Abstract
Rationale
The transient behavioral deficit produced in rodents by typical learned helplessness (LH) procedures limits the utility of LH in identifying the therapeutic mechanisms associated with chronic antidepressant administration. In addition, LH procedures do not differentiate between different antidepressant classes as observed in the forced swim test.
Objectives
To produce both a long lasting and antidepressant reversible behavioral deficit in a modified LH procedure that administers inescapable shock (IS) in the same operant chamber used for shuttle box escape testing.
Results
A single IS session produced a robust increase in the number of escape failures (FR-2 escape contingency) that endured for at least 21 days. This escape deficit was reversed by desipramine (24 mg/kg/day, 6 days) at the first shuttle box session. Fluoxetine (5 mg/kg/day, 6 and 21 days) improved escape performance only after repeated test sessions. In contrast, fluoxetine (5 mg/kg/day, 21 days) completely reversed the first shuttle box test escape deficit induced by exposure to a chronic unpredictable stress procedure devoid of shocks or exposure to operant chambers. These differential drug effects may be due to the presence or absence of contextual cues during escape testing. Repeated re-exposure to the IS context enhanced the FR-2 escape deficit.
Conclusions
These data suggest that performing escape testing and IS in the same environment improves the preclinical modeling of the time-dependency and behavioral pattern of antidepressant response observed clinically. Additionally, contextual information associated with the IS environment modulates escape performance and may interact differentially with discrete antidepressant classes.
Keywords: Depression, Animal model, Uncontrollable stress, Inescapable shock, Learned helplessness, Shuttle box, SSRI, Desipramine, Contextual fear
Introduction
Progress in characterizing the neurobiology of stress-related depressive disorders has relied primarily on the use of animal models. Most of these assess behavioral changes after exposing animals to aversive experience and collectively, constitute a general experimental approach that provides simple simulations of the complex conditions promoting affective illness in humans (Nestler et al. 2002; Willner 1991). For the purposes of drug development and the identification of neurobiological changes underlying depressive disorders, a wide variety of stress and testing conditions have been successfully used to address specific experimental questions. While these models have improved our understanding of cellular and behavioral mechanisms involved in stress and antidepressant action, a cohesive, neurobiologically based understanding of the mechanisms of antidepressant activity has not been developed.
Although chemical antidepressants acutely increase extrasynaptic levels of monoamines, a clinical response is not typically observed until weeks to months later, suggesting that downstream biological events are more relevant effectors of antidepressant efficacy. Commonly used preclinical procedures, such as learned helplessness (LH) and the forced swim test (FST), generally fail to model this ‘therapeutic lag’ and may not be suitable for characterizing the delayed molecular and cellular alterations in neural plasticity associated with chronic drug treatment (Frazer and Morilak 2005). Consequently, reliable experimental procedures modeling the time-dependency of antidepressant action are needed (Schmidt and Duman 2007).
Furthermore, animal models that discriminate between pharmacological classes of antidepressants may allow for the identification of therapeutic mechanisms associated with different neurotransmitter systems (Cryan et al. 2005). For example, in depressed subjects, treatment with drugs that target either serotonergic or noradrenergic neurotransmission may be equally efficacious at distal time points, but they differ in the initial response time and in the pattern of behavioral improvement (Katz et al. 2004). Likewise, in the FST, serotonergic and noradrenergic drugs both decrease the primary behavioral outcome indicative of antidepressant efficacy (immobility), but they also have independent effects on different components of escape behavior (swimming and climbing) (Detke et al. 1995; Lucki 1997). However, when only acute effects of drugs are assessed, the neural changes identified may reflect only the earliest events in a cascade of neurobiological changes responsible for the therapeutic effect. While the differential pattern of behavioral effects in the FST discussed above have also been observed after chronic, but not acute, antidepressant administration when using lower doses than employed in acute studies (Cryan et al. 2005; Detke et al. 1997) it remains unclear whether chronic drug administration is actually a requirement for these behavioral effects (Antelman et al. 1997; Kusmider et al. 2006). Consequently, additional models that reproduce both the pattern of behavioral changes and the temporal course observed clinically may allow for improved characterization of the therapeutically relevant neuroplastic changes associated with chronic exposure to distinct pharmacological classes (Katz et al. 2006).
To this experimental end, the use of an uncontrollable stress-shuttle box escape procedure in which the shuttle box chamber and stress environment share contextual cues may be of utility. Under these conditions, the escape deficit induced by a single uncontrollable stress exposure can last for up to 28 days in contrast to the 48-h deficit observed when IS and shuttle box escape testing are performed in different contexts (Hunziker and Dos Santos 2007; Maier and Watkins 2005; Zazpe et al. 2007). These differences in the duration of the escape deficit may be due to contextual fear processing. Indeed, brief, periodic re-exposures of experimental animals to the IS context can extend the duration of a shuttle box escape deficit from 2 to 46 days, and perhaps indefinitely (Maier 2001). Collectively, these findings suggest that contextual fear information, in addition to the well-established effect of stressor controllability (Maier and Watkins 2005), is associated with the expression of deficits in escape behavior.
The effects of chronic antidepressant treatment on the persistent escape deficit observed when the IS apparatus and shuttle box share contextual cues have not been systematically examined. The primary aim of this paper was to examine whether chronic antidepressant treatment could reverse the persistent shuttle box escape deficit produced when IS and shuttle box testing are performed in the same environment. After establishing experimental parameters that produced an escape deficit lasting at least 3 weeks after a single IS session, the effect of chronic antidepressant treatment on shuttle box performance was examined. To assess whether the experimental endpoint in our IS-shuttle box procedure was pharmacologically dissociable, we compared the behavioral effect of desipramine (DMI), a tricyclic antidepressant with selectivity for blocking norepinephrine reuptake, with that of the serotonin-selective reuptake inhibitors (SSRI’s) fluoxetine (FLX) and citalopram (CTP). In addition, because it has been previously shown that antidepressants improve shuttle box escape performance across repeated test sessions (Martin and Puech 1996; Martin et al. 1990; Tordera et al. 2002), we examined whether this effect would be extended to our IS-shuttle box procedure. Finally, the effect of chronic FLX treatment on shuttle box performance was examined in groups of animals that either received repeated re-exposures to the original IS context or exposure to a chronic unpredictable stress procedure (CUS) designed to minimize generalization of contextually conditioned fear cues during shuttle box escape testing.
Material and methods
Subjects
All experiments were performed on adult male Sprague-Dawley rats (Charles River, MA, USA) that weighed 175–225 g upon arrival to the animal colony and 300–370 g at the end of each experiment. Animals were allowed 7–10 days to habituate to housing conditions before experiments began. Animals were pair-housed in suspended, wire bottom cages under a 12/12-h light–dark cycle with continuous access to food and water in a temperature (25°C) and humidity controlled environment. The provision of food required sliding open the cages to refill stainless steel slotted troughs. Routine cage maintenance consisted of removing trays that collected debris filtering through the cage floor. Therefore, animals were rarely handled at times other than those of direct involvement with various components of experimental procedures. For experimental groups with an even number sample size, each cage contained a randomly assigned pair of animals from the same treatment group. For groups with odd number sample sizes, one animal within each drug treatment group was paired with their respective control. The animals subjected to chronic unpredictable stress were housed in these same conditions when not subjected to the various stressors of the CUS protocol. All procedures followed Yale University care and use of laboratory animal guidelines.
Drug preparation
Desipramine hydrochloride (Sigma, St. Louis, MI, USA), fluoxetine hydrochloride (Eli Lilly, Indanapolis, IN, USA) and citalopram hydrobromide (gift from Eli Lilly, Indianapolis, IN, USA) were dissolved in 0.9% physiological saline and injected intraperitoneally (i.p.). All drug solutions were prepared daily and doses were calculated as mg/kg salt. Solubility was an issue only when DMI needed warming after storage at 4°C. The dosage of DMI was based on previous studies demonstrating reversal of shock-induced shuttle box escape deficits after injections of DMI at 24 mg/kg/day (Martin et al. 1986; Martin and Puech 1996). The dosage of FLX was based on studies demonstrating a reversal of shuttle box escape deficits after injections of FLX at 5 mg/kg/day that were induced by either IS (Chen et al. 2006) or exposure to CUS (Gambarana et al. 2001). The doses of CTP were based on previous studies demonstrating a reversal of shock-induced shuttle box escape deficits by treatment with injections of CTP at 1 mg/kg/day (Martin et al. 1990), decreased immobility in the FST after injections of CTP at 5 and 10 mg/kg/day (Overstreet et al. 2004) and reversal of CUS-induced decreases in sucrose consumption by treatment with CTP injections at 10 mg/kg/day (Papp et al. 2002).
Inescapable shock (IS)
The procedure used in all IS experiments was performed in one chamber of custom built, two-chambered shuttle boxes (Med Associates, VT, USA) with the use of right and left sides counterbalanced within each experiment. The shuttle boxes were situated in sound-attenuating boxes equipped with a fan for ventilation and noise masking. Each chamber consisted of plexiglass walls and a metal grid floor with a grid spacing of 1.5 cm. Animals were allowed to move freely over the grid floor, but prevented from rearing and flipping by hinged plexiglass panels that created a ceiling 10 cm above the grid floor. After a 5-min habituation, animals received 60 unsignaled, randomized foot shocks at an intensity of 0.8 mA. The average duration of each shock was 15 s (range, 5–25 s) with an average inter-trial interval of 45 s (range, 30–60 s). Animals were continuously observed to ensure the absence of escape behavior (i.e., flipping) and to ensure correct system operation during the IS trials.
Escapable shock (ES)
The ES procedure replicated the conditions used for IS except for the following modification. In each shock trial, the mechanical gate separating the two halves of the shuttle box opened 5 s prior to shock onset and remained open for the duration of the shock (30 s). A single crossing event terminated the foot shock in each ES trial.
Shuttle box testing
The shuttle box sessions were run by a PC computer with custom software developed for the system (Med Associates, VT, USA). At the start of each shuttle box session, animals were exposed to a 5-min habituation period in the same chamber where IS or ES was applied. This was followed by 30 escape trials in which the gate separating the two halves of the shuttle box opened 5 s prior to shock onset followed by randomized foot shocks delivered at an intensity of 0.65 mA. The average inter-trial interval was 60 s (range, 20–100 s). In the FR-1 condition, a single crossing terminated the footshock while under the FR-2 condition, the first five escape trials required one crossing, and the remaining 25 trials, two crossings, for shock termination. Crosses were automatically scored by the PC whenever a micro-switch was activated by tilting of the pivoted grid floor after a crossing event.
Time course and drug treaments
In the present study several experimental designs and time courses were utilized. For clarity, each figure illustrates both the time course of and data acquired from each experiment.
Experiment 1
To characterize the effects of crossing condition on shuttle box performance, animals were first subjected to a single IS session, randomly divided into two groups, and tested 24 h later under either an FR-1 or FR-2 escape contingency (n=6 per group).
Experiment 2
To characterize the effect of controllability on shuttle box performance, separate groups of animals received either a single IS or ES session, and were then tested 24 h later under an FR-2 contingency (n=6 per group). The ES and IS animals were not yoked during the shock sessions.
Experiment 3
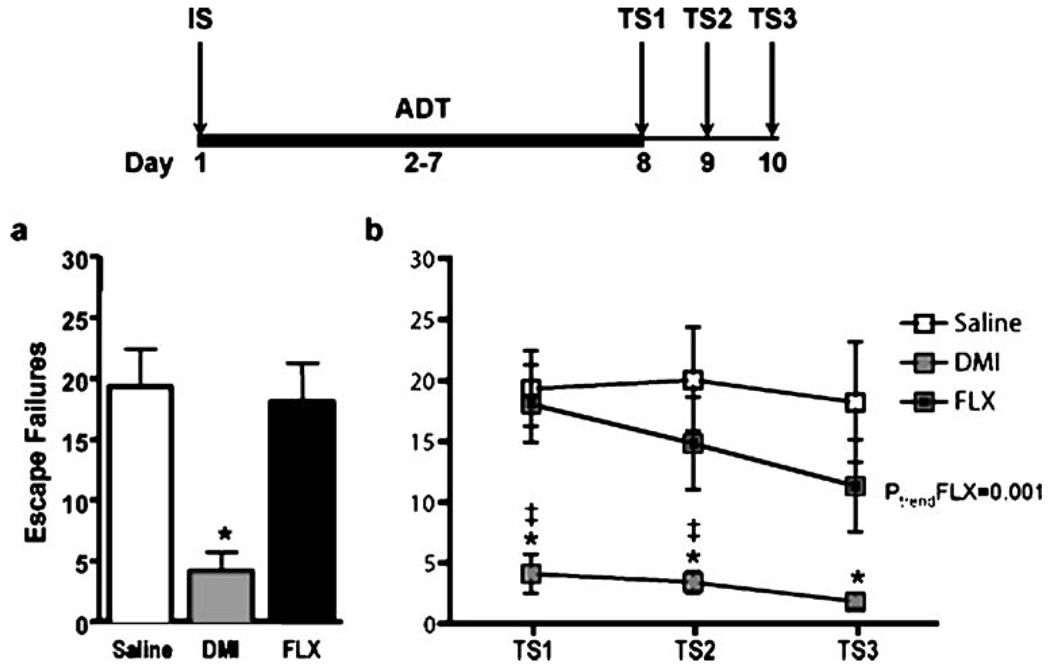
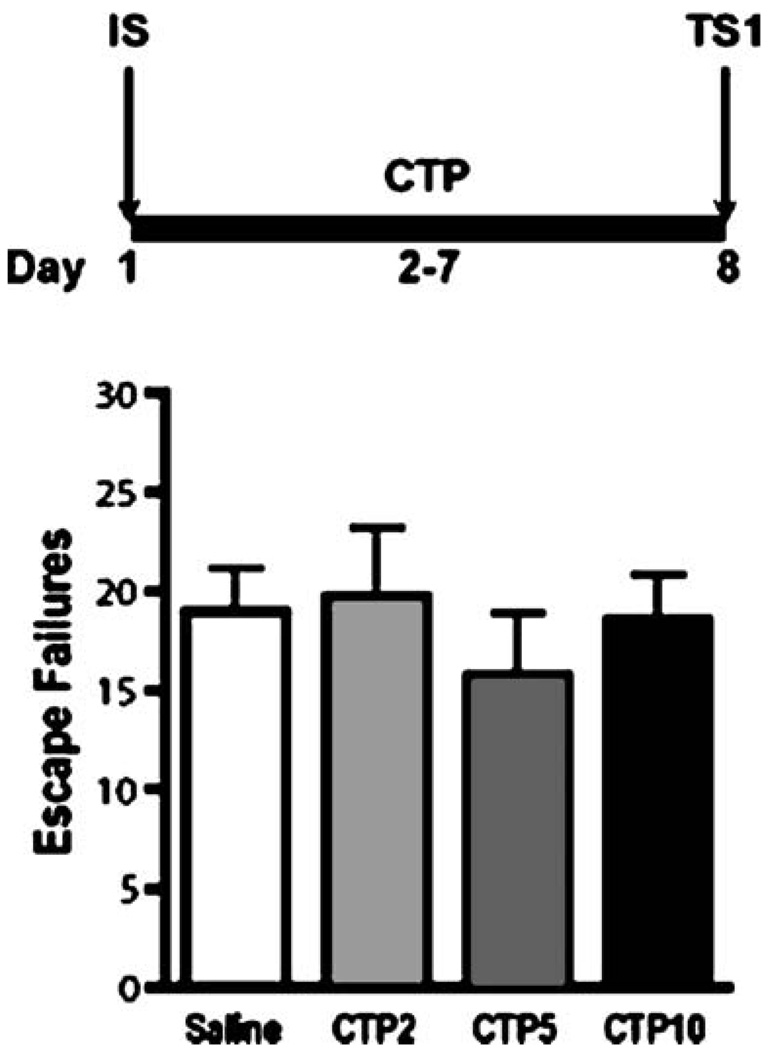
To compare the effects of different classes of chemical antidepressants on shuttle box escape deficits induced by IS, animals were subjected to a single IS session and randomly assigned to three treatment groups (n=7–8 per group). Animals in the SAL and DMI groups received six consecutive days of either of SAL (twice daily) or DMI (12 mg/kg/injection, twice daily). Animals in the FLX group received morning injections of FLX (5 mg/kg/ injection) and evening injections of SAL starting 24 h after the IS session. All animals were subsequently tested under an FR-2 escape contingency for three consecutive days starting 24 h after the last injection of FLX or 16 h after DMI and SAL (Fig. 2). In the CTP experiment, animals were subjected to a single IS session and randomly assigned to four groups receiving six consecutive, twice daily, injections of either SAL or of three different doses of CTP (1, 2.5 and 5 mg/kg/injection). Injections began 24 h after the IS session and shuttle box testing began 16 h after the last dose of SAL or CTP was administered (Fig. 3).
Fig. 2.
Effect of controllability on shuttle box performance. Data are expressed as group mean escape failures±SEM (n=6 per group). Animals exposed to IS had more FR-2 escape failures that ES-exposed animals. *p<0.05
Fig. 3.
FR-2 shuttle box deficits are pharmacologically dissociable. The bold line represents the period of drug administration after IS. TS test session. Data are expressed as group mean escape failures±SEM (n=7–8 per group) a DMI treatment (6 days, 24 mg/kg/day) resulted in significantly fewer escape failures than SAL and FLX (5 mg/kg/day) treatment at the first test session. b DMI treatment resulted in improved escape performance at each test session compared to both SAL and FLX treatment except test session 3 where DMI was not significantly different from FLX. A linear trend for improved escape performance across test sessions was observed in the FLX, but not in DMI- or SAL-treated animals. *p<0.01 compared to SAL, ‡p<0.01 compared to FLX, ptrend<0.001
Experiment 4
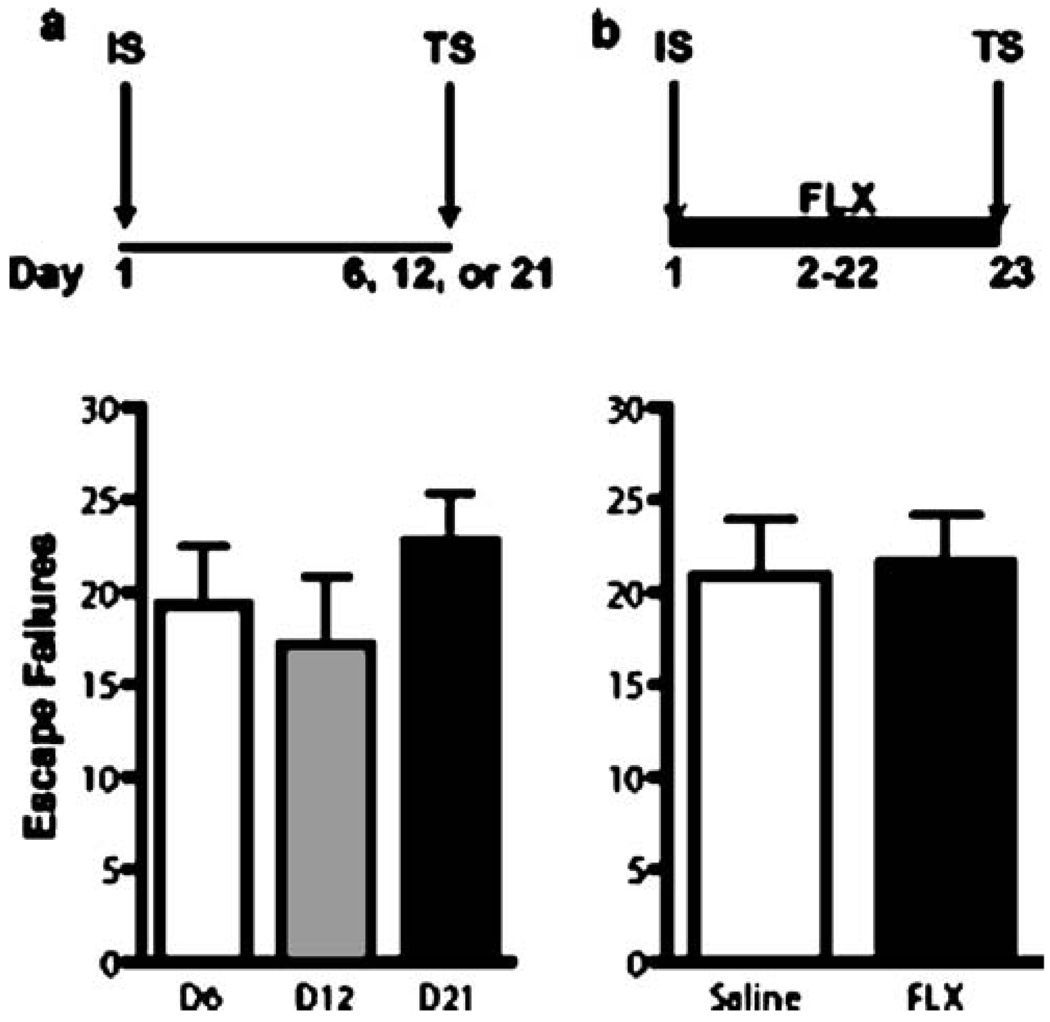
To assess the duration of the shuttle box escape deficit induced by a single IS session, rats were randomly assigned to three groups that were tested at either 6, 12 or 21 days after the initial IS session (n=7–8 per group, Fig. 4a). After IS, animals were returned to their home cage until shuttle box testing. Additional handling after IS was limited to a few instances when animals were briefly handled to clean excessively soiled cages.
Fig. 4.
Dose-response of citalopram (CTP) at first shuttle box test. The bold line represents the period of drug administration after IS. Data are expressed as group mean escape failures±SEM (n=7–15 per group). No significant differences were detected between groups treated with SAL or CTP (2, 5, or 10 mg/kg/day)
Experiment 5
To examine whether the escape deficit present at 21 days could be reversed by chronic FLX treatment, rats were subjected to a single IS session and randomly assigned to two treatment groups (n=7–8 per group). Rats received 21 days of once daily injections of either SAL or FLX (5 mg/kg/injection) starting 24 h after the IS session. Shuttle box escape testing was performed in the morning, 24 h after the last injection (Fig. 4b).
Experiment 6
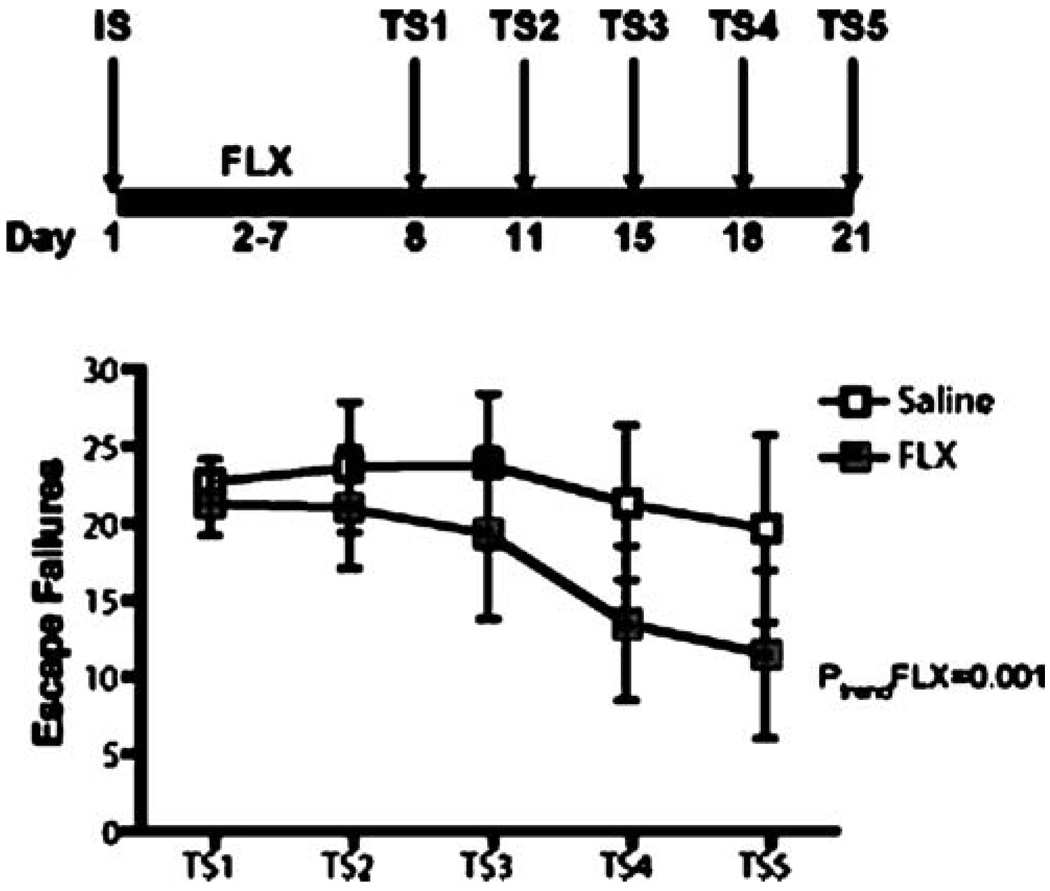
To determine whether chronic treatment with FLX influences shuttle box performance in animals subjected to repeated shuttle box test sessions, rats were first subjected to a single IS session, then randomly assigned to two treatment groups that received daily injections of either SAL or FLX (5 mg/kg/injection) for 21 consecutive days (n=7–8 per group). Both groups of animals were exposed to shuttle box escape testing every third day starting after 6 days of drug treatment for a total of five shuttle box test sessions (Fig. 5). On testing days, injections were given immediately after each shuttle box session.
Fig. 5.
The FR-2 escape deficit induced by a single IS session is long lasting and not reversible by chronic FLX treatment. The bold line represents the period of drug administration after IS. Data are expressed as group mean escape failures±SEM (n=7–8 per group). a The number of escape failures did not differ between independent groups tested at 6, 12, or 21 days after IS. b The escape performance of animals administered FLX (21 days, 5 mg/kg/day) or SAL after a single IS session were not significantly different
Experiment 7
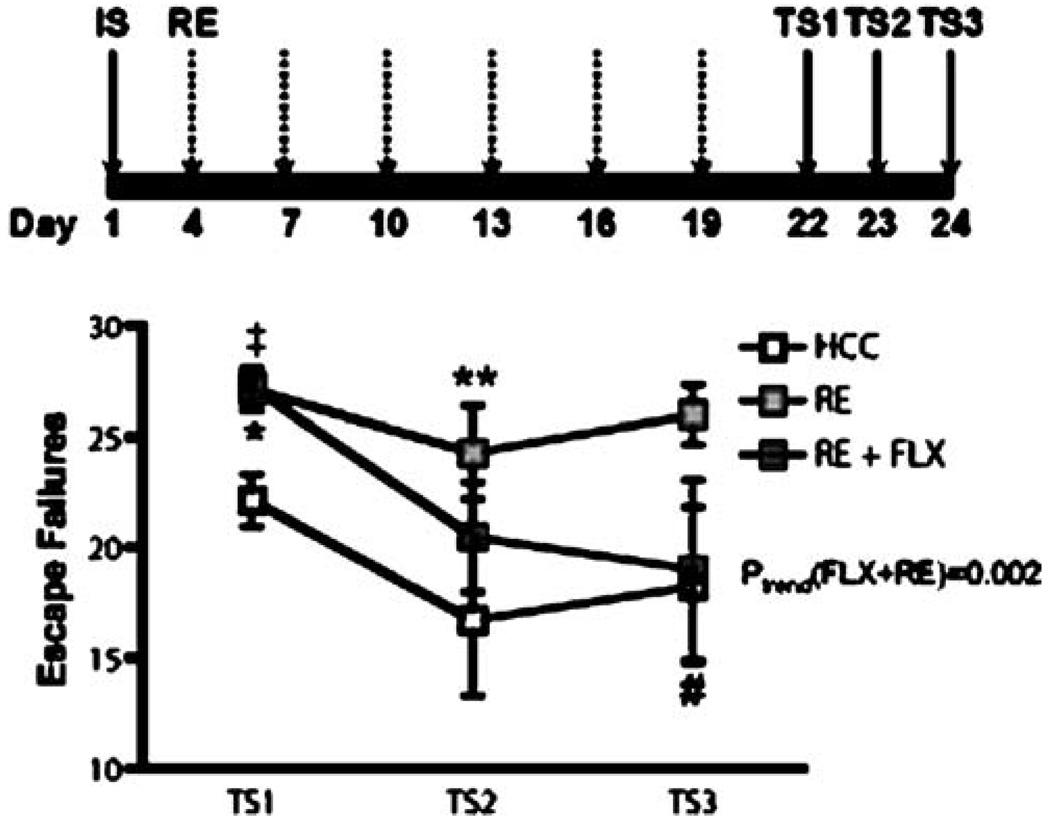
To examine the effect of periodic re-exposure to the IS context on the FR-2 escape deficit and to look for a re-exposure×drug interaction, animals were subjected to a single IS session and randomly assigned to three groups (n=7–8 per group): a control group which received daily injections of SAL for 21 days, but no re-exposure to the IS environment (controls), a re-exposure group and a re-exposure + FLX group. The re-exposure and re-exposure + FLX groups were subjected to a 10-min re-exposure to the same chamber used for IS every third day after the IS session for a total of six re-exposures. On re-exposure days, injections of either SAL or FLX (5 mg/kg/injection) were administered immediately after removal from the IS context. Shuttle box escape testing was then performed for three consecutive days starting 24 h after the last of 21 daily injections (Fig. 6). All injections were given immediately after the shuttle box session on test days.
Fig. 6.
Chronic FLX treatment improved escape performance across repeated test sessions. The bold line represents the period of drug administration after IS. Data are expressed as group mean escape failures±SEM. (n=7–8 per group). After a single IS session, rats were treated with FLX (21 days, 5 mg/kg/day). Rats received their first test session after 6 days of treatment and were re-tested every third day (five sessions; (TS) 1–5). A significant improvement in escape performance across test sessions was detected in FLX-, but not in SAL-treated animals. ptrend<0.001
Experiment 8
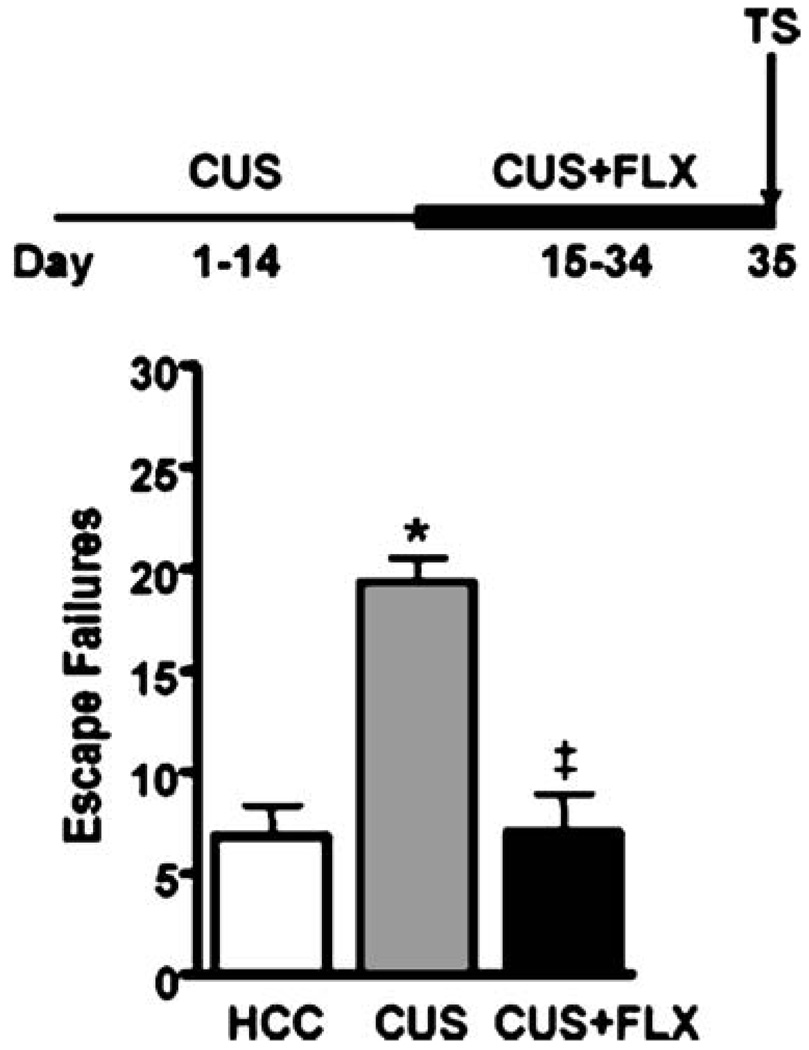
To examine whether the exposure of animals to a chronic unpredictable stress procedure would induce escape deficits in our FR-2 shuttle box escape test, and to examine whether any escape deficit would be reversed by chronic FLX treatment, animals were randomly assigned to three treatment groups (n=5–6 per group). Two animal groups were subjected to the exact same CUS procedure described previously (Banasr et al. 2007). Briefly, rats were exposed to a variable sequence of 12 different stressors, two per day, for 35 days. The stressors included: 1-h cold exposure at 4°C, swim stress at 18°C for 10 min, 1-h cage rotation, overnight social isolation or social crowding (six per cage), overnight food and water deprivation, light–dark cycle interruptions (light on during dark cycle, light off during light cycle and stroboscope overnight), and overnight exposures to wet bedding, odor, and cage tilt. The control group consisted of animals that remained in their home cage for 35 days except for daily handling during SAL injections (controls). CUS treated animals received 21 consecutive days of FLX (5 mg/kg/day) or SAL injections during the last 21 days of CUS. Shuttle box testing began 24 h after the last drug injection (Fig. 7).
Statistics
The primary outcome measure for all experiments was escape failures in the shuttle box escape task. For experiments with two groups, the primary analysis consisted of an unpaired Student’s t-test. Experiments with three or more groups were subjected to one-way analysis of variance (ANOVA), followed by the post hoc Tukey’s multiple comparison test to elucidate patterns of group differences. In experiments with repeated shuttle box sessions, the primary analysis consisted of a 2×5 or 3×3 repeated-measures ANOVA with drug treatment as the between subject factor and test session as the repeated measure. When appropriate, orthogonal contrasts assessed specific group differences. A secondary analysis consisted of linear trend assessments across shuttle box test sessions.
Results
Characterization of the shuttle box escape task
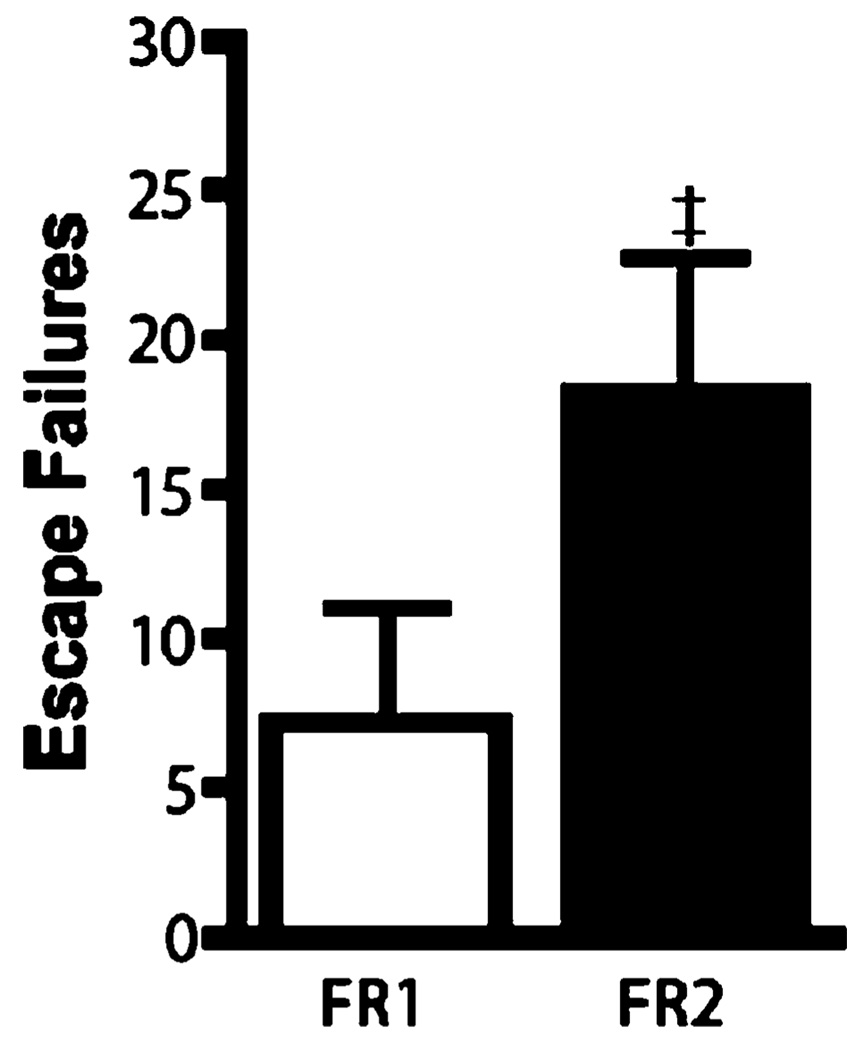
Because the primary purpose of experiments 1 and 2 was to validate our behavioral procedure by reproducing findings of increased interference with escape performance under conditions of stressor uncontrollability and a FR-2 escape contingency, small sample sizes were utilized. As shown in Fig. 1, animals subjected to a single IS session and tested 24 h later had approximately three times more escape failures under an FR-2, as compared to an FR-1 escape contingency. The lack of a significant effect (t=1.83, df= 10, p=0.097) is likely due to insufficient statistical power from the use of small sample sizes. As shown in Fig. 2, animals subjected to IS showed approximately two times more escape failures compared to animals having the option to escape foot shocks by crossing through the gate during the initial shock session (t=3.03, df=10, p=0.013). Collectively, these experiments demonstrated that performing IS and FR-2 shuttle box escape testing in the same apparatus reliably induces a high number of escape failures. Consequently, all subsequent experiments examining the effects of antidepressants on escape performance after IS used a combination of a single IS session and an FR-2 shuttle box escape task.
Fig. 1.
Effect of operant response on shuttle box performance. Data are expressed as group mean escape failures±SEM (n=6 per group). A trend toward a higher number of escape failures was observed in animals under the FR-2 condition ‡p=0.097
Effect of antidepressants on FR-2 shuttle box escape deficits
To determine if antidepressants could reverse the shuttle box escape deficit induced by a single IS session, and to compare the effects of two different pharmacological classes of antidepressants, animals were administered SAL, DMI (24 mg/kg/day) or FLX (5 mg/kg/day) for 6 days after IS, and subsequently subjected to a shuttle box test session (experiment 3). As shown in Fig. 3a, DMI treatment resulted in a significant 75% decrease in escape failures compared to animals treated with either SAL or FLX (F(2,20)=8.7, p<0.01). Because previous experiments have shown that escape performance after IS improves with repeated shuttle box testing (Martin and Puech 1996; Martin et al. 1990; Tordera et al. 2002), we also examined the effects of DMI and FLX on escape performance in two additional shuttle box test sessions. As shown in Fig. 3b, a 3×3 repeated-measures ANOVA of escape failures detected main effects of treatment (F(2,20)=6.8, p=0.006) and test session (F(2,20)=3.58, p=0.037). A treatment×test session interaction was not observed (F(4,20)=0.96, p=0.44). Orthogonal contrasts between groups at each test session demonstrated that the DMI group performed better than both SAL and FLX treated groups at each session except test session 3, where the escape performance of the DMI group did not differ from FLX (test session 1: DMI vs. saline, p<0.01, DMI vs. FLX, p<0.01; test session 2: DMI vs. saline, p<0.01, DMI vs. FLX, p<0.01; test session 3: DMI vs. saline, p<0.01, DMI vs. FLX, p>0.05). A secondary analysis of linear trends detected a highly significant decrease in escape failures in the FLX group across test sessions (F(1,15)=15.7, p<0.01). A linear trend across test sessions was not detected for the SAL (F(1,15)= 0.14, p=0.7) or DMI (F(1,13)=2.73, p=0.12) groups. All animals gained weight during this experiment. A non-significant decrease in mean weight gain in DMI-treated animals, as compared to control and SSRI-treated animals, was observed (data not shown).
To determine if the differential effects of DMI and FLX on FR-2 escape contingency deficits was due to their different pharmacological actions, the behavioral effect of an additional SSRI was also examined. As shown in Fig. 4, the escape performance of independent groups of animals receiving three different doses of CTP (2, 5, or 10 mg/kg/day) for 6 days after a single IS session did not differ form SAL controls (F(3,35)=0.36, p=0.8). Collectively, these findings suggest that a short course of SSRI treatment is unable to reverse the IS-induced escape deficit in our shuttle box test under an FR-2 escape contingency.
Chronic FLX treatment and FR-2 escape contingency deficits
To exclude the possibility that the absence of a FLX effect after 6 days of treatment was not simply the result of a delayed effect compared to the time course of DMI’s effect under our experimental conditions, we examined the effect of 21 days of FLX treatment on shuttle box escape performance (experiment 4 and 5). This was possible because we first established that the escape deficit after a single IS session endured for at least 21 days. As shown in Fig. 5a, the mean number of escape failures did not differ between independent groups of animals tested either 6, 12, or 21 days after IS (F(2,20)=0.81, p=0.5). Similar to the absence of an effect after 6 days of FLX treatment, the escape performance of animals receiving FLX for 21 days after a single IS session was not significantly different from SAL-treated controls (Fig. 4b) (t=0.19, df=14, p=0.9). A nonsignificant decrease in mean weight gain in FLX-treated animals was observed (data not shown). To further characterize our initial finding of a FLX-induced improvement in escape performance after exposure to repeated shuttle box test sessions, we performed an additional experiment examining the behavioral effect of a more prolonged course of FLX treatment across repeated shuttle box sessions (test session 1–5; experiment 6). A 2 × 5 repeated-measures ANOVA with a between-subjects factor of treatment and a repeated-subjects factor of test session detected a trend for a main effect of test session (F(4,40)= 2.33, p=0.07) but not for treatment (F(1,40)=0.78, p=0.4). A treatment × test session interaction was not observed (F(4,40)=0.52, p=0.7). As shown in Fig. 6, a secondary linear trend analysis revealed a highly significant effect of FLX on escape performance over repeated test sessions (F(1,23)=14.0, p<0.01), an effect not observed in the SAL treated animals (F(1,23)=1.13, p=0.3). A nonsignificant decrease in mean weight gain in FLX-treated animals was observed (data not shown).
Chronic FLX treatment and re-exposures to the IS context
To assess the effect of concomitant FLX treatment on escape performance in animals re-exposed to the IS context every third day after a single IS session, groups of animals received either 21 days of SAL (control), periodic 10-min context re-exposures or re-exposures + FLX. As shown in Fig. 7, a 3 × 3 repeated-measures ANOVA (control vs. re-exposure vs. re-exposure + FLX) detected a significant main effect of treatment (F(2,57)=5.96, p<0.01) and of test session (F(2,57)=7.17, p<0.01). A treatment × test session interaction was not detected (F(4,57)=1.23, p=0.3). The re-exposure group had more escape failures averaged over test sessions as compared to the both the control group (p<0.01) and the re-exposure + FLX group, although this difference did not reach statistical significance (p=0.09). No difference was detected between the control and re-exposure + FLX groups averaged over test sessions (p>0.05). Consistent with our previous findings, FLX had no effect on escape performance in the first shuttle box test session, however, orthogonal contrasts between groups at each test session demonstrated that at test session 1, both the re-exposure and re-exposure + FLX groups had more escape failures than the control group (control vs. re-exposure: F(1,57)=13.2, p<0.001, control vs. re-exposure + FLX: F(1,57)=15.5, p<0.001). In addition, at test session 2, the re-exposure group had more escape failures than the control group (control vs. re-exposure: F(1,57)=4.88, p<0.05) and that at test session 3, the re-exposure + FLX group had fewer escape failures than the re-exposure group, although this just missed statistical significance (re-exposure vs. re-exposure + FLX: F(1,57)=3.65, p=0.06). To further analyze the escape performance across test sessions, a secondary analysis of linear trends was performed and revealed a significant improvement in escape performance across test sessions in the re-exposure + FLX group (F(1,15)=13.8, p<0.01) but not in the re-exposure (F(1,13)= 0.46, p=0.5) or control (F(1,13)=1.96, p=0.2) groups.
Fig. 7.
Re-exposure to the IS context enhanced the FR-2 escape deficit. FLX treatment improved escape performance of animals re-exposed to the IS context. The bold line represents the period of drug administration after IS. Data are expressed as group mean escape failures±SEM at each test session. (n=7–8 per group). Following IS, rats were either left in their home cage (controls), re-exposed to the IS context (re-exposure) for 10 min every third day, or re-exposed to the context while receiving daily FLX (5 mg/kg/day) (re-exposure + FLX). Animals re-exposed to the IS context had more escape failures, regardless of treatment, compared to the control group at test session 1. At test session 2, the re-exposure group had significantly more escape failures than controls. At test session 3, the re-exposure + FLX group had fewer escape failures than the re-exposure group. Escape performance improved linearly across test sessions in FLX treated animals. *p<0.001 compared to control, ‡p<0.001 compared to control, **p<0.05 compared to control, #p=0.06 compared to re-exposure, ptrend<0.01
CUS-induced FR-2 escape deficit and reversal by chronic FLX
To identify whether an FR-2 shuttle box deficit could also be induced by exposure to a series of stressors that were unlikely to activate contextually conditioned fear during shuttle box testing, an additional experiment (experiment 8) was performed in which separate groups of animals were either left in their home cages (control), exposed to 35 days of CUS, or were exposed to 35 days of CUS and treated with 21 days of FLX starting on day 15 of the CUS paradigm (CUS + FLX). The series of stressors in the CUS procedure did not include electrical shocks, exposures to shock grids or to the IS context. As shown in Fig. 8, although animals in the CUS had never experienced IS, a significant 75% increase in escape failures was observed in the CUS group as compared to the control group. Furthermore, in contrast to the absence of an effect of 21 days of FLX treatment at the first shuttle box test session in all experiments centered on an initial IS session, the robust CUS-induced FR-2 escape deficit was completely reversed by 21 days of FLX treatment at the first shuttle box test session (F(2,14)=19.4, p<0.001; post-hoc comparisons: control vs. CUS, p<0.001; CUS vs. CUS + FLX, p<0.001).
Fig. 8.
Chronic unpredictable stress (CUS) induced an FR-2 shuttle box escape deficit that was reversed with chronic FLX treatment. The bold line represents the period of drug administration after IS. Data are expressed as group mean escape failures±SEM (n=5–6 per group). Animals exposed to CUS (35 days) had a highly significant increase in escape failures as compared to home cage controls. FLX (21 days, 5 mg/kg/day) completely reversed this behavioral deficit. *p<0.001 compared to controls, #p<0.001 compared to CUS
Discussion
Our primary experimental goal was to examine the effect of chronic antidepressant treatment on escape performance when the initial footshock exposure and subsequent shuttle box escape testing were performed in the same apparatus. Consequently, we first needed to establish experimental conditions that induced strong interference with escape testing so predicted improvements after drug treatment could be detected. One experimental variable that strongly interferes with escape performance in subsequent shuttle box testing is the inability of an animal to exert control over the initial stressor (Maier et al. 1976; Maier and Watkins 2005; Seligman et al. 1967). To maximize uncontrollability, we used unsignaled shocks of variable duration and onset. In addition, behavioral control over stressor exposures was minimized by using customized shuttle boxes that restricted the ability of the animal to rear or flip. Consistent with these previous studies, we found that the ability to exert control over the initial stressors was associated with decreased interference in subsequent shuttle box escape performance.
An additional variable that modulates IS-induced interference with escape performance is the nature of the escape response (Hunziker and Dos Santos 2007; Seligman et al. 1975). In the rat, interference with shuttle box escape performance after IS is not observed when the escape response consists of a single crossing in the shuttle box (Maier et al. 1973). As discussed further below, running to the opposite side of the shuttle box may represent a simple, species-typical defensive response that is independent of learning (Bolles and Bolles 1970; Hunziker and Dos Santos 2007; Maier et al. 1973) In contrast, an additional crossing into the chamber that just previously delivered footshock (FR-2) represents a voluntary, instrumental response that is a sensitive behavioral measure of the effect of inescapable shock (Maier et al. 1973; Maier and Watkins 2005). Consistent with these prior studies, we found that shuttle box escape deficits were more pronounced when animals were tested under an FR-2 vs. FR-1 shuttle box escape contingency.
An additional procedural variable that interferes with operant escape responding in the rat is a large discrepancy between the intensity of shocks used during training and subsequent escape testing (Rosellini et al. 1978). In our modified shuttle box, we initially observed that animals exhibiting helpless-like behavior at 0.8 mA could be ‘forced’ to cross by increasing the shock intensity to 1.2 mA. This occurred when the intensity was ramped during an escape trial or increased after several trials at a lower intensity. These observations are consistent with previous findings that higher shock intensities during escape testing tend to elicit increased escape responding (Rosellini et al. 1978). Our use of a slightly lower shock intensity during escape testing (0.65 mA) than during IS (0.8 mA) was intended to enhance the specificity of our behavioral assay by minimizing any direct contribution of shock intensity to enhanced escape performance while preserving its sensitivity to detect an antidepressant effect.
After establishing experimental conditions that produced strong interference with shuttle box escape performance, we demonstrated that the escape deficit lasted for at least 3 weeks after a single IS session. This finding is consistent with previous reports of prolonged escape deficits when the IS apparatus and shuttle box share contextual cues (Hunziker and Dos Santos 2007; Maier and Watkins 2005; Ruedi-Bettschen et al. 2004; Zazpe et al. 2007). Importantly, this durable escape deficit was reversed by antidepressant treatment. A highly significant improvement in FR-2 escape performance during the first shuttle box test session was observed after six days of DMI treatment while no effect was observed after FLX or CTP treatment. We excluded the possibility that the absence of a FLX effect after 6 days of treatment was due to an insufficient duration of treatment by showing that 21 days of FLX also had no effect on shuttle box escape performance after a single IS session. Consequently, the improvement in escape performance observed after nine, but not 6 days of FLX treatment likely reflects an interaction of FLX and repeated shuttle box testing.
To further characterize this interaction, we measured escape performance in FLX-treated animals first given IS, then exposed to repeated shuttle box test sessions or to re-exposures to the IS context. Again, we observed significantly improved performance in both groups of FLX-treated animals across repeated shuttle box test sessions. Interestingly, in the re-exposure experiment, we observed both an enhanced escape deficit and very small group variability at the first shuttle box test session in both groups re-exposed to the IS context. This suggests that additional neural systems were activated by re-exposure to the IS context that negatively modulated escape performance. Given the procedural similarity to contextual fear conditioning when IS and escape testing are performed in the same context, contextual fear may influence escape behavior. Indeed, in a well-established LH procedure where inescapable tail shocks are performed in a context different from the shuttle box, the escape deficit lasts for only 48 h unless the animals are periodically re-exposed to the IS context (Maier 2001). This effect is thought to be due to ‘reminding’ animals of the initial IS experience thereby activating conditioned fear responses. Although it has been previously shown that shuttle box escape performance in the triadic LH paradigm is not caused by the high levels of fear induced by IS (Maier 1990), this may not apply to experimental situations where the IS apparatus and shuttle box are the same.
In contrast to the absence of an effect of 21 days of FLX on the FR-2 escape deficit at the first shuttle box test session observed in our IS and IS + re-exposure experiments, 21 days of FLX was sufficient to reverse the FR-2 escape deficit induced by CUS at the first test session. Although the constituent stressors and temporal profile of the CUS and IS treated animals were markedly different, the magnitude of the escape deficit produced by both procedures was comparable. This suggests that our FR-2 escape deficit is a shared behavioral consequence of different stress-induced neural changes. The different stress histories may account for the contrasting effects of FLX on the CUS and IS-induced escape deficit. Because it has previously been shown that the 5HT1A agonist 8-OH-DPAT both blocked the development of and reversed FR-2 shuttle box escape deficits when IS was performed in a context different from the shuttle box, but had no effect when IS was administered in the shuttle box used for testing (Maier and Watkins 2005), it is tempting to speculate that the differential activity of FLX on our IS and CUS-induced escape deficit is due to a contextual fear effect. In our CUS protocol, we avoided exposure to shock grids, behavioral chambers and electrical shocks to minimize the contribution of contextual fear to the neural processing mediating shuttle box escape performance (Maier and Watkins 2005). However, it has also been shown that chronic FLX treatment reverses a shuttle box escape deficit induced by a chronic stress procedure that deliberately used re-exposures to contextual cues associated with the IS environment as constituent stressors (Gambarana et al. 2001). However, this study used an FR-1 escape contingency that may be especially sensitive to simple respondent, unconditioned behavior (running when shocked) masking the potential interactions between drug, stress-induced deficits in operant learning, and conditioned fear (Bolles and Bolles 1970; Hunziker and Dos Santos 2007; Maier et al. 1973). Collectively, these findings suggest that the interaction between FLX, stressor controllability, and contextual fear may have complex effects on controlling operant responding when an FR-2 shuttle box escape test is performed in the same environment as IS.
The possibility that the improvement in escape performance we observed after SSRI treatment is due to the modulation of contextual fear processing is supported by several recent studies. Chronic, but not acute, treatment with the SSRI paroxetine suppresses the expression of contextually driven fear responses (Takahashi et al. 2006) and subchronic treatment with FLX has been shown to reduce contextual freezing (Santos et al. 2006). In addition, a prior study examining the effect of chronic FLX given after IS found improved escape performance in adult rats exposed to IS and shuttle box testing in the same apparatus after postnatal isolation (Ruedi-Bettschen et al. 2004). Interestingly, the effect of FLX on escape performance was more pronounced in animals that were exposed to IS as compared to animals that were merely exposed to the same spatial context without footshock (i.e. minimal contextual fear conditioning). Clearly, further studies are needed to more fully characterize the role conditioned fear plays in the durable escape deficit observed when IS and testing are performed in the same environment and the extent to which antidepressant effects on contextual fear processing contributes to the improvement in escape performance observed under these conditions.
Our finding of different behavioral effects with noradrenergic and serotonergic drugs suggests that performing IS and shuttle box testing in the same environment can assist in the identification of mechanisms unique to these pharmacological classes. Although we cannot exclude the possibility that a FLX dose of 5 mg/kg was too low to produce a behavioral effect at the first shuttle box test session as compared to the strong effect observed for DMI, chronic FLX treatment at 5 mg/kg was sufficient to reverse both the FR-2 escape deficit induced by CUS at the first shuttle box test session and reproducibly improve escape performance across repeated shuttle box test sessions in three independent experiments. While a linear trend for improved performance was never observed in SAL or DMI treated groups, it may reflect a floor effect in the DMI group. In addition, a previous study has shown that FLX at 5 mg/kg reversed the shuttle box escape deficit induced by exposure to uncontrollable stressors in the same rat strain used in our experiments (Gambarana et al. 2001). These findings, combined with the absence of an effect of CTP in our model using doses that are behaviorally active in other stress paradigms (Overstreet et al. 2004; Papp et al. 2002), supports the possibility that the strong effect of DMI and the absence of an effect of FLX and CTP at the first shuttle box test session is not a dose response limitation, but reflects differential neural modulation by noradrenergic and serotonergic antidepressants.
Given the behavioral complexity and prolonged duration of the shuttle box escape deficit when performed in the presence of contextual cues from the IS session, multiple neurochemical systems are likely to modulate the behavioral output. In the FST, climbing and swimming represent different escape responses that are selectively modulated by noradrenergic and serotonergic mechanisms respectively (Cryan et al. 2005; Detke et al. 1995; Lucki 1997). Similarly, when the shuttle box escape task is performed in the same context as IS, running and freezing represent different coping strategies, the expression of which may depend on the dynamics between respondent, operant and associative mechanisms interacting with differential pharmacological effects of antidepressants. For example, after exposure to IS, infusion of the SSRI zimeldine into the hippocampus, a brain structure implicated in mediating contextually mediated fear responses, improves shuttle box escape performance while infusion of DMI has no effect (Joca et al. 2006). Importantly, in this study, contextual fear processing was likely to be present during escape testing, as the only attribute that differed between the IS and testing contexts was the gray-scale intensity of the sidewalls (white vs. gray). In addition, after contextual fear conditioning in rats, subchronic treatment with FLX, but not DMI, reduces freezing and increases startle responses while acute treatment with either drug had no effect (Santos et al. 2006). This suggests that the selective action of FLX on freezing was responsible for the expression of an alternative defensive behavior. Collectively, these findings suggest that the duration of treatment and the pharmacological class of antidepressant differentially modulate neural systems that initiate or organize defensive behaviors.
Further work comparing the effects of serotonergic and noradrenergic drugs on the component behaviors of strategies animals use to cope with stressors may have direct clinical relevance (Frazer and Morilak 2005). For example, in clinically depressed subjects, DMI has a faster initial onset of therapeutic effects and selectively on motor retardation, as compared to the slower initial effect of the SSRI paroxetine on anxiety measures (Katz et al. 2004; 2006). Consequently, assessing multiple behavioral components of escape responding when IS and shuttle box testing are performed in the same environment may lead to a more integrated understanding of how competing neural processes modulate stress-induce changes in complex emotional behavior. This approach may be particularly relevant for assessing the behavioral and cellular mechanisms underlying the ineffectiveness of SSRI’s in treatment refractory conditions and help guide the development of novel treatments based on selective pharmacological targeting of different symptom clusters within stress-induced psychiatric disorders (Frazer and Morilak 2005).
Acknowledgement
This work was supported by USPHS grants MH25642, MH4548, by the Connecticut Mental Health Center and by the VA National PTSD Center. The lead author was supported by a NARSAD Young Investigator Award. The authors thank Brian M. Dow, Ph.D. for his assistance with data analysis.
Contributor Information
Gerald Valentine, Email: gerald.valentine@yale.edu, Department of Psychiatry, Yale University, New Haven, CT, USA.
Antonia Dow, Department of Psychiatry, Harvard University, Cambridge, MA, USA.
Mounira Banasr, Department of Psychiatry, Yale University, New Haven, CT, USA.
Brian Pittman, Department of Psychiatry, Yale University, New Haven, CT, USA.
Ronald Duman, Department of Psychiatry, Yale University, New Haven, CT, USA.
References
- Antelman SM, Soares JC, Gershon S. Time-dependent sensitization—possible implications for clinical psychopharmacology. Behav Pharmacol. 1997;8:505–514. doi: 10.1097/00008877-199711000-00006. discussion 515–522. [DOI] [PubMed] [Google Scholar]
- Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007;62:496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Bolles RC. Species-specific defense reactions and avoidance learning. Psychol Rev. 1970;77:32. [Google Scholar]
- Chen H, Pandey GN, Dwivedi Y. Hippocampal cell proliferation regulation by repeated stress and antidepressants. Neuroreport. 2006;17:863–867. doi: 10.1097/01.wnr.0000221827.03222.70. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Johnson J, Lucki I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol. 1997;5:107–112. doi: 10.1037//1064-1297.5.2.107. [DOI] [PubMed] [Google Scholar]
- Frazer A, Morilak DA. What should animal models of depression model? Neurosci Biobehav Rev. 2005;29:515–523. doi: 10.1016/j.neubiorev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Gambarana C, Scheggi S, Tagliamonte A, Tolu P, De Montis MG. Animal models for the study of antidepressant activity. Brain Res Brain Res Protoc. 2001;7:11–20. doi: 10.1016/s1385-299x(00)00056-8. [DOI] [PubMed] [Google Scholar]
- Hunziker MH, Dos Santos CV. Learned helplessness: effects of response requirement and interval between treatment and testing. Behav Processes. 2007;76:183–191. doi: 10.1016/j.beproc.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Joca SR, Zanelati T, Guimaraes FS. Post-stress facilitation of serotonergic, but not noradrenergic, neurotransmission in the dorsal hippocampus prevents learned helplessness development in rats. Brain Res. 2006;1087:67–74. doi: 10.1016/j.brainres.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Katz MM, Tekell JL, Bowden CL, Brannan S, Houston JP, Berman N, Frazer A. Onset and early behavioral effects of pharmacologically different antidepressants and placebo in depression. Neuropsychopharmacology. 2004;29:566–579. doi: 10.1038/sj.npp.1300341. [DOI] [PubMed] [Google Scholar]
- Katz MM, Bowden CL, Berman N, Frazer A. Resolving the onset of antidepressants’ clinical actions: critical for clinical practice and new drug development. J Clin Psychopharmacol. 2006;26:549–553. doi: 10.1097/01.jcp.0000246220.04422.de. [DOI] [PubMed] [Google Scholar]
- Kusmider M, Faron-Gorecka A, Dziedzicka-Wasylewska M. Delayed effects of antidepressant drugs in rats. Behav Pharmacol. 2006;17:641–649. doi: 10.1097/FBP.0b013e3280116ea2. [DOI] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Maier SF. Role of fear in mediating shuttle escape learning deficit produced by inescapable shock. J Exp Psychol Anim Behav Process. 1990;16:137–149. [PubMed] [Google Scholar]
- Maier SF. Exposure to the stressor environment prevents the temporal dissipation of behavioral depression/learned helplessness. Biol Psychiatry. 2001;49:763–773. doi: 10.1016/s0006-3223(00)01095-7. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Maier SF, Albin RW, Testa TJ. Failure to learn to escape in rats previously exposed to inescapable shock depends on nature of escape response. J Comp Physiol Psychol. 1973;85(3):581–592. Dec 1973. [Google Scholar]
- Maier SF, Maier SF, Seligman ME. Learned helplessness: theory and evidence. J Exp Psychol Gen. 1976;105:3. [Google Scholar]
- Martin P, Puech AJ. Antagonism by benzodiazepines of the effects of serotonin-, but not norepinephrine-, uptake blockers in the learned helplessness paradigm in rats. Biol Psychiatry. 1996;39:882–890. doi: 10.1016/0006-3223(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Martin P, Martin P, Soubrie P, Simon P. Shuttle-box deficits induced by inescapable shocks in rats: reversal by the beta-adrenoreceptor stimulants clenbuterol and salbutamol. Pharmacol, Biochem Behav. 1986;24:177. doi: 10.1016/0091-3057(86)90334-5. [DOI] [PubMed] [Google Scholar]
- Martin P, Soubrie P, Puech AJ. Reversal of helpless behavior by serotonin uptake blockers in rats. Psychopharmacology (Berl) 1990;101:403–407. doi: 10.1007/BF02244061. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Keeney A, Hogg S. Antidepressant effects of citalopram and CRF receptor antagonist CP-154,526 in a rat model of depression. Eur J Pharmacol. 2004;492:195–201. doi: 10.1016/j.ejphar.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Papp M, Nalepa I, Antkiewicz-Michaluk L, Sanchez C. Behavioural and biochemical studies of citalopram and WAY 100635 in rat chronic mild stress model. Pharmacol Biochem Behav. 2002;72:465–474. doi: 10.1016/s0091-3057(01)00778-x. [DOI] [PubMed] [Google Scholar]
- Rosellini RA, Rosellini RA, Seligman ME. Role of shock intensity in the learned helplessness paradigm. Anim Learn Behav. 1978;6:143. [Google Scholar]
- Ruedi-Bettschen D, Feldon J, Pryce CR. The impaired coping induced by early deprivation is reversed by chronic fluoxetine treatment in adult fischer rats. Behav Pharmacol. 2004;15:413–421. doi: 10.1097/00008877-200409000-00016. [DOI] [PubMed] [Google Scholar]
- Santos JM, Martinez RC, Brandao ML. Effects of acute and subchronic treatments with fluoxetine and desipramine on the memory of fear in moderate and high-intensity contextual conditioning. Eur J Pharmacol. 2006;542:121–128. doi: 10.1016/j.ejphar.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Seligman ME, Seligman ME, Maier SF. Failure to escape traumatic shock. J Exp Psychol. 1967;74:1. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- Seligman ME, Seligman ME, Beagley G. Learned helplessness in the rat. J Comp Physiol Psychol. 1975;88:534. doi: 10.1037/h0076430. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Morinobu S, Iwamoto Y, Yamawaki S. Effect of paroxetine on enhanced contextual fear induced by single prolonged stress in rats. Psychopharmacology. 2006;189:165–173. doi: 10.1007/s00213-006-0545-6. [DOI] [PubMed] [Google Scholar]
- Tordera RM, Monge A, Del Rio J, Lasheras B. Antidepressant-like activity of VN2222, a serotonin reuptake inhibitor with high affinity at 5-HT1A receptors. Eur J Pharmacol. 2002;442:63–71. doi: 10.1016/s0014-2999(02)01504-2. [DOI] [PubMed] [Google Scholar]
- Willner P. Animal models as simulations of depression. Trends Pharmacol Sci. 1991;12:131–136. doi: 10.1016/0165-6147(91)90529-2. [DOI] [PubMed] [Google Scholar]
- Zazpe A, Artaiz I, Labeaga L, Lucero ML, Orjales A. Reversal of learned helplessness by selective serotonin reuptake inhibitors in rats is not dependent on 5-HT availability. Neuropharmacology. 2007;52:975–984. doi: 10.1016/j.neuropharm.2006.10.014. [DOI] [PubMed] [Google Scholar]