and cancer pain. American Pain Society Quality of Care Committee. JAMA. 1995;274:1874–1880. doi: 10.1001/jama.1995.03530230060032. [DOI] [PubMed] [Google Scholar]
- 17.Agency for Health Care Policy and Research Acute Pain Management Panel. Acute pain management: operative or medical procedures and trauma. Washington: U.S. Dept. of Health and Human Services; 1992. [Google Scholar]
- 18.American Geriatrics Society. The management of persistent pain in older adults. [November 15];2007 Available at: http://www.americangeriatrics.org.
- 19.Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 20.Milisen K, Foreman MD, Abraham IL, et al. A nurse-led interdisciplinary intervention program for delirium in elderly hip-fracture patients. J Am Geriatr Soc. 2001;49:523–532. doi: 10.1046/j.1532-5415.2001.49109.x. [DOI] [PubMed] [Google Scholar]
- 21.Hannan EL, Magaziner J, Wang JJ, et al. Mortality and locomotion 6 months after hospitalization for hip fracture: Risk factors and risk-adjusted hospital outcomes. JAMA. 2001;285:2736–2742. doi: 10.1001/jama.285.21.2736. [DOI] [PubMed] [Google Scholar]
- 22.Burke WJ, Roccaforte WH, Wengel SP. The short form of the Geriatric Depression Scale: A comparison with the 30-item form. J Geriatr Psychiatry Neurol. 1991;4:173–178. doi: 10.1177/089198879100400310. [DOI] [PubMed] [Google Scholar]
- 23.Diehr P, Patrick DL, Spertus J, et al. Transforming self-rated health and the SF-36 scales to include death and improve interpretability. Med Care. 2001;39:670–680. doi: 10.1097/00005650-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Jette AM, Jette DU, Ng J, et al. Are performance-based measures sufficiently reliable for use in multicenter trials? Musculoskeletal Impairment (MSI) Study Group. J Gerontol A Biol Sci Med Sci. 1999;54:M3–6. doi: 10.1093/gerona/54.1.m3. [DOI] [PubMed] [Google Scholar]
- 25.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 27.Rubin DB. The design versus the analysis of observational studies for causal effects: Parallels with the design of randomized trials. Stat Med. 2007;26:20–36. doi: 10.1002/sim.2739. [DOI] [PubMed] [Google Scholar]
- 28.Rubin DB, Thomas N. Matching using estimated propensity scores: Relating theory to practice. Biometrics. 1996;52:249–264. [PubMed] [Google Scholar]
- 29.Gruber-Baldini AL, Zimmerman S, Morrison RS, et al. Cognitive impairment in hip fracture patients: Timing of detection and longitudinal follow-up. J Am Geriatr Soc. 2003;51:1227–1236. doi: 10.1046/j.1532-5415.2003.51406.x. [DOI] [PubMed] [Google Scholar]
- 30.Dolan MM, Hawkes WG, Zimmerman SI, et al. Delirium on hospital admission in aged hip fracture patients: Prediction of mortality and 2-year functional outcomes. J Gerontol A Biol Sci Med Sci. 2000;55:M527–534. doi: 10.1093/gerona/55.9.m527. [DOI] [PubMed] [Google Scholar]
- 31.Stata Corporation. Stata (Version 9) College Station Texas: Stata Corporation; 2005. [Google Scholar]
- 32.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: A Monte Carlo study. Stat Med. 2007;26:734–753. doi: 10.1002/sim.2580. [DOI] [PubMed] [Google Scholar]
- 33.Angell M. The quality of mercy. N Engl J Med. 1982;306:98–99. doi: 10.1056/NEJM198201143060210. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. Cancer Pain Relief. Geneva: World Health Organization; 1986. [Google Scholar]
- 35.Wanzer SH, Federman DD, Adelstein SJ, et al. The physician’s responsibility toward hopelessly ill patients: A second look. N Engl J Med. 1989;320:844–849. doi: 10.1056/NEJM198903303201306. [DOI] [PubMed] [Google Scholar]
- 36.Stratton Hill C. When will adequate pain treatment be the norm? JAMA. 1995;274:1881–1882. [PubMed] [Google Scholar]
- 37.Strassels SA, Chen C, Carr DB. Postoperative analgesia: economics, resource use, and patient satisfaction in an urban teaching hospital. Anesth Analg. 2002;94:130–137. doi: 10.1097/00000539-200201000-00025. table of contents. [DOI] [PubMed] [Google Scholar]
- 38.Whelan C, Jin L, Meltzer D. Pain and satisfaction with pain control in hospitalized medical patients. Arch Int Med. 2004;164:175–180. doi: 10.1001/archinte.164.2.175. [DOI] [PubMed] [Google Scholar]
- 39.Duggleby W, Lander J. Cognitive status and postoperative pain: older adults. J Pain Symptom Manage. 1994;9:19–27. doi: 10.1016/0885-3924(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 40.Lynch EP, Lazor MA, Gellis JE, et al. The impact of postoperative pain on the development of postoperative delirium. Anesth Analg. 1998;86:781–785. doi: 10.1097/00000539-199804000-00019. [DOI] [PubMed] [Google Scholar]
- 41.Brander VA, Stulberg SD, Adams AD, et al. Predicting total knee replacement pain: A prospective, observational study. Clin Orthop Relat Res. 2003:27–36. doi: 10.1097/01.blo.0000092983.12414.e9. [DOI] [PubMed] [Google Scholar]
- 42.McGeary DD, Mayer TG, Gatchel RJ. High pain ratings predict treatment failure in chronic occupational musculoskeletal disorders. J Bone Joint Surg Am. 2006;88:317–25. doi: 10.2106/JBJS.D.02968. [DOI] [PubMed] [Google Scholar]
- 43.Singelyn FJ, Deyaert M, Joris D, et al. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg. 1998;87:88–92. doi: 10.1097/00000539-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 44.Ryu J, Salto S, Yamamoto K, et al. Factors influencing the postoperative range of motion in total knee arthroplasty. Bull Hosp Joint Dis. 1993;53:35–40. [PubMed] [Google Scholar]
- 45.DeFrances CJ, Hall MJ. 2005 National Hospital Discharge Survey. Advance data from vital and health statistics. Hyattsville, Maryland: National Center for Health Statistics; 2007. [PubMed] [Google Scholar]
- 46.Reuben SS, Buvanendran A. Preventing the development of chronic pain after orthopaedic surgery with preventive multimodal analgesic techniques. J Bone Joint Surg Am. 2007;89:1343–1358. doi: 10.2106/JBJS.F.00906. [DOI] [PubMed] [Google Scholar]
- 47.Cousins M, Power I. Acute and postoperative pain. In: Wall PD, Melzack R, editors. Textbook of Pain. fourth edition. London: Churchill Livingstone; 1999. [Google Scholar]


