Abstract
The loss of walking after human spinal cord injury has been attributed to the dominance of supraspinal over spinal mechanisms. The evidence for central pattern generation in humans is limited due to the inability to conclusively isolate the circuitry from descending and afferent input. However, studying individuals following spinal cord injury with no detectable influence on spinal networks from supraspinal centers can provide insight to their interaction with afferent input. The focus of this article is on the interaction of sensory input with human spinal networks in the generation of locomotor patterns. The functionally isolated human spinal cord has the capacity to generate locomotor patterns with appropriate afferent input. Locomotor Training is a rehabilitative strategy that has evolved from animal and humans studies focused on the neural plasticity of the spinal cord and has been successful for many people with acute and chronic incomplete spinal cord injury. However, even those individuals with clinically complete spinal cord injury that generate appropriate locomotor patterns during stepping with assistance on a treadmill with body weight support cannot sustain overground walking. This suggests that although a significant control of locomotion can occur at the level of spinal interneuronal networks the level of sustainable excitability of these circuits is still compromised. Future studies should focus on approaches to increase the central state of excitability and may include neural repair strategies, pharmacological interventions or epidural stimulation in combination with Locomotor Training.
Capacity of functionally isolated human spinal cord to generate locomotor patterns
The loss of standing and walking after human spinal cord injury has been attributed to the dominance of supraspinal over spinal mechanisms in the control of locomotion in primates [34;57;84;106]. The evidence for central pattern generation in humans is limited due to the inability to conclusively isolate the circuitry from descending and afferent input [17-19;21;41;60;68]. In studying the role of the human spinal cord in the generation of locomotor patterns we are restricted not only by our capacity to eliminate afferent input but also the ability to provide drugs or electrical stimulation directly to the spinal cord to activate the networks [10;62]. However, studying individuals following spinal cord injury with no detectable influence on spinal networks from supraspinal centers can provide insight to the their interaction with afferent input [28;39;66;67]. This article will focus on the role of sensory input interacting with human spinal networks to generate locomotor-like patterns in individuals with varying levels of supraspinal input. The design and interpretation of these studies rely extensively on the decades of work detailed in numerous reviews [63;77;89;94;100] and the most recent findings described throughout the other articles in this issue.
We can assess whether the human spinal cord maintains properties of central pattern generation such as the capacity to generate locomotor patterns in the absence of supraspinal input, the interdependence of extensor and flexor motor pools, and interlimb coordination. We can indirectly study the effect of peripheral feedback mechanisms with the interneuronal networks during locomotion by recording electromyographic (EMG) activity [29;101] from the leg muscles during manually assisted stepping using body weight support on a treadmill [11] in individuals with clinically complete spinal cord injury [28;66]. In these individuals the motor patterns observed during stepping are driven by the sensory information available to the interneuronal networks of the spinal cord and are dependent on the current physiological state of the central nervous system [13;32;33;46;68]. We can also study the intrinsic neuronal organization of these networks by examining the relationship of efferent output among ipsilateral and contralateral flexors and extensors. In addition, and most importantly from a clinical perspective, we can assess whether motor output improves with task specific practice.
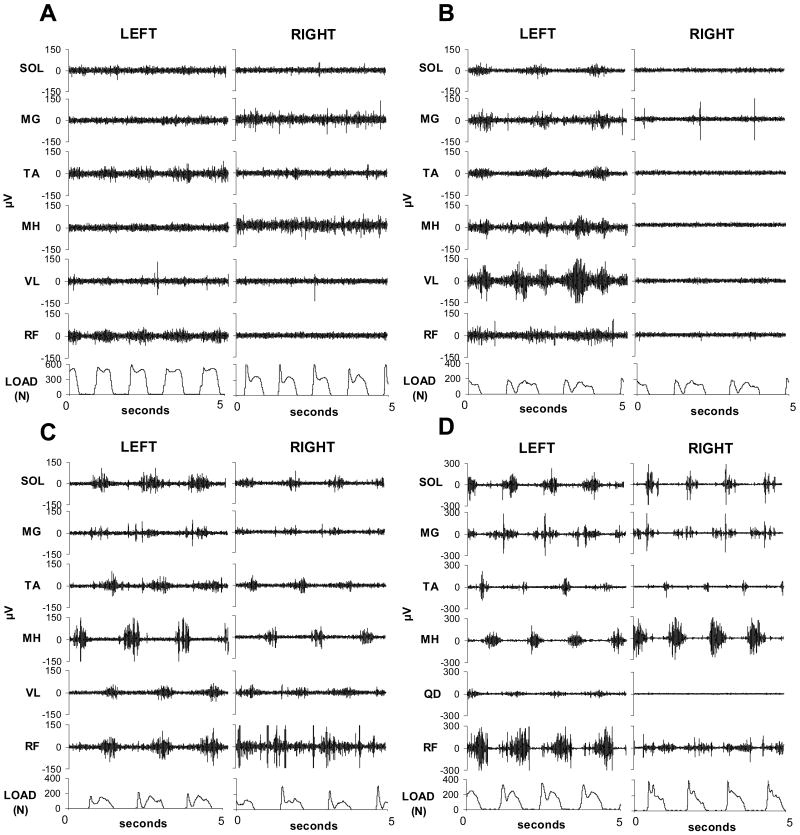
Our research group has studied twenty-nine individuals with clinically complete spinal cord injury and observed motor patterns from leg flexors and extensors during manually assisted stepping using body weight support on a treadmill. Individuals are defined as clinically complete when they are classified using the American Spinal Injury Association scale (ASIA) as A with no sensory or motor function detectable below the level of lesion; and with no response with testing of lower leg sensory evoked potentials [25]. Individuals with clinically complete spinal cord injury show significant variability in m otor patterns during stepping yet at least four distinct patterns can be identified (Fig. 1). Individuals with the first pattern show minimal EMG activity limited to very few muscles regardless of variations in speed and load (Fig. 1a). The second pattern is characterized by predominant activity in several muscles of only one leg with minimal activity in the contralateral leg (Fig. 1b). The individuals who most often show this pattern during stepping show co-activation of alternating flexor and extensor activity of both limbs. The third pattern depicts low to moderate activity from several spinal segments with alternating interlimb activity of extensors and flexors. Co-activation of ipsilateral flexors and extensors is also observed (Fig. 1c). Those individuals with the fourth pattern have moderate to high activity in both legs. There is fluctuation among flexor and extensor alternation within a leg, but interlimb alternation of synergists always occurs (Fig. 1d). These individuals express more than one pattern within a stepping session; limited to the two latter patterns of higher activation (Fig. 1c, Fig. 1d). Alternation of the knee flexors and extensors occurs most often when higher levels of activity are observed in the majority of leg muscles. Co-activation of plantar- and dorsiflexors is routinely observed during the stance phase of stepping [68] and during clonus [14] after spinal cord injury. However, alternation of ankle flexors and extensors can occur, even in individuals with clinically complete spinal cord injury indicating segmental influence of spinal networks can play a significant role in appropriate functional organization of motor output.
Figure 1.
Representative electromyographic (μV, microvolts) patterns during stepping in individuals with clinically complete spinal cord injury. (A) Minimal to no motor output; (B) Moderate motor output unilaterally; (C) Low amplitude motor output bilaterally; (D) Moderate amplitude motor output bilaterally.
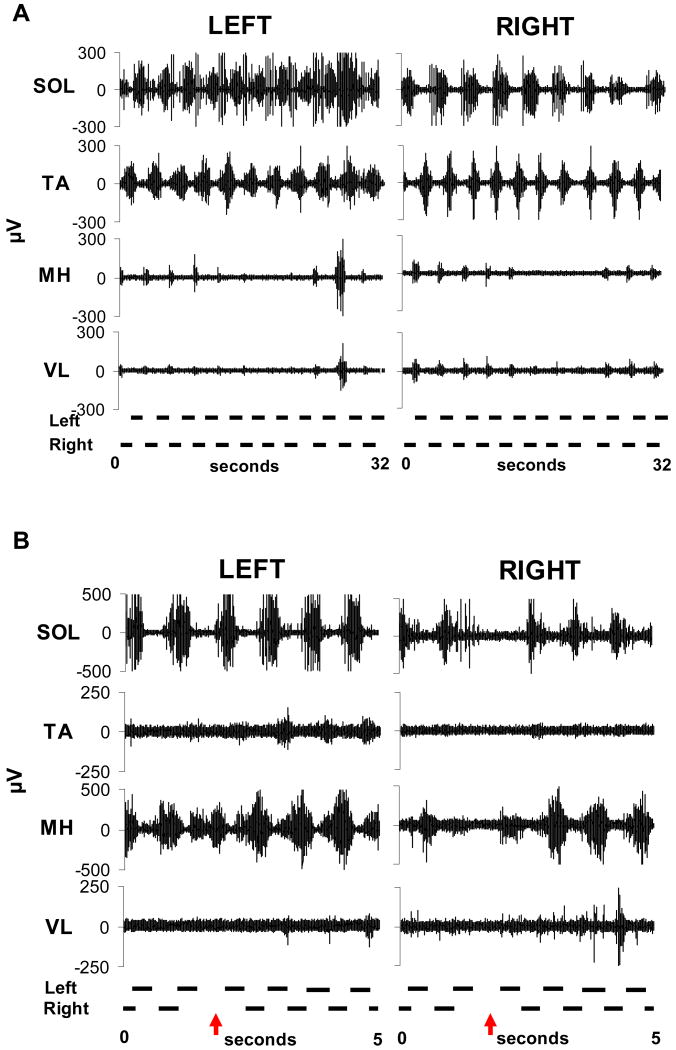
This varied response to similar afferent input generated by alternating leg loading and flexion and extension of the hip, knee and ankles suggests state-dependence of the human spinal networks. The relative amplitude of EMG activity is lower in individuals with complete spinal cord injury when compared to incomplete spinal cord injury and non-disabled individuals and may be a result of the level of activation of remaining supraspinal pathways on the spinal networks. In a series of steps with relatively similar kinetics and kinematics the level of EMG activity can vary with even in the absence of bursts in muscles for several steps (Fig. 2a). Interestingly, when the burst activity re-emerges, the timing and pattern remains the same, suggesting the period of locomotion may be retained even when the motor pool is inactive. This is another similar feature identified as an important component of central pattern generating networks [99].
Figure 2.
Representative electromyographic (μV, microvolts) patterns during stepping in individuals with clinically complete spinal cord injujry. (A) Intermittent rhythmical burst activation of the medial hamstrings (MH) and vastus lateralis (VL) with consistent timing during the stepcycle; (B) Increased activation of the left flexors following unloading and reduction of the contralateral extensors.
The physiological state is likely influenced by the time since injury, extent of ongoing neuromuscular activity below the lesion, the history of anti-spasticity and other medication use [27], as well as other factors that remain undetermined. In addition, the possibility of residual supraspinal input available to the spinal networks not detectable by current clinical or routinely employed neurophysiologic methods may also influence these motor patterns [101]. The observation that the functionally isolated human spinal cord has the capacity to generate locomotor patterns demonstrates that afferent feedback related to locomotion is sufficient to produce oscillatory, rhythmic, alternating efferent output related to the phases of the stepcycle. However, further study is needed to understand the complexity of these patterns and the mechanisms that induce neural plasticity.
Also contributing to the complexity of the locomotor patterns is clonic EMG activity interspersed within the rhythmic bursts observed during stepping in individuals after spinal cord injury [14]. Clonus is oscillatory motor activity that requires non-specific, continuous afferent input [14;40;43;44]. Clonus can be isolated to a single joint and also be distributed among several segments of the lumbosacral spinal cord. It had been well accepted clinically that clonus was mediated by hyperexcitable stretch reflex as a consequence of loss of supraspinal input after neurologic injury [65;72;75;85;95;104], another indication that the requirement of higher centers was critical for human locomotion. Although a rapid stretch to the plantarflexors can induce clonus conceivably by Ia afferent input, a myriad of other afferent inputs can also activate clonus. After spinal cord injury, application of heat or cold, noxious stimuli, as well as simply brushing the skin can elicit clonus at a single segment or multiple segments [42;43]. Further, there is no significant correlation among muscle activity and muscle-tendon stretch during clonus [14]. The burst and intervening silent period durations of the oscillatory pattern of clonus are similar during manual stretch, standing and stepping indicating that the efferent output can be independent of the current proprioceptive environment.
However, clonus at different joint segments when activated simultaneously can be mediated by sensory input. Bilateral ankle clonus elicited by manual stretch in the soleus occurs at the same frequency but in an alternating pattern [14]. However when observed during weight bearing the right and left soleus muscles are activated synchronously and when the load is immediately removed the muscle activation alternates. These results suggest that load related afferent input interacts with the local networks to modulate the timing of their activation and consequently determine the efferent pattern generated. In addition, neurologically intact humans and non-human primates that return from space exhibit clonus in their lower limbs [96] suggesting that the loss of loading, not the loss of supraspinal input is the prominent mechanism of the development of these oscillatory patterns. These results suggest that clonus is generated by internuncial oscillatory networks with inhibitory and excitatory connections that are modulated by afferent input and are capable of interlimb coordination.
Interaction of sensory input with interneuronal networks
The activation of the lower limb motor neurons in humans is effectively modulated by descending supraspinal input and after spinal cord injury the extent of severity of injury is estimated from the movements that individuals can generate volitionally in isolated muscle groups [24;74;108;109]. This assumption implies that the limitation of voluntary control of individual muscle groups is the predominant factor that predicts the capacity for the recovery of walking thus emphasizing supraspinal control of locomotion. Even though the role of peripheral input in human locomotion in individuals without injury has been well documented [29;48;49], the presumption is that the effectiveness of these mechanisms is diminished after loss of supraspinal input [105;106]. However, recent evidence suggests that after human spinal cord injury, sensory input interacting with interneuronal networks plays a significant role in generating locomotor patterns [13;36;51;68;87].
In clinically incomplete and complete individuals with spinal cord injury, stepping resulted in improved motor recruitment and reciprocity between agonists and antagonists when compared to voluntary efforts intended to produce similar movements of flexion and extension of the hip, knee and ankle [87]. The level of activation of the motor pools of the legs during stepping exceeded that generated voluntarily in the absence of weight bearing. Individual muscles that could not be activated during attempts to flex or extend a single-joint or during multi-joint movements became active during stepping. Individuals with incomplete spinal cord injury with minimal residual supraspinal motor input to the spinal cord could initiate entire flexion or extension patterns, but had a limited capacity to selectively activate specific muscles of a single joint or simultaneously inhibit the antagonist. However, during stepping, alternating flexion and extension of the hip, knee and ankle occurred in these individuals indicating that the specific proprioceptive feedback related to stepping improved the efferent output. It cannot be determined in the individuals with incomplete spinal cord injury whether the motor pool recruitment during stepping was due to enhanced supraspinal control, complex processing of interneuronal networks of appropriate sensory input, or interactive spinal and supraspinal mechanisms. However, in individuals with clinically complete spinal cord injury the motor pool activation must have been generated by sensory input derived from the kinematics and kinetics of the limb interacting with spinal networks.
Sensory input provides critical information to facilitate the generation of locomotor-like output by the functionally isolated human spinal cord [13;32;46;51;68;87]. Muscle-tendon length changes influence the amplitude of EMG activity in individuals with compromised supraspinal input [13;14;68;102] but cannot alone generate the level of EMG activity observed during stepping [103] The level of loading during stepping provides specific afferent cues that result in significant increases in the amplitude of motor pool activity of extensors and flexors independent of the level of supraspinal input available to the spinal networks [31;36;68;83]. Even weight bearing without movement of one leg can elicit rhythmic EMG activity timed to the loading pattern during unilateral stepping [51]. These observations are consistent with locomotor dependent group I excitation of extensors extensively studied in other species and attributed to spinally mediated mechanisms [61;88;90].
Modulation of the level of EMG activation also occurs in individuals with spinal cord injury at the same loading condition within a series of steps. This indicates that other afferent systems not closely linked to limb loading such as hip joint position [3;4;64;80-82], contralateral limb information [15;16], and cutaneous stimulation [1;2;47] may also modulate motor pool activation. Temporal and spatial distribution of load related input also plays an important role in the generation of motor patterns during stepping when supraspinal input is limited [13]. Velocity-dependent modulation of locomotor patterns was observed in individuals without detectable supraspinal input available to spinal networks. EMG amplitudes were higher and burst durations shorter in faster stepping velocities in individuals stepping regardless of available input from higher centers to the spinal networks; similar to observations during locomotion in spinalized [3;55;56] and intact animals [59]. These results demonstrate that the rate at which afferent input is delivered to the central nervous system may be important in more complex regulation of efferent patterns.
The spinal networks also have the capacity to modulate interlimb coordination [51]. The human spinal networks can utilize sensory information about contralateral leg movements and loading to increase muscle activation even when there is no limb movement. Movement and loading in one limb can produce rhythmic muscle activity in the other limb even when it is stationary and unloaded in individuals with clinically complete spinal cord injury. Load related information in the contralateral leg is necessary to produce these rhythmic patterns. In addition, when sudden changes occur in one leg there are immediate responses in the EMG pattern of the other leg (Fig. 2b). These results demonstrate that modulated rhythmic motor activity can be generated in the absence of immediate afferent feedback directly from the leg and without detectable supraspinal input. This indicates that spinal networks can integrate complex sensory information and modulate interlimb coordination during stepping suggesting mechanisms that may be similar to those observed in mammalian spinal interneuronal systems [20;77;78;79].
Our finding that EMG amplitude increased with limb loading, not with limb movement, was not observed in another study using robotics to provide the assistance of the hips and legs through the stepping movements on a treadmill [36]. They also studied a unilateral stepping condition with rhythmic limb loading on the stationary contralateral leg and found no rhythmic EMG bursts in the non-stepping leg with individuals with clinically complete spinal cord injury. Two significant differences between the studies were the slower speed of stepping and lower level of loading Faster stepping speeds and greater limb loads enhance sensory feedback to the spinal cord and increase muscle activation amplitudes in humans with clinically complete spinal cord injury [13;68]. This suggests that the closer stepping kinematics and kinetics are to normal walking; the stronger the influence of contralateral locomotor neural pathways on muscle activation. These results indicate that the patterns generated by the functionally isolated human spinal cord during stepping are not produced by a sequential series of responses to immediate afferent feedback but rather the ensemble of the sensory feedback that is continuously integrated and interpreted to culminate in the efferent output.
Functional reorganization of interneuronal networks with task specific practice
Repetitive presentation of specific sensory information can functionally reorganize the spinal networks even in the absence of supraspinal input. However the recovery of walking overground has not been successful in individuals with clinically complete spinal cord injury [23;32;33;66]. Spinal transected cats have the ability to regain hindlimb stepping on a treadmill with repetitive training [7;9;10;26;86] and this training is enhanced with application of perianal stimulation and drug application. The analogous studies in humans involve individuals with clinically complete spinal cord injury using manually assisted stepping using trainers [23;32;33;37] and more recently using robotic devices [22;36;70;76;97;112] without routine use of pharmacological agents or direct sacral stimulation. There appears to be consistency in the report of the literature that sustainable changes in the locomotor pattern are observed in individuals with clinically complete spinal cord injury, however the variability of the initial patterns, the complexity of the reorganization, and the inability to sustain complete independent stepping have made the task of interpreting these changes difficult.
The most consistent result in individuals with clinically complete spinal cord injury is their ability to significantly bear more weight during standing and stepping as the training progresses [23;32;33;37]. Leg extensor EMG has also been reported to increase in some individuals during stepping with training [23;35]. Direct evidence demonstrating whether this effect is due to a fundamental change in the interneuronal networks response to afferent stimuli or simply to the immediate feedback to greater loading on the leg cannot be attained since it is unsafe to test these individuals prior to training at full weight bearing due to muscle atrophy and bone weakness. However, indirect statistical methods suggest that higher extensor EMG amplitudes after locomotor training cannot be solely attributed to immediate response to load receptor feedback [23;35]. These increases in EMG amplitudes were not sustained when repetitive training was discontinued [111].
We recently reported preliminary results indicating more complex reorganization of the interneuronal networks after stand and step training (unpublished observations). Distinct patterns were observed among individuals who were trained to stand unilaterally, stand bilaterally or step providing evidence for activity-dependent plasticity that appears to be dependent on the pattern of repetitive kinematic and kinetic activity that is presented to the spinal networks. Individuals who were trained to either stand or step also showed phase dependent modulation of reflexes in the absence of detectable supraspinal influence during stepping. However, due to the complexity and variability of the initial locomotor patterns, the relatively low numbers of individuals that can be reasonably studied, the inability to control factors such as levels of anti-spasticity medication, time since injury, injury characteristics, age, gender, overall physical condition and other related factors, further investigation is needed. It is remarkable that consistent findings have been reported among individuals in different laboratories given the multitude of variables that cannot be controlled in the human model. Clearly task specific, activity-dependent plasticity can occur in the functionally isolated spinal cord months and even years after injury. Further studies are needed to understand the mechanisms underlying this plasticity and how to drive reorganization most effectively for functional improvement.
Clinical Implications
Locomotor Training is a rehabilitative strategy that has evolved from animal and humans studies focused on the neural plasticity of the spinal cord [5;6;8;28;35]. Task-specific sensorimotor stimulation that functionally reorganizes the central nervous system is the key to the effectiveness of Locomotor Training as a rehabilitative intervention. Evidence from animals and humans provides several practical parameters that can be modified by therapists to influence muscle activation patterns. Optimizing load-related and contralateral sensory input can be used during retraining of the nervous system and in instructing patients to take advantage of these sensory cues to improve motor output when attempting leg movement, standing and stepping. Also, the relationship between voluntary control of specific muscles and the ability to recover standing and walking is much lower than previously considered, especially in conditions where sensory input is optimized for the specific motor task [87]. The presence of clonus and spasticity may also now be considered as a positive indication that neural networks are active and have the potential for functional reorganization, rather than as a consequence of loss of supraspinal input that prohibits recovery of motor function that should be eliminated by pharmacological or surgical interventions [14;30].
Locomotor Training as a rehabilitative strategy has been successful for many people with acute and chronic incomplete spinal cord injury, however varied results are reported [12;38;45;50;52-54;58;71;73;107;110;112]. This variability may be due to the differences among therapists in the relative level of knowledge of the principles underlying retraining of the nervous system, their skill in applying these principles and the effectiveness of the decisions that are made to progress the recovery as well as the intensity and duration of the intervention. Therapists who are aware of the potential of the spinal networks and sensory signals to modulate muscle activation patterns will have the best chance of optimizing Locomotor Training for their patients. Implementing Locomotor Training in the clinic is in the relatively early stages of development and future efforts should focus on education, training and establishing standards. Optimizing the protocols for specific patient populations is continually evolving as we simultaneously learn more from ongoing studies. Efficient and effective translation of scientific and clinical evidence to routine clinical practice will take collaborative efforts among scientists, clinicians and administrators.
The functionally isolated human spinal cord has the capacity to generate locomotor patterns with appropriate afferent input. However, even those individuals with clinically complete spinal cord injury that show alternating and rhythmic EMG in hip, knee and ankle cannot sustain overground walking. Even though some independent steps can be taken on the treadmill with BWS this requires several minutes of manually assisted stepping before an adequate pattern emerges to sustain independent leg movements. Even when independent steps occur on the treadmill they cannot be sustained for longer than a few minutes. Overground it is physically prohibitive to the therapists to be able to introduce a series of manually assisted steps that provides the appropriate sensory inflow to initiate a sufficient locomotor pattern for independence. The relative amplitude of EMG activity is lower and alternation of flexors and extensors of the knee and ankle occur less often when compared to individuals with clinically incomplete spinal cord injury and non-disabled individuals. This suggests that although a significant control of locomotion can occur at the level of spinal interneuronal networks the level of sustainable excitability of these networks is still lacking presumably from the loss of supraspinal influence. Future studies should focus on approaches to increase the central state of excitability and may include neural repair strategies, pharmacological interventions [5;33;58;98;107] or epidural stimulation [69;91-93] in combination with Locomotor Training.
Acknowledgments
This work was supported by Christopher and Dana Reeve Paralysis Foundation HA2-0201-2B, Roman Reed Spinal Cord Injury Research Fund of California RR 04084532, and NIH 49209 and 16333. We gratefully acknowledge Claudia Angeli, Christie Ferreira, and Marie-Pascale Côte for support in manuscript preparation. We thank the research volunteers for their dedication and valuable contribution to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Abraham LD, Marks WB, Loeb GE. The distal hindlimb musculature of the cat cutaneous reflexes during locomotion. Exp Brain Res. 1985;58:594–603. doi: 10.1007/BF00235875. [DOI] [PubMed] [Google Scholar]
- 2.Andersson O, Forssberg H, Grillner S, Lindquist M. Phasic gain control of the transmission in cutaneous reflex pathways to motoneurones during ‘fictive’ locomotion. Brain Res. 1978;149:503–507. doi: 10.1016/0006-8993(78)90493-6. [DOI] [PubMed] [Google Scholar]
- 3.Andersson O, Grillner S. Peripheral control of the cat's step cycle. I. Phase dependent effects of ramp-movements of the hip during “fictive locomotion”. Acta Physiol Scand. 1981;113:89–101. doi: 10.1111/j.1748-1716.1981.tb06867.x. [DOI] [PubMed] [Google Scholar]
- 4.Andersson O, Grillner S. Peripheral control of the cat's step cycle. II. Entrainment of the central pattern generators for locomotion by sinusoidal hip movements during “fictive locomotion”. Acta Physiol Scand. 1983;118:229–239. doi: 10.1111/j.1748-1716.1983.tb07267.x. [DOI] [PubMed] [Google Scholar]
- 5.Barbeau H. Locomotor training in neurorehabilitation: emerging rehabilitation concepts. Neurorehabil Neural Repair. 2003;17:3–11. doi: 10.1177/0888439002250442. [DOI] [PubMed] [Google Scholar]
- 6.Barbeau H, Ladouceur M, Mirbagheri MM, Kearney RE. The effect of locomotor training combined with functional electrical stimulation in chronic spinal cord injured subjects: walking and reflex studies. Brain Res Brain Res Rev. 2002:274–291. doi: 10.1016/s0165-0173(02)00210-2. [DOI] [PubMed] [Google Scholar]
- 7.Barbeau H, Rossignol S. Effects of noradrenergic, serotonergic and dopaminergic drugs on teh initiation of locomotion in the adult spinal cat. Soc Neurocsi Abstracts. 1989;15:393. [Google Scholar]
- 8.Barbeau H, Blunt R. A novel interactive locomotor approach using body weight support to retrain gait in spastic paretic subjects. In: Wernig A, editor. Plasticity of Motorneuronal Connections. Elsevier Science Publishers; 1991. pp. 461–474. [Google Scholar]
- 9.Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- 10.Barbeau H, Rossignol S. Initiation and modulation of the locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs. Brain Res. 1991;546:250–260. doi: 10.1016/0006-8993(91)91489-n. [DOI] [PubMed] [Google Scholar]
- 11.Barbeau H, Wainberg M, Finch L. Description and application of a system for locomotor rehabilitation. Med Biol Eng Comput. 1987;25:341–344. doi: 10.1007/BF02447435. [DOI] [PubMed] [Google Scholar]
- 12.Behrman AK, Lawless-Dixon AR, Davis SB, Bowden MG, Nair P, Phadke C, Hannold EM, Plummer P, Harkema SJ, Behrman AK, Lawless-Dixon AR, Davis SB, Bowden MG, Nair P, Phadke C, Hannold EM, Plummer P, Harkema SJ. Locomotor training progression and outcomes after incomplete spinal cord injury. Phys Ther. 2005;85:1356–1371. [PubMed] [Google Scholar]
- 13.Beres-Jones JA, Harkema SJ. The human spinal cord interprets velocity-dependent afferent input during stepping. Brain. 2004;127:2232–2246. doi: 10.1093/brain/awh252. [DOI] [PubMed] [Google Scholar]
- 14.Beres-Jones JA, Johnson TD, Harkema SJ. Clonus after human spinal cord injury cannot be attributed solely to recurrent muscle-tendon stretch. Exp Brain Res. 2003;149:222–236. doi: 10.1007/s00221-002-1349-5. [DOI] [PubMed] [Google Scholar]
- 15.Berger W, Dietz V, Quintern J. Corrective reactions to stumbling in man: neuronal co-ordination of bilateral leg muscle activity during gait. J Physiol. 1984;357:109–125. doi: 10.1113/jphysiol.1984.sp015492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burke D, Dickson HG, Skuse NF. Task-dependent changes in the responses to low-threshold cutaneous afferent volleys in the human lower limb. J Physiol. 1991;432:445–458. doi: 10.1113/jphysiol.1991.sp018393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bussel B, Roby-Brami A, Azouvi P, Biraben A, Yakovleff A, Held P. Myoclonus in a patient with spinal cord transection. Brain. 1988;111:1235–1245. doi: 10.1093/brain/111.5.1235. [DOI] [PubMed] [Google Scholar]
- 18.Bussel B, Roby-Brami A, Neris OR, Yakovleff A. Evidence for a spinal stepping generator in man. Electrophysiological study. Acta Neurobiol Exp. 1996;56:465–468. doi: 10.55782/ane-1996-1149. [DOI] [PubMed] [Google Scholar]
- 19.Bussel B, Roby-Brami A, Yakovleff A, Bennis N. Late flexion reflex in paraplegic patients. Evidence for spinal stepping generator. Brain Res Bull. 1989;22:53–56. doi: 10.1016/0361-9230(89)90127-5. [DOI] [PubMed] [Google Scholar]
- 20.Cabaj A, Stecina K, Jankowska E. Same spinal interneurons mediate reflex actions of group Ib and group II afferents and crossed reticulospinal actions. J Neurophysiol. 2006;95:3911–3922. doi: 10.1152/jn.01262.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calancie B, Neilson T, Jacobs K, Willer G, Zych G, Green BA. Involuntary stepping after chronic spinal cord injury. Brain. 1994;117:1143–1159. doi: 10.1093/brain/117.5.1143. [DOI] [PubMed] [Google Scholar]
- 22.Colombo G, Joerg M, Schreier R, Dietz V. Treadmill training of paraplegic patients using a robotic orthosis. Journal of Rehabilitation Research and Development. 2000;37:693–700. [PubMed] [Google Scholar]
- 23.Colombo G, Wirz M, Dietz V. Effect of locomotor training related to clinical and electrophysiological examinations in spinal cord injured humans. Ann N Y Acad Sci. 1998;860:536–538. doi: 10.1111/j.1749-6632.1998.tb09097.x. [DOI] [PubMed] [Google Scholar]
- 24.Crozier KS, Cheng LL, Graziani V, Zorn G, Herbison GJ, Ditunno JF. Spinal cord injury: prognosis for ambulation based on quadriceps recovery. para. 1992;30:762–767. doi: 10.1038/sc.1992.147. [DOI] [PubMed] [Google Scholar]
- 25.Curt A, Dietz V. Electrophysiological recordings in patients with spinal cord injury: significance for predicting outcome. Spinal Cord. 1999;37:157–165. doi: 10.1038/sj.sc.3100809. [DOI] [PubMed] [Google Scholar]
- 26.de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Retention of hindlimb stepping ability in adult spinal cats after the cessation of step training. J Neurophysiol. 1999;81:85–94. doi: 10.1152/jn.1999.81.1.85. [DOI] [PubMed] [Google Scholar]
- 27.Dietz V. Neurophysiology of gait disorders: present and future applications. Electroencephalogr Clin Neurophysiol. 1997;103:333–355. doi: 10.1016/s0013-4694(97)00047-7. [DOI] [PubMed] [Google Scholar]
- 28.Dietz V, Harkema SJ. Locomotor activity in spinal cord-injured persons. J Appl Physiol. 2004;96:1954–1960. doi: 10.1152/japplphysiol.00942.2003. [DOI] [PubMed] [Google Scholar]
- 29.Dietz V. Role of peripheral afferents and spinal reflexes in normal and impaired human locomotion. Rev Neurol (Paris) 1987;143:241–254. [PubMed] [Google Scholar]
- 30.Dietz V. Locomotor recovery after spinal cord injury. TINS. 1997;20:346–347. doi: 10.1016/s0166-2236(97)89934-1. [DOI] [PubMed] [Google Scholar]
- 31.Dietz V. Evidence for a load receptor contribution to the control of posture and locomotion. Neuroscience and Biobehavioral Reviews. 1998;22:495–499. doi: 10.1016/s0149-7634(97)00035-3. [DOI] [PubMed] [Google Scholar]
- 32.Dietz V, Colombo G, Jensen L. Locomotor activity in spinal man. The Lancet. 1994;344:1260–1263. doi: 10.1016/s0140-6736(94)90751-x. [DOI] [PubMed] [Google Scholar]
- 33.Dietz V, Colombo G, Jensen L, Baumgartner L. Locomotor capacity of spinal cord in paraplegic patients. Ann Neurol. 1995;37:574–582. doi: 10.1002/ana.410370506. [DOI] [PubMed] [Google Scholar]
- 34.Dietz V, Gollhofer A, Kleiber M, Trippel M. Regulation of bipedal stance: dependency on “load” receptors. Exp Brain Res. 1992;89:229–231. doi: 10.1007/BF00229020. [DOI] [PubMed] [Google Scholar]
- 35.Dietz V, Leenders KL, Colombo G. Leg muscle activation during gait in Parkinson's disease: influence of body unloading. Electroencephalogr Clin Neurophysiol. 1997;105:400–405. doi: 10.1016/s0924-980x(97)00042-8. [DOI] [PubMed] [Google Scholar]
- 36.Dietz V, Muller R, Colombo G. Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain. 2002;125:2626–2634. doi: 10.1093/brain/awf273. [DOI] [PubMed] [Google Scholar]
- 37.Dietz V, Wirz M, Colombo G, Curt A. Locomotor capacity and recovery of spinal cord function in paraplegic patients: a clinical and electrophysiological evaluation. Electroencephalogr Clin Neurophysiol. 1998;109:140–153. doi: 10.1016/s0924-980x(98)00002-2. [DOI] [PubMed] [Google Scholar]
- 38.Dietz V, Wirz M, Curt A, Colombo G. Locomotor pattern in paraplegic patients: training effects and recovery of spinal cord function. Spinal Cord. 1998;36:380–390. doi: 10.1038/sj.sc.3100590. [DOI] [PubMed] [Google Scholar]
- 39.Dietz V, Wirz M, Jensen L. Locomotion in patients with spinal cord injuries. Phys Ther. 1997;77:508–516. doi: 10.1093/ptj/77.5.508. [DOI] [PubMed] [Google Scholar]
- 40.Dimitrijevic MR. Neurophysiology in spinal cord injury. para. 1987;25:205–208. doi: 10.1038/sc.1987.35. [DOI] [PubMed] [Google Scholar]
- 41.Dimitrijevic MR, Gerasimenko Y, Pinter MM. Evidence for a spinal central pattern generator in humans. Ann N Y Acad Sci. 1998;860:360–376. doi: 10.1111/j.1749-6632.1998.tb09062.x. [DOI] [PubMed] [Google Scholar]
- 42.Dimitrijevic MR, Nathan PW. Studies of spasticity in man. 2. analysis of stretch reflexes in spasticity. Brain. 1967;90:333–358. doi: 10.1093/brain/90.2.333. [DOI] [PubMed] [Google Scholar]
- 43.Dimitrijevic MR, Nathan PW, Sherwood AM. Clonus: the role of central mechanisms. Journal of Neurology. 1980;43:321–332. doi: 10.1136/jnnp.43.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dimitrijevic MR, Sherwood AM, Nathan PW. Clonus: peripheral and central mechanisms. Prog Clin Neurophysiol. 1978;5:173–182. [Google Scholar]
- 45.Dobkin B, Apple D, Barbeau H, Basso M, Behrman A, Deforge D, Ditunno J, Dudley G, Elashoff R, Fugate L, Harkema S, Saulino M, Scott M. Weight-supported treadmill vs over-ground training for walking after acute incomplete spinal cord injury. Neurology. 2006:484–493. doi: 10.1212/01.wnl.0000202600.72018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dobkin BH, Harkema SJ, Requejo PS, Edgerton VR. Modulation of locomotor-like EMG activity in subjects with complete and incomplete spinal cord injury. J Neurol Rehabil. 1995;9:183–190. [PubMed] [Google Scholar]
- 47.Duysens J. Reflex control of locomotion as revealed by stimulation of cutaneous afferents in spontaneously walking premammillary cats. J Neurophysiol. 1977;40(4):737–751. doi: 10.1152/jn.1977.40.4.737. [DOI] [PubMed] [Google Scholar]
- 48.Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev. 2000;80:83–133. doi: 10.1152/physrev.2000.80.1.83. [DOI] [PubMed] [Google Scholar]
- 49.Duysens J, van de Crommert HW. Neural control of locomotion; Part 1: The central pattern generator from cats to humans. Gait and Posture. 1998;7:131–141. doi: 10.1016/s0966-6362(97)00042-8. [DOI] [PubMed] [Google Scholar]
- 50.Effing TW, van Meeteren NL, van Asbeck FW, Prevo AJ. Body weight-supported treadmill training in chronic incomplete spinal cord injury: a pilot study evaluating functional health status and quality of life. Spinal Cord. 2006:287–296. doi: 10.1038/sj.sc.3101841. [DOI] [PubMed] [Google Scholar]
- 51.Ferris DP, Gordon KE, Beres-Jones JA, Harkema SJ. Muscle activation during unilateral stepping occurs in the nonstepping limb of humans with clinically complete spinal cord injury. Spinal Cord. 2004;42:14–23. doi: 10.1038/sj.sc.3101542. [DOI] [PubMed] [Google Scholar]
- 52.Field-Fote EC. Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord injury. Arch Phys Med Rehabil. 2001;82:818–24. doi: 10.1053/apmr.2001.23752. [DOI] [PubMed] [Google Scholar]
- 53.Field-Fote EC, Lindley SD, Sherman AL. Locomotor Training Approaches for Individuals with Spinal Cord Injury: A Preliminary Report of Walking-related Outcomes. Journal of Neurological Physical Therapy. 2005;29:127–137. doi: 10.1097/01.npt.0000282245.31158.09. [DOI] [PubMed] [Google Scholar]
- 54.Field-Fote EC, Tepavac D. Improved intralimb coordination in people with incomplete spinal cord injury following training with body weight support and electrical stimulation. Phys Ther. 2002;82:707–15. [PubMed] [Google Scholar]
- 55.Forssberg H, Grillner S. The locomotion of the acute spinal cat injected with clonidine i.v. Brain Res. 1973;50:184–186. doi: 10.1016/0006-8993(73)90606-9. [DOI] [PubMed] [Google Scholar]
- 56.Forssberg H, Grillner S, Halbertsma J, Rossignol S. The locomotion of the low spinal cat. II. interlimb coordination. Acta Physiol Scand. 1980;108:283–295. doi: 10.1111/j.1748-1716.1980.tb06534.x. [DOI] [PubMed] [Google Scholar]
- 57.Fouad K, Pearson K. Restoring walking after spinal cord injury. Prog Neurobiol. 2004:107–126. doi: 10.1016/j.pneurobio.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 58.Fung J, Stewart JE, Barbeau H. The combined effects of clonidine and cyproheptadine with interactive training on the modulation of locomotion in spinal cord injured subjects. J Neurol Sci. 1990;100:85–93. doi: 10.1016/0022-510x(90)90017-h. [DOI] [PubMed] [Google Scholar]
- 59.Gardiner KR, Gardiner PF, Edgerton VR. Guinea pig soleus and gastrocnemius electromyograms at varying speeds, grades, and loads. J Appl Physiol. 1982;52:451–457. doi: 10.1152/jappl.1982.52.2.451. [DOI] [PubMed] [Google Scholar]
- 60.Gerasimenko YP, Makarovskii AN, Nikitin OA. Control of locomotor activity in humans and animals in the absence of supraspinal influences. Neurosci Behav Physiol. 2002:417–423. doi: 10.1023/a:1015836428932. [DOI] [PubMed] [Google Scholar]
- 61.Gossard JP, Floeter MK, Kawai Y, Burke RE, Chang T, Schiff S. Fluctuations of excitability in the monosynaptic reflex pathway to lumbar motoneurons in the cat. J Neurophysiol. 1994;72:1227–1239. doi: 10.1152/jn.1994.72.3.1227. [DOI] [PubMed] [Google Scholar]
- 62.Grillner S. In: Interaction between sensory signals and the central networks controlling locmotion in lamprey, dogfish, and cat. Grillner S, Stein PSG, Stuart DG, Forssberg F, Herman RM, editors. Vol. 45. Macmillan; London: 1986. pp. 505–512. [Google Scholar]
- 63.Grillner S. Handbook of Physiology: The Nervous System II. American Physiological Society; Bestheda, Md: 1981. Control of locomotion in bipeds, tetrapods, and fish; pp. 1179–1236. [Google Scholar]
- 64.Grillner S, Rossignol S. On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res. 1978;146:269–277. doi: 10.1016/0006-8993(78)90973-3. [DOI] [PubMed] [Google Scholar]
- 65.Hagbarth KE, Wallen G, Leofstedt L. Muscle spindle activity in man during voluntary fast alternating movements. J Neurol Neurosurg Psychiat. 1975;38:625–635. doi: 10.1136/jnnp.38.7.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harkema SJ. Neural plasticity after human spinal cord injury: application of locomotor training to the rehabilitation of walking. Neuroscientist. 2001;7:455–468. doi: 10.1177/107385840100700514. [DOI] [PubMed] [Google Scholar]
- 67.Harkema SJ, Dobkin BH, Edgerton VR. Pattern generators in locomotion: implications for recovery of walking after spinal cord injury. Topics in Spinal Cord Injury Rehabilitation. 2000;6:82–96. [Google Scholar]
- 68.Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- 69.Herman R, He J, D'Luzansky S, Willis W, Dilli S. Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord. 2002;40:65–68. doi: 10.1038/sj.sc.3101263. [DOI] [PubMed] [Google Scholar]
- 70.Hesse S, Schmidt H, Werner C, Bardeleben A. Upper and lower extremity robotic devices for rehabilitation and for studying motor control. Curr Opin Neurol. 2003;16:705–710. doi: 10.1097/01.wco.0000102630.16692.38. [DOI] [PubMed] [Google Scholar]
- 71.Hicks AL, Adams MM, Martin Ginis K, Giangregorio L, Latimer A, Phillips SM, McCartney N. Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic spinal cord injury: Effects on functional walking ability and measures of subjective well-being. Spinal Cord. 2005;43:291–298. doi: 10.1038/sj.sc.3101710. [DOI] [PubMed] [Google Scholar]
- 72.Hidler JM, Rymer WZ. A simulation study of reflex instability in spasticity: origins of clonus. IEEE Trans Rehabil Eng. 1999;7:327–340. doi: 10.1109/86.788469. In Process Citation. [DOI] [PubMed] [Google Scholar]
- 73.Hornby TG, Zemon DH, Campbell D. Robotic-assisted, body-weight-supported treadmill training in individuals following motor incomplete spinal cord injury. Phys Ther. 2005;85:52–66. [PubMed] [Google Scholar]
- 74.Hussey RW, Stauffer ES. Spinal cord injury: requirements for ambulation. Arch Phys Med Rehabil. 1973;54:544–547. [PubMed] [Google Scholar]
- 75.Iansek R. The effects of reflex path length on clonus frequency in spastic muscles. J Neurol Neurosurg Psychiat. 1984;47:1122–1124. doi: 10.1136/jnnp.47.10.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Israel JF, Campbell DD, Kahn JH, Hornby TG. Metabolic costs and muscle activity patterns during robotic- and therapist-assisted treadmill walking in individuals with incomplete spinal cord injury. Phys Ther. 2006;86:1466–1478. doi: 10.2522/ptj.20050266. [DOI] [PubMed] [Google Scholar]
- 77.Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- 78.Jankowska E, Edgley SA, Krutki P, Hammar I. Functional differentiation and organization of feline midlumbar commissural interneurones. J Physiol. 2005;565:645–658. doi: 10.1113/jphysiol.2005.083014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jankowska E, Hammar I. Spinal interneurones; how can studies in animals contribute to the understanding of spinal interneuronal systems in man? Brain Res Brain Res Rev. 2002;40:19–28. doi: 10.1016/s0165-0173(02)00185-6. [DOI] [PubMed] [Google Scholar]
- 80.Knikou M. Effects of changes in hip position on actions of spinal inhibitory interneurons in humans. Int J Neurosci. 2006;116:945–961. doi: 10.1080/00207450600675167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knikou M. Hip-phase-dependent flexion reflex modulation and expression of spasms in patients with spinal cord injury. Exp Neurol. 2007;204:171–181. doi: 10.1016/j.expneurol.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Knikou M, Conway BA. Modulation of soleus H-reflex following ipsilateral mechanical loading of the sole of the foot in normal and complete spinal cord injured humans. Neurosci Lett. 2001;303:107–110. doi: 10.1016/s0304-3940(01)01718-9. [DOI] [PubMed] [Google Scholar]
- 83.Kojima N, Nakazawa K, Yano H. Effects of limb loading on the lower-limb electromyographic activity during orthotic locomotion in a paraplegic patient. Neurosci Lett. 1999;274:211–213. doi: 10.1016/s0304-3940(99)00733-8. [DOI] [PubMed] [Google Scholar]
- 84.Kuhn RA. Functional capacity of the isolated human spinal cord. Brain. 1950;73(1):1–51. doi: 10.1093/brain/73.1.1. [DOI] [PubMed] [Google Scholar]
- 85.Latash ML, Penn RD, Corcos DM, Gottlieb GL. Short-term effects of intrathecal baclofen in spasticity. Exp Neurol. 1989;103:165–172. doi: 10.1016/0014-4886(89)90078-2. [DOI] [PubMed] [Google Scholar]
- 86.Lovely RG, Gregor R, Roy RR, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol. 1986;92:421–435. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- 87.Maegele M, Muller S, Wernig A, Edgerton VR, Harkema SJ. Recruitment of spinal motor pools during voluntary movements versus stepping after human spinal cord injury. J Neurotrauma. 2002;19:1217–1229. doi: 10.1089/08977150260338010. [DOI] [PubMed] [Google Scholar]
- 88.McCrea DA. Neuronal basis of afferent-evoked enhancement of locomotor activity. Ann N Y Acad Sci. 1998;860:216–225. doi: 10.1111/j.1749-6632.1998.tb09051.x. [DOI] [PubMed] [Google Scholar]
- 89.McCrea DA. Spinal circuitry of sensorimotor control of locomotion. J Physiol. 2001;533:41–50. doi: 10.1111/j.1469-7793.2001.0041b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCrea DA, Shefchyk SJ, Stephens MJ, Pearson KG. Disynaptic group I excitation of synergist ankle extensor motoneurons during fictive locomotion in the cat. J Physiol. 1995;487.2:527–539. doi: 10.1113/jphysiol.1995.sp020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Minassian K, Jilge B, Rattay F, Pinter MM, Binder H, Gerstenbrand F, Dimitrijevic MR. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord. 2004:401–416. doi: 10.1038/sj.sc.3101615. [DOI] [PubMed] [Google Scholar]
- 92.Minassian K, Jilge B, Rattay F, Pinter MM, Gerstenbrand F, Binder H, Dimitrijevic MR. Proc IFESS. Ljubljana, Slovenia: 2002. Effective spinal cord stimulation (SCS) for evoking stepping movement of paralyzed human lower limbs: study of posterior root muscle reflex responses. [Google Scholar]
- 93.Minassian K, Persy I, Rattay F, Dimitrijevic MR. Peripheral and central afferent input to the lumbar cord. Biocybernetics and Biomedical Engineering. 2005;25:11–29. [Google Scholar]
- 94.Pearson KG. Generating the walking gait: role of sensory feedback. Prog Brain Res. 2004;143:123–129. doi: 10.1016/S0079-6123(03)43012-4. [DOI] [PubMed] [Google Scholar]
- 95.Rack PMH, Ross HF, Thilmann AF. The ankle stretch reflexes in normal and spastic subjects. Brain. 1984;107:637–654. doi: 10.1093/brain/107.2.637. [DOI] [PubMed] [Google Scholar]
- 96.Recktenwald MR, Hodgson JA, Roy RR, Riazanski S, McCall GE, Kozlovskaya I, Washburn DA, Fanton JW, Edgerton VR. Effects of spaceflight on rhesus quadrupedal locomotion after return to 1G. J Neurophysiol. 1999;81:2451–2463. doi: 10.1152/jn.1999.81.5.2451. [DOI] [PubMed] [Google Scholar]
- 97.Reinkensmeyer DJ, Aoyagi D, Emken JL, Galvez JA, Ichinose W, Kerdanyan G, Maneekobkunwong S, Minakata K, Nessler JA, Weber R, Roy RR, de Leon R, Bobrow JE, Harkema SJ, Edgerton VR. Tools for understanding and optimizing robotic gait training. J Rehabil Res Dev. 2006;43:657–670. doi: 10.1682/jrrd.2005.04.0073. [DOI] [PubMed] [Google Scholar]
- 98.Remy-Neris O, Barbeau H, Daniel O, Boiteau F, Bussel B. Effects of intrathecal clonidine on spinal reflexes and on human locomotion in incomplete paraplegic subjects (IPS) Exp Brain Res. 1999;129:433–440. doi: 10.1007/s002210050910. [DOI] [PubMed] [Google Scholar]
- 99.Rybak IA, Shevtsova NA, Lafreniere-Roula M, McCrea DA. Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion. J Physiol. 2006;577:617–639. doi: 10.1113/jphysiol.2006.118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sherrington SCS. On the Innervation of Antagonistic Muscles. Sixth Note. Proc Roy Soc. 1900;66:66–75. [Google Scholar]
- 101.Sherwood AM, Dimitrijevic MR, McKay BW. Evidence of subclinical brain influence in clinically complete spinal cord injury: discomplete spinal cord injury. J Neurol Sci. 1992;110:90–98. doi: 10.1016/0022-510x(92)90014-c. [DOI] [PubMed] [Google Scholar]
- 102.Sinkjaer T, Andersen JB, Larsen B. Soleus stretch reflex modulation during gait in humans. J Neurophysiol. 1996;76:1112–1120. doi: 10.1152/jn.1996.76.2.1112. [DOI] [PubMed] [Google Scholar]
- 103.Stewart JE, Barbeau H, Gauthter L. Modulation of locomotor patterns and spasticity with clonidine in spinal cord injured patients. Can J Neurol Sci. 1991;18:321–332. doi: 10.1017/s0317167100031887. [DOI] [PubMed] [Google Scholar]
- 104.Szumski AJ, Burg D, Struppler A, Velho F. Activity of muscle spindles during muscle twitch and clonus in normal and spastic human subjects. Electroencephalogr Clin Neurophysiol. 1974;37:589–597. doi: 10.1016/0013-4694(74)90072-8. [DOI] [PubMed] [Google Scholar]
- 105.Vilensky JA, O'Connor BL. Stepping in nonhuman primates with a complete spinal cord transection: old and new data, and implications for humans. Ann N Y Acad Sci. 1998;860:528–530. doi: 10.1111/j.1749-6632.1998.tb09095.x. [DOI] [PubMed] [Google Scholar]
- 106.Vilensky JA, O'Connor BL. Stepping in humans with complete spinal cord transection: A phylogenetic evaluation. Motor Control. 1997;1:284–292. [Google Scholar]
- 107.Wainberg M, Barbeau H, Gauthier S. The effects of cyproheptadine on locomotion and on spasticity in patients with spinal cord injuries. J Neurol Neurosurg Psychiat. 1990;53:754–763. doi: 10.1136/jnnp.53.9.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Waters RL, Adkins RH, Yakura JS, Sie Ien. Motor and sensory recovery following incomplete paraplegia. Arch Phys Med Rehabil. 1994;75:67–72. [PubMed] [Google Scholar]
- 109.Waters RL, Yakura JS, Adkins RH. Gait performance after spinal cord injury. Clin Orthop Mar. 1993;288:87–96. [PubMed] [Google Scholar]
- 110.Wernig A, Müller S, Nanassy A, Cagol E. Laufband therapy based on “rules of spinal locomotion” is effective in spinal cord injured persons. Eur J Neurosci. 1995;7:823–829. doi: 10.1111/j.1460-9568.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 111.Wirz M, Colombo G, Dietz V. Long term effects of locomotor training in spinal humans. J Neurol Neurosurg Psychiatry. 2001;71:93–96. doi: 10.1136/jnnp.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wirz M, Zemon DH, Rupp R, Scheel A, Colombo G, Dietz V, Hornby TG. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Arch Phys Med Rehabil. 2005:672–680. doi: 10.1016/j.apmr.2004.08.004. [DOI] [PubMed] [Google Scholar]