Abstract
Oncolytic viruses are novel anticancer agents, currently under investigation in Phase I–III clinical trials. Until recently, most studies have focused on the direct antitumor properties of these viruses, although there is now an increasing body of evidence that the host immune response may be critical to the efficacy of oncolytic virotherapy. This may be mediated via innate immune effectors, adaptive antiviral immune responses eliminating infected cells or adaptive antitumor immune responses. This report summarizes preclinical and clinical evidence for the importance of immune interactions, which may be finely balanced between viral and tumor elimination. On this basis, oncolytic viruses represent a promising novel immunotherapy strategy, which may be optimally combined with existing therapeutic modalities.
Keywords: adaptive, clinical trial, immune response, immunotherapy, innate, oncolytic virus
The anticancer activity of viruses has been reported throughout the 20th century. Developments in virology, genetic manipulation and molecular biology have led to a surge of research investigating viruses with oncolytic or antitumor properties over the last 15 years. Several oncolytic viruses are currently in Phase I–III clinical trials [1]. Until recently, despite the multitude of studies investigating direct viral effects upon cancer cells, relatively little attention had been paid to the interaction between oncolytic viruses and the immune system. We discuss the evidence supporting the view that the host immune response is critical to the efficacy of oncolytic virotherapy. The potential of oncolytic viruses to break immunological tumor tolerance, generating antitumor immunity, represents a novel avenue of immunotherapy.
Oncolytic viruses: background
Oncolytic viruses are self-replicating, tumor selective and directly lyze cancer cells [2]. They may be tumor selective in wild-type or attenuated forms or may be engineered to provide tumor selectivity. Naturally occurring oncolytic viruses include the double-stranded RNA reovirus and single-stranded RNA Newcastle disease virus (NDV) and vesicular stomatitis virus (VSV). By contrast, human DNA viruses, including adenoviruses, vaccinia and herpes simplex viruses (HSV) have been genetically modified in a variety of ways to provide tumor selectivity. A diverse range of mechanisms provide tumor specificity, including inactivation of antiviral defences, such as type I IFN responses in many cancer cells, viral deletions permitting replication only in tumor cells that can substitute for viral defects, tumor-selective uptake via upregulated or mutated receptors, and targeting to tumor promoters.
In the majority of clinical trials performed so far, oncolytic viruses have been administered via intratumoral injection. A smaller number of studies have examined regional or intravenous delivery. Clinical experience has demonstrated a favorable toxicity and safety profile and a number of tumor responses, although overall antitumor efficacy has been limited [1]. For example, ONYX-015, a modified adenovirus, has been used in clinical trials with response rates of 0–14% following intratumoral administration [3]. In view of the short history of oncolytic virotherapy, along with recent scientific advances in methods of viral delivery and enhancing antitumor potency, these low levels of single-agent clinical responses provide encouragement for the future.
Cancer & the immune system
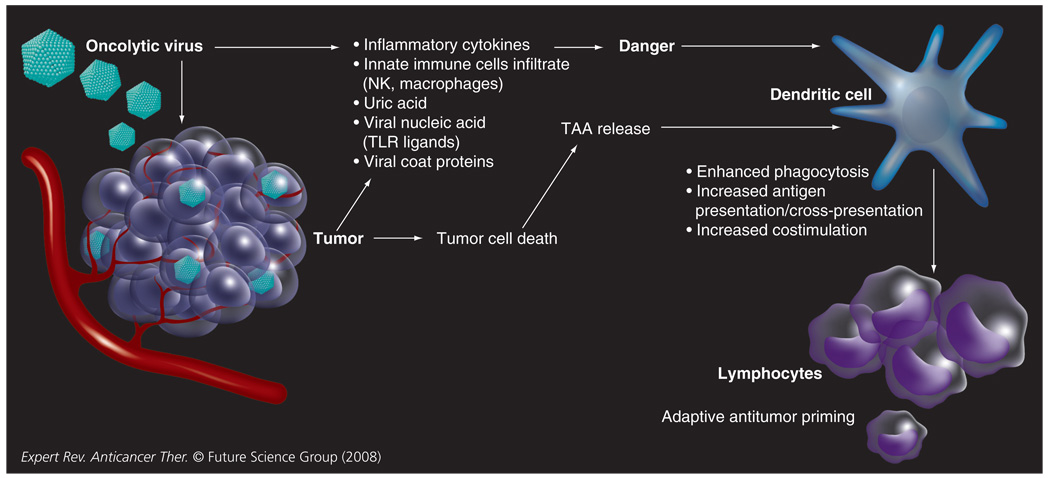
An increasingly powerful body of evidence supports the ability of the immune system to modify the immunogenicity and behavior of tumors [4]. A host of tumor-associated antigens (TAA) have been characterized [5] and in a single tumor, tumor-infiltrating lymphocytes directed towards multiple TAAs can be identified [6]. Despite these antigenic differences, the antitumor immune response is commonly ineffectual. Tumors can subvert antitumor immunity, generating an immunosuppressive tumor microenvironment by a multitude of mechanisms. These include the induction of Treg cells, secretion of soluble immunosuppressive mediators including nitric oxide, IL-10 and TGF-β and recruitment of myeloid suppressor cells [4]. Matzinger’s ‘danger’ hypothesis proposes that the prime role of the immune system is to respond to cellular or tissue distress as opposed to nonself per se [7]. Several danger signals have been identified, including RNA, DNA, IFN-α, heat-shock proteins, uric acid and hyaluron, providing a mechanistic basis for this hypothesis [8]. On this basis, tumor-associated danger signals are critical to the generation of effective antitumor immunity. In addition to their ability to disrupt immune responses, tumors commonly lack such signals and successful tumor immunotherapy will probably to depend upon their provision. Oncolytic virotherapy represents a potent approach to cancer immunotherapy, combining the enhanced release of TAA via tumor cell death, in the context of danger signals (FIGURE 1).
Figure 1. Concept of how oncolytic viral infection of tumor cells may lead to the generation of antitumor immune responses.
NK: Natural killer; TAA: Tumor-associated antigen; TLR: Toll-like receptor.
Oncolytic viruses, the innate immune response & danger signals
The role of the innate immune response to cancer is double-edged. Chronic inflammatory changes can promote tumor progression via proliferative and proangiogenic signals [9], while by contrast, the infiltration of activated innate inflammatory cells can mediate tumor regression in vivo [10]. Manipulation of the immune environment within a tumor is a potentially critical strategy towards successful tumor immunotherapy [11].
Oncolytic viruses represent prime candidates to enhance the immunogenicity of the tumor microenvironment. As detailed below, oncolytic virotherapy may be immunomodulatory via tumor cell death, production of endogenous danger signals, the release of tumor-derived cytokines and direct effects upon cells of the innate immune system. Evidence from preclinical models suggests that an early influx of immune cells, including macrophages and natural killer (NK) cells, occurs in response to tumor viral therapy [12–14]. These changes within the tumor hold the potential to alter the pre-existing immunosuppressive microenvironment, in favor of the generation of therapeutic immune responses. Dendritic cells (DC), the prime antigen-presenting cells and a component of the innate immune response are critical for the subsequent generation of antigen-specific or adaptive immune responses. However, as discussed later, the outcome of the innate response is finely balanced between promotion of tumor clearance and viral clearance limiting efficacy.
Tumor cell death
Virally induced cell death would be expected to enhance the availability of TAA for uptake by DC. Indeed, viral infection of tumors has been reported to enhance the phagocytosis of tumor-derived material [15,16]. The relationship between the mode of cell death and tumor immunogenicity has, however, been controversial; the immunogenicity of tumors has been reported not to be affected by whether tumor cells are alive, apoptotic or necrotic [17]. Even if the mode of cell death is not an immunogenic determinant, the release of intrinsic cell factors, including heat-shock protein [18], uric acid [19] and bradykinin [20], can be identified as danger signals by DC. Oncolytic viral infection may mediate production of these factors. For example, tumor cell infection by a modified oncolytic adenovirus increases intracellular uric acid levels, activating DC [19].
Tumor-derived cytokines
An array of cytokines provides costimulation for T-cell responses, while by contrast, tumor-derived cytokines, including TGF-β and IL-10, have immunosuppressive properties. In addition, the tumor-derived proinflammatory cytokines VEGF, TNF-α and several chemokines have been linked to promotion of tumor growth [21]. Oncolytic viral infection is likely to alter the balance of cytokines produced and the nature of the subsequent immune response. We have investigated the release of cytokines following infection of melanoma cells with reovirus, a naturally occurring double-stranded RNA virus currently in clinical trials [22]. Reovirus was found to induce secretion of IL-8, RANTES and MIP-1α/β, which play a role in the recruitment of DC, neutrophils and monocytes [23], and of IL-6, which can inhibit the immunosuppressive function of Treg cells [24]. Reovirus additionally reduced tumor secretion of the immunosuppressive cytokine IL-10. The immunogenic property of tumor-conditioned media from reovirus-infected tumor cells (filtered to remove viral particles) was confirmed by their ability to activate DC.
DC & the response to viral infection
The immune system is adept at pathogen recognition and a host of receptors specific for pathogen-associated molecular patterns, including the toll-like receptors (TLR), have been identified [25]. Innate viral recognition can center around viral nucleic acids or viral proteins [25]. DC play a critical role in the early innate immune responses, reciprocally interacting with other innate immune cells, including NK cells [26]. In this context, oncolytic viruses can influence the nature of the innate tumor response. Reovirus-infected DC, for example, enhance NK cytotoxicity towards tumor cells [27].
The effect of viruses upon DC is virus specific: measles and a vaccinia virus strain impair DC phenotype and function [28,29], an oncolytic adenovirus has a neutral effect [30], while reovirus is directly stimulatory to DC [27]. Although the immunomodulatory effects of oncolytic viruses have been investigated to a limited degree, it follows that the immune consequences of therapy with different viruses will vary widely. In addition, the genetic modification of viruses to confer oncolytic specificity may involve interference with virulence genes whose function is to modify the antiviral immune response, including type I interferon response genes [2,31]; alteration of such immunomodulatory genes will alter the consequences of the immune interactions of these modified viruses.
Oncolytic viruses & adaptive antitumor immunity
The innate immune response is thought to provide an important link to the generation of adaptive immune responses. DC are key to this link, taking up TAA, integrating danger signals and presenting antigen in an appropriate costimulatory context to the adaptive arm of the immune system. An adaptive antitumor immune response requires activation of cytotoxic CD8 T cells by DC presenting tumor antigen on MHC class I molecules. The presentation of exogenous antigen in a MHC class I context is termed ‘cross-presentation’. Critically, virally infected cells have been shown to be superior at delivering nonviral antigen for cross-presentation and cross-priming adaptive immune responses in vivo [32]. Intriguingly, recent work has defined a role for TLR-4 receptor ligands (bacterially derived lipopolysaccharide) in enhancing cross-presentation [33]; a similar effect of viral as opposed to bacterial TLR ligands has yet to be explored. Inflammatory stimuli have additionally been shown to enhance antigen processing and the generation of MHC class II complexes, required for CD4+ T-cell help in adaptive immune responses [34,35]; such inflammatory stimuli could be provided by viral tumor infection. Oncolytic virotherapy may therefore enhance immune priming via multiple effects upon DC. There is an emerging body of data from murine and human preclinical research supporting the concept that the efficacy of oncolytic virotherapy is at least partially immune mediated and that antitumor immunity can be generated.
Murine models
Adaptive antitumor immune responses following oncolytic virotherapy have been demonstrated in a variety of murine tumor models with several oncolytic viruses. We have demonstrated that effective systemic cellular delivery of VSV in a murine model of melanoma lymph node metastases can generate an antitumor immune response, with adoptive transfer of splenocytes from treated mice providing tumor protection [36]. Additionally, following intratumoral injection of VSV into B16ova tumors, antitumor T-cell responses are generated [12]. Similarly, oncolytic HSV strains have been shown to induce systemic antitumor immune responses in several tumor models [37–40]. In an elegant demonstration of this principle, an attenuated HSV was injected intratumorally into one flank of mice with established bilateral colorectal or melanoma tumors; the contralateral uninjected tumors regressed in association with the generation of TAA-specific CD8 T cells [37]. It should be noted that this study utilized an immunogenic tumor model.
In an intriguing study, NDV was administered locoregionally as therapy for liver metastases from a colon carcinoma cell line that was resistant to the virus in vitro; NDV resulted in a significant delay in tumor growth [41]. Similarly, we have data that B16ova cells are resistant in vitro to reovirus, but sensitive in vivo following intratumoral injection (PRESTWICH ET AL., UNPUBLISHED DATA). These data imply a critical role for antitumor host immune responses in oncolytic virotherapy.
In vitro human systems
Similarity between the mouse and human immune systems is limited and human in vitro studies are therefore important in this field. Tumor cell lysates induced by an oncolytic virus, parvovirus H-1, stimulate DC maturation and cross-presentation of melanoma antigens to cytotoxic lymphocyte clones, providing ‘proof of principle’ that virus-induced cell death can lead to cross-presentation of TAAs [15]. Greiner et al. demonstrated that a melanoma cell line infected with an attenuated vaccinia virus was able to prime an adaptive response towards a candidate TAA, MelanA [16]. Similarly, we have demonstrated that loading DC with reovirus-infected melanoma cells can efficiently prime an antitumor response and cross-prime an expansion of MART-1-reactive CD8 T cells [42].
Oncolytic viruses & the role of antiviral immune interactions: detrimental or beneficial for virotherapy?
The interaction between oncolytic virotherapy and the immune system, including innate immunity and adaptive antitumor and antiviral responses, is complex and may be detrimental as opposed to beneficial to therapeutic outcome. Soluble mediators including complement [43,44], preimmune IgM [44] and specific neutralizing antiviral antibodies [45] limit viral efficacy following systemic delivery. Circulating viral particles are additionally susceptible to nonspecific binding to blood cells [46], nontarget tissues and uptake by the reticuloendothelial system. Overcoming these immune and nonimmune barriers is critical to the success of systemic virotherapy and may also be key to harnessing any beneficial immune interactions. A host of promising strategies are under investigation to enhance viral delivery to tumors, shielding viral particles within the circulation. These include:
Modification of the viral coat by lipid encapsulation, polymer coating [47] and polyethylene glycol [48]
Bispecific fusion proteins or antibodies [49]
Serotype switching utilizing multiple viral serotypes to evade specific antibody neutralization [50]
Delivery utilizing cell carriers to chaperone viral particles in the circulation [51,52]
Arterial delivery [53]
The cellular antiviral immune response may limit the efficacy of virotherapy by eliminating tumor infection via clearance of infected tumor cells. Alternatively, clearance of infected tumor cells may play a key role in tumor regression, augmenting any direct cytolytic activity. In an intracranial murine model of metastatic melanoma, lymphocytes have been shown to be critical to tumor regression mediated by a neuroattenuated HSV [40]; the lymphocyte response was found to include virally specific cytotoxic activity in addition to antitumor reactivity. In a B16ova melanoma model, the efficacy of intratumorally injected VSV has been shown to be dependent upon NK and CD8 T cells [12]. CD8 T cells were detected towards both viral epitopes and the SIINFEKL epitope of the model TAA, OVA. In this study, it remains an open question whether the CD8 T cells critical for virotherapy were directed towards tumor or viral epitopes. Thus, local immune reactivity towards virally infected cells may be critical for the efficacy of virotherapy in this system.
Overall, the antiviral humoral and cellular immune responses may have contrasting consequences. Methods of enhancing viral delivery to tumors or immunomodulation provide an opportunity to alter this balance in favor of therapeutic benefit.
Clinical trials & the immune response
Although preclinical studies have provided support for the concept that the efficacy of oncolytic virotherapy may be dependent upon the host immune response, there are limited data on the immune response following virotherapy from early clinical trials.
Studies of intratumoral administration have provided direct evidence of a cellular immunological response. In a Phase I trial of a second-generation oncolytic HSV expressing GM–CSF injected into subcutaneous metastases from a variety of tumor types, post-treatment biopsies revealed an extensive immune cell infiltrate [54]. Additionally, suggestive of an immune-mediated antitumor effect, was the observation of inflammation in uninjected tumor deposits in four of 30 treated patients. Similarly, in a study of intratumoral administration of a recombinant vaccinia–GM–CSF virus in patients with melanoma deposits, treated lesions were shown to have a dense immune cell infiltrate. The generation of antitumor immunity was implied by the regression of noninjected regional dermal metastases in association with an immune infiltrate in four of seven treated patients [55]. A Phase I study of injection of JX-594, a targeted poxvirus armed with GM–CSF, into primary and metastatic liver tumors has recently been reported with encouraging evidence of activity, with a partial response in three and stable disease in six of ten evaluable patients by Response Evaluation Criteria in Solid Tumors (RECIST) [56]. Consistent with a possible antitumor immune response was the durability of tumor responses. Notably, there was evidence of functional response in noninjected tumors in three of seven evaluable patients by Choi criteria for reduction in Hounsfield units (n = 2) and by reduced 18F-fluorodeoxyglucose (18FDG)-PET signal (n = 1). There was evidence of viral dissemination to noninjected tumor tissue. The responses in injected and noninjected tumor tissue could therefore have been mediated by direct viral oncolysis, antiviral immune responses towards virally infected cells or antitumor immune responses established in the injected lesions.
Oncolytic viruses have been combined with tumor vaccines in an attempt to exploit viral danger signals. Vaccinia virus–melanoma cell lysate vaccines were used in an adjuvant Phase III study of 700 patients following melanoma resection, with no improvement in recurrence or overall survival [57]. A series of clinical studies has been performed by Schirrmacher et al. using a live autologous tumor vaccine infected by NDV irradiated to render tumor cells nonviable [58]. A significant proportion of patients developed antitumor immune responses as assessed by a delayed-type hypersensitivity response to skin prick tests. Phase II studies have been performed in glioblastoma multiforme, melanoma, breast and colorectal cancer with improvements in overall survival by 20–36% at 2–5-year follow-up compared with historical controls. These studies suggest that oncolytic viruses can break immunological tumor tolerance, although Phase III studies are needed to confirm these findings.
Oncolytic virotherapy & immunomodulation
Clinical trials have demonstrated the production of antiviral neutralizing antibodies following both intratumoral and intravenous viral therapy [56,59]. Although neutralizing antibody levels do not consistently appear to correlate with clinical response [3,56], preclinical studies have suggested that suppression of antibody production with cyclophosphamide can enhance viral delivery [60]. Suppression of the innate immune response, including NK cells and macrophages, using cyclophosphamide has also been shown to enhance intravascular delivery of HSV to rat gliomas [44]. Although immunosuppression would not intuitively appear to favor the generation of antitumor immunity, enhanced tumor infection followed by recovery from immunosuppression may be beneficial. In addition, cyclophosphamide may selectively deplete Treg cells, which suppress antitumor immunity [61]. The overall immunological consequences of viral therapy with immunomodulating doses of cyclophosphamide remain unclear and clinical trials are currently planned.
Future directions: combination therapy
Combination therapy may be the optimal context in which to exploit the immunotherapeutic potential of oncolytic viruses. A rationale exists for combination with existing immunotherapy strategies, along with conventional therapy.
Adoptive cellular therapy & viral delivery
The use of cell carriers to chaperone viral particles to the tumor is a promising innovation [51]. Cells of the immune system have proven particularly adept, including cytokine-activated killer cells [52] and T lymphocytes [36]. Adoptive cellular therapy has met with some clinical success, but has been limited by the trafficking to and survival of T cells in the tumor microenvironment [62]. In a mouse model, the combination of oncolytic virus delivery with antigen-specific adoptive T-cell therapy has been shown to improve upon either treatment modality alone [63]. Although yet to be tested in clinical trials, these findings are of significant translational potential.
Immunotherapy combinations
Immunotherapy approaches may be logically combined with virotherapy to enhance antitumor responses. For example, IL-2 in combination with Treg cell depletion has been shown to facilitate therapy with systemic VSV [64]. This combination induces a degree of vascular permeability that may increase viral access to the tumor, but also generates ‘hyperactivated’ NK cells with antitumor activity. As an alternative to combination with systemic cytokine therapy, oncolytic viruses have been designed to express cytokines that may facilitate the generation of antitumor immunity. For example, vaccinia viruses have been engineered to express GM–CSF [65] and IFN-β [66]. The in vivo immunological response to these viruses compared with nontransduced viruses has yet to be fully elucidated. Several monoclonal antibodies with immunoregulatory properties, such as anti-cytotoxic T-lymphocyte antigen (CTLA)4 and anti-VEGF antibodies, are currently in clinical trials. There is potential to combine these agents with virotherapy, although this remains to be tested.
Radiotherapy
Radiotherapy has been shown to act synergistically with oncolytic reovirus in vitro and in vivo [67]. This combination is also promising immunotherapeutically as radiotherapy has been shown to enhance T-cell trafficking [68], and antigen presentation and T-cell recognition of tumor cells [69]. However, radiotherapy is also locally immunosuppressive, killing lymphocytes, and the optimal combination to enhance antitumor immune responses will require careful consideration of dose fractionation and treatment scheduling.
Chemotherapy
Chemotherapy combinations with oncolytic viral therapy may have additive or synergistic effects in terms of direct oncolysis. Post-chemotherapy myelosuppression means that the combination as an immunotherapeutic regimen is problematic, although chemotherapy has been reported to augment immunotherapy [70].
Clinical trial design
Clinical trials of oncolytic virotherapy should be designed to assess immunological outcomes in addition to more traditional end points. Importantly, immunologically mediated responses may take longer to develop than the cytotoxic effects of therapeutic agents. As for other trials of immunotherapy, outcome measures should include appropriate immunological read-outs and appropriate follow-up periods to detect immunologically mediated clinical responses [71]. Patient selection is important, as heavily pretreated end-stage patients may already be immunosuppressed, while patients with rapid disease progression may not have time to generate an effective immune response.
Expert commentary
The host immune response will probably be critical to the efficacy of oncolytic virotherapy, although it is a fine balance between rapid viral elimination and innate and adaptive responses, which may mediate tumor regression. The rational design of combination therapy, modulating the immunological outcome, may hold the key to fulfilling the potential of these novel agents. Clinical trials should be designed to include specific assessment of immune responses to both tumor and viral antigens, and recognize the immunotherapeutic potential of virotherapy in terms of clinical end points and patient selection.
Five-year view
The next few years will see progress in terms of viral delivery, in particular the use of immune cell carriers that have yet to enter clinical trials. The promising strategy of combining existing antitumor adoptive cellular therapy with oncolytic viral delivery is likely to be explored. Combinations of viral therapy with chemotherapy, radiotherapy, transient immunosuppression and other immunotherapy strategies will probably be tested in early-phase clinical trials. Viruses engineered to enhance antitumor immune responses already appear promising and will enable determination of the consequences of manipulation of the immune response when administered via effective delivery vehicles.
Key Issues
Oncolytic viruses have direct anticancer properties.
The host immune response is likely to be critical to the efficacy of in vivo oncolytic virotherapy, via innate, antitumor and/or antiviral adaptive immunity.
Tumor infection can induce tumor cell death with release of tumor-associated antigens in combination with endogenous danger signals, cytokines and direct effects on dendritic cells.
Preclinical and clinical observations suggest that oncolytic virotherapy can induce adaptive antitumor immunity.
The immune response is finely balanced between viral elimination and antitumor effects.
Different oncolytic viruses have varying immunotherapeutic potential, some being immunosuppressive and others having immunostimulatory properties.
Viruses are being engineered to enhance their potential to generate antitumor immunity.
Clinical trials should be designed with immunological end points in mind.
The future involves novel methods of viral delivery and combination with immunotherapy or conventional therapy.
Acknowledgments
Financial & competing interests disclosure
Robin J Prestwich, Kevin J Harrington, Fiona Errington and Alan A Melcher are supported by grants from Cancer Research UK. Richard G Vile is supported by a US National Institutes of Health grant CA R01107032-02, Mayo Foundation, Richard M Schulze Family Foundation.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Robin J Prestwich, Cancer Research UK Clinical Centre, Leeds Institute of Molecular Medicine, University of Leeds, Leeds, LS9 7TF, UK, Tel.: +44 113 343 8450, Fax: +44 113 242 9886, r.j.d.prestwich@leeds.ac.uk.
Kevin J Harrington, Targeted Therapy Laboratory, The Institute of Cancer Research, Cancer Research UK, Centre for Cell and Molecular Biology, Chester Beatty, Laboratories, 237 Fulham Road, London, SW3 6JB, UK, Tel.: +44 207 153 5157, Fax: +44 207 808 2235, kevin.harrington@icr.ac.uk.
Hardev S Pandha, Oncology, Postgraduate Medical School, University of Surrey, Guildford, GU2 7XX, UK, Tel.: +44 148 368 8618, Fax: +44 148 368 8558, h.pandha@surrey.ac.uk.
Richard G Vile, Cancer Research UK Clinical Centre, Leeds Institute of Molecular Medicine, University of Leeds, Leeds, LS9 7TF, UK; Molecular Medicine Program; Department of Immunology, Mayo Clinic, Rochester, Minnesota, 55905, USA, Tel.: +1 507 284 9941, Fax: +1 507 266 2122, vile.richard@mayo.edu.
Alan A Melcher, Cancer Research UK Clinical Centre, Leeds Institute of Molecular Medicine, University of Leeds, Leeds, LS9 7TF, UK, Tel.: +44 113 343 8450, Fax: +44 113 242 9886, a.a.melcher@surrey.ac.uk.
Fiona Errington, Cancer Research UK Clinical Centre, Leeds Institute of Molecular Medicine, University of Leeds, Leeds, LS9 7TF, UK, Tel.: +44 113 343 8450, Fax: +44 113 242 9886, f.errington@leeds.ac.uk.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Aghi M, Martuza RL. Oncolytic viral therapies – the clinical experience. Oncogene. 2005;24(52):7802–7816. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 2.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. Cancer. 2005;5(12):965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 3.Kirn D. Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Ther. 2001;8(2):89–98. doi: 10.1038/sj.gt.3301377. [DOI] [PubMed] [Google Scholar]
- 4.Prestwich RJ, Errington F, Hatfield P, et al. The immune system – is it relevant to cancer development, progression and treatment? Clin. Oncol. (R. Coll. Radiol.) 2008;20(2):101–112. doi: 10.1016/j.clon.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol. Immunother. 2005;54(3):187–207. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411(6835):380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 7.Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 8.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr. Opin. Immunol. 2001;13(1):114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 9.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer. 2006;6(1):24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 10.Xue W, Zender L, Miething C, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445(7128):656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diaz RM, Galivo F, Kottke T, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67(6):2840–2848. doi: 10.1158/0008-5472.CAN-06-3974.. •• Demonstrates the critical role of immune response to oncolytic virotherapy.
- 13.Miller CG, Fraser NW. Role of the immune response during neuro-attenuated herpes simplex virus-mediated tumor destruction in a murine intracranial melanoma model. Cancer Res. 2000;60(20):5714–5722. [PubMed] [Google Scholar]
- 14.Benencia F, Courreges MC, Conejo-Garcia JR, et al. HSV oncolytic therapy upregulates interferon-inducible chemokines and recruits immune effector cells in ovarian cancer. Mol. Ther. 2005;12(5):789–802. doi: 10.1016/j.ymthe.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Moehler MH, Zeidler M, Wilsberg V, et al. Parvovirus H-1-induced tumor cell death enhances human immune response in vitro via increased phagocytosis, maturation, and cross-presentation by dendritic cells. Hum. Gene Ther. 2005;16(8):996–1005. doi: 10.1089/hum.2005.16.996. [DOI] [PubMed] [Google Scholar]
- 16.Greiner S, Humrich JY, Thuman P, Sauter B, Schuler G, Jenne L. The highly attenuated vaccinia virus strain modified virus Ankara induces apoptosis in melanoma cells and allows bystander dendritic cells to generate a potent anti-tumoral immunity. Clin. Exp. Immunol. 2006;146(2):344–353. doi: 10.1111/j.1365-2249.2006.03177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartholomae WC, Rininsland FH, Eisenberg JC, Boehm BO, Lehmann PV, Tary-Lehmann M. T cell immunity induced by live, necrotic, and apoptotic tumor cells. J. Immunol. 2004;173(2):1012–1022. doi: 10.4049/jimmunol.173.2.1012. [DOI] [PubMed] [Google Scholar]
- 18.Manjili MH, Park J, Facciponte JG, Subjeck JR. HSP110 induces “danger signals” upon interaction with antigen presenting cells and mouse mammary carcinoma. Immunobiology. 2005;210(5):295–303. doi: 10.1016/j.imbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Endo Y, Sakai R, Ouchi M, et al. Virus-mediated oncolysis induces danger signal and stimulates cytotoxic T-lymphocyte activity via proteasome activator upregulation. Oncogene. 2008;27(17):2375–2381. doi: 10.1038/sj.onc.1210884. [DOI] [PubMed] [Google Scholar]
- 20.Aliberti J, Viola JP, Vieira-de-Abreu A, Bozza PT, Sher A, Scharfstein J. Cutting edge: bradykinin induces IL-12 production by dendritic cells: a danger signal that drives Th1 polarization. J. Immunol. 2003;170(11):5349–5353. doi: 10.4049/jimmunol.170.11.5349. [DOI] [PubMed] [Google Scholar]
- 21.Ichim CV. Revisiting immunosurveillance and immunostimulation: implications for cancer immunotherapy. J. Transl. Med. 2005;3(1):8. doi: 10.1186/1479-5876-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Errington F, White CL, Twigger KR, et al. Inflammatory tumour cell killing by oncolytic reovirus for the treatment of melanoma. Gene Ther. 2008;15(18):1257–1270. doi: 10.1038/gt.2008.58.. • Demonstration of inflammatory consequences of tumor infection by an oncolytic virus.
- 23.Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25(2):75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Detournay O, Mazouz N, Goldman M, Toungouz M. IL-6 produced by type I IFN DC controls IFN-γ production by regulating the suppressive effect of CD4+ CD25+ regulatory T cells. Hum. Immunol. 2005;66(5):460–468. doi: 10.1016/j.humimm.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27(3):370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Kalinski P, Giermasz A, Nakamura Y, et al. Helper role of NK cells during the induction of anticancer responses by dendritic cells. Mol. Immunol. 2005;42(4):535–539. doi: 10.1016/j.molimm.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 27.Errington F, Steele L, Prestwich R, et al. Reovirus activates human dendritic cells to promote innate antitumor immunity. J. Immunol. 2008;180(9):6018–6026. doi: 10.4049/jimmunol.180.9.6018. [DOI] [PubMed] [Google Scholar]
- 28.Jenne L, Hauser C, Arrighi JF, Saurat JH, Hugin AW. Poxvirus as a vector to transduce human dendritic cells for immunotherapy: abortive infection but reduced APC function. Gene Ther. 2000;7(18):1575–1583. doi: 10.1038/sj.gt.3301287. [DOI] [PubMed] [Google Scholar]
- 29.Grosjean I, Caux C, Bella C, et al. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J. Exp. Med. 1997;186(6):801–812. doi: 10.1084/jem.186.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schierer S, Hesse A, Muller I, et al. Modulation of viability and maturation of human monocyte-derived dendritic cells by oncolytic adenoviruses. Int. J. Cancer. 2008;122(1):219–229. doi: 10.1002/ijc.23074. [DOI] [PubMed] [Google Scholar]
- 31.Stojdl DF, Lichty BD, tenOever BR, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4(4):263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 32.Schulz O, Diebold SS, Chen M, et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433(7028):887. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 33.Burgdorf S, Scholz C, Kautz A, Tampe R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat. Immunol. 2008;9(5):558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 34.Turley SJ, Inaba K, Garrett WS, et al. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288(5465):522–527. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- 35.Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299(5611):1400–1403. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 36.Qiao J, Kottke T, Willmon C, et al. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat. Med. 2008;14(1):37–44. doi: 10.1038/nm1681. [DOI] [PubMed] [Google Scholar]
- 37. Toda M, Rabkin SD, Kojima H, Martuza RL. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum. Gene Ther. 1999;10(3):385–393. doi: 10.1089/10430349950018832.. • Clear demonstration of generation of adaptive antitumor immunity.
- 38.Li H, Dutuor A, Fu X, Zhang X. Induction of strong antitumor immunity by an HSV-2-based oncolytic virus in a murine mammary tumor model. J. Gene Med. 2007;9(3):161–169. doi: 10.1002/jgm.1005. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Dutuor A, Tao L, Fu X, Zhang X. Virotherapy with a type 2 herpes simplex virus-derived oncolytic virus induces potent antitumor immunity against neuroblastoma. Clin. Cancer Res. 2007;13(1):316–322. doi: 10.1158/1078-0432.CCR-06-1625. [DOI] [PubMed] [Google Scholar]
- 40.Miller CG, Fraser NW. Requirement of an integrated immune response for successful neuroattenuated HSV-1 therapy in an intracranial metastatic melanoma model. Mol. Ther. 2003;7(6):741–747. doi: 10.1016/S1525-0016(03)00120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Apostolidis L, Schirrmacher V, Fournier P. Host mediated anti-tumor effect of oncolytic Newcastle disease virus after locoregional application. Int. J. Oncol. 2007;31(5):1009–1019.. • Demonstration of in vivo efficacy of oncolytic virus, despite in vitro sensitivity, implying immune-mediated tumor regression.
- 42.Prestwich RJ, Errington F, Ilett EJ, et al. Tumor infection by oncolytic reovirus primes adaptive anti-tumor immunity. Clin. Cancer Res. 2008 doi: 10.1158/1078-0432.CCR-08-0831. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ikeda K, Wakimoto H, Ichikawa T, et al. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J. Virol. 2000;74(10):4765–4775. doi: 10.1128/jvi.74.10.4765-4775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ikeda K, Ichikawa T, Wakimoto H, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat. Med. 1999;5(8):881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 45.Tsai V, Johnson DE, Rahman A, et al. Impact of human neutralizing antibodies on antitumor efficacy of an oncolytic adenovirus in a murine model. Clin. Cancer Res. 2004;10(21):7199–7206. doi: 10.1158/1078-0432.CCR-04-0765. [DOI] [PubMed] [Google Scholar]
- 46.Lyons M, Onion D, Green NK, et al. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol. Ther. 2006;14(1):118–128. doi: 10.1016/j.ymthe.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Green NK, Herbert CW, Hale SJ, et al. Extended plasma circulation time and decreased toxicity of polymer-coated adenovirus. Gene Ther. 2004;11(16):1256–1263. doi: 10.1038/sj.gt.3302295. [DOI] [PubMed] [Google Scholar]
- 48.Croyle MA, Chirmule N, Zhang Y, Wilson JM. PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum. Gene Ther. 2002;13(15):1887–1900. doi: 10.1089/104303402760372972. [DOI] [PubMed] [Google Scholar]
- 49.Bian H, Fournier P, Moormann R, Peeters B, Schirrmacher V. Selective gene transfer to tumor cells by recombinant Newcastle disease virus via a bispecific fusion protein. Int. J. Oncol. 2005;26(2):431–439. [PubMed] [Google Scholar]
- 50.Bangari DS, Mittal SK. Current strategies and future directions for eluding adenoviral vector immunity. Curr Gene Ther. 2006;6(2):215–226. doi: 10.2174/156652306776359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Power AT, Bell JC. Taming the Trojan horse: optimizing dynamic carrier cell/oncolytic virus systems for cancer biotherapy. Gene Ther. 2008;15(10):772–779. doi: 10.1038/gt.2008.40. [DOI] [PubMed] [Google Scholar]
- 52.Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311(5768):1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- 53.Reid T, Galanis E, Abbruzzese J, et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): Phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62(21):6070–6079. [PubMed] [Google Scholar]
- 54.Hu JC, Coffin RS, Davis CJ, et al. A Phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin. Cancer Res. 2006;12(22):6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 55. Mastrangelo MJ, Maguire HC, Jr, Eisenlohr LC, et al. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther. 1999;6(5):409–422. doi: 10.1038/sj.cgt.7700066.. •• Evidence of virotherapy generating antitumor immunity in a clinical trial.
- 56.Park BH, Hwang T, Liu TC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a Phase I trial. Lancet Oncol. 2008;9(6):533–542. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- 57.Hersey P, Coates AS, McCarthy WH, et al. Adjuvant immunotherapy of patients with high-risk melanoma using vaccinia viral lysates of melanoma: results of a randomized trial. J. Clin. Oncol. 2002;20(20):4181–4190. doi: 10.1200/JCO.2002.12.094. [DOI] [PubMed] [Google Scholar]
- 58.Schirrmacher V. Clinical trials of antitumor vaccination with an autologous tumor cell vaccine modified by virus infection: improvement of patient survival based on improved antitumor immune memory. Cancer Immunol. Immunother. 2005;54(6):587–598. doi: 10.1007/s00262-004-0602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White CL, Twigger KR, Vidal L, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a Phase I clinical trial. Gene Ther. 2008;15(12):911–920. doi: 10.1038/gt.2008.21. [DOI] [PubMed] [Google Scholar]
- 60.Qiao J, Wang H, Kottke T, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin. Cancer Res. 2008;14(1):259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J. Immunol. 2004;34(2):336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 62.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat. Rev. Immunol. 2006;6(5):383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Qiao J, Wang H, Kottke T, et al. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther. 2008;15(8):604–616. doi: 10.1038/sj.gt.3303098.. • Preclinical evidence for combining oncolytic virotherapy and adoptive cell transfer.
- 64.Kottke T, Galivo F, Wongthida P, et al. Treg depletion-enhanced IL-2 treatment facilitates therapy of established tumors using systemically delivered oncolytic virus. Mol. Ther. 2008;16(7):1217–1226. doi: 10.1038/mt.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thorne SH, Hwang TH, O’Gorman WE, et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J. Clin. Invest. 2007;117(11):3350–3358. doi: 10.1172/JCI32727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kirn DH, Wang Y, Le Boeuf F, Bell J, Thorne SH. Targeting of interferon-β to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4(12):E353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Twigger K, Vidal L, White CL, et al. Enhanced in vitro and in vivo cytotoxicity of combined reovirus and radiotherapy. Clin. Cancer Res. 2008;14(3):912–923. doi: 10.1158/1078-0432.CCR-07-1400. [DOI] [PubMed] [Google Scholar]
- 68.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J. Immunol. 2005;174(12):7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 69.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006;203(5):1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63(15):4490–4496. [PubMed] [Google Scholar]
- 71.Hoos A, Parmiani G, Hege K, et al. A clinical development paradigm for cancer vaccines and related biologics. J. Immunother. 2007;30(1):1–15. doi: 10.1097/01.cji.0000211341.88835.ae. [DOI] [PubMed] [Google Scholar]