Abstract
Growth factors in the brain are important to depression and it’s treatment and we assessed the ability of peripherally administered insulin-like growth factor-I (IGF-I) to influence behavior related to depression. We found that mice that received chronic IGF-I treatment showed antidepressant-like behavior in forced-swim and novelty-induced hypophagia (NIH) tests and increased sucrose consumption after chronic mild unpredictable stress exposure. Additionally, peripheral anti-IGF-I administration blocked exercise-induced antidepressant effects in the forced-swim test (FST). These results support the functional relevance of neurotrophic mechanisms to depression and extend this idea to include neurotrophic factors in the periphery.
Keywords: Antidepressant, IGF-I, Behavior, Mouse, Exercise, Forced-swim test, Novelty-induced hypophagia
1. Introduction
Neurotrophic factors were originally identified for their roles in growth and development of the nervous system, but are now also considered important mediators of neuronal growth, survival and function in the adult [4,27].Roles for neurotrophic factors have been demonstrated in learning and memory, synaptic facilitation and other plasticity-related processes [5,32,33]. Many diseases of the nervous system including depression are hypothesized to involve dysregulation of growth factor processes, and the therapeutic application of treatments that regulate neurotrophic actions is an active area of investigation [40,54].
Long-term antidepressant treatment increases the expression of neurotrophic factors in hippocampus [19]. This has been suggested to be functionally relevant due to the converse, down-regulation of neurotrophic factors by stress, which can be a precipitating factor for depression [20]. A loss of neurotrophic support could contribute to atrophy and loss of neurons observed in pre-clinical models, as well as volumetric and cellular abnormalities reported in postmortem studies of depressed patients [7,41,47].
Insulin-like growth factor-I (IGF-I) is among the growth factors reported to be up-regulated in rat brain by antidepressant treatment [29]. IGF-I is a trophic factor that mediates actions of growth hormone and regulates cell growth and metabolism in the periphery [50]. IGF-I is expressed in many tissues, including the brain, where it is important to nerve growth and differentiation and neurotransmitter synthesis and release [1,14]. Actions of IGF-I are mediated primarily through the IGF-I type I receptor and are modulated by IGF-I binding proteins [12,50]. The adult brain contains high levels of IGF-I receptors, but unlike the case in developing brain, expression levels of IGF-I are low suggesting that the adult brain may utilize IGF-I from sources outside of the brain [6].
Circulating IGF-I, derived primarily from liver, can gain entry into the brain via transport across the blood–brain and blood–cerebrospinal fluid (CSF) barriers [42,43]. Physical exercise stimulates the expression and release of liver IGF-I and results in elevated brain uptake of IGF-I in rodents [8,56]. Moreover, peripheral IGF-I is necessary for exercise-induced hippocampal neurogenesis and for functional recovery after brain injury in rodent models [9,52]. These findings implicate peripheral IGF-I in the beneficial consequences of exercise on brain function and suggest that peripheral IGF-I might also be important to other central effects of exercise.
Chronic exercise can result in improvements in depression in humans and in antidepressant-like responses in rodent behavioral models [2,22,23,26]. In the experiments reported here, we aimed to assess the role of peripheral IGF-I in mediating antidepressant-like behavior under resting physiological conditions. We also investigated the extent to which IGF-I might contribute to antidepressant-like behavior in exercising mice.
2. Methods
2.1. Animals
Male C57Bl/6 mice were obtained at 10 weeks of age from Jackson Laboratories (Bar Harbor, ME) and were acclimated to the facility for 1 week before use in experiments. Mice were singly housed in standard mouse cages (Nalgene) with ad libitum access to food and water. Mice were maintained on a 12-h light-dark cycle with lights on at 7 am. Animal maintenance and use procedures were in accordance with the NIH Guide for Care and Use of Laboratory Animals and were approved by the Yale University Animal Care and Use Committee.
For exercise experiments, the cages of exercising mice were equipped with running wheels (34.5cm diameter) attached to mechanical counters. The counters were connected to a CLOCKLAB data collection system (Actimetrics, Evanston, IL) and wheel-running activity was recorded continuously throughout the experiment. Chronic exercising mice were given wheel access for 4 weeks. Behavioral testing was performed 24 h following the last wheel access.
2.2. Treatments
Human recombinant IGF-I (GroPep Limited, Alelaide, Australia) was administered to mice via continuous subcutaneous osmotic minipump infusion. For chronic IGF-I treatments, Alzet model 1002 minipumps (Durect Corp., Cupertino, CA) were filled to deliver 50µg/kg of IGF-I per day over a 14-day period. Control mice were implanted with osmotic minnipumps containing vehicle. Anti-IGF-I polyclonal antibody was a kind gift of Dr. Ignacio Torres-Aleman. This antibody effectively recognized IGF-I by Western blot analysis and has been used in published reports [9,31,52]. For the exercise experiments, anti-IGF-Iwas administered to mice throughout a 28-day period of running wheel access. Anti-IGF-I (20% in saline)was delivered by subcutaneous osmotic minipump infusion (Alzet 2004) at a rate of 6µl/day. Control mice received similar minipump infusion of 20% normal rabbit serum in saline. Desipramine hydrochloride (Sigma, St. Louis,MO)was administered as a single acute dose of 15 mg/kg, i.p., given 30 min prior to testing and fluoxetine hydrochloride (gift of Eli Lily, Indianapolis IN) was administered in the drinking water at a dose of 20 mg/kg/day. Separate groups of mice were used for the antidepressant-responsive behavioral paradigms (forced-swim test (FST) and novelty-induced hypophagia (NIH)), the stress-exposure experiment and for the exercise experiment.
2.3. Behavioral procedures
2.3.1. Forced-swim test
The forced-swim test was performed according to standard published procedures with minor modifications [13,21].Mice were placed in a glass cylinder (12 cm diameter) filled to a depth of 10 cm with water (23 °C). A 6 min test session was conducted and videotaped. Time spend immobile was defined as the absence of active/escape directed movements and was scored by a blind observer for the last 4 min of the test period.
2.3.2. Novelty-induced hypophagia
The novelty-induced hypophagia paradigm was conducted as described by Dulawa et al. [18]. After 3 days of habituation to a palatable solution (Carnation sweetened condensed milk 1:3 in water), mice were individually presented with the solution in the home cage and latencies to approach and drink were recorded as a control. On the subsequent day, mice were presented with the milk solution in a novel cage with bright lighting and the latency to approach and drink was recorded.
2.3.3. Chronic mild stress exposure
The chronic mild unpredictable stress (CUS) paradigm is a modification of published procedures [3,36]. The stress regimen consisted of exposure to the following physical and/or psychological stressors: confinement (1 h), cold exposure (4 °C, 45 min), new cagemates (3 h), exposure to rat odor (3 h), wet bedding (3 h), swim stress (10 min), periods of darkness during the light cycle (3 h), cage rotation (1 h), crowding (1 or 3 h), stroboscopic lighting (2 h), cage tilt (12 h), food deprivation (12 h), light on (overnight). Two different stressors were applied during each 24 h period, in a pseudo-random, and changing sequence. Non-stressed control mice were housed in a separate room.
2.3.4. Sucrose consumption
The consumption of a sucrose solution was conducted as previously published for mice [25].Mice were habituated to sucrose over a 48 h period by replacing water bottles with bottles containing sucrose solution (1%). Sucrose consumption was then measured by presenting sucrose (1%) in the home cage for a 1 h test period that followed overnight water deprivation. Mice were tested in single-bottle tests in their home cages while their cagemates were temporarily removed. All mice were tested between 7:00 and 11:00 am. Sucrose consumption was quantified by weighing bottles before and after the test periods.
2.3.5. Locomotor activity
Locomotor activity was measured by video tracking (Ethovision Pro,Noldus Inc.) in standard cages. Datawere analyzed with Ethovision behavioral analysis software.
2.4. Measurement of growth factors
Animals were decapitated at the end of the experiment and brains were rapidly removed and hemisected. Prefrontal cortex was dissected from one hemisphere and frozen on dry ice. The remaining hemisphere was frozen on dry ice and used for in situ hybridization analysis.
2.4.1. IGF-I protein
IGF-I in brain tissue was quantified by ELISA (R&D Systems, Minneapolis, MN) according to manufacturers instructions. Tissue homogenates were treated with an acidic dissociation solution in order to remove any IGF-I binding proteins and then aliquots of pre-treated samples and standards were added to a microplate precoated with amonoclonal antibody specific for IGF-I which binds IGF-I contained in the sample. Following washing, an enzyme-linked polyclonal antibody specific for IGF-I was added to the wells. After an additional wash, substrate solution that uses tetramethylbenzidine as a chromagen was added which develops color in proportion to the amount of IGF-I bound in the initial step. Color development was stopped by addition of sulfuric acid solution and optical density for individual samples was determined using a microplate reader set to 450 nm. Samples were run in duplicate.
2.4.2. In situ hybridization
In situ hybridization for IGF-I or for brain derived neurotrophic factor (BDNF) mRNA was carried out as described previously [37,38]. Cryostat cut coronal sections (14 mm thickness) were fixed in 4% paraformaldehyde, acetylated and dried. Slides were incubated with 35S-labeled antisense riboprobes against the coding exon V (rat BDNF cDNA was obtained from Regeneron, Tarrytown, NY) or against IGF-I (RTPCR synthesis; primer sequences; forward – TCTTCTACCTGGCGCTCTGC and reverse – TCTTGGGCATGTCAGTGTGG; the reverse primer included a T7 template sequence (TAATACGACTCACTATAGGGAGA) on the 5’end). Riboprobe was verified by sequencing of the PCR product. After hybridization, sections were washed, dried and exposed to BioMax Film (Kodak, Rochester, NY). Levels of IGF-I or BDNF mRNA were analyzed using Scion Image Analyzer program. Hippocampal subregions were analyzed by outlining the area of interest; an equivalent area was outlined for each sample. For each animal, the optical density measurements from two individual hemisections were analyzed, from which the mean was calculated. To correct for non-linearity, 14C step standards were used for calibration.
2.5. Statistics
For analysis of two groups, data were subjected to Student’s t-test. For analysis of the effect of IGF-I antibody administration in exercising mice, data were analyzed by one-way analysis of variance (ANOVA) and Fischer’s protected least significant difference was used for post-hoc comparisons of group means.
3. Results
3.1. Effect of IGF-I on depression-related behavior
In order to determine if peripheral IGF-I can produce changes in depression-related behavior we administered human IGF-I to mice via continuous subcutaneous osmotic minipump infusion (50 µg/kg/day) for 14 days). This administration paradigm has been shown to produce central effects including increased glucose uptake and stimulation of angiogenesis in the brain of adult mice in published reports [9,31]. In the current study, we found that this paradigm resulted in detectable levels of recombinant IGF-I in brain tissue (46.3 pg/mg protein, determined by ELISA).We tested IGF-I-treated mice in the forced-swim test (FST), a standard and widely used behavioral paradigm for assessing antidepressant activity in rats and mice [13]. IGF-I-treated mice had significantly decreased immobility in the FST compared with vehicle-treated mice (Fig. 1a). This effect is similar to the action of antidepressant drugs as shown for the drug desipramine (Fig. 1b). Antidepressant-like effects in the FST consist of less immobility and it is possible that a treatment-induced up-regulation of general activity could contribute to antidepressant-like profiles in this test. Chronic-IGF-I treatment did not alter locomotor activity compared with vehicle-treated mice (Fig. 1c), indicating that the decreased immobility in the FST is not due to IGF-I-induced up-regulation of baseline activity.
Fig. 1.
Peripheral IGF-I administration results in an antidepressant-like behavioral response in the forced-swim test (FST). IGF-I (50 ug/kg/day) was administered subcutaneously via osmotic minipump for 14 days. (A) IGF-I-treated mice showed decreased immobility in the FST compared with mice that received similar administration of vehicle. Student’s t-test, *p = 0.01, n = 9–10. (B) Antidepressant response to desipramine in the FST is shown for comparison. Desipramine (DMI) (20 mg/kg, i.p.) was administered in a single acute dose 30 min prior to test. Student’s t-test, *p = 0.01, n = 8/group. (C) Baseline locomotor activity of IGF-I-treated mice was not different from the activity of control mice. Horizontal distance traveled was measured by video tracking in standard mouse cages. Bars represent the mean±SEM for each experiment.
IGF-I-treated mice were also assessed in the NIH test. The NIH test provides a behavioral measure of anxiety that is sensitive to chronic but not acute antidepressant treatment, consistent with the time lag for the therapeutic action of antidepressants [17]. This NIH test does not rely on prior food deprivation and is therefore appropriate to use with treatments that could conceivably affect feeding behavior. When mice were treated chronically with IGF-I (subcutaneous osmotic minipump, 50 ug/kg/day), the consumption latencies measured in the novel environment were significantly decreased in IGF-I-treated mice compared to vehicle-treated mice (Fig. 2a). The latencies for consumption in the homecage did not differ between IGF-I-treated and vehicle-treated mice. Homecage consumption latency is a control for feeding behavior in a nonanxiogenic condition and the equivalent homecage consumption latencies indicate that IGF-I treatment did not alter feeding drive. IGF-I treatment did not alter body weight (25.4±0.4 vs. 26.6±0.5, p = 0.1, for control and IGF-I groups, respectively), providing further evidence that IGF-I did not influence feeding. The ability of IGF-I to specifically decrease consumption latencies in the novel environment is similar to the effect of chronic antidepressant administration in this test [18], (Fig. 2b).
Fig. 2.
Peripheral IGF-I administration results in an antidepressant-like behavioral response in the novelty-induced hypophagia test (NIH). IGF-I (50µg/kg/day) was administered via osmotic minipump (s.c.). (A) Chronic IGF-I treatment resulted in decreased NIH. IGF-I-treated mice had significantly decreased consumption latencies in the novel environment compared with control mice when tested after 13 days of IGF-I treatment. Student’s t-test, *p = 0.03, n = 7–9. Consumption latencies in the home cage environment were not significantly different for vehicle- and IGF-I-treated mice. (B) Response to fluoxetine treatment in the novelty-induced hypophagia test is shown for comparison. Fluoxetine (20 mg/kg/day) was administered in the drinking water during a 21-day period of chronic mild stress exposure. Student’s t-test, +p = 0.07, n = 7–8/group.
3.2. Effect of IGF-I in stress-exposed mice
We also tested the influence of IGF-I treatment in groups of mice that were exposed to a modified CUS paradigm as an additional measure of potential antidepressant effectiveness. IGF-I was infused at 50µg/kg/day via subcutaneous osmotic minipump, during a 2-week CUS exposure period. Consumption of a 1% sucrose solution was measured as an index of anhedonia at the end of the 2-week treatment period. Mice receiving peripheral administration of IGF-I during the CUS exposure period showed increased sucrose consumption compared with vehicle-treated mice that were also exposed to CUS (Table 1). IGF-I treatment did not alter sucrose consumption in control mice (Table 1). This is similar to the case with antidepressant drugs where the increase in sucrose consumption is seen in stressed but not in unstressed rodents [55]. Although the decrease in sucrose consumption for CUS-exposed vs. non-stressed vehicle-treated mice was not statistically significant for this CUS exposure (Table 1), the significant increase in sucrose consumption in IGF-I-treated mice indicates an antidepressant-like effect.
Table 1.
Sucrose consumption (ml/kg body weight) following chronic mild unpredictable stress (CUS) exposure.
p = 0.1 vs. vehicle-treated control, n = 6–7/group.
p = 0.007 vs. vehicle-treated CUS, n = 6–7/group.
3.3. BDNF and IGF-I in Brain
Peripheral administration of IGF-I could influence behavior via a number of different pathways, including the induction of neurotrophic/growth factor expression in the brain. To examine this possibility, levels of IGF-I and BDNF mRNA in the hippocampus and prefrontal cortex were determined by in situ hybridization analysis of brain sections from mice that received chronic infusions of IGF-I or vehicle. The results indicate that chronic IGF-I administration did not significantly influence the expression of either BDNF or IGF-I mRNA in subfields of the hippocampus (dentate gyrus granule cell layer, CA1 and CA3 pyramidal cell layers) or in the medial prefrontal cortex (Table 2).
Table 2.
Levels of IGF-I or BDNF mRNA following chronic IGF-I administration.
| BDNF mRNA | IGF-I mRNA | |||
|---|---|---|---|---|
| Vehicle | IGF-I | Vehicle | IGF-I | |
| CA1 | 100 ± 4 | 94 ± 4 | 100 ± 10 | 91 ± 7 |
| CA3 | 100 ± 5 | 100 ± 8 | 100 ± 8 | 91 ± 8 |
| DG | 100 ± 4 | 93 ± 5 | 100 ± 12 | 97 ± 7 |
| PFC | 100 ± 10 | 79 ± 20 | 100 ± 8 | 94 ± 6 |
Levels of IGF-I or BDNFmRNAwere determined by in situ hybridization analysis. The results are expressed as percent of vehicle and are the mean±S.E.M (n = 5/group).
3.4. Effect of anti-IGF-I administration on exercise-induced antidepressant behavior
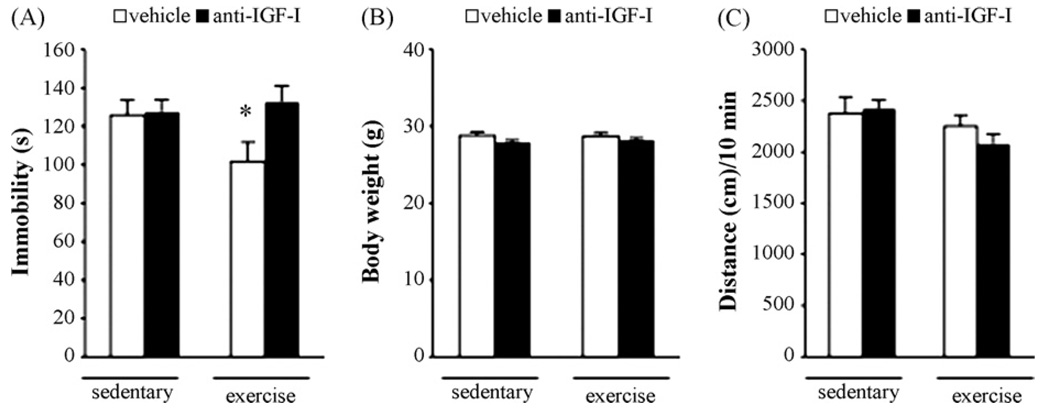
Chronic wheel-running exercise produces antidepressant-like behavior in mice and rats [22,26]. In order to assess a contribution of peripheral IGF-I to antidepressant behavioral responses of exercising mice, we administered an anti-IGF-I antibody to neutralize IGF-I in the periphery in chronic wheel-running mice. This antibody has been effectively used to block peripheral IGF-I in published reports [9,52]. In our study, groups of mice were implanted with osmotic minipumps containing either anti-IGF-I antibody or control serum and were given chronic access to running wheels. After chronic running wheel access, mice that received a control serum infusion showed a significant antidepressant-like response when tested in the FST as compared with their sedentary counterparts (Fig. 3a), in agreement with our study of exercise in mice [22]. In contrast, exercising mice that received the anti-IGF-I antibody infusion did not show this antidepressant-like effect and had immobility values that were not different from sedentary mice (Fig. 3a). The immobility values of sedentary mice were not affected by anti-IGF-I infusion indicating that peripheral IGF-I does not contribute to the baseline immobility behavior in the FST in non-exercising mice. Locomotor activity and body weights were not significantly altered by exercise or by anti-IGF-I antibody treatment (Fig. 3b and c) and anti-IGF-I antibody administration did not alter the average daily running distances of exercising mice (data not shown). We did not assess behavior of exercising mice in the NIH paradigm due to the potentially confounding effect of exercise on feeding and consumption parameters.
Fig. 3.
Anti-IGF-I antibody administration blocks the antidepressant-like effect of chronic exercise in the forced-swim test (FST). (A) Exercising mice given free access to running wheels in their home cages for 4 weeks show an antidepressant-like decrease in immobility and this effect is blocked in exercising mice that concurrently received continuous peripheral anti-IGF-I infusion. ANOVA: F(3, 58) = 2.72, p = 0.05. Fisher’s protected least significant difference (PLSD) post-hoc test: *p = 0.04 vs. sedentary control and p = 0.01 vs. exercise + anti-IGF-I. Bars represent the mean±SEM from two separate experiments, n = 15–16/group. (B) Body weights and (C) baseline locomotor activity were not significantly altered by exercise or by anti-IGF-I treatment. Bars represent the mean±SEM from two separate experiments, n = 15–16/group.
4. Discussion
The neurotrophic hypothesis of depression postulates that reductions in the expression and function of neurotrophic/growth factors contribute to some types of depressive illness and that antidepressant treatments act in part by reversing these decrements. Research addressing this hypothesis has typically focused on growth factors and growth factor-mediated processes within the brain [19]. The present study demonstrates regulation of depression-related behavior by a peripheral growth factor, providing further support for the idea that neurotrophic mechanisms are functionally significant to depression and extending this concept to include growth factors in the periphery. Peripheral IGF-I was previously demonstrated to be important to the influence of exercise on hippocampal neurogenesis and on recovery after brain injury [9,52]. The results of the present study indicate that peripheral IGF-I can alter behavior independent of exercise or injury and can produce antidepressant-like behavioral responses. Additionally, the results demonstrate that peripheral IGF-I contributes to the antidepressant-like behavioral effect of chronic exercise in a rodent model.
We found that peripheral administration of IGF-I produced antidepressant-like behavior in the mouse models tested, indicating that IGF-I derived from sources outside the brain can have functional behavioral consequences relevant to depression. The decreased immobility of IGF-I-treated mice in the FST indicates an antidepressant-like effect similar to that observed with antidepressant drugs. The decrease in immobility was not a consequence of a general increase in activity, as IGF-I administration did not alter levels of locomotor activity determined in an independent test. Our results are consistent with the reported antidepressant-like effect of IGF-I following central administration and with a depressant-like effect seen in mutant mice that are deficient in serum IGF-I [28,34,53]. We also found that peripheral IGF-I administration resulted in a behavioral effect that is similar to that of chronic antidepressant drug treatment in the NIH test. The NIH test displays predictive validity for the time-course of antidepressant drugs, showing an anxiolytic response to chronic, but not to acute antidepressant administration. The NIH test has been used to study mechanisms thought to be important to time-dependent processes critical to antidepressant action [17,46]. The effectiveness of IGF-I in the NIH test in the present study suggests that chronic IGF-I administration might induce time-dependent adaptive changes similar to those induced by antidepressant treatment. These results further suggest that peripheral IGF-I can influence behavior under conditions that are not expected to alter blood–brain-barrier function or transport.
We also found that peripheral administration of IGF-I increased sucrose consumption in mice exposed to CUS. Decreased sucrose consumption after CUS is thought to reflect a stress-induced state of anhedonia, a core symptom of depression in humans, and is sensitive to chronic but not acute antidepressant treatment [55]. Although the decrement in sucrose consumption did not reach statistical significance in the vehicle-treated animals, the increase induced by peripheral IGF-I compared with vehicle treatment in CUS mice is suggestive of an antidepressant-like effect of peripheral IGF-I. Given the clinical relevance of anhedonia and the requirement for chronic treatment for reversal of sucrose consumption deficits, the ability of chronic peripheral IGF-I to increase sucrose consumption in stressed animals suggests an action of IGF-I relevant to depression. Since it is likely that multiple limbic brain regions, including the hippocampus, prefrontal cortex, and nucleus accumbens, are involved in the behavioral models tested, it will be interesting in future studies to examine which of these regions are important in the actions of IGF-I.
Our study also demonstrates a role for IGF-I in the antidepressant effects of exercise. For these studies we used an anti-IGF-I antibody that binds to and neutralizes IGF-I, thereby preventing the entrance of IGF-I into brain. We confirmed that this antiserum effectively recognized IGF-I by Western blot analysis (D. Russell, unpublished observation). Moreover, this approach has been verified by previous studies showing that the IGF-I antiserum used in the current report inhibits the interaction of IGF-I with it’s receptor in neuronal culture and blocks the exercise-induced increase in IGF-I labeling in brain [9,52]. In the present study, peripheral administration of anti-IGF-I antibody blocked the effect of chronic exercise in the FST, indicating a requirement for peripheral IGF-I in this antidepressant-like behavior in wheel-running mice.
Exercise-induced enhancement of BDNF in the hippocampus has been shown to depend on serum IGF-I, in experiments that used the anti-IGF-I antibody approach [11]. Because hippocampal BDNF can promote antidepressant behavioral responses [48], this suggests the possibility that an IGF-I-hippocampal BDNF interaction could be involved in the antidepressant effect of exercise. In contrast, a recent study reported that mutant mice deficient in serum IGF-I were still responsive to the antidepressant-like effect of exercise in the FST [53]. It is possible that the mutant mice could have changes in the periphery or in brain that functionally compensate for the deficiency in serum IGF-I. Our data in combination with studies that demonstrated blockade of exercise-induced neurogenesis and neuroprotection after similar anti-IGF-I antibody administration implicate peripheral IGF-I as important to brain processes that are affected by exercise and involve neuroplasticity [9,52].
In addition to increasing IGF-I uptake into the brain, exercise has been shown to increase the IGF-I mRNA expression in hippocampus [8,15]. It is possible that the effects of peripheral IGF-I could be mediated, in part, by induction of the central expression of this, or another growth factor. In the current study, we found that peripheral IGF-I administration did not influence the expression of IGF-I mRNA in the prefrontal cortex or subfields of the hippocampus. In addition, IGF-I administration did not influence the expression of prefrontal or hippocampal BDNF, which has been associated with behavioral actions of antidepressants [20]. Our studies indicate that the actions of peripheral IGF-I may not be mediated by central induction of IGF-I or BDNF, although the possibility of central induction of other neurotrophic/growth factors requires further study. It is also possible that the actions of peripheral IGF-I occur via regulation of other factors in peripheral tissues or regulation of metabolic processes. This point highlights the requirement for additional studies to elucidate the functional importance of central vs. peripheral actions of IGF-I to the behavioral changes induced by exercise.
The influence of IGF-I on function in exercising animals could be related to exercise-induced alterations in blood–brain-barrier parameters. Similarly, it is possible that the influence of IGF-I on recovery after brain injury is facilitated by alterations of the blood–brain-barrier and transport processes, in this case resulting from the injury [24,44]. However, the current study also shows behavioral effects of peripherally administered IGF-I in an otherwise undisturbed physiological condition. We were able to detect recombinant IGF-I in brain after peripheral administration, suggesting that IGF-I entry into the brain can occur under normal physiological conditions and could contribute to the behavioral effects observed.
The uptake of IGF-I into the CNS and the mechanisms underlying this process have been the subject of several studies. Saturable transport of IGF-I at the blood–brain-barrier and megalin-mediated transport of IGF-I in the choroids plexus have been reported [10,39,43]. These findings provide evidence for mechanisms that could potentially underlie the ability of peripherally administered IGF-I to enter the brain and produce behavioral responses in the models tested in the current study. Although speculative, it is interesting to consider the possibility that in some cases, depression as well as other disorders could result from dysfunction of the peripheral expression and/or transport of IGF-I into the brain.
Therapeutic use of IGF-I administration may have relevance in the context of age-related deficits. Effectiveness of growth hormone/IGF-I replacement strategies has been shown for age-related reductions in hippocampal neurogenesis and cognitive function in pre-clinical studies [30,35,49]. The therapeutic use of IGF-I in neurodegenerative disease has also received attention [16,45,51]. Such findings raise the possibility of a novel therapeutic approach for depression that might be particularly relevant for IGF-I-deficient individuals.
Acknowledgments
We thank Dr. Ignacio Torres-Aleman for the kind gift of anti-IGF-I antibody. This work is supported by USPHS grant nos.MH45481 and 2PO1 MH25642, a Veterans Administration National Center Grant for PTSD and by the Connecticut Mental Health Center.
References
- 1.Anlar B, Sullivan KA, Feldman EL. Insulin-like growth factor-I and central nervous system development. Horm Metab Res. 1999;31(2–3):120–125. doi: 10.1055/s-2007-978708. [DOI] [PubMed] [Google Scholar]
- 2.Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, Craighead WE, Baldewicz TT, Krishnan KR. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62(5):633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Banasr M, Valentine GW, Li XY, Gourley S, Taylor J, Duman RS. Chronic stress decreases cell proliferation in adult cerebral cortex of rat: Reversal by antidepressant treatment. Biol Psychiatry. 2007;62:496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Barde YA. The nerve growth factor family. Prog Growth Factor Res. 1990;2(4):237–248. doi: 10.1016/0955-2235(90)90021-b. [DOI] [PubMed] [Google Scholar]
- 5.Black IB. Trophic regulation of synaptic plasticity. J Neurobiol. 1999;41(1):108–118. [PubMed] [Google Scholar]
- 6.Bondy CA, Lee WH. Patterns of insulin-like growth factor and IGF receptor gene expression in the brain. Functional implications. Ann N Y Acad Sci. 1993;692:33–34. doi: 10.1111/j.1749-6632.1993.tb26203.x. [DOI] [PubMed] [Google Scholar]
- 7.Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157(1):115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 8.Carro E, Nunez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20:2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor Imediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21:5678–5684. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carro E, Spuch C, Trejo JL, Antequera D, Torres-Aleman I. Choroid plexusmegalin is involved in neuroprotection by serum insulin-like growth factor I. J Neurosci. 2005;25(47):10884–10893. doi: 10.1523/JNEUROSCI.2909-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen MJ, Russo-Neustadt AA. Running exercise- and antidepressant-induced increases in growth and survival-associated signaling molecules are IGF-dependent. Growth Factors. 2007;25(2):118–131. doi: 10.1080/08977190701602329. [DOI] [PubMed] [Google Scholar]
- 12.Cohick W, Clemmons DR. The insulin-like growth factors. Annu Rev Physiol. 1993;55:131–153. doi: 10.1146/annurev.ph.55.030193.001023. [DOI] [PubMed] [Google Scholar]
- 13.Cryan J, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 14.D’Ercole AJ, Ye P, Calikoglu AS, Gutierrez-Ospina G. The role of insulin-like growth factors in the central nervous system. Mol Neurobiol. 1996;13(3):227–255. doi: 10.1007/BF02740625. [DOI] [PubMed] [Google Scholar]
- 15.Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006;140:823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- 16.Dore S, Kar S, Quirion R. Rediscovering an old friend. IGF-I: potential use in the treatment of neurodegenerative diseases. Trends Neurosci. 1997;20(8):326–331. doi: 10.1016/s0166-2236(96)01036-3. [DOI] [PubMed] [Google Scholar]
- 17.Dulawa S, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Dulawa S, Holick KA, Gunderson B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- 19.Duman R. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromol Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- 20.Duman R, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Duman C, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAPK signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 22.Duman C, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunn A, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez AM, de la Vega AG, Torres-Aleman I. Insulin-like growth factor I restores motor coordination in a rat model of cerebellar ataxia. Proc Natl Acad Sci USA. 1998;95(3):1253–1258. doi: 10.1073/pnas.95.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gourley S, Wu FJ, Kiraly DD, Ploski JE, kedves AT, Duman RS, Taylor JR. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry. 2008;63(4):353–359. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenwood B, Foley TF, Day HEW, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/ behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hefti F, Hartikka J, Knusel B. Function of neurotrophic factors in the adult and aging brain and their possible use in the treatment of neurodegenerative diseases. Neurobiol Aging. 1989;10(5):515–533. doi: 10.1016/0197-4580(89)90118-8. [DOI] [PubMed] [Google Scholar]
- 28.Hoshaw B, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005;1037:204–208. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Khawaja X, Xu J, Liang J-J, Barrett JE. Proteomic analysis of protein changes developing in rat hippocampus after chronic antidepressant treatment: impliations for depressive disorders and future therapies. J Neurosci. 2004;75:451–460. doi: 10.1002/jnr.10869. [DOI] [PubMed] [Google Scholar]
- 30.Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107(4):603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Lopez C, leRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci USA. 2004;101:9833–9838. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu B, Chow A. Neurotrophins and hippocampal synaptic transmission and plasticity. J Neurosci Res. 1999;58(1):76–87. [PubMed] [Google Scholar]
- 33.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malberg J, Platt B, Rizzo SJS, Ring RH, Lucki I, Schechter LE, Rosenzweig-Lipson S. Increasing the levels of insulin-like growth factor-1 by an IGF binding inhibitor produces anxiolytic and antidepressant-like effects. Neuropsychopharmacology. 2007;32:2360–2368. doi: 10.1038/sj.npp.1301358. [DOI] [PubMed] [Google Scholar]
- 35.Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87(3):559–569. doi: 10.1016/s0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- 36.Monleon S, D’Aquila P, Parra A, Simon VM, Brain PF, Willner P. Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology. 1995;117:453–457. doi: 10.1007/BF02246218. [DOI] [PubMed] [Google Scholar]
- 37.Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E, Duman RS. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J Neurosci. 2003;23(34):10841–10851. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15(11):7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan W, Kastin AJ. Interactions of IGF-I with the blood-brain-barrier in vivo and in situ. Neuroendocrinology. 2000;72:171–178. doi: 10.1159/000054584. [DOI] [PubMed] [Google Scholar]
- 40.Patel NK, Gill SS. GDNF delivery for Parkinson’s disease. Acta Neurochir Suppl. 2007;97(Pt 2):135–154. doi: 10.1007/978-3-211-33081-4_16. [DOI] [PubMed] [Google Scholar]
- 41.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33(1):88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 42.Pulford BE, Ishii DN. Uptake of circulating insulin-like growth factors (IGFs) into cerebrospinal fluid appears to be independent of the IGF receptors as well as IGF-binding proteins. Endocrinology. 2001;142(1):213–220. doi: 10.1210/endo.142.1.7894. [DOI] [PubMed] [Google Scholar]
- 43.Reinhardt R, Bondy CA. Insulin-like growth factors cross the blood-brain barrier. Endocrinology. 1994;135:1753–1761. doi: 10.1210/endo.135.5.7525251. [DOI] [PubMed] [Google Scholar]
- 44.Saatman KE, Contreras PC, Smith DH, Raghupathi R, McDermott KL, Fernandez SC, Sanderson KL, Voddi M, McIntosh TK. Insulin-like growth factor-1 (IGF-1) improves both neurological motor and cognitive outcome following experimental brain injury. Exp Neurol. 1997;147(2):418–427. doi: 10.1006/exnr.1997.6629. [DOI] [PubMed] [Google Scholar]
- 45.Sakowski SA, Schuyler AD, Feldman EL. Insulin-like growth factor-I for the treatment of amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2008;3:1–11. doi: 10.1080/17482960802160370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 47.Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;1;54(3):338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- 48.Shirayama Y, Chen A, Nakagawa C-H, Russell S, Duman RS. Brain derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2003;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev. 2005;4(2):195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Stewart CE, Rotwein P. Growth, differentiation, and survival: multiple physiological functions for insulin-like growth factors. Physiol Rev. 1996;76(4):1005–1026. doi: 10.1152/physrev.1996.76.4.1005. [DOI] [PubMed] [Google Scholar]
- 51.Torres-Aleman I. Targeting insulin-like growth factor-1 to treat Alzheimer’s disease. Expert Opin Ther Targets. 2007;11(12):1535–1544. doi: 10.1517/14728222.11.12.1535. [DOI] [PubMed] [Google Scholar]
- 52.Trejo J, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trejo JL, Llorens-Martín MV, Torres-Alemán I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci. 2008;37(2):402–411. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 54.Tuszynski MH. Nerve growth factor gene delivery: animal models to clinical trials. Dev Neurobiol. 2007;67(9):1204–1215. doi: 10.1002/dneu.20510. [DOI] [PubMed] [Google Scholar]
- 55.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 56.Zanconato S, Moromisato DY, Moromisato MY, Woods J, Brasel JA, Leroith D, Roberts CT, Jr, Cooper DM. Effect of training and growth hormone suppression on insulin-like growth factor I mRNA in young rats. J Appl Physiol. 1994;76(5):2204–2209. doi: 10.1152/jappl.1994.76.5.2204. [DOI] [PubMed] [Google Scholar]