Abstract
To protect viral particles from neutralization, sequestration, nonspecific adhesion, and mislocalization following systemic delivery, we have previously exploited the natural tumor-homing properties of antigen-specific CD8+ T cells. Thus, OT-I T cells, preloaded in vitro with the oncolytic vesicular stomatitis virus (VSV), can deliver virus to established B16ova tumors to generate significantly better therapy than that achievable with OT-I T cells, or systemically delivered VSV, alone. Here, we demonstrate that preconditioning immune-competent mice with Treg depletion and interleukin-2 (IL-2), before adoptive T-cell therapy with OT-I T cells loaded with VSV, leads to further highly significant increases in antitumor therapy. Therapy was associated with antitumor immune memory, but with no detectable toxicities associated with IL-2, Treg depletion, or systemic dissemination of the oncolytic virus. Efficacy was contributed by multiple factors, including improved persistence of T cells; enhanced delivery of VSV to tumors; increased persistence of OT-I cells in vivo resulting from tumor oncolysis; and activation of NK cells, which acquire potent antitumor and proviral activities. By controlling the levels of virus loaded onto the OT-I cells, adoptive therapy was still effective in mice preimmune to the virus, indicating that therapy with virus-loaded T cells may be useful even in virus-immune patients. Taken together, our data show that it is possible to combine adoptive T-cell therapy, with biological therapy (Treg depletion+IL-2), and VSV virotherapy, to treat established tumors under conditions where none of the individual modalities alone is successful.
INTRODUCTION
To protect viral vectors from the hazards of exposure to the circulation,1–8 we,9,10 and others,4,11–14 have proposed the use of cells to chaperone viral vectors into tumors. Melanomas are often infiltrated with T cells with specificity for tumor antigens,15–18 which can be expanded in vitro and adoptively transferred back to the patient where they traffic, at least to some degree, to tumors and directly kill tumor cells.16,18,19 Therefore, we have exploited the natural tumor-homing ability of antigen-specific T cells to carry replication-defective retroviral vectors encoding suicide9,20 or chemokine10 genes to target established tumors.21 As a murine model of adoptive T-cell therapy, we have used OT-I CD8+ T cells. These cells express a transgenic T-cell receptor specific for the SIINFEKL epitope of the ovalbumin protein which is presented in the context of the H-2 kb major histocompatibility complex class I molecule expressed by B16ova tumor cells.9,22 We went on to show that OT-I T cells could deliver a replication-competent, oncolytic virus to B16ova tumors on the basis that, at least in theory, only low levels of viral delivery would be required to initiate spreading infections to cover the tumor comprehensively.2,23,24 In these studies, we used vesicular stomatitis virus (VSV), a negative strand RNA Rhabdovirus, which replicates in the cytoplasm and is highly lytic.25,26 VSV infection of normal cells induces a potent type I interferon (IFN) response (IFN-α/β), which blocks viral replication and extinguishes the infection. However, many tumor cells have defects in their IFN response and are nonresponsive to exogenous IFN;25,27 hence, VSV infection induces little, or no IFN response, allowing free ranging spread, infection, and lysis of tumors.28–30 We showed that OT-I cells can deliver VSV to established B16ova tumors to achieve significantly better therapy than that achievable with OT-I T cells, or systemically delivered VSV alone.31 In addition, VSV loaded onto naive T cells could purge lymphoid organs of metastatic disease through viral release and oncolysis of metastatic B16 cells.32
Biological therapies which provide cytokine support for adoptively transferred T cells, as well lymphodepletion regimens which allow for their selective expansion, have shown great promise in both preclinical and clinical settings.17,18,33 Although interleukin-2 (IL-2) has been developed to support adoptive T-cell therapy, it is also associated with endothelial cell injury leading to vascular leak syndrome,34,35 mediated in part by effector lymphocytes36,37 which bind, and lyse, endothelial cells.38,39 On the basis of the hypothesis that IL-2-induced vascular leak syndrome would enhance access of systemically delivered viruses into tumors, we demonstrated that nontoxic doses of IL-2 led to improved localization of intravenously delivered VSV to subcutaneous tumors.40 Moreover, depletion of regulatory T cells (Treg) before IL-2 significantly enhanced tumor regressions.40 Therapy was mediated by “hyper-activated” NK/LAK cells, which induced vascular leak syndrome, had direct antitumor activity, and conditioned the tumor to facilitate increased viral replication, spread, and oncolysis.40
Here, we test the hypothesis that preconditioning with Treg depletion and IL-2 (ref. 40) will also enhance adoptive T-cell therapy using OT-I T cells loaded with VSV.31 The rationale for this is based on the proposal that (i) Treg depletion enhances adoptive T-cell therapy;17,18,33 (ii) IL-2 can support adoptive T-cell therapy, although dose-related toxicity can be severely limiting;34–37 (iii) the moderate vascular leak syndrome induced by this low-dose, nontoxic IL-2 (ref. 40) may enhance accumulation of T cells into the tumor, along with improved virus delivery;40 (iv) NK cell activation induced by Treg depletion/IL-2 may enhance efficacy through both direct NK-mediated antitumor effects, as well as by enhancing replication and spread of OT-I-released VSV through B16ova tumors.40
RESULTS
Treg depletion+IL-2 enhances therapy of antigen-specific cells preloaded with VSV
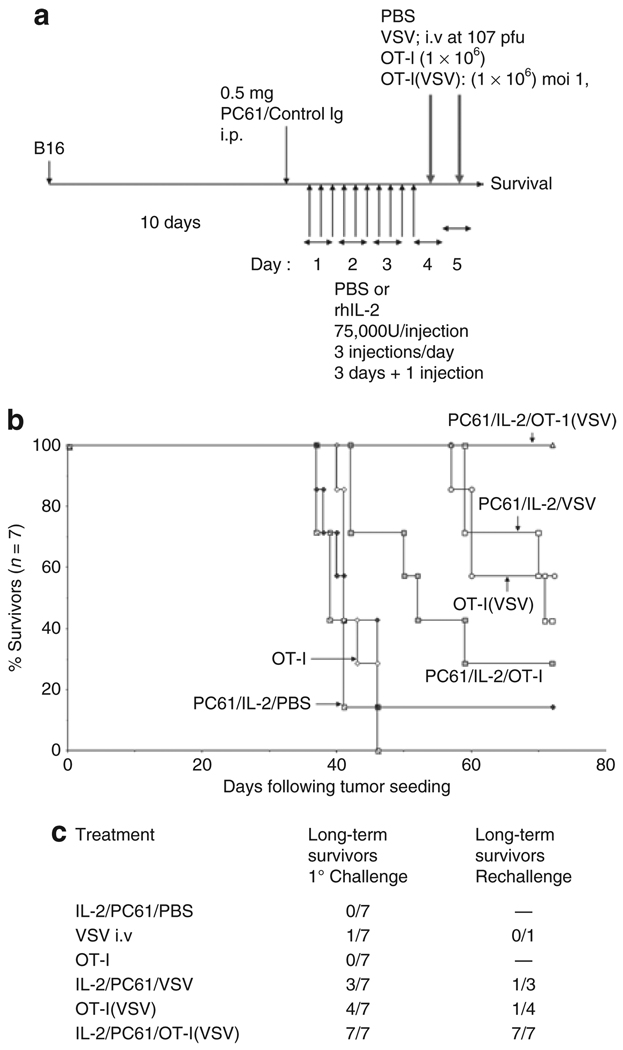
We modeled clinically suboptimal adoptive T-cell therapy with OT-I T cells by transferring levels of OT-I T cells that are noncurative for established B16ova tumors9,10,31,41 (Figure 1a, b). Consistent with our previous findings,31 adoptive transfer of OT-I T cells, preloaded with VSV (MOI 1) (OT-I(VSV)), generated significantly enhanced therapy compared to either the same dose of OT-I, or systemically administered VSV, alone (P < 0.01) (Figure 1a, b). Preconditioning with Treg depletion and IL-2 (ref. 40) (Figure 1a) (PC-61/IL-2/OT-I(VSV)) significantly improved efficacy of adoptive transfer of VSV-loaded OT-I T cells (P < 0.001) such that, in the experiment of Figure 1b, 100% of treated mice were cured. Treatment of mice with IgG/IL-2 or PC-61/PBS generated no significant therapy over OT-I alone, PC-61/IL-2/PBS, or untreated controls40 (not shown). Consistent also with our previous observations, although using a dose of VSV 1 log lower than reported in those studies,40 preconditioning with Treg depletion and IL-2 also significantly enhanced cell-free delivery of VSV (IL-2/PC-61/VSV) compared to VSV therapy alone (P < 0.02) or the PC-61/IL-2 regimen alone (P < 0.001) (Figure 1b). Over several experiments, OT-I associated delivery of 1 × 106 pfu of VSV, by 1 × 106 OT-I cells, was consistently significantly more effective than intravenous delivery of 107 pfu cell-free VSV with preconditioning with Treg depletion and IL-2 (Figure 1b). In addition, Treg depletion+IL-2 also significantly improved therapy with OT-I T cells alone (Figure 1b). In separate experiments, adoptive therapy with OT-I(VSV) was never significantly improved by either Control IgG/IL-2, or by PC-61/PBS, preconditioning (not shown), indicating that both Treg depletion and IL-2 are required for these therapeutic effects.
Figure 1. Treg depletion+IL-2 enhances tumor therapy using VSV-loaded OT-I T cells.
(a) C57Bl/6 mice were seeded with s.c. B16ova tumors. Ten days later, mice received an intraperitoneal injection of anti-CD25 antibody PC-61 or a control IgG. Twenty-four hours later, mice were injected intraperitoneally with PBS or with rhIL-2 at a dose of 75,000 U/injection three times a day for 3 days. On the fourth day, a single further injection of IL-2 was given. Two and twenty-four hours after this last injection of IL-2/PBS, mice received an intravenous injection of PBS; 107 pfu of VSV-GFP (VSV); 1 × 106 untreated OT-I (OT-I), 1 × 106 OT-I loaded with VSV at a MOI of 1 (OT-I(VSV)). The dose of 1 × 106 OT-I was selected as this is known to be nontherapeutic in this B16ova model.31,41 (b) Survival of tumor-bearing mice treated as shown (n = 7/group), and as described in a earlier, is shown with time following tumor seeding. Data are representative of three separate experiments. (c) Long-term survivors cured of tumor by the treatments shown (long-term survivors, 1° challenge) were rechallenged 60 days following the initial tumor challenge with 5 × 105 B16ova tumor cells subcutaneously along with a group of naive C57Bl/6 mice. All of the naive mice succumbed to tumor by day 35 after challenge. Numbers of mice surviving this rechallenge in each group are shown (long-term survivors, rechallenge).
Treg depletion+IL-2 enhances antitumor memory following OT-I(VSV) therapy
Whereas only a proportion of the long-term survivors from Figure 1b treated with OT-I(VSV) or PC-61/IL-2/VSV rejected a rechallenge with B16ova, 100% (seven of seven) of long-term survivors treated with PC-61/IL-2/OT-I(VSV) were protected (Figure 1c). None of the five naive mice challenged with the same cells survived long term in this experiment.
Treg depletion+IL-2 enhances persistence of CD8+ T cells in tumors
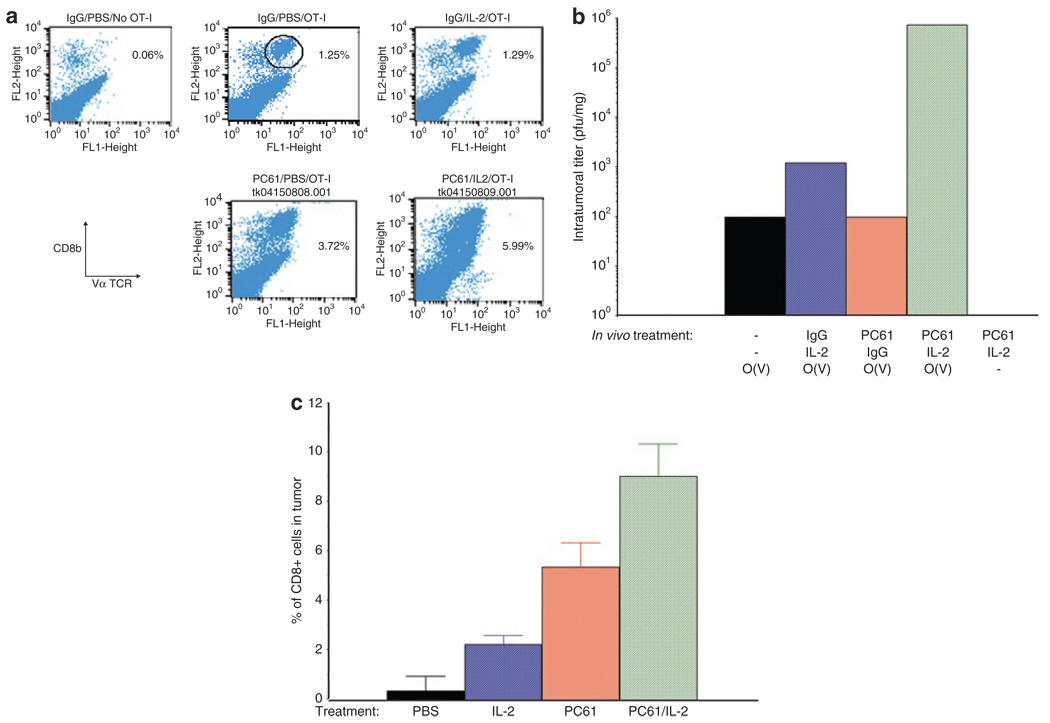
Although IL-2 alone did not significantly enhance levels of OT-I T-cell accumulation/persistence in the tumors, compared to mice treated with phosphate-buffered saline (PBS), Treg depletion induced about a twofold increase in levels of OT-I in subcutaneous (s.c.) B16ova tumors (Figure 2a). PC-61+IL-2 further enhanced OT-I levels by about fourfold over control levels (Figure 2a). Consistent with increased intratumoral levels of OT-I cell carriers (Figure 2a), the levels of VSV recovered from s.c. B16ova tumors were significantly enhanced by up to two logs, over two separate experiments, in mice preconditioned with Treg depletion and IL-2 compared to any other treatment (P < 0.001) (Figure 2b).
Figure 2. Treg depletion+IL-2 increases T-cell persistence and virus delivery.
(a) B16ova tumors were established subcutaneously in C57Bl/6 mice. Ten days later, following the regimen described in Figure 1a, mice received either a control IgG or anti-CD25 antibody PC-61; 1 day later this was followed by 10 i.p. injections of phosphate-buffered saline (PBS) or of rhIL-2 at a dose of 75,000 U/injection; 24 hours after the last injection of PBS or IL-2, mice received an intravenous injection of either PBS or of 106 OT-I cells. Tumors were harvested 48 hours later, dissociated and analyzed by flow cytometry for OT-I T cells, expressed as the percentage of cells from the tumors which labeled double positive for both CD8 and the Vα2 T-cell receptor expressed by OT-I transgenic mice. Data shown are from individual mice from groups of three mice/treatment and are representative of two separate experiments. (b) The experiment of a was repeated with mice treated with PC-61 and/or IL-2 as shown; 24 hours after the last injection of IL-2/PBS, groups received an intravenous injection of 106 OT-I loaded with VSV at an MOI of 1 (O(V)) or PBS (−). Forty-eight hours later, mice were euthanized and viral titers recovered from the freeze thaw/lysates of the tumors. Viral titers are the mean of two mice per group. Data shown are representative of two separate experiments. (c) The experiment of a was repeated with mice (3/group) treated with PBS, IL-2 alone, PC-61 alone, or PC-61+IL-2. Seventy-two hours after the last injection of IL-2/PBS, tumors were harvested, dissociated, and analyzed by flow cytometry for CD3+, CD8+ T cells. Values are expressed as the percentage of cells from the tumors which labeled double positive for both CD8 and CD3. Data are representative of two separate experiments.
We also observed highly significant increases in levels of endogenous CD8+ T cells within B16ova tumors following Treg depletion+IL-2 treatment compared to PBS-treated mice (P < 0.005) (Figure 2c). Unlike the accumulation of adoptively transferred OT-I, IL-2 alone increased levels of endogenous CD8+ T cells in B16ova tumors (Figure 2c). Similar to results of Figure 2a, predepletion of Treg also significantly increased endogenous CD8+ T cells in B16ova tumors (Figure 2c).
Treg depletion+IL-2 induces persistence of adoptively transferred OT-I cells
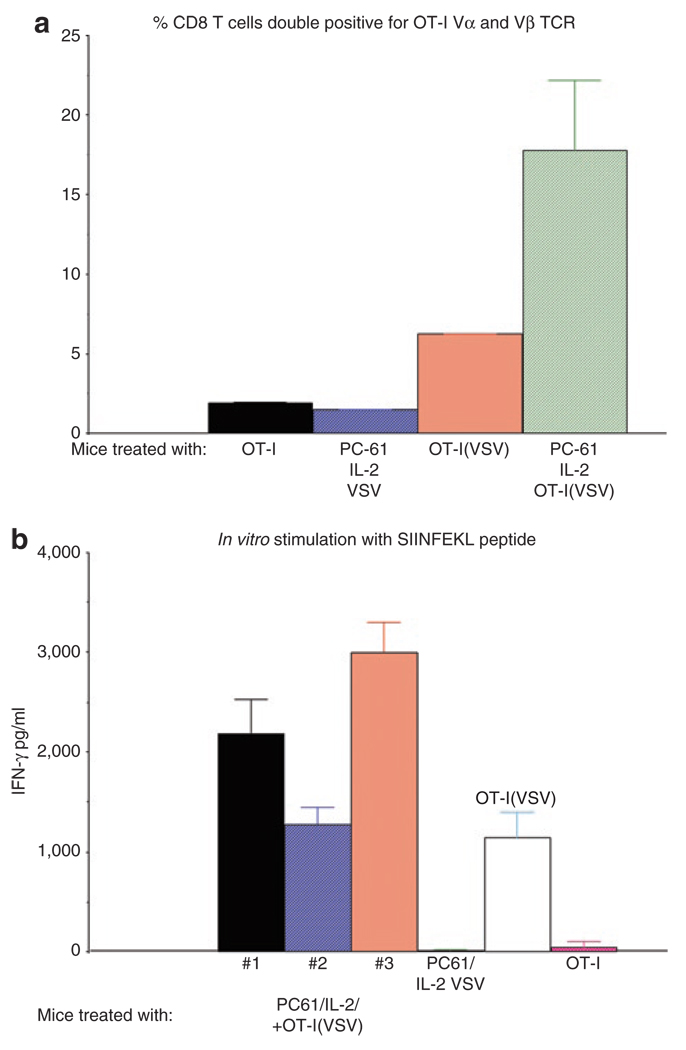
Consistent with the concept that lymphodepletion can enhance efficacy of adoptive T-cell therapy at least in part through depletion of Treg,17,33 we observed that mice preconditioned with Treg depletion+IL-2 before adoptive transfer of OT-I(VSV) cells consistently became reconstituted with OT-I T cells. Thus, only about 1% of all CD8+ T cells from nontumor-bearing mice treated with OT-I cells were double positive for the Vα2 and Vβ5 T-cell receptor chains which are expressed by the adoptively transferred OT-I T cells (Figure 3a); in contrast, between 15–25% of all CD8+ T cells from mice cured of B16ova tumors with PC-61/IL-2/OT-I(VSV) were Vα2+ve, Vβ5+ve, CD8+ (Figure 3a) (P < 0.001 relative to all other groups in Figure 3a). These data are consistent with these cells being derived from the adoptively transferred Vα2+ve, Vβ5+ve, CD8+ OT-I cells. Interestingly, adoptive transfer of OT-I(VSV) cells in the absence of Treg depletion and IL-2 treatment also led to partial reconstitution of long-term survivor mice, to levels of about 10% of all CD8+ T cells being OT-I-derived (Figure 3a) (P < 0.05 with respect to mice treated with OT-I alone). Long-term reconstitution with ova-reactive T cells following PC-61/IL-2/OT-I(VSV) therapy was confirmed by the observation of very high levels of reactivity to the class I major histocompatibility complex–restricted SIINFEKL epitope of OVA in splenocytes over 60 days following adoptive transfer, as evidenced by IFN-γ release upon in vitro stimulation (Figure 3b). Consistent with the FACS data of Figure 3a, splenocytes from one of the mice treated with OT-I(VSV) also contained ova-specific T cells (Figure 3b). However, splenocytes from mice which had received OT-I T cells alone (no tumor), or which had been treated with PC-61+IL-2 and received no OT-I, contained background levels of SIINFEKL-reactive T cells (Figure 3b).
Figure 3. PC-61+IL-2 promotes reconstitution of mice with adoptively transferred OT-I.
(a) C57Bl/6 mice were seeded with s.c. B16ova tumors. Ten days later, mice were treated as described in Figure 1a with the combinations of PC-61 + IL-2 + 1 × 106 OT-I loaded with VSV at an MOI of 1 (PC-61/IL-2/OT-I(VSV)); PC-61 + IL-2 + 107 pfu of cell-free VSV (PC-61/IL-2/VSV); or with control IgG + PBS + 1 × 106 OT-I loaded with VSV at a MOI of 1 (OT-I(VSV)). A further group was not seeded with tumor and received 1 × 106 OT-I cells (OT-I). Splenocytes were recovered from these mice at least 14 days following adoptive transfer and analyzed by FACS using antibodies which recognize the Vα and Vβ T-cell receptor chains expressed by OT-I cells. CD8+ cells were gated and values shown represent the % of CD8+ T cells which were double positive for the OT-I T-cell receptor (TCR) chains. (b) Splenocytes from the mice described in a were recovered from treated mice and, in triplicates, 250,000 were pulsed with the synthetic, H-2Kb-restricted Ova SIINFEKL peptide. Supernatants were assayed by ELISA for IFN-γ 48 hours later. Similar triplicates of splenocytes pulsed with an irrelevant control peptide did not secrete IFN-γ above background levels (not shown). Splenocytes of all three mice treated with PC-61/IL-2/OT-I(VSV) were positive and are shown; only the single mouse from the group treated with IgG/PBS/OT-I(VSV) which contained SIINFEKL-reactive splenocytes is shown; none of the mice treated with PC-61/IL-2/VSV, or with no tumor and OT-I alone, contained SIINFEKL-reactive splenocytes and representative values are shown from one mouse per group.
NK cells from PC-61+IL-2 treated mice enhance virus spread through intact, excised tumors
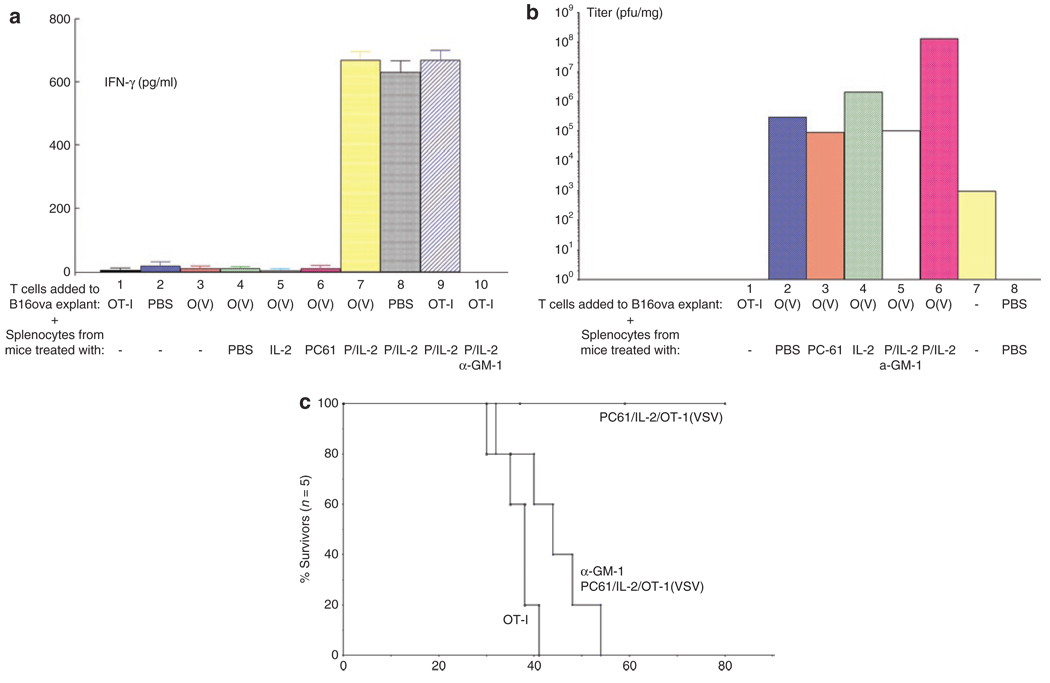
We established an in vitro model to test for antitumor activity of immune cells generated in vivo by treatment with PC-61+IL-2. Typically, in vitro–activated OT-I T cells, cocultured with target B16ova cells in vitro, secrete large amounts of IFN-γ (>1,000 pg/ml/106 OT-I/48 hours). However, when these in vitro–activated OT-I cells were cocultured with 15-day established B16ova tumors freshly explanted directly from C57Bl/6 mice, they secreted negligible levels of IFN-γ. (Figure 4a, lane 1), irrespective of whether the T cells were preloaded with VSV (Figure 4a, lane 3). Next, we investigated the effects of adding in splenocytes from mice treated in different ways on these OT-I/B16ova tumor explant cultures. When splenocytes were added to these cocultures from mice treated with PBS, IL-2, or PC-61 alone, no IFN-γ was detected (Figure 4a, lanes 4–6). In contrast, when splenocytes from mice treated with PC-61+IL-2 were cocultured with explanted B16ova tumors, IFN-γ was readily detected at high levels, irrespective of the presence (Figure 4a, lanes 7 and 9), or absence (Figure 4a, lane 8), of added OT-I cells. This antitumor activity disappeared when the splenocyte cultures came from mice previously depleted of NK cells (Figure 4a, lane 10). Thus NK cells in mice treated with PC-61+IL-2 secrete high levels of IFN-γ when cocultured with intact B16ova tumors under assay conditions where the tumor explants heavily suppress activated T cells (OT-I).
Figure 4. PC-61+IL-2 Generates antitumor effectors and promotes intratumoral viral replication.
(a) C57Bl/6 mice were seeded with s.c B16ova tumors. Fifteen days later, established tumors were excised and, without being dissociated, placed intact into wells of a 24-well tissue culture plate in DMEM. 106 OT-I cells (OT-I) (Lane 1), PBS (Lane 2), or 106 OT-I loaded with VSV at an MOI of 10 (O(V)) (lanes 3–7) were added to the wells containing explanted B16ova tumors. Wells also received 107 splenocytes recovered from separate C57Bl/6 mice, which had received an intraperitoneal injection of anti-CD25 antibody PC-61 (P) (lanes 6–10), or a control IgG (lanes 4 and 5), followed by 10 injections of rhIL-2 (IL-2) at a dose of 75,000 U/injection (lanes 5, 7–10). One group of mice, which was treated with PC-61+IL-2, also received an injection of the NK cell–depleting asialo GM-1 antibody 24 hours before treatment with PC-61 (lane 10). Forty-eight hours later, supernatants were removed from the wells containing the explanted B16ova tumors and assayed by ELISA for IFN-γ. (b) In a similar experiment to that described in a, explanted B16ova tumors were recovered from wells following coculture with OT-I alone (lane 1), or with OT-I cells loaded with VSV and splenocytes from mice treated with PBS (lane 2), PC-61 (lane 3), IL-2 (lane 4), or PC-61+IL-2 with (lane 5) or without (lane 6) prior NK cell depletion. The tumor explants were then dissociated and subjected to three rounds of freeze–thaw treatment. Two explanted tumors were treated with 107 pfu of cell-free VSV as a control for viral replication (VSV) without any added OT-I or splenocytes (lane 7). Mean virus titers from these freeze–thaw lysates are shown as pfu/mg tumor (2/3 per group). Representative of three separate experiments. (c) C57Bl/6 mice were seeded with s.c. B16ova tumors. Nine days later, one group of mice received an injection of the NK cell–depleting asialo GM-1 antibody (α-GM-1). Two other groups received a control IgG injection. Twenty-four hours later mice received an intraperitoneal injection of anti-CD25 antibody PC-61 or a control IgG. Twenty-four hours later, mice were injected intraperitoneally with PBS or with rhIL-2 at a dose of 75,000 U/injection three times a day for 3 days. On the fourth day, a single further injection of IL-2 was given. Two and twenty-four hours after this last injection of IL-2/PBS, mice received an intravenous injection of 1 × 106 untreated OT-I (OT-I) or 1 × 106 OT-I loaded with VSV at a MOI of 1 (OT-I(VSV)). Survival of tumor-bearing mice treated as shown (n = 5/group).
In the same assay, coculture of freshly explanted B16ova tumors with in vitro–activated OT-I cells, preloaded with VSV, led to significantly increased virus replication within the tumors (Figure 4b, lane 2) compared to that generated by simple coculture with cell-free virus (Figure 4b, lane 7) over three separate experiments (P < 0.002) (Figure 4b). Addition of splenocytes from IL-2 treated (Figure 4b, lane 4), but not PC-61-treated (Figure 4b, lane 3), mice could increase virus replication within the explanted B16ova tumors by a small, but significant extent (P < 0.05) (Figure 4b, lanes 2 and 4). However, addition of splenocytes from PC-61+IL-2;treated mice (Figure 4b, lane 6) greatly potentiated replication of OT-I-delivered VSV within explanted B16ova tumors compared to that seen in B16ova tumors cocultured with splenocytes from either IL-2-treated (P < 0.01) or PBS-treated mice (P < 0.001) (Figure 4b, lanes 2 and 4). Splenocytes from mice depleted of NK cells (Figure 4b, lane 5) no longer increased viral replication in explanted B16ova tumors over that seen with OT-I(VSV) alone (Figure 4b, lane 2). Therefore, both activated OT-I cells (Figure 4b, lane 2) and NK cells from mice treated with PC-61+IL-2 (Figure 4b, lanes 5 and 6) promote replication of VSV within tumor explants. Consistent with these data suggesting a role for host-derived NK cells in mediating potential antitumor effects, the curative effects of PC-61/IL-2/OT-I(VSV) treatment were completely lost in NK-depleted mice (Figure 4c).
Low MOI of virus loading evades neutralizing antibody responses
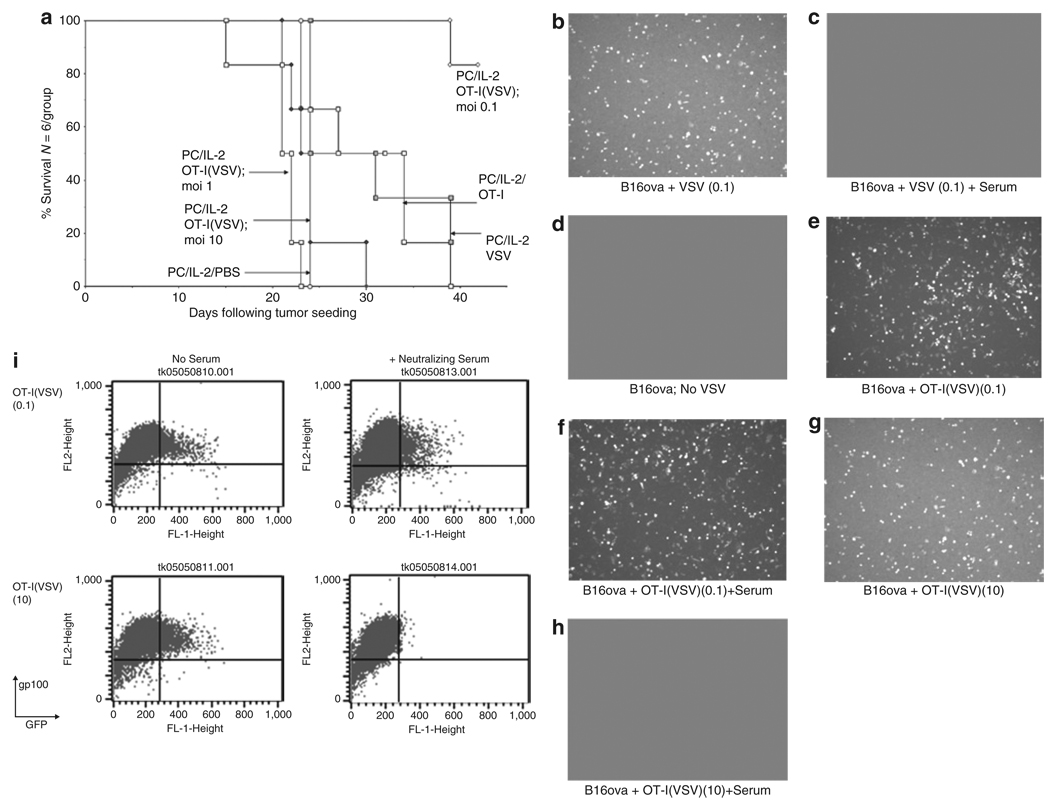
Finally, we tested the efficacy of T-cell mediated delivery in virus-immune hosts when high levels of anti-VSV NAb were present. In contrast to Figure 1b, antitumor therapy was completely lost in virus-immune mice treated with PC-61/IL-2/OT-I(VSV) in which virus was loaded onto the T cells at an MOI of either 10 or 1 (Figure 5a). In fact, therapy was significantly reduced compared to that with the same dose of PC-61/IL-2/OT-I T cells alone (Figure 5a), suggesting that preexisting viral immunity led to inactivation of any T cells upon which virus was exposed to circulating NAb. However, adoptive transfer of OT-I T cells preloaded with VSV at the lowest MOI of 0.1 was still able to generate significant antitumor therapy, despite the presence of high levels of antiviral immunity (Figure 5a). These data suggested that OT-I T cells may protect VSV particles from neutralization by NAb if they are present at a low enough threshold on the cells. To test this hypothesis, B16ova tumor cells were cocultured in vitro with OT-I cells preloaded with VSV in the presence or absence of NAb serum. Cell-free VSV infection of B16ova in vitro was completely neutralized by anti-VSV serum as assessed by transfer of GFP expression to gp100+ve B16ova tumor cells (Figure 5b–d). As reported previously,31 OT-I cells preloaded with VSV can release virus for productive infection of cocultured B16ova tumor cells (Figure 5e, f). Consistent with the in vivo results of Figure 5a, preincubation with neutralizing anti-VSV serum of OT-I cells loaded with VSV at a low MOI (0.1) did not significantly reduce transduction of B16ova tumor cells (Figure 5g, i). In contrast, preincubation with neutralizing anti-VSV serum of OT-I cells loaded with VSV at a higher MOI (10) completely abolished transduction of B16ova tumor cells (Figure 5h, i).
Figure 5. OT-I(VSV) are therapeutically effective in virus-immune mice at low MOI of virus loading.
(a) C57Bl/6 mice were vaccinated i.v. with 5 × 108 pfu VSV which induces high levels of both anti-VSV neutralizing antibody and anti-VSV T cells.32 Twenty-one days later, these virus-immune mice were seeded with s.c. B16ova tumors. Ten days later, all mice received an intraperitoneal injection of anti-CD25 antibody PC-61 followed 24 hours later by i.p. injections of rhIL-2 (75,000 U/injection) three times a day for 3 days. On the fourth day, a single further injection of IL-2 was given. Two and twenty-four hours after this last injection of IL-2, mice received an intravenous injection of PBS; or 107 pfu of VSV-GFP (VSV); or 1 × 106 untreated OT-I (OT-I); or 1 × 106 OT-I loaded with VSV at an MOI of 0.1, 1, or 10 (OT-I(VSV) moi 0.1, 1, or 10). Survival with time following initial tumor seeding is shown. (b–i) 2 × 104 pfu of VSV, 2 × 105 OT-I T cells, or 2 × 105 OT-I loaded with VSV at a MOI of 0.1 or 10 (OT-I(VSV) moi 0.1 or 10) were incubated at 37 °C for 1 hour in the presence of 100 µl of mouse control, or anti-VSV (neutralizing titer 1:2560), serum. Virus or cells were then added to 2 × 105 B16ova cells plated 24 hours previously. After 12 hours, cultures were examined for GFP expression by fluorescence microscopy (b–h) or by flow cytometry (i) for GFP and gp100, a melanoma-specific antigen32 not expressed by OT-I T cells. In all samples, <0.1% of the total cell populations analyzed were GFP+ve, gp100−ve, confirming our previous observation that VSV-GFP infection of OT-I T cells is minimal.31 Therefore, fluorescence shown in b–h represents infection of B16ova target cells by VSV-GFP rather than infected OT-I. At later time points after infection (>24 hours), B16ova cultures showed extensive cytotoxicity associated with VSV oncolysis in cultures b, e, f, and g. Minimal VSV-associated cytotoxicity was observed in cultures c, d, and h.
DISCUSSION
Here, we show that preconditioning of a fully immune-competent host with a regimen of Treg depletion and low, nontoxic, dose, IL-2 (ref. 40) leads to a highly significant increase in antitumor therapy using adoptive transfer of OT-I(VSV) cells compared to OT-I alone, OT-I(VSV), or OT-I cell therapy combined with Treg depletion+IL-2 (Figure 1b). Indeed, using levels of OT-I which alone have no significant therapy compared to PBS, 100% of treated mice were cured of established B16ova tumors by combining T-cell therapy with PC-61+IL-2 biological therapy and with VSV virotherapy (Figure 1b). Moreover, all of the cured mice developed effective antitumor immune memory (Figure 1c). Importantly, mice treated with PC-61+IL-2, along with OT-I (not loaded with VSV), survived significantly longer than mice treated with OT-I alone (Figure 1b). Therefore, efficacy of adoptive T-cell therapy is itself enhanced by Treg depletion+IL-2 and this contributes a significant component to the therapy seen in Figure 1b when adoptively transferred T cells are additionally loaded with oncolytic VSV.
PC-61+IL-2 treatment led to a fourfold increase in the levels of adoptively transferred OT-I T cells in subcutaneous tumors (Figure 2a). Interestingly, Treg depletion alone also significantly increased OT-I accumulation/persistence, perhaps mimicking lymphodepletion regimens in which Treg suppression of activated T-cell activity is removed.33 Increased persistence of OT-I induced by PC-61+IL-2 correlated well with increased levels of VSV recovered from B16ova tumors following adoptive transfer of OT-I(VSV) (Figure 2b). These intratumoral virus levels were also probably enhanced as a result of the activation of NK cells in vivo by PC-61/IL-2, which promote viral spread through the tumor (see following text and Figure 4b). Therefore, it seems likely that a significant component of the therapy is due to both better viral delivery to s.c. B16ova tumors and better intratumoral viral replication, spread, and oncolysis.
Treg depletion+IL-2 also significantly increased levels of endogenous CD8+ T cells infiltrating s.c. B16ova tumors (Figure 2c). Both IL-2 alone and Treg depletion alone also caused significant increases in CD8+ T-cell infiltration. Thus, IL-2-mediated expansion of endogenous CD8+ T cells, along with removal of Tregmediated suppression of their activity, may combine in vivo to enhance infiltration of endogenous T cells into tumors. Depletion studies are underway to determine the therapeutic role of these tumor-infiltrating CD8+ T cells.
The data of Figure 3a, b indicate that preconditioning with Treg depletion+IL-2, along with T cell–mediated delivery of VSV, generated long-term reconstitution of C57Bl/6 mice with the adoptively transferred T cells. Thus, up to 25% of all CD8+ T cells in the spleens of long-term tumor survivors expressed the Vα2 and Vβ5 T-cell receptor chains as expressed by the adoptively transferred OT-I T cells (Figure 3a). We have shown previously that VSV-mediated killing of B16ova tumors in situ generates a proinflammatory environment that both reverses the immunosuppressive environment of the tumor42 and leads to the release, and subsequent cross-presentation, of tumor-associated antigens, including OVA.42 We hypothesize that enhanced presentation of OVA by activated APC leads to persistent activation/proliferation of the adoptively transferred OT-I T cells, as well as their subsequent differentiation into a pool of memory cells, as reflected by the persistence of OT-I even in the absence of Treg depletion (Figure 3a). However, the long-term persistence of OT-I cells in mice treated with PC-61/IL-2 and OT-I(VSV) was significantly further enhanced(Figure 3a), presumably due to the decreased suppression of T-cell activity, and expansion, induced by additional Treg depletion (Figure 3a). It seems probable, therefore, that the continued persistence of these cells in vivo contribute significantly both to the therapeutic clearance of B16ova tumors (Figure 1b) and to the long-term immune protection observed in tumor survivors (Figure 1c).
The data from Figure 4a indicate that a population of NK cells, generated in mice treated with PC-61+IL-2, secrete high levels of IFN-γ when cocultured with intact B16ova tumors (Figure 4a, lane 8)—even though these tumor explants heavily suppress the ability of in vitro–activated T cells (OT-I) to secrete IFN-γ under the same assay conditions (Figure 4a, lane 1). This elevated NK cell activity may contribute to therapy in at least two ways. First, we have previously shown that PC-61/IL-2 treatment increases the infiltration of tumors with NK cells,40 and our current data suggest that these NK cells will have direct antitumor activity within infiltrated tumors. Second, it may be that expression of IFN-γ. in situ from NK cells will help to rescue effector functions of the OT-I T cells, which are suppressed by immunosuppressive tumors, and we are currently investigating this possibility. Moreover, the data from Figure 4b indicate that both activated OT-I cells (Figure 4b, lane 2) and NK cells generated in mice treated with PC-61+IL-2 (Figure 4b, lanes 5 and 6) promote the replication of VSV within intact tumor explants over that seen with cell-free virus (Figure 4b, lane 7). These findings are consistent with our finding that NK cells from PC-61+IL-2-treated mice secrete factors, such as Matrix Metalloproteinase-2, which disrupt tumor architecture and promote the replication of VSV, and its spread through intact B16 tumors.40 The importance of these NK-associated functions to overall therapy is underscored by the in vivo data showing that depletion of NK cells before PC-61/IL-2 conditioning abolished the therapy induced by OT-I(VSV) adoptive T-cell therapy (Figure 4c).
After a single administration of T cells carrying VSV, it is likely that NAb to the virus will be induced, which might inhibit the efficacy of subsequent doses of T cell–delivered VSV. Here, we demonstrate that VSV can be protected by the T cell from neutralizing serum both in vitro (Figure 5b–i) and in vivo (Figure 5a), but only if the virus is loaded at a level below a certain threshold concentration. We have previously confirmed that, under the loading conditions used here, <5% of OT-I T cells become infected with VSV and that there is no productive infection of the OT-I T cells as judged by release of viral progeny or de novo synthesis of new viral proteins.31 We have also speculated that VSV loaded onto the T cell becomes available for infection of tumor cells following dissociation from the surface of the cell once it has arrived at the tumor site.31,32 It may be, therefore, that virus is shielded from NAb by components of the T-cell surface, and that the binding sites that afford this protection can be saturated by high levels of virus—at which point NAb have access to the virus and neutralize both the virus and the carrier T cell. Consistent with this hypothesis, therapy with OT-I(VSV) at an MOI of 10 or 1 was significantly reduced compared to therapy with the same dose of PC-61/IL-2/OT-I T cells alone (Figure 5a), suggesting that preexisting viral immunity led to inactivation of the T cells upon which virus was exposed to circulating NAb. Although the MOI of virus loading onto OT-I is determined at the level of plaque-forming units in vitro, this is almost certainly an underrepresentation of the true level of viral particles/antigen present on the surface of loaded cells. Electron microscopy studies in our laboratory suggest that virus clumping may sequester multiple replication-competent particles within a single plaque-forming unit; in addition, we are investigating what levels of defective viral particles are present in our stocks of VSV. Therefore, the actual number of viral particles loaded on the T-cell surface, which triggers immune recognition in our studies, is probably significantly higher than the apparent level of between MOI 0.1 and 1.0. Studies to understand the mechanisms by which the T cell can protect the virus from a hostile neutralizing environment are ongoing. Nonetheless, these results indicate that adoptive T-cell therapy with virus-loaded T cells may be useful for repeated administrations even in patients who either have preexisting immunity to the virus or develop immunity following the first dose(s) of adoptive therapy.
In summary, we describe here a protocol that includes biological, cellular, and virological therapies. Significantly, although we observed tumor cures in 100% of mice in some experiments, we did not observe any toxicities associated with either IL-2, Treg depletion or systemic dissemination of the oncolytic virus. The fact that OT-I carrying low levels of virus can still be therapeutic, even in virus-immune mice (Figure 5a), suggests that direct viral oncolysis of target B16ova tumors is just one component, albeit critically important, of the overall therapeutic effects (Figure 1b, c and Figure 5a). Therefore, we hypothesize that therapeutic efficacy of the PC-61/IL-2/OT-I(VSV) regimen is contributed by multiple factors, including improved persistence of both OT-I and endogenous T cells in the tumor; enhanced delivery of VSV to tumors; cross presentation of tumor-derived antigens as a result of viral oncolysis leading to persistence of OT-I cells; and the activation of additional host factors, including NK cells, which acquire potent antitumor and proviral activities. For clinical translation it will be important to define effective reagents which can be used in humans to achieve the multiple separate effects that are important for therapy in this model. However, increasing experience with lymphodepleting regimens in conjunction with adoptive T-cell transfer,17,33 chemotherapeutic modulation of Treg,43 cytokine therapies to better support adoptively transferred T cells,44,45 and virotherapies23 suggest that such translation will soon be possible.
MATERIALS AND METHODS
Cells and viruses
Murine B16ova melanoma cells (H2-Kb) were derived from B16 cells by transduction with a cDNA encoding the chicken ovalbumin gene.46 Cell lines were grown in Dulbecco’s modified Eagle’s minimal essential medium (Life Technologies, Gaithersburg, MD) supplemented with 10% (vol/vol) fetal calf serum (Life Technologies) and L-glutamine (Life Technologies) and were free of Mycoplasma infection.
VSV-GFP was generated by cloning the cDNA for GFP into the plasmid pVSV-XN2, as described in ref. 28. VSV-GFP is referred to as VSV. Monoclonal VSV was obtained by plaque purification on BHK-21 cells. Concentration and purification were performed by sucrose gradient centrifugation. Titers were measured by standard plaque assays on BHK-21 cells.28
Preparation of activated OT-I cells
The OT-I mouse strain is on a C57BL background (H-2Kb) and expresses a transgenic T-cell receptor Vα2 specific for the SIINFEKL peptide of ovalbumin in the context of major histocompatibility complex class I, H2-Kb.22 Preparation of activated OT-I cells was done as described previously.9 Briefly, naive OT-I cells were isolated from spleen and lymph nodes. Red blood cells were lysed using ACK buffer (0.15 mol/l NH4Cl, 1.0 mmol/l KHCO3, 0.1 mmol/l EDTA, pH 7.2–7.4), and the dissociated single-cell suspension was grown in Iscove’s modified Dulbecco’s medium plus 5% FBS in the presence of 1 µg/ml SIINFEKL peptide, 50 µmol/l β-mercaptoethanol, 1% Pen/Strep, and 50 IU rIL-2/ml. Three days after activation, cells were harvested and purified through centrifugation in Lympholyte-M density gradient (Cedarlane). The cells were used for in vivo injection or for in vitro assays. These populations consisted typically of >98% CD8+ T cells, of which >90% of the cells expressed the Vα2 chain of the transgenic OT-I T cell receptor.
Loading of OT-I cells with VSV
OT-I cells were pelleted and incubated with VSV in 100 µl for 4 hours at 4 °C. Cells were washed three times in ice-cold PBS and either plated in tissue culture or used directly for in vivo adoptive transfer.
Treg depletion and IL-2 treatment
The regimen of Treg depletion and IL-2 treatment was described by us previously.40 Briefly, for Treg depletion, 0.5 mg of PC-61 antibody (Monoclonal Antibody Core Facility, Mayo Clinic, Rochester, MN) per mouse was given intraperitoneally as described.40,47 FACS analysis of spleens and lymph nodes confirmed depletion of CD4+, FoxP3+, CD25+ cells. The control for PC-61 treatment was i.p. injection of IgG control (ChromPure Rat IgG, Jackson ImmunoResearch, West Grove, PA). For mice treated with Treg depletion and IL-2, 24 hours following the PC-61 Ab treatment, mice were injected intraperitoneally with rhIL-2 at a dose of 75,000 U/injection (Proleukin, Novartis) three times a day for 3 days. On the fourth day, a single further injection of IL-2 was given. The control for IL-2 treatment was i.p. injections of 100 µl of PBS.
Flow cytometry
For analysis of phenotype, organs/tumors were recovered from mice and dissociated in vitro to achieve single-cell suspensions. 1 × 106 cells were washed in PBS containing 0.1 % bovine serum albumin (wash buffer), resuspended in 50 µl of wash buffer, and exposed to directly conjugated primary Abs for 30 min at 4 °C. Cells were then washed and resuspended in 500 µl of PBS containing 4% formaldehyde. Cells were analyzed by flow cytometry and data were analyzed using CellQuest software (BD Biosciences, Franklin Lakes, NJ). Anti-CD8b PE, APC and FITC, anti-Vα2 PE, anti-Vβ2 PE, and the isotype controls were purchased from BD Pharmingen (San Diego, CA).
ELISA analysis for IFN-γ secretion
Splenocytes enriched in lymphocytes were prepared from spleens from treated/vaccinated animals by standard techniques.48 Freshly purified splenocyte populations were washed in PBS and 250,000 splenocytes were plated in 24-well plates and incubated at 37 °C with the indicated peptides at 5 µg/ml in triplicate. Cell-free supernatants were collected after 48 hours and tested by specific ELISA for IFN-γ (BD OptEIA IFN-γ; BD Biosciences). The synthetic, H-2Kb-restricted ova SIINFEKL peptide was synthesized at the Mayo Foundation Core Facility.
In vivo tracking of adoptively transferred T cells
Purified OT-I lymphocytes were adoptively transferred into mice. At the indicated times, animals were euthanized, tumors were recovered and dissociated in vitro. Cells were washed in PBS containing 0.1% bovine serum albumin (wash buffer) and resuspended in 500 µl PBS containing 4% formaldehyde. Cells were analyzed by flow cytometry and data were analyzed using CellQuest software (BD Biosciences).
Virus titration from tumor
Tumors recovered from mice were harvested, weighed, and lysed by three cycles of freeze/thawing. Virus was recovered from the lysates and titers were determined on BHK-21 cells as described earlier and expressed as pfu of VSV/mg tissue.
In vivo studies
All procedures were approved by the Mayo Foundation Institutional Animal Care and Use Committee. C57Bl/6 mice were purchased from Jackson Laboratories at 6–8 weeks of age. To establish subcutaneous tumors, 2 × 105 B16ova cells in 100 µl of PBS were injected into the flank of mice. For adoptive transfer experiments, mice were intravenously administered OT-I, or OT-I preloaded with VSV, typically at 2 × 105 cells in 100 µl. For survival studies, tumor diameter in two dimensions was measured three times weekly using calipers and mice were killed when tumor size was ~1.0 × 1.0 cm2 in two perpendicular directions.
NK cell depletion
Anti-asialo-GM1 from Cedarlane was resuspended in 1 ml of PBS. Twenty-five microliters (~0.75 mg/mouse) were injected intraperitoneally once at the time indicated in the text. Mice treated with isotype received the same protein concentration of Rabbit IgG (Jackson ImmunoResearch Laboratories). NK depletion was verified by spleen NK1.1 Flowcytometry analysis (RDP-84; R&D Systems, Minneapolis, MN).
Statistics
Survival data from the animal studies were analyzed using the log rank test,49 and two-sample unequal variance Student’s t-test analysis was applied for in vitro assays. Statistical significance was determined at the level of P < 0.05.
ACKNOWLEDGMENTS
This work was supported by The Richard M Schulze Family Foundation, the Mayo Foundation, and by NIH grants CA RO1107082-02 and RO1130878. We thank Toni L Higgins for expert secretarial assistance.
REFERENCES
- 1.Fisher K. Striking out at disseminated metastases: the systemic delivery of oncolytic viruses. Curr Opin Mol Ther. 2006;8:301–313. [PubMed] [Google Scholar]
- 2.Bell JC. Oncolytic viruses: what's next? Curr Cancer Drug Targets. 2007;7:127–131. doi: 10.2174/156800907780058844. [DOI] [PubMed] [Google Scholar]
- 3.Harrington K, Alvarez-Vallina L, Crittenden M, Gough M, Chong H, Diaz RM, et al. Cells as vehicles for cancer gene therapy: the missing link between targeted vectors and systemic delivery? Hum Gene Ther. 2002;13:1263–1280. doi: 10.1089/104303402760128504. [DOI] [PubMed] [Google Scholar]
- 4.Power AT, Wang J, Falls TJ, Paterson JM, Parato KA, Lichty BD, et al. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol Ther. 2007;15:123–130. doi: 10.1038/sj.mt.6300039. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda K, Wakimoto H, Ichikawa T, Jhung S, Hochberg FH, Louis DN, et al. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J Virol. 2000;74:4765–4775. doi: 10.1128/jvi.74.10.4765-4775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci USA. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman A, Tian JP, Fulci G, Chiocca EA, Wang J. Glioma virotherapy: effects of innate immune suppression and increased viral replication capacity. Cancer Res. 2006;66:2314–2319. doi: 10.1158/0008-5472.CAN-05-2661. [DOI] [PubMed] [Google Scholar]
- 8.Qiao J, Wang X, Kottke T, White C, Twigger K, Diaz RM, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of Reovirus. Clin Cancer Res. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole C, Qiao J, Kottke T, Diaz RM, Ahmed A, Sanchez-Perez L, et al. Tumor-targeted, systemic delivery of therapeutic viral vectors using hitchhiking on antigen-specific T cells. Nat Med. 2005;11:1073–1081. doi: 10.1038/nm1297. [DOI] [PubMed] [Google Scholar]
- 10.Thanarajasingam U, Sanz L, Diaz RM, Qiao J, Sanchez-Perez L, Kottke T, et al. Delivery of CCL-21 to metastatic disease improves the efficacy of adoptive T-cell therapy. Cancer Res. 2007;67:300–308. doi: 10.1158/0008-5472.CAN-06-1017. [DOI] [PubMed] [Google Scholar]
- 11.Yotnda P, Savoldo B, Charlet-Berguerand N, Rooney C, Brenner M. Targeted delivery of adenoviral vectors by cytotoxic T cells. Blood. 2004;104:2272–2280. doi: 10.1182/blood-2003-11-3803. [DOI] [PubMed] [Google Scholar]
- 12.Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311:1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- 13.Ong HT, Hasegawa K, Dietz AB, Russell SJ, Peng KW. Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene Ther. 2007;14:324–333. doi: 10.1038/sj.gt.3302880. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Navarro J, Contreras JL, Arafat W, Jiang XL, Krisky D, Oligino T, et al. Genetically modified CD34+ cells as cellular vehicles for gene delivery into areas of angiogenesis in a rhesus model. Gene Ther. 2000;7:43–52. doi: 10.1038/sj.gt.3301054. [DOI] [PubMed] [Google Scholar]
- 15.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–676. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kottke T, Qiao J, Diaz RM, Ahmed A, Vroman B, Thompson J, et al. The perforin-dependent immunological synapse allows T-cell activation-dependent tumor targeting by MLV vector particles. Gene Ther. 2006;13:1166–1177. doi: 10.1038/sj.gt.3302722. [DOI] [PubMed] [Google Scholar]
- 21.Harrington K, Vile R. Virus smuggling, tax evasion and tumor assassination. Nat Med. 2006;12:507–509. doi: 10.1038/nm0506-507. [DOI] [PubMed] [Google Scholar]
- 22.Hogquist KA, Jameson SC, Health WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonistic peptides induce positive selection. Cell. 1994;76:17. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 23.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineeringc. Mol Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 24.Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat Med. 2001;7:781–787. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
- 25.Lichty BD, Power AT, Stojdl DF, Bell JC. Vesicular stomatitis virus: reinventing the bullet. Trends Mol Med. 2004;10:210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Barber GN. Review: vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004;17:516–527. doi: 10.1089/vim.2004.17.516. [DOI] [PubMed] [Google Scholar]
- 27.Barber GN. VSV tumor selective replication and protein translation. Oncogene. 2005;24:7710–7719. doi: 10.1038/sj.onc.1209042. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez M, Porosnicu M, Markovic D, Barber GN. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J Virol. 2002;76:895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebert O, Harbaran S, Shinozaki K, Woo SL. Systemic therapy of experimental breast cancer metastases by mutant vesicular stomatitis virus in immune-competent mice. Cancer Gene Ther. 2005;12:350–358. doi: 10.1038/sj.cgt.7700794. [DOI] [PubMed] [Google Scholar]
- 30.Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 31.Qiao J, Wang H, Kottke T, Diaz RM, Willmon C, Hudacek A, et al. Loading of oncolytic vesicular stomatitis virus onto antigen specific T cells enhances the efficacy of adoptive T cell therapy of tumors. Gene Ther. 2008;15:604–616. doi: 10.1038/sj.gt.3303098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiao J, Kottke T, Willmon C, Galivo F, Wongthida P, Diaz RM, et al. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat Med. 2008;14:37–44. doi: 10.1038/nm1681. [DOI] [PubMed] [Google Scholar]
- 33.Muranski P, Boni A, Wreszinski C, Citrin DE, Rosenberg SA, Childs R, et al. Increased intensity lymphodepletion and adoptive immunotherapy—how far can we go? Nat Clin Pract Oncol. 2006;3:668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baluna R, Vitetta ES. Vascular leak syndrome: a side effect of immunotherapy. Immunopharmacology. 1997;37:117–132. doi: 10.1016/s0162-3109(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg SA, Mule JJ, Spiess PJ, Reichert CM, Schwarz SL. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin-2. J Exp Med. 1985;161:1169–1188. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakajima T, Schulte S, Warrington KJ, Kopecky SL, Frye RL, Goronzy JJ, et al. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002;105:570–575. doi: 10.1161/hc0502.103348. [DOI] [PubMed] [Google Scholar]
- 37.Yoneda O, Imai T, Goda S, Inoue H, Yamauchi A, Okazaki T, et al. Fractalkine-mediated endothelial cell injury by NK cells. J Immunol. 2000;164:4055–4062. doi: 10.4049/jimmunol.164.8.4055. [DOI] [PubMed] [Google Scholar]
- 38.Melencio L, McKallip RJ, Guan H, Ramakrishnan R, Jain R, Nagarkatti PS, et al. Role of CD4(+)CD25(+) T regulatory cells in IL-2-induced vascular leak. Int Immunol. 2006;18:1461–1471. doi: 10.1093/intimm/dxl079. [DOI] [PubMed] [Google Scholar]
- 39.Renkonen R, Ristimaki A, Havry P. Interferon-γ protects human endothelial cells from lymphokine-activated killer cell-mediated lysis. Eur J Immunol. 1998;18:1839–1842. doi: 10.1002/eji.1830181129. [DOI] [PubMed] [Google Scholar]
- 40.Kottke T, Galivo F, Wongthida P, Diaz RM, Thompson J, Jevremovic D, et al. Treg depletion-enhanced IL-2 treatment facilitates therapy of established tumors by systemically delivered oncolytic virus. Mol Ther. 2008;16:1217–1226. doi: 10.1038/mt.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez-Perez L, Gough M, Qiao J, Thanarajasingam U, Kottke T, Ahmed A, et al. Synergy of adoptive T-cell therapy with intratumoral suicide gene therapy is mediated by host NK cells. Gene Ther. 2007;14:998–1009. doi: 10.1038/sj.gt.3302935. [DOI] [PubMed] [Google Scholar]
- 42.Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 43.Di Paolo NC, Tuve S, Ni S, Hellstrom KE, Hellstrom IE, Lieber A. Effects of adenovirus-mediated heat shock protein expression and oncolysis in combination with low-dose cyclophosphamide treatment on antitumor immune responses. Cancer Res. 2006;66:960–969. doi: 10.1158/0008-5472.CAN-05-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linardakis E, Bateman A, Phan V, Ahmed A, Gough M, Olivier K, et al. Enhancing the efficacy of a weak allogeneic melanoma vaccine by viral fusogenic membrane glycoprotein-mediated tumor cell-tumor cell fusion. Cancer Res. 2002;62:5495–5504. [PubMed] [Google Scholar]
- 47.Daniels G, Sanchez-Perez L, Kottke T, Diaz RM, Thompson J, Lai M, et al. A simple method to cure established tumors by inflammatory killing of normal cells. Nat Biotechnol. 2004;22:1125–1132. doi: 10.1038/nbt1007. [DOI] [PubMed] [Google Scholar]
- 48.Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W. Current Protocols in Immunology. New York: Wiley; 1998. [Google Scholar]
- 49.Altman DG. Practical Statistics for Medical Research. London: Chapman Hall; 1991. Analysis of survival times; pp. 365–395. [Google Scholar]