Abstract
Prospective assessment of cardiovascular control in individuals with spinal cord injury (SCI) in response to active stand training. Cardiovascular parameters were measured at rest and in response to orthostatic challenge before and after training in individuals with clinically complete SCI. The goal of this study was to evaluate the effect of active stand training on arterial blood pressure and heart rate and changes in response to orthostatic stress in individuals with SCI. Measurements were obtained in individuals with SCI (n = 8) prior to and after 40 and 80 sessions of the standing component of a locomotor training intervention (stand LT). During standing, all participants wore a harness and were suspended by an overhead, pneumatic body weight support (BWS) system over a treadmill. Trainers provided manual facilitation as necessary at the trunk and legs. All individuals were able to bear more weight on their legs after the stand LT training. Resting arterial blood pressure significantly increased in individuals with cervical SCI after 80 training sessions. At the end of the training period, resting systolic blood pressure (BP) in individuals with cervical SCI in a seated position, increased by 24% (from 84 ± 5 to 104 ± 7 mmHg). Furthermore, orthostatic hypotension present in response to standing prior to training (decrease in systolic BP of 24 ± 14 mmHg) was not evident (decrease in systolic BP of 0 ± 11 mmHg) after 80 sessions of stand LT. Hemodynamic parameters of individuals with thoracic SCI were relatively stable prior to training and not significantly different after 80 sessions of stand LT. Improvements in resting arterial blood pressure and responses to orthostatic stress in individuals with clinically complete cervical SCI occurred following intensive stand LT training. These results may be attributed to repetitive neuromuscular activation of the legs from loading and/or conditioning of cardiovascular responses from repetitively assuming an upright posture.
Key words: cardiovascular control, orthostatic stress, spinal cord injury, stand training
Introduction
Cardiovascular control is severely disrupted after cervical and high thoracic spinal cord injury (SCI). Individuals with SCI can experience low resting arterial blood pressure (BP) or episodes of severe hypertension from autonomic dysreflexia throughout their lifetime (Krassioukov et al., 2006a; Mathias et al., 2002; Sheel et al., 2005; Teasell et al., 2000). These individuals can also experience episodes of orthostatic hypotension, characterized by significant decreases in systolic arterial BP in response to postural changes (Krassioukov et al., 2006a; Mathias, 1995). Symptoms of orthostatic hypotension commonly include fatigue or weakness, light-headedness, dizziness, blurred vision, dyspnea, and restlessness associated with cerebral hypoperfusion. This cardiovascular dysfunction can cause discomfort, and interfere with rehabilitation efforts and the ability to perform activities of daily living (Illman et al., 2000).
The effects of exercise on cardiovascular function are well known; however, individuals with clinically complete SCI are limited in the ability to activate the neuromuscular system below the level of lesion thus potentially reducing the cardiovascular benefits of exercise. Furthermore, cardiovascular responses that were deconditioned from lack of orthostatic challenges (assumption of a standing position) may also contribute to cardiovascular impairments. Pharmacological and other interventions including, abdominal binders, pressure stockings and functional electrical stimulation have been used to combat hypotension and low tolerance to orthostatic stress with limited success (Barber et al., 2000; Faghri et al., 2001, 2002; Mukand et al., 2001; Raymond et al., 1999).
Locomotor training (LT) is a rehabilitation strategy that allows individuals with SCI to repetitively practice standing and stepping using body weight support on a treadmill (BWST) with manual facilitation from therapists (Behrman et al., 2007). Animal and human studies have shown that spinal interneuronal networks specific to standing and stepping are highly dependent on continuous afferent feedback and can activate the neuromuscular system below the level of lesion (Barbeau et al., 1998, 2001, 2003; Barbeau, 2003; Behrman et al., 2000; Beres-Jones et al., 2004; Dietz et al., 2004; Dobkin et al., 2006; Edgerton et al., 2001; Harkema, 2008; Harkema et al., 1997; Maegele et al., 2002; Pratt et al., 1994; Rossignol et al., 1996). Previous studies have demonstrated that cats with a complete thoracic spinal transection were able to independently stand with activation of hind-limb extensors and flexors after repetitive task specific stand training (de Leon et al., 1998; Pratt et al., 1994). In the present study, we evaluated the changes in cardiovascular function resulting from intensive repetitive stand LT training in individuals with a clinically complete SCI.
Methods
Research participants
Eight individuals with SCI volunteered for this study (Table 1). The University of California, Los Angeles Institutional Review Board approved all experiments and each participant signed an informed consent before participating in the study. A clinician assessed the level and extent of injury according to the International Standards for Neurological Classification of SCI and the American Spinal Injury Association impairment scale (AIS) (Marino et al., 2003; Maynard, Jr., et al., 1997). Somatosensory evoked potential (SSEP) responses were recorded at the cortex during unilateral posterior tibial nerve electrical stimulation at the ankle. All individuals were classified with a clinically complete SCI, AIS grade A (no motor or sensory function below the lesion including the sacral segments S4–S5) and an absent SSEP. Participants had a stable medical condition and did not have a cardiopulmonary disease unrelated to their SCI. Individuals were not taking any antispasticity or vasoactive medication during the course of this study.
Table 1.
Subject Characteristics
| Subject ID | Sex | Age (years) | Injury level | AIS | Years post-injury | Stand LT |
|---|---|---|---|---|---|---|
| A19 | M | 55 | C5 | A | 14.7 | Unilateral |
| A20 | M | 24 | C5 | A | 0.7 | Bilateral |
| A21 | M | 27 | C5 | A | 4.9 | Bilateral |
| A26 | M | 48 | C6 | A | 29.5 | Unilateral |
| A24 | M | 41 | T4 | A | 2.1 | Bilateral |
| A23 | F | 26 | T4 | A | 7.4 | Bilateral |
| A22 | M | 28 | T6 | A | 0.8 | Unilateral |
| A28 | M | 21 | T6 | A | 0.8 | Unilateral |
ID, identification; C, cervical; T, thoracic; AIS, ASIA Impairment Scale; Stand LT, stand locomotor training.
Experimental design
Hemodynamic parameters (BP and heart rate [HR]) were recorded before (0 sessions), during (40 sessions), and after (80 sessions) stand LT. Measurements were obtained in three different positions: sitting, sitting with a harness and standing with a harness using body weight support (BWS). SCI individuals were fitted with a harness according to a previously established protocol that reported the effects of the harness application on the hemodynamic parameters (Krassioukov et al., 2006b).
Stand LT intervention
During the stand LT training session (60 min, five times weekly), SCI individuals wore a harness and were suspended by an overhead, pneumatic BWS system on a treadmill (BWST) (Innoventer, St. Louis, MO). The BWST was used to provide the maximum load possible while avoiding knee and trunk flexion. BWS was monitored and recorded from a load cell placed in series with the cable attached to the harness. BWS was continuously reduced over the course of the training as the individual increased the ability to bear weight with minimal or no assistance at the trunk, pelvis and legs. Four SCI individuals completed the bilateral stand LT (A20, A21, A23, and A24) and four individuals completed the unilateral stand LT (A19, A22, A26, and A28; Table 2).
Table 2.
Changes in Systolic (SBP), Diastolic (DBP), and Mean (MBP) Blood Pressures in Individuals with Cervical and Thoracic SCI in the Seated Position with a Harness (S-H) and Then Standing Position with a Harness (ST-H) Before and During Stand LT (Assessments at 40 and 80 Sessions)
| |
|
Prior to the training |
Session 40 |
Session 80 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SBP | DBP | MBP | SBP | DBP | MBP | SBP | DBP | MBP | ||
| Cervical SCI | S-H | 105 ± 12 | 66 ± 6 | 79 ± 8 | 104 ± 12 | 70 ± 10* | 81 ± 11 | 108 ± 8 | 75 ± 4 | 86 ± 4 |
| ST-H | 82 ± 7* | 57 ± 9* | 65 ± 8 | 100 ± 8*# | 64 ± 10* | 76 ± 9 | 108 ± 12# | 74 ± 9# | 85 ± 10 | |
| Thoracic SCI | S-H | 111 ± 12 | 76 ± 8 | 87 ± 9 | 117 ± 16 | 82 ± 6 | 93 ± 9 | 113 ± 11 | 79 ± 4 | 90 ± 4 |
| ST-H | 112 ± 9 | 82 ± 3 | 92 ± 5 | 117 ± 11 | 81 ± 8 | 93 ± 9 | 114 ± 5 | 79 ± 12 | 90 ± 9 | |
p < 0.05, comparison of cervical versus thoracic SBP, DBP, and MBP at each session interval (0, 40, and 80 sessions).
p < 0.05, within group (cervical or thoracic) comparisons of session interval (40 and 80 sessions) versus baseline (0 sessions).
SCI, spinal cord injury; LT, locomotor training.
A trainer positioned behind the subject assisted with pelvis and trunk stabilization by applying anterior forces at the pelvis and/or posterior forces at the shoulders while ensuring that the trunk and pelvis were not flexed or hyper-extended. Trainers assisted in maintaining dynamic knee extension by applying pressure at the tibial tuberosity and stimulation of the patellar and Achilles tendons to facilitate extensor activity of weight-bearing legs. Trainers promoted slight knee flexion in the extended limbs in order to avoid locking of the hips and knees as well as to stimulate neuromuscular activity. To further promote extensor muscle activation, the BWS was rapidly increased and decreased periodically during the training session.
During bilateral standing, load was distributed between both limbs, while during unilateral standing the ipsilateral limb assumed full loading in an extended position and the contralateral limb was maintained in a flexed position, similar to mid-swing.
During unilateral standing, flexor muscle activation was facilitated when needed by the unloaded leg stimulating the hamstrings and tibialis anterior tendons. All trainers provided facilitation only when needed to promote extensor muscle activity in both legs (bilateral group) or extensor activity in one leg and flexor activity in the other leg (unilateral group). Individuals actively participated in the stand LT session by maintaining an upright trunk, shifting their body to transfer load between limbs (bilateral standing) and maintaining knee extension.
EMG data acquisition and analyses
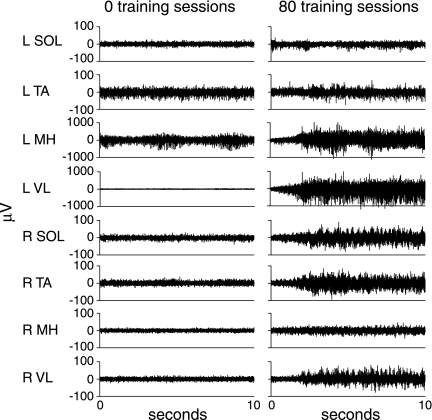
Following standard skin preparation, disposable bipolar recording electrodes of fixed inter-electrode distance (Multi BioSensors, El Paso, TX) were applied and secured with surgical adhesive tape. Bilateral surface EMG was recorded from the soleus (SOL), medial gastrocnemius (MG), tibialis anterior (TA), medial hamstrings (MH), and vastus lateralis (VL) in all individuals before and after training (Fig. 1). EMG signals were filtered with a cut-off frequency of 20–1000 Hz and sampled by an on-line analog to digital (A–D) acquisition system (National Instruments, Austin, TX) at 1000 Hz. The EMG amplifier system (Konigsberg Instruments, Pasadena, CA) was coupled to a pulse interval modulator that relayed data to decoding electronics. A customized LabVIEW (National Instruments, Austin, TX) software was used for acquiring and analyzing EMG data.
FIG. 1.
Representative electromyographic (μV) activity from the soleus (SOL), tibialis anterior (TA), medial hamstrings (MH), and vastus lateralis (VL) during standing in one individual (C5, AIS A) with cervical spinal cord injury (A20) before (0 sessions, 59% body weight support [BWS]) and after (80 sessions, 34% BWS) the stand locomotor training intervention.
Hemodynamic data acquisition and analyses
BPs were manually measured by the same examiner using a standard calibrated sphygmomanometer (Tycos 509 Mobile Aneroid, Welch Allyn, Inc.). HR was obtained using an oximeter (Nonin Onyx 9500, Nonin Medical Inc.) on the index finger of the opposite arm from the BP measurements. Individuals did not exercise, walk or propel their wheelchair for at least 5 min before the sitting hemodynamic measurement. During each event (i.e., sitting, sitting with harness, and standing with harness), BP and HR were recorded at 1-min intervals for a total of 5 min. Mean arterial pressure (MAP) was calculated for each BP measurement using the formula [DBP + (SBP − DBP)/3]. Orthostatic hypotension was identified as a reduction of at least 20 mmHg in systolic BP or 10 mmHg in diastolic BP within 3 min of standing (Consensus Committee, 1996).
Statistical analysis
A linear mixed effects (LME) model was fit for systolic and diastolic BP as measured in the sitting position. The fixed effects terms in the model were number of treatment sessions, patient type (cervical or thoracic), and the interaction of the two. The number of treatment sessions was treated as a factor rather than a regression parameter, to allow for the hypothesis tests to be conducted among and within the levels of the fixed effects terms. The intercepts in the model were treated as random effects. Models with the identical effect specification were fit for the change in systolic and diastolic pressure and HR under assumption of a standing position. All hypothesis tests derived from the linear mixed effects model were conducted at a significance level of 0.05. Statistical analysis was conducted using the R software package (R v. 2.6.2, The R Foundation for Statistical Computing, Vienna, Austria). Data is presented as mean ± standard deviation.
Results
Demographic data
Eight individuals with a cervical (n = 4) or thoracic level (n = 4) SCI participated in the study (Table 1). The majority of SCI individuals were male (88%), with an age range of 21–55 years (average: 33.8 ± 12.6 years). All individuals sustained a traumatic, clinically complete SCI, and the average time post-injury was 7.6 ± 10.1 years.
Effects of stand LT on body weight load and muscle activation
Leg loading increased with training (% BWS decreased) in the bilateral stand trained group (36 ± 11% BWS pre-training, 8 ± 6% BWS post-training) and the unilaterally stand trained group (58 ± 15% BWS pre-training, 34 ± 20% BWS post-training). All individuals increased extensor neuromuscular activity of the leg muscles during bilateral standing after the stand LT intervention as shown by exemplary data from a research participant with a cervical SCI (A20, Fig. 1).
Effect of stand LT on hemodynamic parameters at rest
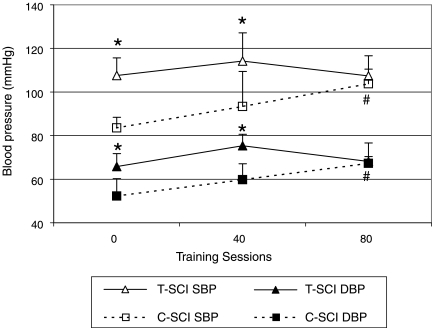
Cardiovascular parameters before (0 sessions), during (40 sessions), and after (80 sessions) the stand LT intervention were compared within a group (i.e., within the cervical or within the thoracic group) and across groups (i.e., cervical compared to thoracic individuals) during the seated position without a harness (Fig. 2). Average systolic and diastolic BPs were significantly lower in the cervical group when compared to the thoracic group before training (SBP/DBP 84 ± 5/52 ± 8 mmHg and 108 ± 8/66 ± 6 mmHg, respectively; p < 0.05) and after 40 training sessions (SBP/DBP 93 ± 16/60 ± 7 mmHg and 114 ± 13/75 ± 5 mmHg, respectively; p < 0.05). However, there were no significant differences after 80 training sessions. There were significant increases in systolic and diastolic BPs within the cervical group before and after 80 training sessions, however there were no significant changes from 0 to 40 sessions. At the end of the stand LT intervention, the sitting resting systolic BP in the cervical group increased by 20% (from pre- of 84 ± 5 mmHg, to post- of 104 ± 7 mmHg). The thoracic group did not exhibit significant changes in BP during sitting at any time point comparison (i.e., 0–40, or 40–80 sessions). Seated resting HR was not significantly different between the cervical and thoracic groups before (65 ± 5 and 75 ± 17 bpm, respectively) or after training (72 ± 12 and 75 ± 17 bpm, respectively). Furthermore, there were no significant differences in HR within a group across the different time points.
FIG. 2.
Effect of training on resting systolic and diastolic blood pressure in sitting position without the harness for individuals with cervical and thoracic spinal cord injury (SCI). Hemodynamic parameters were recorded prior to the training (0 sessions) and at the middle (40 sessions) and end of the active training (80 sessions). *p < 0.05, comparison of cervical versus thoracic systolic and diastolic blood pressures at each session interval (0, 40, and 80 sessions); #p < 0.05, within group (cervical or thoracic) comparisons of session interval (40 and 80 sessions) versus baseline (0 sessions).
Effect of training on postural changes in hemodynamic parameters
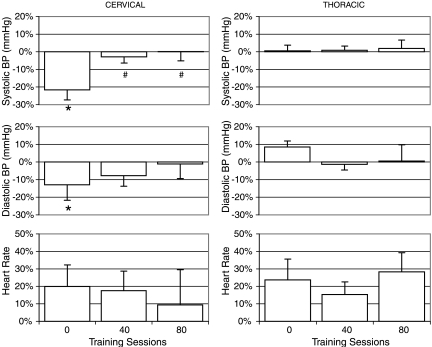
The harness application increases the resting BP parameters in the seated position only in individuals with a cervical SCI with no significant differences in HR (Krassioukov et al., 2006b). The degree of increase in systolic BP in sitting following harness application declined from 26 ± 13% (22 ± 11 mmHg) before training to 4 ± 6% (5 ± 5mmHg) after 80 training sessions.
Regardless of the effect of the harness application, individuals with a cervical injury also had a significant decrease in systolic BP (24 ± 14 mmHg) and diastolic BP (9 ± 13 mmHg) during orthostatic stress before the stand LT intervention (Fig. 3 and Table 2, compare S-H with ST-H). Following the stand LT intervention there was significant improvement of cardiovascular responses to standing in individuals with cervical SCI (Fig. 3 and Table 2). For example, in individuals with a cervical SCI, the average decrease of systolic BP was only 0 ± 11 mmHg, ranging from −10 to +15mmHg after 80 training sessions. However, individuals with a thoracic SCI demonstrated minimal changes in BP during orthostatic stress before (0 ± 6 mmHg) and after (2 ± 10 mmHg) training. There were no significant differences in HR between the cervical and thoracic group at any time point or within a group after 40 or 80 training sessions.
FIG. 3.
Changes in systolic and diastolic blood pressure and heart rate in individuals with cervical and thoracic spinal cord injury (SCI) during assumption of standing position (sit to stand with harness) at different intervals of training. Hemodynamic parameters were recorded prior to the training (0 sessions) and at the middle (40 sessions) and end of active training (80 sessions). *p < 0.05, comparison of cervical versus thoracic systolic and diastolic blood pressures at each session interval assessment (0, 40, and 80 sessions); #p < 0.05, comparison of systolic and diastolic blood pressures within group (cervical or thoracic) comparisons of session interval (40 and 80 sessions) versus baseline (0 sessions).
Case presentation
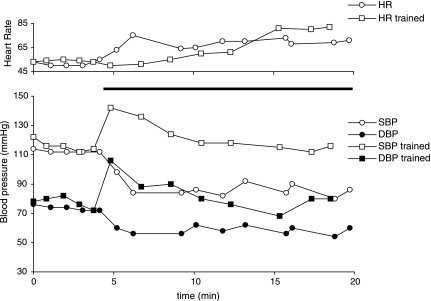
A 24-year-old, male, AIS A, with a C5 traumatic injury was enrolled in the study and had pronounced orthostatic hypotension prior to training (Fig. 4). His systolic BP decreased by 28 mmHg after 3 min of standing with the harness using the BWST. He also exhibited compensatory tachycardia of 20 bpm. When he started the stand LT intervention, he frequently experienced light headedness and dizziness and had to be returned to a seated or horizontal position. After 80 stand LT sessions, he demonstrated significant improvement in his cardiovascular response to orthostatic stress. Upon assumption of an upright position with the harness in the BWST, he exhibited a brief initial increase of systolic and diastolic BPs and was able to maintain stable arterial BPs throughout the 60 min of stand training session (data shown for first 20 min, Fig. 4). After training, this individual did not report any clinical signs of orthostatic intolerance that he experienced before the stand LT intervention.
FIG. 4.
Changes in systolic and diastolic blood pressure and heart rate in a 24-year-old man with AIS A, C5 injury during active standing before and after completion of course of training. Black bar indicates the period of assumption of the standing position with harness and body weight support (BWS).
Discussion
This study demonstrated that active stand LT can significantly improve hemodynamic parameters in individuals with chronic clinically complete cervical SCI. Specifically, we found that, following the training intervention, individuals with a cervical SCI demonstrated an increase in resting arterial BP. Stand LT also eliminated the orthostatic instability during their transfer from a seated to a standing position in all individuals with a cervical SCI. Furthermore, by the end of the training all individuals were able to maintain their BP while standing with BWS for one hour. Individuals with a midthoracic injury had stable hemodynamic parameters prior to training with no significant changes following stand LT.
Cardiovascular control is severely disrupted following SCI (Krassioukov et al., 2006b; Mathias et al., 2002; Teasell et al., 2000). Previously, it was demonstrated that low systolic BP is common in the acute period and could persist in individuals with chronic cervical SCI; and it has an inverse linear relationship with the level of SCI (Claydon et al., 2006a; Mathias, 1995; Mathias et al., 2002; Sidorov et al., 2008). The initial basal resting systolic BP was significantly lower in individuals with a cervical SCI (84 ± 5 mmHg) when compared to individuals with a thoracic SCI (108 ± 8 mmHg), consistent with previous studies (Claydon et al., 2006c; Dela et al., 2003; Krassioukov et al., 2006a,b). Furthermore, individuals with cervical SCI consistently demonstrated the presence of orthostatic hypotension following assumption of an upright position using the BWST prior to stand LT. The decrease in systolic BP of 24 ± 14 mmHg from sitting to standing with BWST was consistent with the orthostatic hypotension definition by the American Autonomic Society and the American Academy of Neurology (Consensus Committee, 1996). Orthostatic hypotension is a common problem in individuals with SCI, particularly in the acute phase (Corbett et al., 1971; Sidorov et al., 2008). Illman and colleagues reported that 73.6% of individuals with a SCI suffer from orthostatic hypotension during mobilization treatments (Illman et al., 2000). However orthostatic hypotension continues to be prominent in chronic SCI. In our recent study we documented that 50% of individuals with a chronic cervical SCI (>1 year after injury) still experience symptomatic orthostatic hypotension (Claydon et al., 2006a; Krassioukov et al., 2006a).
It is likely that persistent low resting BP, with episodes of orthostatic hypotension that are common in individuals with a cervical SCI, would have a deleterious effect upon their health. Illman and colleagues reported that the presence of orthostatic hypotension is not only a cause of discomfort for individuals with SCI but is also associated with a delay of rehabilitation (Illman et al., 2000). Furthermore, Duschek et al. (2003) demonstrated that even moderate resting hypotension in able bodied subjects (systolic pressure below 110 mmHg) is associated with deficits in cognitive performance. Finally, the presence of orthostatic hypotension is commonly associated with fatigue in the able bodied population (Freeman, 2003; Wessely et al., 1990) and neck pain in individuals with SCI (Cariga et al., 2002). Both of these factors can adversely impact an individual's quality of life and participation in rehabilitation.
Previously, numerous mechanisms that could contribute to the unstable cardiovascular control following SCI, including orthostatic hypotension, were identified. In our recent review, we discussed possible mechanisms of developing orthostatic hypotension following SCI and reported that the patho-physiology of this condition is likely to be multifactorial (Claydon et al., 2006b). First, the loss of the descending sympatho-excitatory control to the spinal autonomic circuits and reduction in sympathetic drive below the level of SCI could be identified as a leading cause of low resting BP and orthostatic hypotension following SCI. The venous pooling may be greater in paralyzed limbs of individuals with SCI than in able-bodied subjects in whom skeletal muscle pumping activity promotes venous return and maintains BP (Guy-ton et al., 1964). There is also an effect of cardiovascular deconditioning from inactivity in individuals with SCI, especially individuals with tetraplegia. Finally, the alterations in salt and water balance could lead to hypovolemia and predispose these individuals to orthostatic hypotension (Claydon et al., 2006b; Frisbie et al., 1997).
In a study by Dela et al. (2003), investigators reported that seated resting mean arterial BP in individuals with tetraplegia was remarkably low and estimated at 57 mmHg in tetra-plegics, 90 mmHg in paraplegics, and 96 mmHg in controls. Following passive leg movements while seated in a bike, arterial BP increased significantly in all groups (p < 0.05). The most pronounced effect was observed in individuals with tetraplegia, where BP reached levels comparable to the control group. However, this effect was only transient as the arterial BP dramatically declined despite the initiation of FES exercise in the lower extremities. Although there are obvious differences in their experimental paradigm with our study, there are some interesting comparisons between the observed hemodynamic responses. Similar to our study, the majority of individuals from the Dela study sustained a severe motor complete injury (AIS A and B). The baseline arterial BP of cervical and thoracic SCI individuals were similar between the two studies. Furthermore, similar to the tetraplegics in the Dela study, the individuals with cervical SCI in our study were unable to maintain the arterial BP following standing prior to repetitive stand LT (Fig. 3 and Table 2).
Beneficial effect of stand LT
A sedentary lifestyle with limited physical activity results in general deconditioning with pronounced deleterious effects on the cardiovascular system (Erikssen et al., 1998). Furthermore, Erikssen et al. reported that the level of physical fitness in healthy middle-aged men is a strong predictor of mortality and that even minor improvements are associated with a significantly lowered risk of death (Erikssen et al., 1998). Unfortunately, individuals with SCI, especially those with a high cervical injury, must adapt to a sedentary lifestyle because of paralysis. A sedentary lifestyle affects the already compromised cardiovascular system in SCI individuals, and could potentially lead to premature death. Numerous studies demonstrated that cardiovascular disease is now among the leading cause of death in individuals with SCI (DeVivo et al., 1999; Garshick et al., 2005). Therefore, increased physical activity achieved with stand LT that provides improvements in cardiovascular function may lead to improved health outcomes. Eng and co-investigators evaluated mailed survey questionnaires from 142 individuals with SCI regarding the effect of prolonged standing on their perceived health and well-being (Eng et al., 2001). Individuals with SCI who engaged in prolonged standing reported positive perceived health benefits that included circulation, bladder and bowel functions, pain control, and sleep. However, the most prevalent benefit was the feeling of well-being reported by 87% of the participants. The individuals in our study verbally reported less fatigue as well as increased well-being; however, we did not directly assess these parameters in our study.
One study of individuals with thoracic and cervical SCI reported increased femoral artery compliance but did not show improvements in baseline cardiovascular parameters after 4 months of body weight supported step training on a treadmill (Ditor et al., 2005). Several factors may have contributed to the difference in our results. In our study, we analyzed the thoracic and cervical SCI in separate groups (n = 4, thoracic; n = 4 cervical) while the other investigators combined the individuals (n = 6) with different injury levels. We found significant differences in resting baseline systolic and diastolic BPs between thoracic and cervical SCI individuals prior to training in this study and in previous studies (Claydon et al., 2006a; Krassioukov et al., 2006a), suggesting that combining these two SCI populations confounds the interpretation. Furthermore, the intensity of weight bearing was dramatically higher in our study with 60 min of weight bearing, five times weekly compared to only 15 min of weight bearing, three times weekly in their study (Ditor et al., 2005). The stand LT protocol in our study resulted in the ability of the SCI individuals to bear more weight while there was no improvement during the body weight supported step training protocol. This suggests that the intensity of weight bearing may be an important factor in improvement of cardiovascular function after SCI.
Improvement in orthostatic hypertensive responses during active (with functional electrical stimulation) standing but not passive standing using a static standing frame has been shown in individuals with SCI (Faghri et al., 2001; Jacobs et al., 2003). Faghri and co-investigators reported that the cervical SCI group had significantly lower systolic BPs and mean arterial BPs in response to orthostatic stress (passive standing) than the thoracic SCI group (Faghri et al., 2001). However, this difference was not detectable during active standing with functional electrical stimulation that activated the neuromuscular system below the level of lesion. Jacobs et al. (2003) reported lower HRs during passive standing than active standing, suggesting a higher cardiovascular demand during active standing. We have found similar results in the cervical and thoracic group in our study with stand LT that also activates the leg muscles by providing task specific sensory cues related to the kinematics and kinetics of weight bearing that activates spinal circuits to increase efferent motor activity. Activating large leg muscles conceivably increases venous return which is compromised after SCI. Whether spinal autonomic pathways are also activated by stand LT needs to be determined.
Limitations and future directions
One limitation of this study was the use of manual BP recordings. However, this is a standard, clinically acceptable and easily available method to assess cardiovascular function. Future studies should use continuous beat to beat monitoring to provide more precise evaluation of changes in cardiovascular parameters. The limited number of research participants is not optimal, however, it is common among human studies due to availability of SCI individuals at a given rehabilitation center. However, detailed quantitative information on relatively few individuals can provide insights to understanding the complexity of the complications of SCI. Another limitation is that we classified the severity of injury by motor and sensory function using the AIS (Marino et al., 2003) rather than assessment of the integrity of the spinal autonomic pathways (Krassioukov et al., 2007). However, this assessment is not presently established as a standard neurologic evaluation of SCI patients but should be considered in future studies. There are potential challenges to the widespread utilization of stand LT when considering the economic investment of training of clinicians, the BWST equipment required and that two to three trained individuals are needed to provide the activity-based intervention. However, LT for standing is less expensive than for stepping and the long-term benefits of improved cardiovascular function may exceed the cost to the health care system over the life span of the individual with a SCI. Future studies need to address the cost utility measures of stand LT and other activity-based therapies that require high intensity interventions.
Conclusion
Standing is considered beneficial for people with SCI who are confined to a wheelchair, as immobilization can contribute to secondary pathologies such as osteoporosis, leg muscle contractures, pressure sores, and muscle atrophy (Saitoh et al., 1996; Tsuzuku et al., 1999). Standing frames are the most viable option for long term use in individuals with clinically complete SCI; however, improvements in cardiovascular control have not been reported, potentially because of minimal leg muscle activation. In our study, intensive stand LT that produced neuromuscular activity below the lesion improved cardiovascular function in individuals after cervical SCI. Activity-based therapies such as LT, that emphasize repetitive weight bearing and activation of the neuromuscular system below the level of lesion, may be important for improvements of secondary complications related to clinically complete SCI, even when recovery of walking is not realized.
Acknowledgments
We thank the research participants for their dedication and valuable contribution to this study. We would also like to acknowledge the expertise and support during the conduction of this study from Amy Budovitch, Janell Jones, Carie Zuzick, and other personnel from the Human Locomotion Research Center at UCLA. We acknowledge Douglas J. Lorenz for his expertise with statistical analysis. This work was supported by the Christopher and Dana Reeve Foundation (HA2-02012B), Roman Reed Spinal Cord Injury Research Fund of California (RR 04084532), and NIH (49209 and 16333; PI Dr. Harkema). We acknowledge the support of collaboration between our laboratories by the grant initiative of Christopher and Dana Reeve Foundation (PI Dr. Krassioukov).
Author Disclosure Statement
No competing financial interests exist.
References
- Barbeau H. Locomotor training in neurorehabilitation: emerging rehabilitation concepts. Neurorehabil. Neural Repair. 2003;17:3–11. doi: 10.1177/0888439002250442. [DOI] [PubMed] [Google Scholar]
- Barbeau H. Norman K. Fung J. Visintin M. Ladouceur M. Does neurorehabilitation play a role in the recovery of walking in neurological populations? Ann. N. Y. Acad. Sci. 1998;860:377–392. doi: 10.1111/j.1749-6632.1998.tb09063.x. [DOI] [PubMed] [Google Scholar]
- Barbeau H. Visintin M. Optimal outcomes obtained with body-weight support combined with treadmill training in stroke subjects. Arch. Phys. Med. Rehabil. 2003;84:1458–1465. doi: 10.1016/s0003-9993(03)00361-7. [DOI] [PubMed] [Google Scholar]
- Barbeau H. Fung J. The role of rehabilitation in the recovery of walking in the neurological population. Curr. Opin. Neurol. 2001;14:735–740. doi: 10.1097/00019052-200112000-00009. [DOI] [PubMed] [Google Scholar]
- Barber D.B. Rogers S.J. Fredrickson M.D. Able A.C. Midodrine hydrochloride and the treatment of orthostatic hypotension in tetraplegia: two cases and a review of the literature. Spinal Cord. 2000;38:109–111. doi: 10.1038/sj.sc.3100959. [DOI] [PubMed] [Google Scholar]
- Behrman A.L. Harkema S.J. Physical rehabilitation as an agent for recovery after spinal cord injury. Phys. Med. Rehabil. Clin. North Am. 2007;18:183–202. doi: 10.1016/j.pmr.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Behrman A.L. Harkema S.J. Locomotor training after human spinal cord injury: a series of case studies. Phys. Ther. 2000;80:688–700. [PubMed] [Google Scholar]
- Beres-Jones J.A. Harkema S.J. The human spinal cord interprets velocity-dependent afferent input during stepping. Brain. 2004;127:2232–2246. doi: 10.1093/brain/awh252. [DOI] [PubMed] [Google Scholar]
- Cariga P. Ahmed S. Mathias C.J. Gardner B.P. The prevalence and association of neck (coat-hanger) pain and orthostatic (postural) hypotension in human spinal cord injury. Spinal Cord. 2002;40:77–82. doi: 10.1038/sj.sc.3101259. [DOI] [PubMed] [Google Scholar]
- Claydon V.E. Krassioukov A.V. Orthostatic hypotension and autonomic pathways after spinal cord injury. J. Neurotrauma. 2006a;23:1713–1725. doi: 10.1089/neu.2006.23.1713. [DOI] [PubMed] [Google Scholar]
- Claydon V.E. Steeves J.D. Krassioukov A. Orthostatic hypotension following spinal cord injury: understanding clinical pathophysiology. Spinal Cord. 2006b;44:341–351. doi: 10.1038/sj.sc.3101855. [DOI] [PubMed] [Google Scholar]
- Claydon V. Hol A. Eng J. Krassioukov A. Cardiovascular responses and postexercise hypotension after arm cycling exercise in subjects with spinal cord injury. Arch. Phys. Med. Rehabil. 2006c;87:1106–1114. doi: 10.1016/j.apmr.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Consensus Committee. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology. 1996;46:1470. doi: 10.1212/wnl.46.5.1470. [DOI] [PubMed] [Google Scholar]
- Corbett J.L. Frankel H.L. Harris P.J. Cardiovascular responses to tilting in tetraplegic man. J. Physiol. 1971;215:411–431. doi: 10.1113/jphysiol.1971.sp009477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon R.D. Hodgson J.A. Roy R.R. Edgerton V.R. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J. Neurophysiol. 1998;80:83–91. doi: 10.1152/jn.1998.80.1.83. [DOI] [PubMed] [Google Scholar]
- Dela F. Mohr T. Jensen C.M. Haahr H.L. Secher N.H. Biering-Sørensen F. Kajær M. Cardiovascular control during exercise: insights from spinal cord-injured humans. Circulation. 2003;107:2127–2133. doi: 10.1161/01.CIR.0000065225.18093.E4. [DOI] [PubMed] [Google Scholar]
- DeVivo M.J. Krause J.S. Lammertse D.P. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch. Phys. Med. Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- Dietz V. Harkema S.J. Locomotor activity in spinal cord-injured persons. J. Appl. Physiol. 2004;96:1954–1960. doi: 10.1152/japplphysiol.00942.2003. [DOI] [PubMed] [Google Scholar]
- Ditor D.S. Kamath M.V. Macdonald M.J. Bugaresti J. McCartney N. Hicks A.L. Effects of body weight-supported treadmill training on heart rate variability and blood pressure variability in individuals with spinal cord injury. J. Appl. Physiol. 2005;98:1519–1525. doi: 10.1152/japplphysiol.01004.2004. [DOI] [PubMed] [Google Scholar]
- Dobkin B. Apple D. Barbeau H. Basso M. Behrman A. Deforge D. Ditunno J. Dudley G. Elashoff R. Fugate L. Harkema S. Saulino M. Scott M. Weight-supported treadmill vs. over-ground training for walking after acute incomplete SCI. Neurology. 2006;66:484–493. doi: 10.1212/01.wnl.0000202600.72018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duschek S. Weisz N. Schandry R. Reduced cognitive performance and prolonged reaction time accompany moderate hypotension. Clin. Auton. Res. 2003;13:427–432. doi: 10.1007/s10286-003-0124-4. [DOI] [PubMed] [Google Scholar]
- Edgerton V.R. de Leon R.D. Harkema S.J. Hodgson J.A. London N. Reinkensmeyer D.J. Roy R.R. Talmadge R.J. Tillakaratne N.J. Timoszyk W. Tobin A. Topical Review: retraining the injured spinal cord. J. Physiol. 2001;533:15–22. doi: 10.1111/j.1469-7793.2001.0015b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng J.J. Levins S.M. Townson A.F. Mah-Jones D. Bremner J. Huston G. Use of prolonged standing for individuals with spinal cord injuries. Phys. Ther. 2001;81:1392–1399. doi: 10.1093/ptj/81.8.1392. [DOI] [PubMed] [Google Scholar]
- Erikssen G. Liestol K. Bjornholt J. Thaulow E. Sandvik L. Erikssen J. Changes in physical fitness and changes in mortality. Lancet. 1998;352:759–762. doi: 10.1016/S0140-6736(98)02268-5. [DOI] [PubMed] [Google Scholar]
- Faghri P.D. Yount J. Electrically induced and voluntary activation of physiologic muscle pump: a comparison between spinal cord-injured and able-bodied individuals. Clin. Rehabil. 2002;16:878–885. doi: 10.1191/0269215502cr570oa. [DOI] [PubMed] [Google Scholar]
- Faghri P.D. Yount J.P. Pesce W.J. Seetharama S. Votto J.J. Circulatory hypokinesis and functional electric stimulation during standing in persons with spinal cord injury. Arch. Phys. Med. Rehabil. 2001;82:1587–1595. doi: 10.1053/apmr.2001.25984. [DOI] [PubMed] [Google Scholar]
- Freeman R. Treatment of orthostatic hypotension. Semin. Neurol. 2003;23:435–442. doi: 10.1055/s-2004-817727. [DOI] [PubMed] [Google Scholar]
- Frisbie J.H. Steele D.J. Postural hypotension and abnormalities of salt and water metabolism in myelopathy patients. Spinal Cord. 1997;35:303–307. doi: 10.1038/sj.sc.3100436. [DOI] [PubMed] [Google Scholar]
- Garshick E. Kelley A. Cohen S.A. Garrison A. Tun C.G. Gagnon D. Brown R. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43:408–416. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton A.C. Richardson T.Q. Langston J.B. Regulation of cardiac output and venous return. Clin. Anesth. 1964;3:1–34. [PubMed] [Google Scholar]
- Harkema S.J. Plasticity of interneuronal networks of the functionally isolated human spinal cord. Brain Res. Rev. 2008;57:255–264. doi: 10.1016/j.brainresrev.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema S.J. Hurley S.L. Patel U.K. Requejo P.S. Dobkin B.H. Edgerton V.R. Human lumbosacral spinal cord interprets loading during stepping. J. Neurophysiol. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- Illman A. Stiller K. Williams M. The prevalence of orthostatic hypotension during physiotherapy treatment in patients with an acute spinal cord injury. Spinal Cord. 2000;38:741–747. doi: 10.1038/sj.sc.3101089. [DOI] [PubMed] [Google Scholar]
- Jacobs P.L. Johnson B. Mahoney E.T. Physiologic responses to electrically assisted and frame-supported standing in persons with paraplegia. J. Spinal Cord Med. 2003;26:384–389. doi: 10.1080/10790268.2003.11753710. [DOI] [PubMed] [Google Scholar]
- Krassioukov A.V. Claydon V.E. The clinical problems in cardiovascular control following spinal cord injury: an overview. Prog. Brain Res. 2006a;152:223–229. doi: 10.1016/S0079-6123(05)52014-4. [DOI] [PubMed] [Google Scholar]
- Krassioukov A.V. Harkema S.J. Effect of harness application and postural changes on cardiovascular parameters of individuals with spinal cord injury. Spinal Cord. 2006b;44:780–786. doi: 10.1038/sj.sc.3101952. [DOI] [PubMed] [Google Scholar]
- Krassioukov A.V. Karlsson A.K. Wecht J.M. Wuermser L.A. Mathias C.J. Marino R.J. Assessment of autonomic dysfunction following spinal cord injury: rationale for additions to International Standards for Neurological Assessment. J. Rehabil. Res. Dev. 2007;44:103–112. doi: 10.1682/jrrd.2005.10.0159. [DOI] [PubMed] [Google Scholar]
- Maegele M. Muller S. Wernig A. Edgerton V.R. Harkema S.J. Recruitment of spinal motor pools during voluntary movements versus stepping after human spinal cord injury. J. Neurotrauma. 2002;19:1217–1229. doi: 10.1089/08977150260338010. [DOI] [PubMed] [Google Scholar]
- Marino R.J. Barros T. Biering-Sorensen F. Burns S.P. Donovan W.H. Graves D.E. Haak M. Hudson L.M. Priebe M.M. International standards for neurological classification of spinal cord injury. J. Spinal Cord Med. 2003;26(Suppl 1):S50–S56. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- Mathias C.J. Orthostatic hypotension: causes, mechanisms, and influencing factors. Neurology. 1995;45:S6–S11. [PubMed] [Google Scholar]
- Mathias C.J. Frankel H.L. Autonomic disturbances in spinal cord lesions. In: Mathias C.J., editor; Bannister R., editor. Oxford University Press; Oxford, UK: 2002. pp. 494–513. [Google Scholar]
- Maynard F.M., Jr. Bracken M.B. Creasey G. Ditunno J.F., Jr. Donovan W.H. Ducker T.B. Garber S.L. Marino R.J. Stover S.L. Tator C.H. Waters R.L. Wilberger J.E. Young W. International Standards for Neurological and Functional Classification of Spinal Cord Injury. American Spinal Injury Association. Spinal Cord. 1997;35:266–274. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- Mukand J. Karlin L. Barrs K. Lublin P. Midodrine for the management of orthostatic hypotension in patients with spinal cord injury: a case report. Arch. Phys. Med. Rehabil. 2001;82:694–696. doi: 10.1053/apmr.2001.22350. [DOI] [PubMed] [Google Scholar]
- Pratt C.A. Fung J. MacPherson J.M. Stance control in the chronic spinal cat. J. Neurophysiol. 1994;71:1981–1985. doi: 10.1152/jn.1994.71.5.1981. [DOI] [PubMed] [Google Scholar]
- Raymond J. Davis G.M. Bryant G. Clarke J. Cardiovascular responses to an orthostatic challenge and electrical-stimulation-induced leg muscle contractions in individuals with paraplegia. Eur. J. Appl. Physiol. Occup. Physiol. 1999;80:205–212. doi: 10.1007/s004210050583. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Chau C. Brustein E. Belanger M. Barbeau H. Trevor D. Locomotor capacities after complete and partial lesions of the spinal cord. Acta Neurobiol. Exp. 1996;56:449–463. doi: 10.55782/ane-1996-1148. [DOI] [PubMed] [Google Scholar]
- Saitoh E. Suzuki Y. Sonoda S. Fujitani J. Tomita Y. Chino N. Clinical experience with a new hip-knee-ankle-foot orthotic system using a medial single hip joint for paraplegic standing and walking. Am. J. Phys. Med. Rehabil. 1996;75:198–203. doi: 10.1097/00002060-199605000-00010. [DOI] [PubMed] [Google Scholar]
- Sheel A.W. Krassioukov A.V. Inglis J.T. Elliott S.L. Autonomic dysreflexia during sperm retrieval in spinal cord injury: influence of lesion level and sildenafil citrate. J. Appl. Physiol. 2005;99:53–58. doi: 10.1152/japplphysiol.00154.2005. [DOI] [PubMed] [Google Scholar]
- Sidorov E.V. Townson A.F. Dvorak M.F. Kwon B.K. Steeves J. Krassioukov A. Orthostatic hypotension in the first month following acute spinal cord injury. Spinal Cord. 2008;46:65–69. doi: 10.1038/sj.sc.3102064. [DOI] [PubMed] [Google Scholar]
- Teasell R.W. Arnold J.M. Krassioukov A. Delaney G.A. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch. Phys. Med. Rehabil. 2000;81:506–516. doi: 10.1053/mr.2000.3848. [DOI] [PubMed] [Google Scholar]
- Tsuzuku S. Ikegami Y. Yabe K. Bone mineral desity differences between paraplegic and quadriplegic patients: a cross-sectional study. Spinal Cord. 1999;37:358–361. doi: 10.1038/sj.sc.3100835. [DOI] [PubMed] [Google Scholar]
- Wessely S. Nickson J. Cox B. Symptoms of low blood pressure: a population study. BMJ. 1990;301:362–365. doi: 10.1136/bmj.301.6748.362. [DOI] [PMC free article] [PubMed] [Google Scholar]