Abstract
Estrogen has diverse and powerful effects in the brain, including actions on neurons, glia, and the vasculature. It is not surprising, therefore, that there are many changes in the female brain as serum estradiol levels rise and fall during the normal ovarian cycle. At times of life when estradiol levels change dramatically, such as puberty, postpartum, or menopause, there also are dramatic changes in the central nervous system. Changes that occur because of fluctuations in serum estrogen levels are potentially relevant to neurological disorders because symptoms often vary with the time of the ovarian cycle. Moreover, neurological disorders (eg, seizures and migraine) often increase in frequency in women when estradiol levels change. In this review, the contribution of 2 growth factors targeted by estrogen, the neurotrophin brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF), will be discussed. Estrogen-sensitive response elements are present on the genes for both BDNF and VEGF, and they are potent modulators of neuronal, glial, and vascular function, making them logical candidates to mediate the multitude of effects of estrogen. In addition, BDNF induces neuropeptide Y, which has diverse actions that are relevant to estrogen action and to the same neurological disorders.
Keywords: brain-derived neurotrophic factor, catamenial epilepsy, estrogen, migraine, neuropeptide Y, vascular endothelial growth factor
Introduction
Estrogen is a potent neuromodulator that influences brain structure and function in diverse ways. As a result, changes in central nervous system (CNS) function would be expected with fluctuations in estradiol. Such fluctuations in estradiol levels occur at specific times of life, such as puberty, pregnancy, postpartum, and menopause. Fluctuations in estradiol levels also occur during the ovarian cycle. Nevertheless, it remains unclear how much the changes in estradiol levels—either during the ovarian cycle or at particular times of life—are responsible for the changes in brain function that have been reported in women. One reason for the lack of a clear understanding is that levels of other serum hormones with effects on the brain, such as progesterone, also fluctuate. The roles of estradiol relative to progesterone are difficult to clarify because the complex fluctuations of estradiol and progesterone during the ovarian cycle are difficult to simulate in an ovariectomized animal. Nor is there a well-accepted approach to objectively evaluate the role of estrogen in puberty, pregnancy, the postpartum period, or menopause.
Clarifying how changes in estradiol levels impact brain function is important not only for the normal female but also for women with neurological disorders such as seizures or migraine, because changes in estradiol may modify symptoms. Moreover, some seizures or migraine episodes may be precipitated by changes in estrogen levels. For example, there is a substantial rise in estrogen levels at puberty and during pregnancy. At postpartum or at menopause, there is a sudden loss of estrogen.
Another problem in trying to clarify the role of physiologic changes in estradiol is that the targets of estrogen are diverse. In this review, 2 targets of estrogen will be considered that may mediate several effects in a manner that can explain the ways physiologic fluctuations in estrogen levels influence neurological disorders. These 2 examples are termed “growth factors,” but they do much more to the CNS than initiate growth. One is brain-derived neurotrophic factor (BDNF) and the other is vascular endothelial growth factor (VEGF). In addition, the possible consequences of BDNF induction of the synthesis of neuropeptide Y (NPY), which has powerful effects in the CNS, will be discussed. The sequential induction of growth factors and peptides by estrogen may provide insight into the ways estrogen influences neurological disease during the menstrual cycle and during the lifespan.
Estrogen
Estrogen and Its Receptors
The primary source of serum estradiol is the ovaries. Estrogen also is synthesized in other areas of the body but to a much lesser extent. In the brain, estrogen is synthesized from cholesterol in astrocytes, where biosynthetic enzymes such as aromatase are located. This review will focus on the effects of serum estradiol, which is the major physiologic source of estrogen. In addition, discussion is confined to the female because of the dynamic nature of estrogen levels in women. However, estrogen is present in the male, and some of the effects of estrogen may be similar in males and females.
There are 3 physiologic estrogens in the body: estrone (E1), 17β-estradiol (estradiol, E2), and estriol (E3). Estradiol is thought to be the primary bioactive estrogen under most physiologic conditions. Estrogens are thought to act via 2 known receptors, estrogen receptor (ER)α and β.1 Estrogen binds to these receptors in the plasma membrane, in the cytoplasm of the cell, as well as in the cell nucleus. In the nucleus, dimerization of the receptors occurs, allowing the ER to bind to specific segments of DNA and activate target genes or to work with other transcriptional regulatory molecules, such as activating protein 1, a transcription factor. Estrogen actions via membrane-associated ERs, as well as in mitochondria, are thought to lead to actions on a more rapid time scale, involving activation of signal transduction pathways, including the mitogen-activated protein kinase pathway and the phosphatidylinositol 3-kinase/Akt pathway.2
The Ovarian Cycle
The ovarian cycle in women (the menstrual cycle) and in rodents (the estrous cycle) is depicted schematically in Figure 1.3 In women, the 28-day cycle includes a follicular and a luteal phase. At the transition between the 2 phases, immediately prior to ovulation, estradiol secretion from the ovaries surges (Fig. 1A). It then falls, but estradiol levels do not remain low for long. There is a subsequent smaller and slower rise in estradiol levels during the luteal phase. The luteal phase is accompanied by a rise in progesterone levels. The end of the luteal phase is a time when both estrogen and progesterone fall to low levels, and menses begins.
Fig 1.
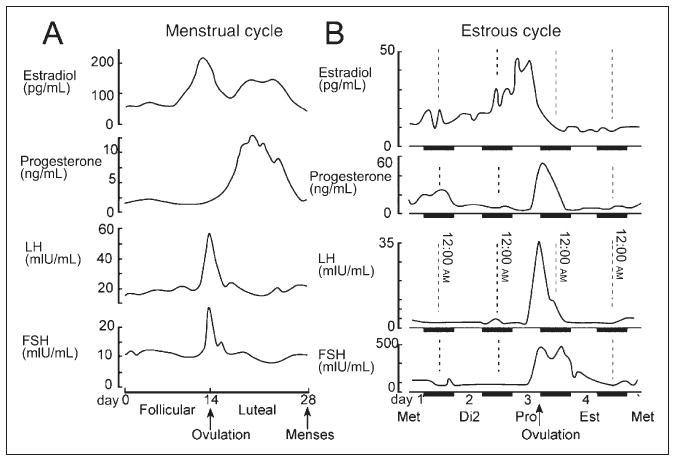
The ovarian cycle in women (the menstrual cycle) and rodents (the estrous cycle). (A) In women, the menstrual cycle is 28 days. The serum levels of estradiol, progesterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) are plotted as a function of the time during the cycle. (B) The estrous cycle in the rodent is 4 days. The time when estrogen levels surge before ovulation is the morning of proestrus (Pro). That afternoon and evening, progesterone levels begin to rise and then fall just before the morning of the next day, the day of estrus (Est). The subsequent 2 days are collectively termed “diestrus” and begin with diestrus 1 (also called “metestrus” [Met]), followed by diestrus 2 (Di2). Adapted with permission from Scharfman HE & MacLusky NJ (2006).3
Figure 1B depicts the estrous cycle in rodents, a primary animal model for the study of the ovarian cycle. The estrous cycle typically lasts 4 days.4,5 On the morning of proestrus, estrogen surges to reach a peak by midmorning and then subsides. The time of ovulation is late in the day of proestrus and is accompanied by rising progesterone levels. During the evening of proestrus, sexual behavior is initiated (ie, behavioral estrus). During the night, progesterone levels peak and then rapidly fall during the early morning of the next day, which is called “estrus.” Levels of both hormones then remain relatively low for 2 days, which are collectively referred to as “diestrus.”4,5
Actions of Estrogen in the Brain
The actions of estrogen in the brain are difficult to summarize because they are numerous and they are not the same for all brain regions. This paper will focus on some of the actions of estrogen that are particularly relevant to seizure disorders and migraine. However, even with this narrow focus, it is difficult to review all of the neurological effects of estrogen during the ovarian cycle because outcomes of studies of estrogen action do not always agree, at times for technical reasons, such as the variation in estrogen treatments among studies. In addition, the physiologic effects of estrogen in the intact rat are not as commonly studied as are the effects of pharmacologic doses of estrogen in ovariectomized rats. Yet the intact rat provides the greatest insight into the ovarian cycle. Finally, some animal models of disorders lead to a loss of estrous cyclicity because of changes to the hypothalamic-pituitary-gonadal axis. Nevertheless, potential insights into the ways that cyclic fluctuations in estrogen levels are relevant to clinical conditions have been provided by experimental studies of estrogen action.
Role of Estrogen in Epilepsy and Migraine
It has been known for decades that estrogen increases neuronal excitability, and estrogen application to the brain can induce seizure activity.3 However, in animal models of epilepsy, estrogen is not necessarily pro-convulsant.3 In women with epilepsy, there can be an increase in seizure frequency and/or severity at times of the menstrual cycle, a condition termed “catamenial epilepsy.”3,6-8 Often seizures increase with the rise in estrogen levels (ie, during the periovulatory period).6 On the other hand, some women experience an increased incidence of seizures not when estrogen levels rise before ovulation, but at the end of the cycle when estrogen levels are low.6-8
It also has been reported that women with a history of migraine may experience migraine during particular times of the menstrual cycle, or that migraine is more severe during the perimenstrual period relative to the mid-luteal phase,9-13 although this is controversial.14 Some reasons for an increase in migraine may be the increase in neuronal excitability that occurs at specific times during the menstrual cycle, particularly in women with catamenial epilepsy. In fact, there is evidence that catamenial epilepsy increases the risk of migraine.15 Vasodilation also may play a role because vasodilation accompanies seizures, and vasodilation accompanies migraine.16 Estrogen may mediate vasodilation in this context because ERs exist on blood vessels, and estrogen exposure induces vasodilation.17
Progesterone
Although the focus of this paper is on estrogen, it is important to recognize the potent actions of progesterone in the brain. In some conditions, estrogen and progesterone act synergistically, and, in other situations, it appears that progesterone counteracts effects of estrogen. For example, progesterone downregulates ERs in some areas of the brain.18-21 Any discussion of estrogen, therefore, must take into consideration the potential ways that estrogen and progesterone interact.
As shown in Figure 1, progesterone levels rise during the luteal phase of the ovarian cycle in women, which occurs after the preovulatory surge in estradiol. In rodents, the timing is more rapid. After the estrogen surge on proestrous morning, which is analogous to the preovulatory period, progesterone levels begin to rise and continue to increase in the afternoon and evening. Although actions of estradiol and progesterone may seem independent, the actions initiated by estradiol may be potentiated by the subsequent elevation of progesterone levels.3 However, over a longer time scale, progesterone antagonizes estradiol action by at least 2 mechanisms: first, progesterone antagonizes transcriptional responses to estradiol22; second, one of the metabolites of progesterone, allopregnanolone, is a potent allosteric modulator of the γ-aminobutyric acid (GABA)A receptor.23 Therefore, exposure to progesterone typically has inhibitory effects on neurons. This action appears to oppose the potentiating effects of estrogen on glutamatergic transmission.
In women, many of the changes that occur during the perimenstrual period that are related to excitability or mood have been attributed to the decline in levels of progesterone and allopregnanolone. This effect is often termed “progesterone withdrawal” and is used to explain the increase in neuronal excitability that occurs at menses. The rapid decline in progesterone levels also leads to a change in GABAA receptor subunit composition, which decreases the efficacy of GABAA-receptor–mediated inhibition.7,24,25
Although the evidence that progesterone withdrawal underlies perimenstrual changes in excitability is compelling, its role in migraine seems less well established. In fact, reducing the fall in progesterone levels at the end of the menstrual cycle does not appear to reduce menstrual migraine.26 There appears to be more evidence that the fall in estrogen levels could have a role in the perimenstrual period. One reason to suggest a role of estrogen is that levels of estrogen fall at a similar time as the fall in progesterone levels during the perimenstrual period. Therefore, at the end of the menstrual cycle, a decline in estradiol levels occurs that could lead to an “estrogen withdrawal.” Clinical studies would suggest that interventions that blunt the fall in serum estradiol levels during the perimenstrual period are effective in preventing menstrual migraine.26-29 It is also important to consider that target genes induced by estrogen could have effects long after estrogen levels subside. One example is BDNF. Thus, the preovulatory surge in estrogen could lead to a delayed elevation in BDNF levels that leads to mid-cycle migraine, and the mid-luteal rise in estrogen levels could lead to elevated BDNF levels during the perimenstrual period, possibly leading to menstrual migraine.
BDNF
In 1995, it was shown that estrogen can induce BDNF expression via an estrogen-sensitive response element on the BDNF gene.30 It was subsequently shown that BDNF mRNA levels increase in many brain areas of ovariectomized rats treated with estradiol.30-32 These studies suggested that BDNF might be a mediator of estrogen action.
BDNF is a member of the neurotrophin family, which includes nerve growth factor, neurotrophin-3, and neurotrophin-4/5. All neurotrophins have potent actions at tropomyosin receptor kinases (trk). BDNF binds with high specificity to trkB, and all neurotrophins bind to p75 (Fig. 2).33 Both trkB and receptor p75 are coupled to an array of signal transduction pathways.34-37 Although historically BDNF was studied in the context of development, more recent studies have shown important actions in the adult CNS.38-41 In addition, studies of neurological disorders and psychiatric illness indicate a potential role for BDNF in pathological conditions.42-51 For example, BDNF has been implicated in epilepsy.50
Fig 2.
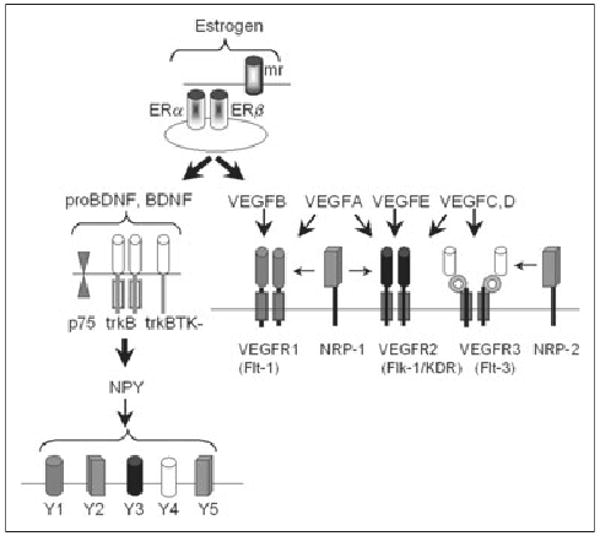
Receptors for estrogen, BDNF, VEGF, and NPY. Estrogen receptors are associated with the membrane (membrane receptors, mr) or are cytoplasmic (ERα, ERβ). ProBDNF and BDNF can bind to p75 as well as full-length trkB (trkB) or truncated trkB (trkBTK-). BDNF induces NPY by trkB activation, and NPY binds to 5 receptor subtypes. VEGF signaling is mediated by 5 receptors, VEGFR1, VEGFR2, and VEGFR3 and neuropilin (NRP)-1 and NRP-2. VEGF isoforms include VEGFA through VEGFE. BDNF, brain-derived neurotrophic factor; VEGF, vascular endothelial growth factor; NPY, neuropeptide Y. Adapted with permission from Cross MJ et al (2003).33
Some of the most detailed studies of BDNF have used hippocampal neurons. BDNF potentiates synaptic transmission in hippocampal area CA1, area CA3, and the dentate gyrus, and is critical to the late phase of long-term potentiation.52-54 In area CA3, BDNF potentiates a major glutamatergic input to pyramidal cells, the mossy fiber pathway.51,55 Remarkably, BDNF exposure leads to a predisposition for epileptiform activity in area CA3, because brief stimulation of the mossy fibers activates repetitive synchronous firing of CA3 pyramidal cells when BDNF is present or overexpressed.51,55,56 Spreading depression episodes often follow a brief period of mossy fiber stimulation in the presence of BDNF (Fig. 3).51,55,56
Fig 3.
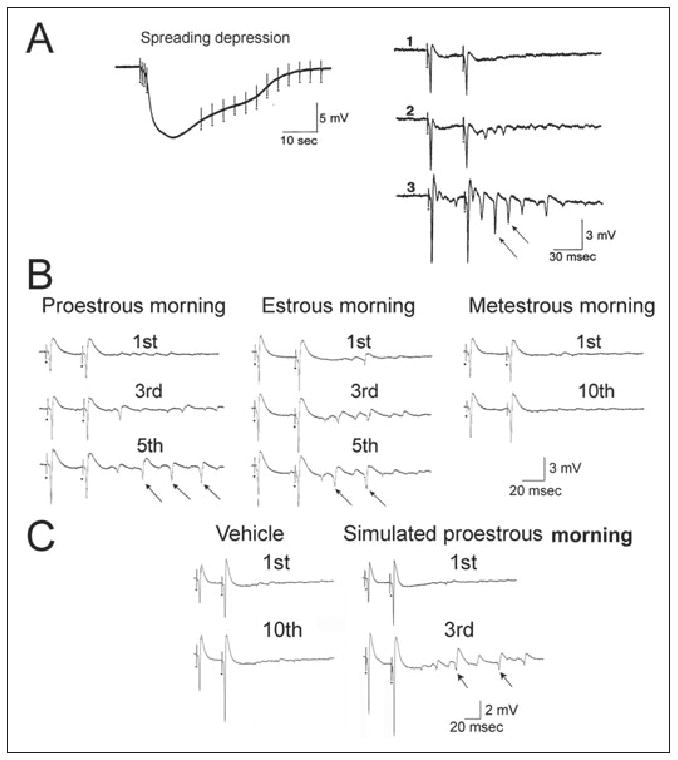
Mossy fiber transmission increases in response to brain-derived neurotrophic factor (BDNF) and estrogen. (A) The mossy fibers were stimulated at 1 Hz in the presence of recombinant BDNF, and spreading depression occurred in area CA3. The response to the first 3 stimulus pairs is shown on the right, illustrating that repetitive population spikes were induced by the stimulation. Reprinted with permission from Scharfman HE (1997).55 (B) In slices from rats on proestrous morning or estrous morning, similar effects as those with BDNF exposure occurred when mossy fibers were stimulated; however, BDNF was not added. Adapted with permission from Scharfman HE et al (2003).57 (C) In slices from rats that were ovariectomized and treated with estradiol to simulate proestrus morning, the results were similar to those obtained from rat slices exposed to BDNF. Adapted with permission from Scharfman HE et al (2007).58
Taken together, these studies suggest that estrogen might exert some of its actions on excitability by inducing BDNF. This hypothesis was tested using immunocytochemistry in sections of rats at different stages of the estrous cycle. First, it was confirmed that BDNF expression increased in the hippocampus during the rodent estrous cycle as estradiol levels increased. Animals were perfusion-fixed midmorning of proestrus, when serum estradiol peaks, and compared with animals perfused at the same time of day at times of the estrous cycle when serum estradiol is low (estrous and metestrous morning). BDNF protein levels were high on proestrous morning, especially in the mossy fibers. BDNF levels remained elevated through the morning of estrus and declined by the morning of metestrus (Fig. 4).57 These results were replicated using ovariectomized rats that were treated with estradiol to simulate proestrous morning.58 These data suggest that estrogen leads to prolonged increases in BDNF that outlast the serum rise in estradiol levels.
Fig 4.
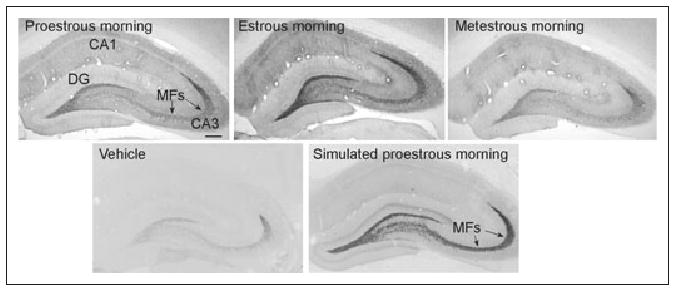
Brain-derived neurotrophic factor (BDNF) expression increases in hippocampus in response to estrogen. (Top) Animals were compared after perfusion either midmorning of proestrus, estrus, or metestrus, and BDNF levels were elevated in mossy fibers (MF) on proestrus and estrus. (Bottom) Another comparison was made between ovariectomized animals treated with vehicle or 3 injections of estradiol to simulate the preovulatory surge of estrogen in the rat (simulated proestrous morning). BDNF levels increased after estrogen, similar to those in the intact rat. Reprinted with permission from Scharfman HE et al (2003).57
To address the physiologic ramifications of the immunocytochemical data, hippocampal slices from proestrous and estrous females (ie, animals with high levels of mossy fiber BDNF) were compared with those from metestrous or ovariectomized rats (ie, animals with lower levels of mossy fiber BDNF). In addition, ovariectomized rats that were treated with estrogen to simulate the preovulatory surge were compared with those treated with vehicle. Mossy fiber responses of CA3 neurons in slices that were made from female rats at proestrous and estrous morning demonstrated increased excitability (Fig. 3). The increased excitability was similar to what had been reported for the effect of BDNF in area CA3 using male rats that had been perfused with recombinant BDNF51,55 or male transgenic mice that overexpressed BDNF.56 The increased excitability in area CA3 that was observed at proestrous and estrous morning was not detected in slices from female rats at metestrous morning or ovariectomized rats.57 Evoked responses in area CA1 also demonstrated increased excitability. Notably, when estradiol levels were raised above normal physiologic levels at proestrous morning, epileptiform activity occurred in area CA3 without any stimulation.58
In summary, the data suggest that the preovulatory estrogen surge in the rat is followed by a period of time when BDNF levels are elevated in the hippocampus, and excitability in the hippocampus is increased. If this were to occur in women during the analogous time of the ovarian cycle (ie, the periovulatory period), it might explain an increased incidence of seizures. Migraine incidence might increase because of heightened excitability and, in addition, elevated risk of spreading depression. Another reason that migraine incidence might increase could be the fact that BDNF levels are increased in the trigeminal ganglion; BDNF levels may contribute to the effects of calcitonin gene-related peptide in the trigeminal ganglion that promote migraine.59 In addition, others have shown that there are changes in the response to capsaicin in the trigeminal nucleus caudalis and dorsal horn of C1-C3 during the proestrous and estrous stages ofthe rat estrous cycle.60 These studies and others have led to the suggestion that BDNF may lead to an increased response to glutamate in the trigeminal system, and this could be a potential mechanism for changes in migraine that occur in response to ovarian hormones.11
As mentioned above, the rise in BDNF levels that is induced by estrogen appears to be long lasting (Fig. 4),57 suggesting that BDNF levels may remain elevated long after estrogen levels subside. This is potentially important because it would lead to the prediction that estrogen would increase BDNF levels during the perimenstrual period, as well as during the periovulatory period (Fig. 5). High levels of BDNF could explain the increase in incidence of seizures and migraine at these times of the ovarian cycle. The perimenstrual period might be a time of highest risk because declining progesterone levels may also increase excitability, in addition to the effects of BDNF (Fig. 5). Indeed, in catamenial epilepsy and migraine, more women appear to report changes in their seizures or migraine at the perimenstrual time of the cycle.6,61
Fig 5.
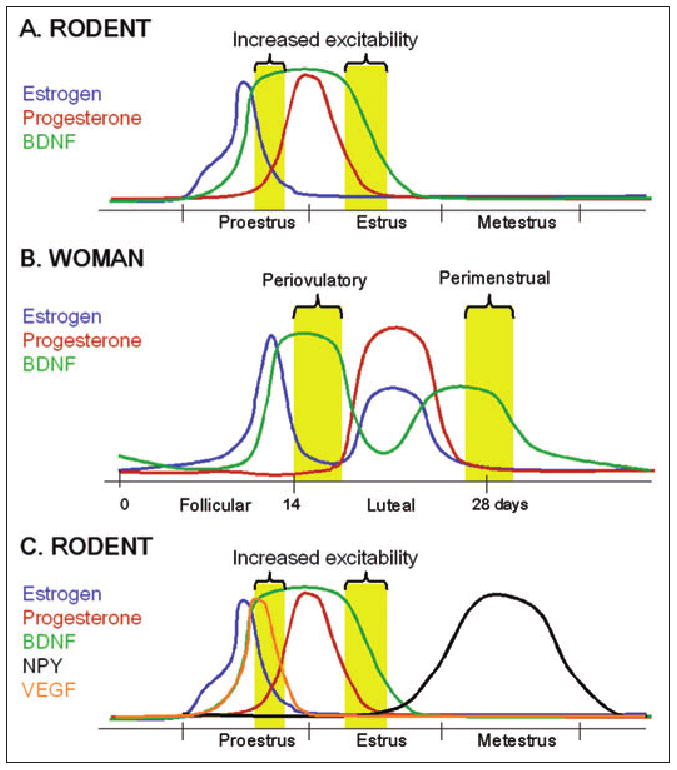
Modulation of excitability during the ovarian cycle by neuromodulators induced by estrogen. (A and B) A hypothesis for the changes in excitability and, possibly, migraine risk that occur during the ovarian cycle of the rodent (A) and woman (B). Increased excitability and migraine risk (yellow bars) may develop because brain-derived neurotrophic factor (BDNF; green) is induced by estrogen (blue; see text for further discussion and references). Progesterone (red) may dampen excitability induced by BDNF during times of the ovarian cycle when progesterone levels rise, such as during the luteal phase. It is notable that, at least in the rodent, the rise in BDNF levels that occurs in response to estrogen appears to be long lasting, because this would explain an increase in excitability just after the preovulatory estrogen surge and the mid-luteal rise in estrogen levels, when migraine risk appears to increase. (C) Levels of inhibitory modulators such as vascular endothelial growth factor (VEGF; orange) and neuropeptide Y (NPY; black) are also induced by estrogen (see text for discussion and references), leading to decreased excitability at specific times of the ovarian cycle. Together, this pattern of hormone and neuromodulator secretion and expression can account for the times of the ovarian cycle when migraine risk increases. For simplicity, the hypothesis is illustrated only for the rodent estrous cycle.
VEGF
VEGF is a growth factor that is known to control angiogenesis and vascular permeability in the peripheral vasculature. Figure 2 diagrams the known members of the VEGF family and their receptors and shows the potential signaling pathways activated by VEGF receptors.33,62,63
Although less is known about VEGF in the brain than in the peripheral vasculature, a very important action has been identified: VEGF is neuroprotective. VEGF protects motor neurons and has been suggested to play a critical neuroprotective role in slowing down the neuronal degenerative process in amyotrophic lateral sclerosis.64-66 VEGF also is neuroprotective in experimental ischemia67-69 and in models of seizure-induced neuronal damage.70
VEGF also inhibits synaptic transmission. This action appears to be common for the hippocampus and brainstem71,72 and appears to be mediated by VEGF receptors on glia.73 The effect on synaptic transmission may explain why VEGF blocks epileptiform activity in animals with recurrent seizures.71 Reducing glutamatergic transmission may also contribute to the neuroprotective effects of VEGF.73
In 2000, Hyder and colleagues reported an estrogen-sensitive response element on the gene for VEGF.74 Taken together with the previously discussed studies and the reports that estrogen is protective by interactions with glia75 and the vasculature,17 the neuroprotective effects of estrogen could be due to its induction of VEGF. Thus, estrogen may induce VEGF synthesis, and VEGF would then bind to VEGF receptors on glia. VEGF would presumably inhibit neurotransmission and possibly have other effects that would be protective. VEGF would also bind to the vasculature, leading to vasodilation. Therefore, estrogen-induced vasodilation17 may also be mediated by VEGF. If vasodilation mediated by VEGF does occur, then actions of VEGF might improve seizure control; one would predict, however, that migraine risk would increase because VEGF-induced vasodilation and consequent plasma protein extravasation would be expected to increase the risk of migraine.16
Consideration of the induction of BDNF and VEGF synthesis by estrogen leads to several questions. If estrogen induces both BDNF and VEGF, why would seizure susceptibility increase during the periovulatory period after estrogen levels rise? One might expect BDNF and VEGF effects to oppose one another with respect to seizures. A possible explanation is that BDNF exerts a dominant effect because it is elevated for a longer time than is VEGF. This would seem likely given that the time course of expression of BDNF seems prolonged, as previously discussed. In contrast, one might expect VEGF levels or the effects of VEGF to rapidly return to normal after its induction by estrogen, because it appears to be primarily bound to glial receptors,73 rather than residing within neurons or glia.
Another question relates to the reports that estrogen therapy improves migraine. How could this be the case if estrogen induces both BDNF and VEGF, and both BDNF and VEGF seem to promote migraine? One possibility is that estrogen administration would acutely increase levels of these growth factors, but, afterward, it would improve migraine because BDNF induces a potent vasoconstrictor, NPY. After a few days, therefore, estrogen therapy may provide relief from migraine because of increased levels of NPY.
NPY
NPY is a ligand for 5 receptors, with diverse expression in the CNS and periphery (Fig. 2). In the hippocampus, NPY has been shown to act on Y1 and Y2 receptors.76 Notably, NPY is synthesized in the same pathway that also produces BDNF, the mossy fiber pathway.76 In addition, NPY acts to inhibit glutamate release from mossy fibers.76 Therefore, NPY seems to be in an ideal position to antagonize effects of BDNF. Moreover, NPY is expressed in GABAergic neurons of the hippocampus, which innervate other glutamatergic afferents and inhibit glutamate release from those inputs.76,77 Therefore, NPY can inhibit other glutamatergic pathways that are potentiated by BDNF. Use of several complementary approaches, such as viral vector delivery, infusion, and transgenic mice, has shown that NPY has both anticonvulsant and neuroprotective effects.78-80
During the estrous cycle, the hippocampal interneurons that synthesize NPY increase their protein levels.81 It is likely that the same is true in the trigeminal ganglion because NPY mRNA levels are elevated in the trigeminal ganglion at estrus.82 Although protein content was not evaluated in the same experiments, one would predict that a rise in NPY protein levels would follow a rise in NPY mRNA levels, leading to elevated NPY protein levels at diestrus. Taken together, it appears that NPY protein levels are lowest during the times of the cycle when seizure and migraine risk is high. This may occur because estrogen induces BDNF on proestrus, BDNF then induces NPY, and NPY levels ultimately rise after that time, which would be late on estrus and during diestrus. Estrogen, therefore, may not normally induce NPY fast enough to antagonize the effects of BDNF and VEGF (Fig. 5). This hypothesis could explain why estrogen therapy for several days in a row would improve migraine. In addition, it may explain the efficacy of triptans in women with migraine because the vasoconstriction induced by these drugs would compensate for the lack of endogenous NPY during the periovulatory and perimenstrual time periods (Fig. 5).
Conclusions
To completely understand the effects of estrogen, it is suggested that estrogen–growth factor interactions need to be considered. In addition, the time course of growth factor induction in response to estrogen seems particularly important. For example, although estrogen levels surge and fall rapidly during the preovulatory period, it appears to initiate an increase in growth factor expression that continues long after estrogen levels subside. It is also important to consider that induction of one growth factor may not follow a similar time course to that of another growth factor. In the case of BDNF, protein elevation appears persistent; in the case of VEGF, however, protein elevation may be transient. Estrogen and BDNF also induce neuropeptide expression, such as NPY. NPY is potentially important because of its neuroprotective and anticonvulsant effects, and its influence throughout the periphery. We propose that a detailed understanding of the time-dependent induction of growth factors and peptides by estrogen will help explain changes in brain function during the ovarian cycle.
There is potential to use this framework to develop new therapeutic strategies for patients. Decreasing levels of BDNF or antagonists of trkB during the periovulatory or perimenstrual period may improve symptoms. Inducing NPY could improve seizure and migraine control. Ultimately, the receptors and signaling mechanisms associated with estrogen—BDNF, VEGF, and NPY—may provide new targets for drug development for epilepsy and migraine, and possibly for other clinical conditions as well.
Acknowledgments
Research supported by NIH NINDS 37562.
Abbreviations
- CNS
central nervous system
- BDNF
brain-derived neurotrophic factor
- VEGF
vascular endothelial growth factor
- NPY
neuropeptide Y
- ER
estrogen receptor
- GABAA
γ-aminobutyric acid A
- trk
tropomyosin receptor kinase
- trkB
tropomyosin receptor kinases B
Contributor Information
Helen E. Scharfman, Nathan Kline Institute for Psychiatric Research & New York University School of Medicine, Orangeburg, NY, USA.
Neil J. MacLusky, Department of Biomedical Sciences, University of Guelph, Guelph, ON, Canada.
References
- 1.Levin ER. Nuclear receptor versus plasma membrane oestrogen receptor. Novartis Found Symp. 2000;230:41–50. doi: 10.1002/0470870818.ch5. [DOI] [PubMed] [Google Scholar]
- 2.Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–376. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- 3.Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia. 2006;47:1423–1440. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knobil E, Neill JD. The Physiology of Reproduction. 2nd. New York, NY: Raven Press; 1994. [Google Scholar]
- 5.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: Prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 6.Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 7.Rogawski MA. Progesterone, neurosteroids, and the hormonal basis of catamenial epilepsy. Ann Neurol. 2003;53:288–291. doi: 10.1002/ana.10534. [DOI] [PubMed] [Google Scholar]
- 8.Reddy DS. Role of neurosteroids in catamenial epilepsy. Epilepsy Res. 2004;62:99–118. doi: 10.1016/j.eplepsyres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Martin V, Houle TT, Bean J. Mid-cycle migraines: Do they exist? Headache. 2007;47:788. Abstract S12. [Google Scholar]
- 10.Beckham JC, Krug LM, Penzien DB, et al. The relationship of ovarian steroids, headache activity and menstrual distress: A pilot study with female migraineurs. Headache. 1992;32:292–297. doi: 10.1111/j.1526-4610.1992.hed3206292.x. [DOI] [PubMed] [Google Scholar]
- 11.Martin VT, Behbehani M. Ovarian hormones and migraine headache: Understanding mechanisms and pathogenesis – part 2. Headache. 2006;46:365–386. doi: 10.1111/j.1526-4610.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 12.Ashkenazi A, Silberstein S. Menstrual migraine: A review of hormonal causes, prophylaxis and treatment. Expert Opin Pharmacother. 2007;8:1605–1613. doi: 10.1517/14656566.8.11.1605. [DOI] [PubMed] [Google Scholar]
- 13.Martin VT, Penzien DB, Houle TT, Andrew ME, Lofland KR. The predictive value of abbreviated migraine diagnostic criteria. Headache. 2005;45:1102–1112. doi: 10.1111/j.1526-4610.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 14.Stewart WF, Lipton RB, Chee E, Sawyer J, Silberstein SD. Menstrual cycle and headache in a population sample of migraineurs. Neurology. 2000;55:1517–1523. doi: 10.1212/wnl.55.10.1517. [DOI] [PubMed] [Google Scholar]
- 15.Velioglu SK, Ozmenoglu M. Migraine-related seizures in an epileptic population. Cephalalgia. 1999;19:797–801. doi: 10.1046/j.1468-2982.1999.1909797.x. [DOI] [PubMed] [Google Scholar]
- 16.Silberstein SD. Migraine pathophysiology and its clinical implications. Cephalalgia. 2004;24(Suppl 2):2–7. doi: 10.1111/j.1468-2982.2004.00892.x. [DOI] [PubMed] [Google Scholar]
- 17.Duckles SP, Krause DN. Cerebrovascular effects of oestrogen: Multiplicity of action. Clin Exp Pharmacol Physiol. 2007;34:801–808. doi: 10.1111/j.1440-1681.2007.04683.x. [DOI] [PubMed] [Google Scholar]
- 18.Godwin J, Hartman V, Grammer M, Crews D. Progesterone inhibits female-typical receptive behavior and decreases hypothalamic estrogen and progesterone receptor messenger ribonucleic acid levels in whiptail lizards (genus Cnemidophorus) Horm Behav. 1996;30:138–144. doi: 10.1006/hbeh.1996.0017. [DOI] [PubMed] [Google Scholar]
- 19.Bethea CL, Brown NA, Kohama SG. Steroid regulation of estrogen and progestin receptor messenger ribonucleic acid in monkey hypothalamus and pituitary. Endocrinology. 1996;137:4372–4383. doi: 10.1210/endo.137.10.8828498. [DOI] [PubMed] [Google Scholar]
- 20.Simerly RB, Carr AM, Zee MC, Lorang D. Ovarian steroid regulation of estrogen and progesterone receptor messenger ribonucleic acid in the anteroventral periventricular nucleus of the rat. J Neuroendocrinol. 1996;8:45–56. doi: 10.1111/j.1365-2826.1996.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 21.Brown TJ, MacLusky NJ. Progesterone modulation of estrogen receptors in microdissected regions of the rat hypothalamus. Mol Cell Neurosci. 1994;5:283–290. doi: 10.1006/mcne.1994.1033. [DOI] [PubMed] [Google Scholar]
- 22.Brann DS, Rao IM, Mahesh VB. Antagonism of estrogen-induced prolactin release by progesterone. Biol Reprod. 1988;39:1067–1073. doi: 10.1095/biolreprod39.5.1067. [DOI] [PubMed] [Google Scholar]
- 23.Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: Correlation with gamma-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- 24.Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABA(A) receptors: Focus on the alpha4 and delta subunits. Pharmacol Ther. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 26.Somerville BW. The role of progesterone in menstrual migraine. Neurology. 1971;21:853–859. doi: 10.1212/wnl.21.8.853. [DOI] [PubMed] [Google Scholar]
- 27.Somerville BW. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology. 1972;22:355–365. doi: 10.1212/wnl.22.4.355. [DOI] [PubMed] [Google Scholar]
- 28.MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A. Prevention of menstrual attacks of migraine: A double-blind placebo-controlled crossover study. Neurology. 2006;67:2159–2163. doi: 10.1212/01.wnl.0000249114.52802.55. [DOI] [PubMed] [Google Scholar]
- 29.de Lignières B, Vincens M, Mauvais-Jarvis P, Mas JL, Touboul PJ, Bousser MG. Prevention of menstrual migraine by percutaneous oestradiol. Br Med J (Clin Res Ed) 1986;293:1540. doi: 10.1136/bmj.293.6561.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sohrabji F, Miranda RC, Toran-Allerand CD. Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92:11110–11114. doi: 10.1073/pnas.92.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- 33.Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci. 2003;28:488–494. doi: 10.1016/S0968-0004(03)00193-2. [DOI] [PubMed] [Google Scholar]
- 34.Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci (Lond) 2006;110:167–173. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- 35.Bibel M, Barde YA. Neurotrophins: Key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- 36.Chao MV. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 37.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pezet S, McMahon SB. Neurotrophins: Mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 39.Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: On the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu B, Gottschalk W. Modulation of hippocampal synaptic transmission and plasticity by neurotrophins. Prog Brain Res. 2000;128:231–241. doi: 10.1016/S0079-6123(00)28020-5. [DOI] [PubMed] [Google Scholar]
- 41.Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scharfman H, Schwarcz R. Neuromodulation of seizures, epileptogenesis, and epilepsy. In: Engel J, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. New York, NY: Lippincott-Raven; 2007. pp. 289–306. [Google Scholar]
- 43.Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- 44.Allen SJ, Wilcock GK, Dawbarn D. Profound and selective loss of catalytic TrkB immunoreactivity in Alzheimer's disease. Biochem Biophys Res Commun. 1999;264:648–651. doi: 10.1006/bbrc.1999.1561. [DOI] [PubMed] [Google Scholar]
- 45.Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- 46.Matsushita S, Arai H, Matsui T, et al. Brain-derived neurotrophic factor gene polymorphisms and Alzheimer's disease. J Neural Transm. 2005;112:703–711. doi: 10.1007/s00702-004-0210-3. [DOI] [PubMed] [Google Scholar]
- 47.Altar CA. Neurotrophins and depression. Trends Pharmacol Sci. 1999;20:59–61. doi: 10.1016/s0165-6147(99)01309-7. [DOI] [PubMed] [Google Scholar]
- 48.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Fumagalli F, Racagni G, Riva MA. Shedding light into the role of BDNF in the pharmacotherapy of Parkinson's disease. Pharmacogenomics J. 2006;6:95–104. doi: 10.1038/sj.tpj.6500360. [DOI] [PubMed] [Google Scholar]
- 50.Scharfman HE. Brain-derived neurotrophic factor and epilepsy – a missing link? Epilepsy Curr. 2005;5:83–88. doi: 10.1111/j.1535-7511.2005.05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scharfman HE. Brain-derived neurotrophic factor (BDNF) and the dentate gyrus mossy fibers: Implications for epilepsy. In: Stanton PK, Bramham C, Scharfman HE, editors. Synaptic Plasticity and Trans-synaptic Signaling. New York, NY: Springer; 2005. pp. 201–220. [Google Scholar]
- 52.Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 53.Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: Role of secreted proteins tPA and BDNF. Ageing Res Rev. 2004;3:407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 55.Scharfman HE. Hyperexcitability in combined entorhinal/hippocampal slices of adult rat after exposure to brain-derived neurotrophic factor. J Neurophysiol. 1997;78:1082–1095. doi: 10.1152/jn.1997.78.2.1082. [DOI] [PubMed] [Google Scholar]
- 56.Croll SD, Suri C, Compton DL, et al. Brain-derived neurotrophic factor transgenic mice exhibit passive avoidance deficits, increased seizure severity and in vitro hyperexcitability in the hippocampus and entorhinal cortex. Neuroscience. 1999;93:1491–1506. doi: 10.1016/s0306-4522(99)00296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: A potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23:11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scharfman HE, Hintz TM, Gomez J, et al. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. Eur J Neurosci. 2007;26:2595–2612. doi: 10.1111/j.1460-9568.2007.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buldyrev I, Tanner NM, Hsieh HY, Dodd EG, Nguyen LT, Balkowiec A. Calcitonin gene-related peptide enhances release of native brain-derived neurotrophic factor from trigeminal ganglion neurons. J Neurochem. 2006;99:1338–1350. doi: 10.1111/j.1471-4159.2006.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin VT, Lee J, Behbehani MM. Sensitization of the trigeminal sensory system during different stages of the rat estrous cycle: Implications for menstrual migraine. Headache. 2007;47:552–563. doi: 10.1111/j.1526-4610.2007.00714.x. [DOI] [PubMed] [Google Scholar]
- 61.MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A. Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology. 2006;67:2154–2158. doi: 10.1212/01.wnl.0000233888.18228.19. [DOI] [PubMed] [Google Scholar]
- 62.Leal SM, Neckameyer WS. Talking the talk: The role of VEGF proteins in cell signaling. Trends Endocrinol Metab. 2002;13:319–320. doi: 10.1016/s1043-2760(02)00675-6. [DOI] [PubMed] [Google Scholar]
- 63.Neufeld G, Kessler O, Herzog Y. The interaction of neuropilin-1 and neuropilin-2 with tyrosine-kinase receptors for VEGF. Adv Exp Med Biol. 2002;515:81–90. doi: 10.1007/978-1-4615-0119-0_7. [DOI] [PubMed] [Google Scholar]
- 64.Storkebaum E, Lambrechts D, Dewerchin M, et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8:85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- 65.Lambrechts D, Storkebaum E, Carmeliet P. VEGF necessary to prevent motoneuron degeneration, sufficient to treat ALS? Trends Mol Med. 2004;10:275–282. doi: 10.1016/j.molmed.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 66.Azzouz M, Ralph GS, Storkebaum E, et al. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- 67.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: Direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci USA. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hayashi T, Abe K, Itoyama Y. Reduction of ischemic damage by application of vascular endothelial growth factor in rat brain after transient ischemia. J Cereb Blood Flow Metab. 1998;18:887–895. doi: 10.1097/00004647-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 69.Sun Y, Jin K, Xie L, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nicoletti JN, Shah SK, McCloskey DP, Elkady A, Scharfman HE, Croll SD. Vascular endothelial growth factor is up-regulated after status epilepticus and protects against seizure-induced neuronal loss in hippocampus. Neuroscience. 2008;151:232–241. doi: 10.1061/j.neuroscience.2007.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCloskey DP, Croll SD, Scharfman HE. Depression of synaptic transmission by vascular endothelial growth factor in adult rat hippocampus and evidence for increased efficacy after chronic seizures. J Neurosci. 2005;25:8889–8897. doi: 10.1523/JNEUROSCI.2577-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McClosky DP, Hintz TM, Scharfman HE. Modulation of vascular endothelial growth factor (VEGF) expression in motor neurons and its electrophysiological effects. Brain Res Bull. 2008;76:36–44. doi: 10.1016/j.brainresbull.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Croll SD, McCloskey DP, Nicoletti JN, Scharfman HE. VEGF as a novel seizure therapeutic: Killing two birds with one stone. In: Binder DK, Scharfman HE, editors. Growth Factors and Epilepsy. New York, NY: Nova Sciences Publishers; 2005. pp. 145–162. [Google Scholar]
- 74.Hyder SM, Nawaz Z, Chiappetta C, Stancel GM. Identification of functional estrogen response elements in the gene coding for the potent angiogenic factor vascular endothelial growth factor. Cancer Res. 2000;60:3183–3190. [PubMed] [Google Scholar]
- 75.Dhandapani KM, Brann DW. Role of astrocytes in estrogen-mediated neuroprotection. Exp Gerontol. 2007;42:70–75. doi: 10.1016/j.exger.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 76.Sperk G, Hamilton T, Colmers WF. Neuropeptide Y in the dentate gyrus. Prog Brain Res. 2007;163:285–297. doi: 10.1016/S0079-6123(07)63017-9. [DOI] [PubMed] [Google Scholar]
- 77.Houser CR. Interneurons of the dentate gyrus: An overview of cell types, terminal fields and neurochemical identity. Prog Brain Res. 2007;163:217–232. doi: 10.1016/S0079-6123(07)63013-1. [DOI] [PubMed] [Google Scholar]
- 78.Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: Emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- 79.Silva AP, Xapelli S, Grouzmann E, Cavadas C. The putative neuroprotective role of neuropeptide Y in the central nervous system. Curr Drug Targets CNS Neurol Disord. 2005;4:331–347. doi: 10.2174/1568007054546153. [DOI] [PubMed] [Google Scholar]
- 80.Veliskova J, Velisek L. Beta-estradiol increases dentate gyrus inhibition in female rats via augmentation of hilar neuropeptide Y. J Neurosci. 2007;27:6054–6063. doi: 10.1523/JNEUROSCI.0366-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: Complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol. 2006;27:415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Puri V, Cui L, Liverman CS, et al. Ovarian steroids regulate neuropeptides in the trigeminal ganglion. Neuropeptides. 2005;39:409–417. doi: 10.1016/j.npep.2005.04.002. [DOI] [PubMed] [Google Scholar]


