Abstract
West Nile virus (WNV) has remained epidemic in Kern County, CA, since its introduction in 2004 through 2007 when the human case annual incidence increased from 6 – 8 to 17 per 100,000, respectively. The 2007 increase in human infection was associated with contradicting surveillance indicators, including severe drought, warm spring but cool summer temperature anomalies, decreased rural and urban mosquito abundance but increased early season infection in urban Culex quinquefasciatus Say, moderate avian “herd immunity,” and declines in the catch of competent (western scrub-jay and house finch) and noncompetent (California quail and mourning dove) avian species. The decline in these noncompetent avian hosts may have increased contact with competent avian hosts and perhaps humans. The marked increase in home foreclosures and associated neglected swimming pools increased urban mosquito production sites, most likely contributing to the urban mosquito population and the WNV outbreak within Bakersfield. Coalescing five surveillance indicators into a risk assessment score measured each half month provided 2- to 6-wk early warning for emergency planning and was followed consistently by the onset of human cases after reaching epidemic conditions. St. Louis encephalitis virus (SLEV) antibody was detected rarely in wild birds but not mosquitoes or sentinel chickens, indicating that previously infected birds were detected in Kern County, but SLEV reintroduction was not successful. In contrast, western equine encephalitis virus (WEEV) was detected during 3 of 5 yr in Culex tarsalis Coquillett, sentinel chickens, and wild birds, but failed to amplify to levels where tangential transmission was detected in Aedes mosquitoes or humans. A comparison of transmission patterns in Kern County to Coachella Valley in the southeastern desert of California showed the importance of mosquito phenology and spatial distribution, corvids, or other avian “super spreaders” and anthropogenic factors in WNV epidemiology.
Keywords: West Nile virus, western equine encephalomyelitis virus, Culex tarsalis, Culex quinquefasciatus, epidemiology
West Nile virus (Flaviviridae, Flavivirus, WNV) invaded southern California during the summer of 2003 (Reisen et al. 2004b) but initially was confined to areas south of the Tehachapi Mountains (Hom et al. 2004). WNV overwintered successfully in this area during 2003–2004 (Reisen et al. 2006b), amplified rapidly to epidemic levels in Los Angeles (Wilson et al. 2005), and invaded the Central Valley and the remainder of California during 2004 (Hom et al. 2005). Unlike the 3-yr pattern of silent introduction, epidemic amplification, and subsidence repeated throughout much of North America (NA) (Hayes et al. 2005), epidemic transmission occurred in the vicinity of Bakersfield in Kern County when introduced during the summer of 2004 and annually during the subsequent 3 yr, culminating in 2007 in the largest mosquito-borne encephalitis outbreak since the 1952 epidemic of western equine encephalomyelitis virus (Togaviridae, Alphavirus, WEEV) (Reeves and Hammon 1962). Historically, both WEEV and St. Louis encephalitis virus (Flaviviridae, Flavivirus, SLEV) were endemic in Kern County and the southern Central Valley (Reeves 1990) but have declined as public and veterinary health problems during the last 25 yr, with the last recorded outbreak of 34 SLEV human cases occurring in 1989 (Reisen et al. 1992a). This paper explores factors that may have enabled the continued epidemic transmission of WNV while limiting WEEV and SLEV amplification.
WNV persists within both rural and urban transmission cycles involving different vector mosquitoes in different parts of the United States. Kern County provides the opportunity to compare the importance of rural transmission by Culex tarsalis Coquillett to urban transmission by Culex quinquefasciatus Say. Cx. tarsalis tends to be a more competent vector of WNV in the laboratory (Reisen et al. 2005a, 2008a), but Cx. quinquefasciatus exploits peridomestic environments and therefore may feed more frequently on humans in urban habitats (Reisen et al. 1992b). Historically, climate variation has altered the relative abundance of these two species in Kern County, with Cx. tarsalis dominating during cool wet years and Cx. quinquefasciatus dominating during hot dry years (Smith et al. 1995). Our research explores the notion that hot dry weather enables Cx. quinquefasciatus population increases and WNV amplification in urban environments while concurrently decreasing Cx. tarsalis abundance and WNV transmission in rural environments. The California Mosquito-Borne Virus Surveillance and Response Plan recently has been modified to segregate rural and urban transmission risk to humans based on abundance and infection rates in Cx. tarsalis and Cx. quinquefasciatus, respectively (Kramer 2008). Our current data provided the opportunity to evaluate the plan and to determine whether separating these species was an effective method of separating urban and rural risk.
The Bakersfield Metropolitan Statistical Area has a population >800,000, making it the third largest inland city of California after Fresno and Sacramento. The economy relies on agriculture, petroleum extraction and refining, and limited manufacturing. Bakersfield is the fastest growing city in the United States, with a population of >250,000 and provides an interesting ecological contrast to Palm Springs and the Coachella Valley, also one of the fastest-growing areas in the United States. The Coachella Valley contains nine incorporated cities with a combined population of >611,000 and an economy based mainly on agriculture in the southeast part of the Valley, but transitioning to retirement housing and tourism in the northwest near Palm Springs. In marked contrast to Kern County, there have been relatively few human cases of WNV reported from the Coachella Valley during 2004 –2007, despite our documentation of continued enzootic transmission (Reisen et al. 2008c). Our current research used similar methods to compare the epidemiology of WNV in these two areas of California with similar human population size, economies with a strong agricultural base, and well-coordinated vector control programs, but with different avifauna and climate.
Materials and Methods
Description of Study Area
Kern is a large county (area = 21,138 km2 or about the size of the State of Connecticut) at the southern end of the Central Valley, extending from the Temblor Mountains of the Coastal Range east across the San Joaquin Valley floor and into the Sierra Nevadas and the Mojave Desert. The southern boundary is formed by the Tehachapi Mountains that separate the Central Valley from the Los Angeles basin. The county has a large agricultural economic base dominated by almonds, grapes, milk products, carrots, and citrus, and is a significant producer of oil, natural gas, hydro-electric power, wind-turbine power, and geothermal power. Kern remains California’s top oil-producing county, with >85% of the state’s 43,000 active wells, and accounts for 1/10th of U.S. domestic oil production.
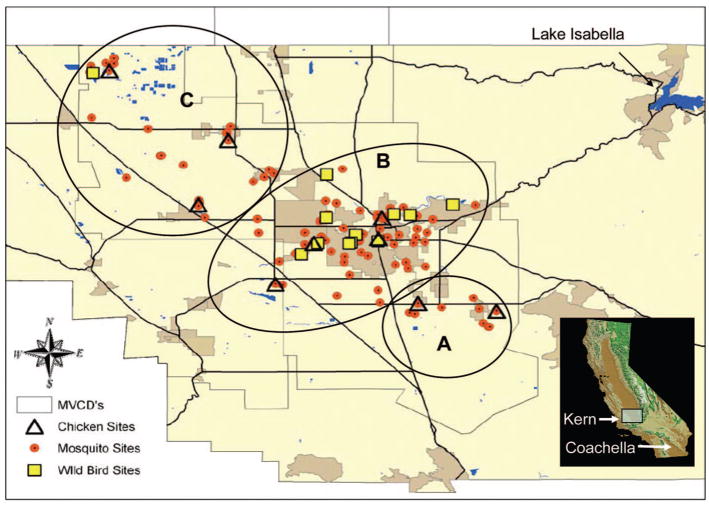
Our study area was delimited by the boundary of the Kern Mosquito and Vector Control District that covers 4,275 km2 on the floor of the San Joaquin Valley (Fig. 1). Mosquito control efforts focus on the detection and treatment of larval sources, with adulticides applied infrequently and only after larval control fails to prevent virus transmission. Based on landscape (rural versus urban) and mosquito and virus activity, sampling was stratified geographically into three zones: (A) northwest encompassing wetlands managed for migratory waterfowl at the Kern National Wildlife Refuge, extensive rural farmland, and the towns of Lost Hills, Buttonwillow, Wasco, and Shafter; (B) greater Bakersfield area including the adjacent cities of Oildale and Rosedale, wetlands immediately to the southwest for aquifer recharge, and riparian habitats along the Kern River that transect the city of Bakersfield; and (C) southeast encompassing extensive agricultural areas and the towns of Arvin and Lamont.
Fig. 1.
Map of the Kern Mosquito and Vector Control District within Kern County, showing the locations of sampling sites within zones A–C. Inset shows the locations of the current map and the Coachella Valley in the state of California. (Online figure in color.)
Sampling
Methods were identical to those used during our concurrent study in Coachella Valley (Reisen et al. 2008c). The locations of fixed mosquito sampling sites during 2007 are shown in Fig. 1. During the 5-yr period, mosquitoes were collected using dry ice-baited CDC-style traps (CO2 traps) (Newhouse et al. 1966) that were operated for one night biweekly without lights at 50 –70 sites. In residential settings, especially within the greater Bakersfield area, gravid Cx. quinquefasciatus females were collected using hay infusion-baited up-draft gravid female traps (Cummings 1992) operated at 20 –30 sites. Collections were returned alive to the laboratory where mosquitoes were anesthetized with triethylamine, identified to species, sex and reproductive condition, enumerated into pools of ≤50 females each, frozen at −80°C, and shipped on dry ice to the Center for Vectorborne Diseases BSL3 Arbovirus Laboratory at University of California, Davis (CVEC) for testing. Viral culture and testing was approved under Biological Use Authorization 0554 issued by the University of California Davis Environmental Health and Saftety committee.
Ten white Leghorn laying hens, 16 –18 wk old, were prebled, banded, and deployed as sentinels each April at nine sites within the Kern MVCD (Fig. 1). Hens were bled biweekly by lancet prick of the comb, with the blood collected on filter paper strips (Reisen et al. 1993), dried at air temperature, and shipped to the Viral and Rickettsial Diseases Laboratory of the California Department of Public Health (VRDL) for testing. Hens seroconverting or dying were replaced until replacements were no longer available. Care and bleeding of sentinel chickens was carried out under University of California Davis Institutional Animal Care and Use Committee (IACUC) protocol 12878.
Dead birds reported by the public to the Dead Bird Program hot line (McCaughey et al. 2003) were identified to species, geocoded, and mapped. Birds collected within 24 h of death were shipped to the California Animal Health and Food Safety laboratory at University of California, Davis, for necropsy, and kidney or other tissue samples were forwarded to CVEC for testing for WNV RNA by singleplex RT-polymerase chain reaction (PCR).
From January 2003 through November 2007, wild birds were collected weekly or biweekly at fixed mist netting sites (8 –10 2.6 by 12-m nets with size 36 –38 mm mesh operated for 3– 4 h starting at dawn) and at grain-baited traps. Initially, birds were netted and trapped biweekly in rural habitats at two sites along the Kern River SW of Bakersfield, Jerry Slough at the Tracy Ranch, and at the Kern NWR. However, drought conditions left the Kern River dry, and extensive vegetative clearing at our study area along Jerry Slough caused us to abandon these netting sites during 2006 and 2007. Modified Australian crow traps and walk-in traps on the ground or on pedestals at 1 m height were baited continuously with mixed wild bird seed and a watering device and closed biweekly. Bird collection sites were situated within 0.5 km of the chicken flocks shown in Fig. 1. Birds were identified to species, sex, and age, banded using USGS bands, bled (0.1 ml blood into 0.9 ml saline), and immediately released near the point of capture. Blood samples were held at ambient conditions until transported to the laboratory where they were clarified by centrifugation and frozen at −80°C until shipped to CVEC for testing. The collection of wild birds was done under IACUC Protocol 12880, CA Resident Scientific Collection Permit 801049 – 02 by the State of California Department of Fish and Game, Federal Fish and Wildlife Permit MB082812– 0, and Master Station Banding Marking and Salvage Permit 22673 by the USGS.
Human and equine cases were diagnosed by local health care providers and reported to county and state health departments. Data were made available by the Kern County Health Department and were summarized by date of reporting.
Diagnostics
Mosquito pools were triturated in a Spex Mixer Mill (Spex CertiPrep, Metuchen, NJ) in diluent (phosphate-buffered saline [PBS], 15% fetal bovine serum, antibiotics), and the RNA was extracted using either an robotic ABI 6700 or an ABI 6100 workstation (Applied Biosystems, Foster City, CA). Samples were screened for WNV, SLEV, and WEEV RNA using real-time multiplex RT-PCR with an ABI Prism 7900 TaqMan, using published (Lanciotti and Kerst 2001) and unpublished primers from the envelope gene. All multiplex RT-PCR positives were confirmed by virus isolation on Vero cell culture, in situ enzyme immunoassay (EIA) (Graham et al. 1986), and/or a second singleplex RT-PCR using primer sets from the NS region (Chiles et al. 2004). Confirmation rates were excellent if the critical threshold (Ct) scores were <30, and therefore during 2006 and 2007, confirmation by the second primer set was done only on samples with screening Ct values >30 and <40. Dead bird tissues were screened and confirmed only for WNV using a singleplex RT-PCR following the same paradigm as indicated above for mosquito pools. Previous studies indicated that few birds succumb from infection with WEEV or SLEV (Reisen et al. 2003b) and therefore dead birds were not tested for these viruses.
Chicken sera were screened for antibody by WEEV and WNV/SLEV antigens using an EIA (Reisen et al. 1994). Wild bird sera were screened using an indirect sandwich EIA with a polyclonal anti-bird detector antibody (Chiles and Reisen 1998, Ebel et al. 2002). Presumptive positives were confirmed using a plaque reduction neutralization test (PRNT), with positives producing >80% reduction of 50 –90 plaque forming units (PFU) at a ≥1:20 dilution. Viruses were considered identified when the end point titers were at least four times the competing virus. Because of antigenic similarity between WNV and SLEV, definitive determination was not always possible, so wild bird sera were scored as being negative (EIA positive/negative well ratio < 2), unconfirmed Flavivirus (P/N > 2, but PRNT < 1:20), Flavivirus confirmed (P/N > 2 and PRNT ≥ 1:20, but PRNT less than four times competing virus), and WNV or SLEV (P/N > 2, PRNT titer at least four times the competing virus). Data were analyzed based on EIA positives, because previously we showed that EIA, but not PRNT, antibody persists into the following season (Reisen et al. 2003a, 2004a). Therefore, we assumed most of the unconfirmed EIA positives during spring were old infections from the previous transmission season.
Statistics
Abundance in counts per species per trap per night was transformed to ln(y + 1) to normalize the distribution and control the variance. Comparisons among the three study zones, years, and months were done using a repeated-measures analysis of variance (ANOVA), with means tested by posteriori least significance difference procedures (Hintze 1998). Means presented in tables or figures were back transformed or geometric means. Infection incidence per 1,000 mosquitoes tested was calculated as maximum likelihood estimates (MLEs) (Biggerstaff 2003).
Risk
Five surveillance factors from Kern County were coalesced into a WNV risk score calculated from the California Mosquito-Borne Virus Surveillance and Response Plan risk assessment model (Kramer 2008) as currently loaded into the California Surveillance Gateway program (Park et al. 2008). Average risk ranged from 1.0 to 2.5 for a normal season with limited enzootic activity, 2.6 to 4.0 for emergency planning with human cases anticipated, and 4.1 to 5.0 for epidemic conditions with human cases expected. Quintile ranges for each of the five factors are summarized in Table 1. Data were as follows. (1) Temperature: mean daily temperature for Kern County was downloaded from the NASA Terrestrial Observation and Prediction System (http://ecocast.arc.nasa.gov/content/view/76/133/) and risk ranked in quintiles from ≤13 to >26°C based on the duration of the extrinsic period of WNV in Cx. tarsalis (Reisen et al. 2006c). (2) Abundance: mosquito data were separated into Cx. tarsalis and Cx. quinquefasciatus as indicators of rural and urban risk, respectively. Female abundance was ranked from <50 to >300% as an anomaly of the average abundance over the five previous years. (3) Infection incidence: MLEs of incidence for each species were ranked from 0 to ≥5 per 1,000. (4) Sentinel seroconversions: data were ranked based on the number of seroconversions per flock and the number of flocks seroconverting, from zero flocks with zero seroconversions to multiple flocks with at least two seroconversions. The risk model uses a spatial parameter of “general area” and “specific region” for which we used Kern County and the city of Bakersfield, respectively. (5) Dead birds: dead birds reported by the public and testing positive for WNV were ranked from zero in Kern County to at least five in the Bakersfield area using the same spatial criteria listed above. The number of factors averaged each half month depended on data availability because of sampling protocols (see above), so risk was calculated based on temperature alone in midwinter through all five factors during the transmission season.
Table 1.
Surveillance factors and their risk assessment quintiles
| Factor | Risk assessment value |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| 1. Temperature (°C) | ≤13 | 14–18 | 19–22 | 23–26 | >26 |
| 2. Adult Culex abundance (%) | ≤50 | 51–90 | 91–150 | 151–300 | >300 |
| 3. Virus infection incidence per 1,000 | 0 | 0.1–1.0 | 1.1–2.0 | 2.1–5.0 | >5.0 |
| 4. Number of sentinel chicken seroconversions | 0 | ≥1 Kern | 1–2 in 1 flock | >2 in > 1 flocks | >2 in >2 flocks |
| 5. Number of dead birds positive | 0 | ≥1 Kern | ≥1 BKSF | 2–5 BKSF | >5 BKSF |
Kern, throughout Kern County; BKSF, specifically in the Bakersfield area.
Results
Climate
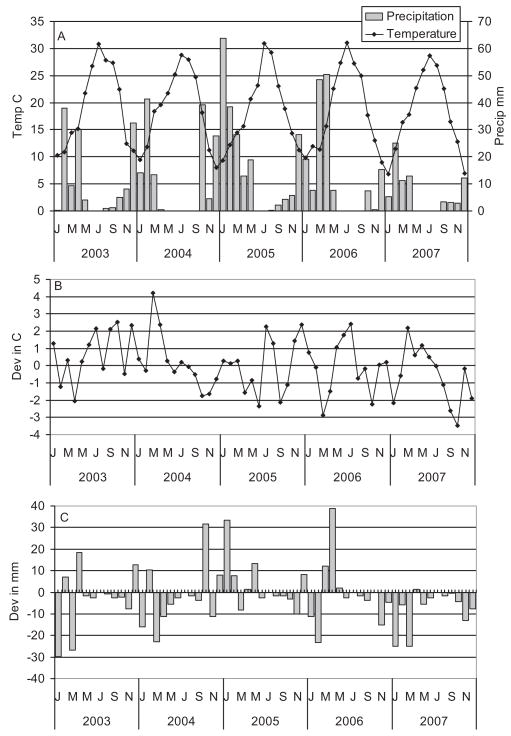
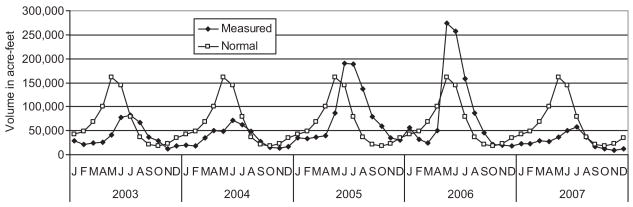
Climate variation affects mosquito abundance and determines periods conducive to virus transmission. The Mediterranean climate of Kern County was characterized by cool damp winters and hot dry summers (Fig. 2), with most rain falling on the valley floor between November and April. Additional precipitation fell as snow on the western slope of the Sierra Nevadas, melted during the spring and either ran off onto the valley floor in unmanaged small streams such as Poso Creek or was stored in Lake Isabella along the Kern River and released according to municipal and agricultural needs. Discharge of the Kern River through Bakersfield and into Lake Buena Vista to the west was dependent on snow pack and water allotments used for agriculture (Fig. 3).
Fig. 2.
Climate variation during 2003–2007 measured at Bakersfield Airport, Kern County. (A) Total precipitation and average temperature. (B) Deviation of mean temperatures from 30-yr normals. (C) Deviation of total monthly precipitation from 30-yr normals.
Fig. 3.
Monthly flow of the Kern River at Bakersfield compared with the 30-yr average (normal).
Total precipitation per day did not differ significantly among years (F = 1.78; df = 4,1766; P = 0.13); however, there was significant month by year interaction (F = 5.04; df = 44,1766; P = 0.008), indicating that the pattern of rainfall among months varied among years (Fig. 2A). Examination of deviations from the 30-yr normals indicated that the epidemic year of 2007 was exceptionally dry (Fig. 2C) and that the Kern River flowing through Bakersfield was completely dry for most of the summer, contrasting the two previous summers (Fig. 3). Temperature varied significantly among years (F = 9.33; df = 4,1766; P < 0.001), and there was significant month by year interaction (F = 8.97; df = 44,1766; P < 0.001), indicating that the pattern of warming and cooling varied among years (Fig. 2A). Overall, 2003 was significantly hotter (mean = 19.3°C) and 2007 significantly cooler (mean = 17.9°C; Fisher least significant difference [LSD], P = 0.05) than other years in our study. The epidemic year of 2007 was warmer than normal during spring but became cooler than normal during summer and fall compared with 30-yr normals for same weather station in Bakersfield (Fig. 2B).
Mosquito Abundance
Trap counts provided a measure of mosquito population size and species composition. Over the 5-yr period, 752,429 female and 18,035 male mosquitoes comprising 18 species were collected during 6,085 trap nights. Culex tarsalis was most abundant (40% of total), followed by Aedes melanimon Dyar (28%), Cx. quinquefasciatus (21%), and Cx. erythrothorax Dyar (9%) (Table 2). The remaining 16 species comprised <2% of the total. In the Central Valley of California, the Culex pipiens complex consists of both Cx. pipiens L. and quinquefasciatus Say and their hybrids (Cornel et al. 2003). Designation of specimens from Bakersfield as Cx. quinquefasciatus was based on their southern distribution, failure to diapause (Reisen et al. 1986), and male morphology in previous studies (Urbanelli et al. 1997, Tabachnick and Powell 1983), with the understanding that there probably was pipiens genetic introgression. Overall, 91% of total mosquitoes were collected by dry ice– baited (CO2) traps. Only Cx. quinquefasciatus was collected frequently at gravid female traps, comprising 99% of the total females collected by this method. Because of their abundance and frequent infection with WNV (see below), the remaining analyses focused on Cx. tarsalis and Cx. quinquefasciatus.
Table 2.
Number of female mosquitoes collected in Kern County during 2003–2007
| Species | CO2 traps |
Gravid traps |
Grand total | ||
|---|---|---|---|---|---|
| Total | Mean | Total | Mean | ||
| Trap nights | 4,252 | 1,833 | 6,085 | ||
| Ae. melanimon | 212,532 | 49.98 | 0 | 0.00 | 212,532 |
| Ae. nigromaculis | 430 | 0.10 | 0 | 0.00 | 430 |
| Ae. sierrensis | 110 | 0.03 | 2 | 0.00 | 112 |
| An. franciscanus | 178 | 0.04 | 0 | 0.00 | 178 |
| An. freeborni | 1,616 | 0.38 | 4 | 0.00 | 1,620 |
| An. punctipennis | 1,585 | 0.37 | 1 | 0.00 | 1,586 |
| Cs. incidens | 16 | 0.00 | 82 | 0.04 | 98 |
| Cs. inornata | 2,894 | 0.68 | 2 | 0.00 | 2,896 |
| Cs. particeps | 3 | 0.00 | 0 | 0.00 | 3 |
| Cx. apicalis | 2 | 0.00 | 0 | 0.00 | 2 |
| Cx. boharti | 18 | 0.00 | 140 | 0.08 | 158 |
| Cx. erythrothorax | 71,011 | 16.70 | 46 | 0.03 | 71,057 |
| Cx. quinquefasciatus | 95,115 | 22.37 | 63,762 | 34.79 | 158,877 |
| Cx. restuans | 83 | 0.02 | 1 | 0.00 | 84 |
| Cx. stigmatosoma | 512 | 0.12 | 18 | 0.01 | 530 |
| Cx. tarsalis | 301,897 | 71.00 | 233 | 0.13 | 302,130 |
| Cx. thriambus | 133 | 0.03 | 1 | 0.00 | 134 |
| Or. signifera | 2 | 0.00 | 0 | 0.00 | 2 |
| Total | 688,137 | 64,292 | 752,429 | ||
Culex tarsalis females were significantly (F = 27.2; df = 2,99; P < 0.001) more abundant in rural areas (northwest zone A = geometrc mean 16.2 females per trap-night, n = 1,135 and southeast zone C = 20.1 females per trap-night, n = 499 trap-nights) than in Bakersfield (zone B = 4.5 females per trap-night, n = 2,618 trap-nights). Variation in abundance among years approached significance (F = 1.92; df = 4,99; P = 0.06), with abundance during wet years (2005 = 17.1 and 2006 = 13.7 females per trap-night) significantly greater (Fisher LSD, P = 0.05) than during dry years (2003 = 10.3, 2004 = 9.5, and 2007 = 8.8 females per trap-night).
Culex quinquefasciatus females exhibited a different abundance pattern, with significantly (F = 24.9; df = 2,99; P < 0.001) more collected by CO2 trap in the southeast zone C (19.8 female per trap-night) than in northwest zone A (2.0 per trap-night) or Bakersfield zone B (2.7 per trap-night). Although there were no overall significant differences among years (P > 0.05), abundance during 2003 (8.8 females per trap-night) was significantly greater (Fisher LSD, P = 0.05) than the remaining years (range, 3.9 –5.0 females per trap-night). Zone C contained several old dairies and a farm using sewage treatment plant effluent for irrigation that produced large numbers of Cx. quinquefasciatus until the arrival of WNV and a drought year stimulated more judicious water management. Within Bakersfield, gravid traps (mean = 6.2 females per trap-night) collected significantly (F = 128.4; df = 1,71; P < 0.001) more Cx. quinquefasciatus females during the 2004 –2007 period than CO2 traps (mean = 1.8 females per trap-night), and this difference changed over the course of the season, because the trap type by month interaction term in the repeated-measures ANOVA was significant (P = 16.7; df = 9,71; P < 0.001; Fig. 4B). In general, gravid trap abundance increased rapidly during spring and early summer, whereas CO2 trap abundance peaked in late summer and fall. Although not directly comparable, Cx. quinquefasciatus gravid trap abundance exceeded Cx. tarsalis CO2 trap abundance in Bakersfield (Fig. 4B), indicating this species was more abundant.
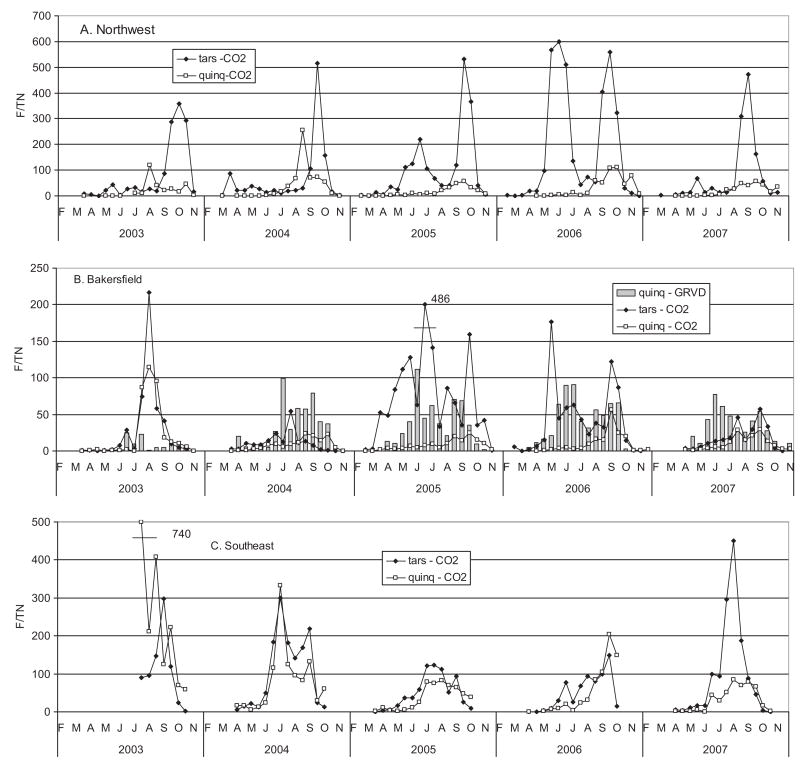
Fig. 4.
Geometric mean abundance of Cx. tarsalis and Cx. quinquefasciatus per CO2 or gravid trap night in three geographical zones of Kern County.
Mosquito Infection
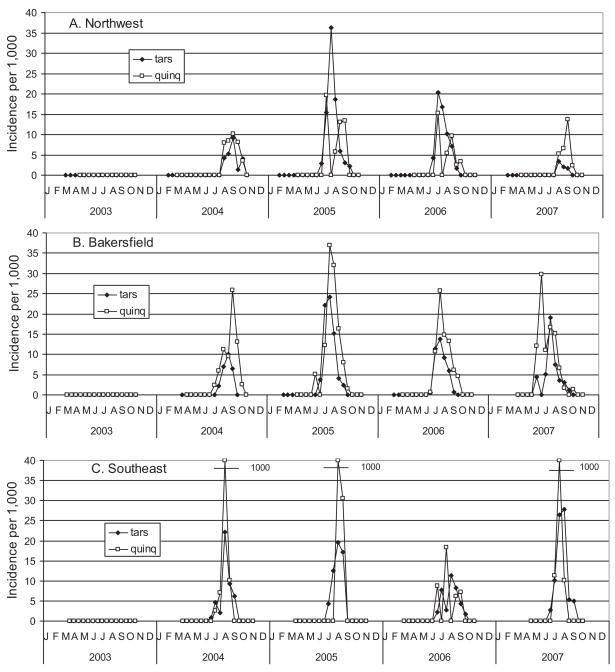
Detection of infected mosquitoes provided direct evidence that WNV had been acquired by the vector population, whereas the incidence of infection provided a measure of the extent, timing, and distribution of amplification. A total of 256,132 female mosquitoes were tested for WEEV, SLEV, and WNV RNA in 6,526 pools, of which 817 pools were positive for WNV (MLE per 1,000 = 3.51) and 58 for WEEV (MLE per 1,000 = 0.60). None were positive for SLEV. Of the 817 WNV positive pools, 328 were from Cx. tarsalis, 482 were from Cx. quinquefasciatus, 4 were from Ae. melanimon, and 3 were from Cx. erythrothorax (Table 3). As mentioned in the introduction, WNV was not detected north of the Tehachapi Mountain Range in 2003, and all pools tested during that year were negative (Fig. 5). Infection incidence in both Culex species exceeded the 5 per 1,000 epidemic threshold (Kramer 2008) in all three zones during all summers, peaking in July and August during wet years (2005 (2006) and in August and September (2004 and in August and September (2007) during dry years. A noted exception was the early increase in infection in Cx. quinquefasciatus in Bakersfield during 2007.
Table 3.
Maximum likelihood estimates of WNV infection incidence for mosquitoes collected in each of three locations with Kern County, California, 2004–2007
| Location | Species | Pools | Positives | Infection incidence per 1,000 |
|||
|---|---|---|---|---|---|---|---|
| Total | MLE | LL | UL | ||||
| Northwest | Cx. tarsalis | 973 | 104 | 41,524 | 2.66 | 2.18 | 3.20 |
| Cx. quinquefasciatus | 459 | 65 | 17,392 | 4.09 | 3.19 | 5.19 | |
| Ae. melanimon | 803 | 4 | 36,063 | 0.11 | 0.04 | 0.27 | |
| Cx. erythrothorax | 197 | 3 | 8,497 | 0.36 | 0.09 | 0.96 | |
| Bakersfield | Cx. tarsalis | 1,259 | 144 | 50,685 | 3.04 | 2.57 | 3.57 |
| Cx. quinquefasciatus | 1,960 | 385 | 64,821 | 6.82 | 6.16 | 7.53 | |
| Ae. melanimon | 149 | 0 | 6,013 | ||||
| Others | 110 | 0 | 3,990 | ||||
| Southeast | Cx. tarsalis | 385 | 80 | 17,190 | 5.26 | 4.20 | 6.52 |
| Cx. quinquefasciatus | 230 | 32 | 9,946 | 3.48 | 2.42 | 4.86 | |
| Ae. melanimon | 0 | 0 | 0 | ||||
| Others | 1 | 0 | 11 | ||||
| Totals | 6,526 | 817 | 256,132 | 3.51 | 3.27 | 3.76 | |
MLE, maximum likelihood estimates; LL and UL, lower and upper 95% confidence intervals.
Fig. 5.
Incidence of WNV infection per 1,000 Cx. tarsalis or Cx. quinquefaciatus females tested in three zones of Kern County.
WEEV was not detected during 2003 and 2004, but was detected repeatedly in Cx. tarsalis pools collected to the west of Bakersfield during the wet years of 2005 (24 positive pools) and 2006 (19 positive pools) and in southeast zone C during 2007 (24 positive pools). Increases in WEEV typically have been associated with wet El Niño years such as 1983 (Wegbreit and Reisen 2000).
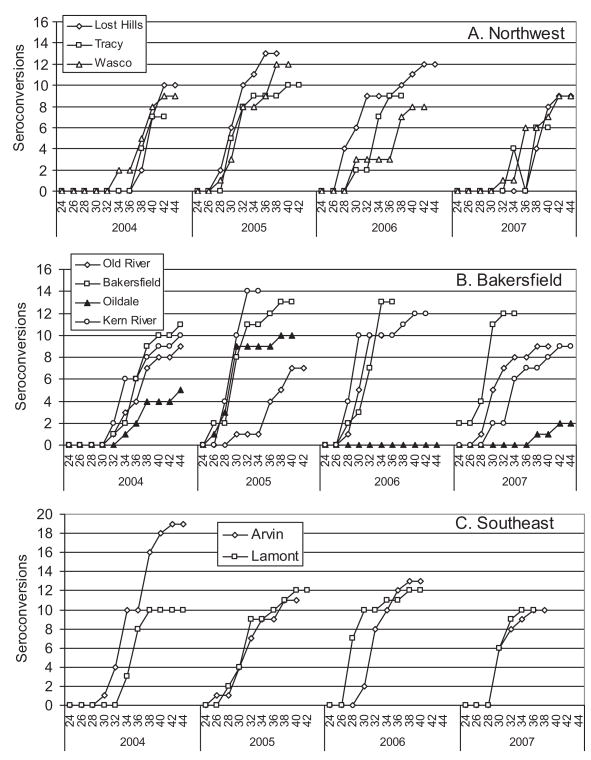
Sentinel Chicken Seroconversions
Sentinel seroconversions provided evidence that virus had been transmitted to birds by vector mosquitoes at specific flock locations. Nine flocks of 10 sentinel hens were maintained at the same locations during each year of our study (Fig. 1). Hens were bled biweekly starting in May and ending in November or when all the birds had seroconverted (Fig. 6). The mean number of seroconversions to WNV per flock per year averaged 9.9, and all flocks had multiple seroconversions per year except for the Oildale flock during 2006. When available, positive birds were replaced as soon as possible with seronegative birds, and therefore, the number of seroconversions per year frequently exceeded 10 (Fig. 6). Chickens require 10 –14 d after infection to a develop a diagnostic rise in IgG antibody (Reisen et al. 1994), and in agreement the first seroconversions, each season lagged slightly behind the first detection of virus in mosquito pools (Table 4). In agreement with our mosquito infection data, 26 hens seroconverted to WEEV: 10 during 2005, 7 during 2006, and 9 during 2007. Most WEEV seroconversions occurred along the Kern River (11 seroconversions) during the wet years of 2005 and 2006.
Fig. 6.
Cumulative number of sentinel chickens seroconverting to WNV during each biweekly period in three zones of Kern County.
Table 4.
Dates when first positives were detected by each surveillance method in southeast (SE), Bakersfield (BK), and northwest (NW) locations of Kern County, California
| Location | Surveilance method | Date of first positive per zone |
|||
|---|---|---|---|---|---|
| 2004 | 2005 | 2006 | 2007 | ||
| SE | Mosquito pools | 30-Jun | 7-Jul | 28-Jun | 20-Jun |
| SE | Sentinel chickens | 19-Jul | 11-Jul | 3-Jul | 6-Aug |
| SE | Dead birds | None | 15-Aug | None | None |
| BK | Mosquito pools | 1-Jul | 10-Jun | 20-Jun | 5-Jun |
| BK | Sentinel chickens | 2-Aug | 27-Jun | 3-Jul | 11-Jun |
| BK | Dead birds | 22-Jun | 25-Feba | 4-Jul | 25-May |
| NW | Mosquito pools | 12-Aug | 21-Jun | 28-Jun | 21-Jun |
| NW | Sentinel chickens | 16-Aug | 27-Jun | 3-Jul | 23-Jul |
| NW | Dead birds | 28-Jul | 9-Aug | None | None |
First American robin on 16-Jun.
Dead Birds
Bird common names and classification follow the American Ornithologist’s Union (American Ornithologists’ Union 1998). The NA variant of WNV (WN02; Davis et al. 2005) routinely kills susceptible passeriform birds during acute infection, and therefore, monitoring dead birds provides a measure of recent transmission. During 2004 –2007, 823 birds comprising 76 taxa were reported by the public or collected by our field personnel and shipped to the California Animal Health and Food Safety laboratory for necropsy, and kidney and other tissues were sent to CVEC for testing, of which 299 (34%) comprising 31 species tested positive for WNV RNA by singleplex RT-PCR (Table 5). None of the 13 specimens from 10 species submitted during 2003 were positive. Overall, 74% of the dead birds were collected within the Bakersfield area, which had the large human population necessary to find and report dead birds. The species diversity of dead birds was high and relatively equitable, with no one species comprising >15% of the total tested. The most frequently submitted species was the house sparrow (15% of total), followed closely by western scrub-jay (11%) and American crow (11%). With the exception of five infrequently submitted species (<1% of total), western scrub-jays and American crows had the highest percentage WNV RNA positive (Table 5). Of the 30 species testing positive, 8 were carnivores or scavengers that feed on living or dead birds and may have been infected orally and by mosquito bite. Peridomestic species such as the house finch and American robin were 36 and 40% positive, respectively. In contrast, house sparrows were only 11% positive, indicating that WNV was not a frequent cause of death in this species. Of interest, 14% of rock doves (domestic pigeons) and 13% of mourning doves tested positive at necropsy, even though these species are not competent hosts for WNV and do not succumb during experimental infection (Komar et al. 2003, Reisen et al. 2005a). These positive birds were either recently infected and vire-mic or had chronic infections of the kidney when they died of other causes and were submitted for testing.
Table 5.
Dead birds reported by the public in Kern County, submitted for necropsy, and tested for WNV RNA by singleplex RT-PCR
| Species | 2004 |
2005 |
2006 |
2007 |
Totals |
% total collected | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested | Pos | Tested | Pos | Tested | Pos | Tested | Pos | Tested | Pos | % | ||
| American kestrel | 1 | 1 | 2 | 2 | 3 | 3 | 100 | 0.4 | ||||
| Canary | 2 | 2 | 2 | 2 | 100 | 0.2 | ||||||
| Pacific-slope flycatcher | 1 | 1 | 1 | 1 | 100 | 0.1 | ||||||
| Rufous hummingbird | 2 | 2 | 2 | 2 | 100 | 0.2 | ||||||
| Swainson’s thrush | 1 | 1 | 1 | 1 | 100 | 0.1 | ||||||
| American crow | 32 | 26 | 18 | 12 | 8 | 6 | 33 | 26 | 91 | 70 | 77 | 11.1 |
| Western scrub-jay | 32 | 28 | 20 | 12 | 10 | 7 | 29 | 23 | 91 | 70 | 77 | 11.1 |
| Cooper’s hawk | 8 | 6 | 2 | 0 | 1 | 0 | 2 | 1 | 13 | 7 | 54 | 1.6 |
| Steller’s jay | 3 | 3 | 3 | 0 | 6 | 3 | 50 | 0.7 | ||||
| Western bluebird | 2 | 1 | 2 | 1 | 50 | 0.2 | ||||||
| House finch | 23 | 5 | 8 | 2 | 26 | 16 | 57 | 23 | 40 | 6.9 | ||
| American robin | 2 | 2 | 9 | 2 | 6 | 3 | 8 | 2 | 25 | 9 | 36 | 3.0 |
| House sparrow | 7 | 3 | 33 | 4 | 23 | 2 | 60 | 4 | 123 | 13 | 11 | 14.9 |
| Acorn woodpecker | 2 | 1 | 1 | 0 | 3 | 1 | 33 | 0.4 | ||||
| California towhee | 1 | 0 | 2 | 1 | 3 | 1 | 33 | 0.4 | ||||
| Hermit thrush | 1 | 1 | 1 | 0 | 1 | 0 | 3 | 1 | 33 | 0.4 | ||
| Western tanager | 1 | 0 | 1 | 0 | 1 | 1 | 3 | 1 | 33 | 0.4 | ||
| Barn owl | 7 | 3 | 14 | 4 | 5 | 1 | 4 | 0 | 30 | 8 | 27 | 3.6 |
| Black-headed Grosbeak | 1 | 0 | 2 | 1 | 1 | 0 | 4 | 1 | 25 | 0.5 | ||
| Lesser nighthawk | 2 | 1 | 2 | 0 | 4 | 1 | 25 | 0.5 | ||||
| Western kingbird | 2 | 0 | 2 | 1 | 4 | 1 | 25 | 0.5 | ||||
| Red-tailed hawk | 2 | 1 | 3 | 1 | 4 | 0 | 9 | 2 | 22 | 1.1 | ||
| Brewer’s blackbird | 5 | 4 | 16 | 0 | 2 | 0 | 5 | 1 | 28 | 5 | 18 | 3.4 |
| Northern mockingbird | 3 | 2 | 3 | 0 | 5 | 0 | 7 | 1 | 18 | 3 | 17 | 2.2 |
| Sparrow | 1 | 0 | 5 | 1 | 6 | 1 | 17 | 0.7 | ||||
| Rock dove | 1 | 0 | 20 | 3 | 21 | 3 | 14 | 2.6 | ||||
| Mourning dove | 3 | 0 | 7 | 0 | 10 | 0 | 44 | 8 | 64 | 8 | 13 | 7.8 |
| Common raven | 23 | 3 | 12 | 1 | 5 | 1 | 2 | 0 | 42 | 5 | 12 | 5.1 |
| Unidentified bird | 1 | 0 | 2 | 0 | 5 | 1 | 3 | 0 | 11 | 1 | 9 | 1.3 |
| European starling | 1 | 0 | 15 | 0 | 7 | 0 | 19 | 3 | 42 | 3 | 7 | 5.1 |
| 45 negative species | 20 | 0 | 23 | 0 | 15 | 0 | 53 | 0 | 111 | 0 | 0 | 13.5 |
| Grand total | 159 | 87 | 213 | 44 | 118 | 24 | 333 | 96 | 823 | 251 | 30 | 100.00 |
Pos, positive.
The number of birds submitted for testing varied among years from 118 in 2006 to 333 in 2007. Variation probably depended on public interest, because mosquito infection rates were relatively similar among years (Fig. 5). Percent testing positive varied significantly among years being 55% in 2004 and ranging from 20 to 29% during 2005–2007. Changes here were related to the percentage of American crows and western scrub-jays tested each year that decreased from 40% in 2004 to 15–18% during 2005–2007 (Table 5). Similar to the incidence of infection in mosquitoes (Fig. 5), the percentage of dead birds testing positive for WNV increased markedly during the course of each season, peaking in August during 2004 –2006 but in June and July during 2007 (Fig. 7). In Bakersfield, the first detection of WNV-positive birds usually preceded mosquito and sentinel chicken positives (Table 4).
Fig. 7.
Percentage of dead birds testing positive for WNV RNA and number of birds reported by the public and tested each month from Kern County.
Live Bird Serology
Seroprevalence among free ranging birds provided an overview of the frequency of infection among bird species available for capture that survived acute infection and provided our only measure of avian “herd immunity.” Overall, 15,346 birds were collected by mist netting or grain baited traps and bled, and their sera were tested for antibodies by EIA (Table 6). Of these, 1,543 collected during 2004 –2007 were positive against our SLEV antigen, of which 924 were confirmed and identified by endpoint PRNT80 to be against WNV; 4 were positive against SLEV. The four confirmed SLEV positives were all detected in after hatching year (AHY) birds, including a white-crowned sparrow, a California quail, and a tricolored blackbird collected during 2005 and a mourning dove collected in 2007. Because SLEV has not been detected in California since 2003 (Reisen et al. 2008c), we presumed that these few positives were in older birds infected before 2004 or in birds infected elsewhere. Mourning doves especially are known to disperse considerable distances, and birds banded in California have been detected in southern Mexico (McClure et al. 1962) and Florida (our unpublished banding records). These data show that birds possibly were entering California from areas supporting SLEV transmission or had survived the period since SLEV was active in California. The remaining 615 EIA-positive sera had PRNT titers = 1:20 and were too low to identify WNV or SLEV or were negative by PRNT (titers < 1:20). We based subsequent analyses on the EIA results, because experimentally infected birds lose neutralizing antibody during winter and often test negative during spring. These birds were still protected from reinfection (Reisen et al. 2001), indicating that PRNT data may underestimate residual herd immunity acquired during previous transmission seasons. In addition to WNV and SLEV, 55 sera from 12 species were positive by EIA for antibodies against WEEV during the summers of 2004 –2007. Of these, 36 (65%) were detected in 2004 and 34 were in HY birds, including one juvenile house finch just off the nest. White-crowned sparrows (14 positive), mourning doves (13), and California quail (9) were the species most frequently testing positive for WEEV.
Table 6.
Live birds collected by mist netting or grain baited traps, bled, and tested for antibodies against WNV by EIA
| Species | Resa | 2003 |
2004 |
2005 |
2006 |
2007b |
2004 –2007 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Total | % positive | Total | % positive | Total | % positive | Total | % positive | Total | % positive | ||
| Western scrub-jay | YR | 164 | 161 | 13.7 | 209 | 47.4 | 155 | 55.5 | 27 | 44.4 | 552 | 39.7 |
| Mourning dove | YR | 237 | 130 | 10.8 | 383 | 30.5 | 303 | 38.0 | 204 | 33.3 | 1,020 | 30.8 |
| California quail | YR | 247 | 458 | 11.6 | 259 | 27.8 | 1,153 | 38.4 | 57 | 40.4 | 1,927 | 30.7 |
| Cooper’s hawk | YR | 1 | 9 | 22.2 | 2 | 50.0 | 0 | 0 | 11 | 27.3 | ||
| House finch | YR | 110 | 340 | 7.4 | 326 | 17.8 | 533 | 20.5 | 182 | 22.0 | 1,381 | 16.8 |
| Loggerhead shrike | YR | 24 | 24 | 4.2 | 9 | 33.3 | 5 | 20.0 | 1 | 100.0 | 39 | 15.4 |
| Ash-throated flycatcher | S | 3 | 8 | 0.0 | 9 | 22.2 | 4 | 25.0 | 1 | 0.0 | 22 | 13.6 |
| California thrasher | YR | 107 | 68 | 7.4 | 10 | 10.0 | 8 | 62.5 | 5 | 20.0 | 91 | 13.2 |
| Rock pigeon | YR | 140 | 6.4 | 129 | 7.8 | 0 | 0 | 269 | 7.1 | |||
| Black-headed grosbeak | M | 3 | 7 | 28.6 | 5 | 0.0 | 35 | 0.0 | 1 | 100.0 | 48 | 6.3 |
| Brewer’s blackbird | YR | 18 | 46 | 0.0 | 25 | 16.0 | 0 | 1 | 0.0 | 72 | 5.6 | |
| House sparrow | YR | 17 | 57 | 0.0 | 160 | 7.5 | 444 | 4.1 | 311 | 6.4 | 972 | 5.1 |
| Bewick’s wren | YR | 39 | 39 | 2.6 | 30 | 0.0 | 13 | 23.1 | 5 | 0.0 | 87 | 4.6 |
| Brown-headed Cowbird | YR | 17 | 18 | 0.0 | 79 | 3.8 | 71 | 5.6 | 29 | 0.0 | 197 | 3.6 |
| Western kingbird | S | 22 | 39 | 0.0 | 18 | 0.0 | 13 | 15.4 | 2 | 0.0 | 72 | 2.8 |
| Common yellowthroat | S | 13 | 14 | 7.1 | 17 | 0.0 | 9 | 0.0 | 1 | 0.0 | 41 | 2.4 |
| Bullock’s oriole | S | 76 | 123 | 0.0 | 40 | 10.0 | 17 | 0.0 | 0 | 180 | 2.2 | |
| Tricolored blackbird | YR | 3 | 0 | 44 | 2.3 | 1 | 0.0 | 0 | 45 | 2.2 | ||
| Northern mockingbird | YR | 49 | 56 | 1.8 | 21 | 0.0 | 14 | 7.1 | 2 | 0.0 | 93 | 2.2 |
| Song sparrow | YR | 194 | 238 | 0.4 | 312 | 2.2 | 119 | 3.4 | 25 | 0.0 | 694 | 1.7 |
| Golden-crowned sparrow | W | 106 | 124 | 1.6 | 123 | 1.6 | 134 | 1.5 | 32 | 0.0 | 413 | 1.5 |
| White-crowned sparrow | W | 577 | 855 | 2.0 | 963 | 1.2 | 1,035 | 1.1 | 292 | 0.0 | 3,145 | 1.3 |
| Red-winged blackbird | YR | 128 | 110 | 0.0 | 45 | 4.4 | 16 | 0.0 | 0 | 171 | 1.2 | |
| Marsh wren | YR | 40 | 45 | 0.0 | 26 | 3.8 | 19 | 0.0 | 0 | 90 | 1.1 | |
| Lincoln’s sparrow | W | 48 | 65 | 1.5 | 49 | 0.0 | 24 | 0.0 | 7 | 0.0 | 145 | 0.7 |
| European starling | YR | 3 | 72 | 0.0 | 14 | 0.0 | 5 | 0.0 | 2 | 0.0 | 93 | 0.0 |
| Audubon’s warbler | W | 16 | 14 | 0.0 | 21 | 0.0 | 28 | 0.0 | 7 | 0.0 | 70 | 0.0 |
| Orange-crowned Warbler | M | 30 | 11 | 0.0 | 21 | 0.0 | 15 | 0.0 | 7 | 0.0 | 54 | 0.0 |
| Black phoebe | YR | 14 | 18 | 0.0 | 23 | 0.0 | 9 | 0.0 | 1 | 0.0 | 51 | 0.0 |
| Lark sparrow | YR | 9 | 0.0 | 32 | 0.0 | 1 | 0.0 | 5 | 0.0 | 47 | 0.0 | |
| Fox sparrow | W | 16 | 8 | 0.0 | 7 | 0.0 | 9 | 0.0 | 11 | 0.0 | 35 | 0.0 |
| Wilson’s warbler | M | 27 | 13 | 0.0 | 13 | 0.0 | 8 | 0.0 | 0 | 34 | 0.0 | |
| Hermit thrush | M | 11 | 7 | 0.0 | 17 | 0.0 | 7 | 0.0 | 0 | 31 | 0.0 | |
| Tree swallow | S | 54 | 4 | 0.0 | 1 | 0.0 | 19 | 0.0 | 0 | 24 | 0.0 | |
| Savannah sparrow | W | 3 | 7 | 0.0 | 1 | 0.0 | 12 | 0.0 | 0 | 20 | 0.0 | |
| Ruby-crowned Kinglet | S | 10 | 10 | 0.0 | 4 | 0.0 | 3 | 0.0 | 1 | 0.0 | 18 | 0.0 |
| American robin | S | 7 | 9 | 0.0 | 4 | 0.0 | 0 | 0 | 13 | 0.0 | ||
| Spotted towhee | S | 4 | 3 | 0.0 | 1 | 0.0 | 7 | 0.0 | 0 | 11 | 0.0 | |
| Bushtit | S | 8 | 7 | 0.0 | 0 | 2 | 0.0 | 1 | 0.0 | 10 | 0.0 | |
| Forty-seven species with <10 collected | 232 | 44 | 6.8 | 56 | 0.0 | 27 | 3.7 | 17 | 23.5 | 380 | 2.1 | |
| Total | 2,678 | 3,410 | 4.6 | 3,508 | 11.8 | 4,277 | 18.8 | 1,237 | 13.5 | 12,432 | 12.4 | |
| Hatching year birds | 786 | 1,092 | 5.1 | 900 | 13.1 | 1,099 | 11.1 | 325 | 13.5 | 3,416 | 10.1 | |
Residency: YR, year round; S, summer; W, winter; M, migrant.
Collections emphasized grain-baited traps.
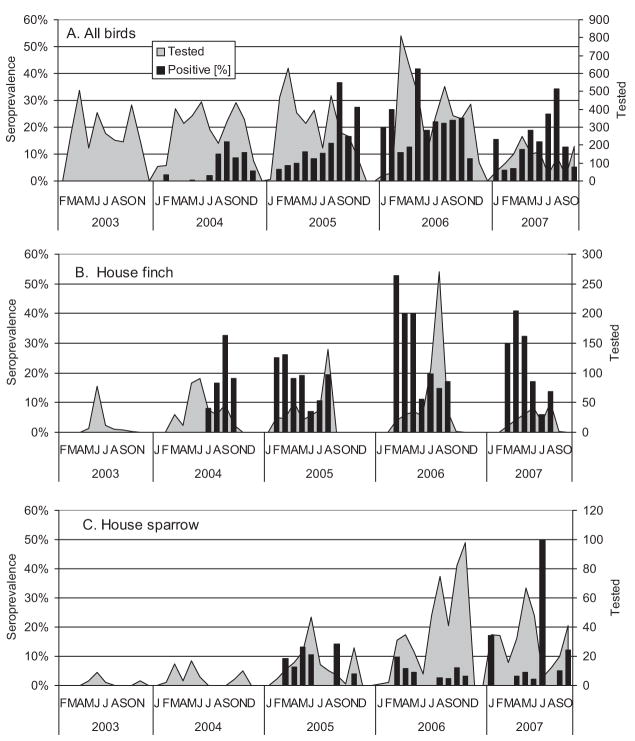
During 2004 –2007, 12,668 sera from 86 species of birds were tested, of which 1,543 (12.4%) tested positive from 39 species (Table 6). Overall, seroprevalence in AHY birds approximately doubled each season during 2004 –2006, increasing from 4.4% in 2004 to 13.8% in 2005 to 23.8% in 2006 but declined to 14.3% in 2007, perhaps reflecting the marked decline in the California quail population because of drought conditions. Annual seroprevalence in AHY birds during the 2004 –2007 period was significantly greater (χ2 = 45.6, df = 3, P < 0.001) than in HY birds (Table 6), because of multiple years of exposure to infection. Seroprevalence in HY birds increased from 2004 (5.1%) to 2005 (13.3%) but remained relatively constant during 2006 (11.1%) and 2007 (13.5%).
The western scrub-jay had the highest overall seroprevalence (39.7% of total sera), followed by mourning dove (30.8%) and California quail (30.7%). Western scrub-jays frequently succumb during experimental infection (Reisen et al. 2005a) and frequently were positive at necropsy (Table 5), so the high percentage of living birds that were antibody positive was unexpected, but indicated that most individuals in the Bakersfield population probably were infected each season and that relatively few would be susceptible to infection until after the next nesting season. Although seroprevalence was lower, a similar scenario was observed for the house finch (16.8% positive) that also frequently succumbed to infection (Fang and Reisen 2006) and was frequently positive at necropsy in the Dead Bird program. In contrast, mourning doves and California quail do not succumb after experimental infection (Reisen et al. 2005a, 2006d), and these species were expected to have high seroprevalence with WNV (Reisen et al. 2008c) and other arboviruses (Reisen et al. 2000). Although abundant and widely distributed in Kern County, overall seroprevalence in house sparrows (5.1%) was significantly lower (χ2 = 65.1, df = 1, P < 0.001) than in house finches (16.8%). In agreement with the notion that more house finches were dying of WNV, the age structure of house finches contained significantly more (χ2 = 18, df = 1, P < 0.001) HY year birds (68%) than did house sparrows (55%), even though, in Kern County, house sparrows have several broods per year.
Seroprevalence and therefore herd immunity generally increased during each season as additional birds became infected (Fig. 8A). During the initial year of the invasion, seroprevalence remained relatively low (<15%), but during the second year (2005) increased gradually during the course of the season, eventually exceeding 15% during September. In subsequent years, our measures of seroprevalence frequently were elevated in the spring but declined as HY birds left the nest. Because of their repeated infection and high host competence, we felt that the immune status of the house finch and house sparrow populations may be critical for virus amplification (Fig. 8B and C). Vernal seroprevalence for house finches during 2006 and 2007 exceeded 30% but declined rapidly in early summer when collections were dominated by HY birds. In marked contrast, house sparrow annual seroprevalence during 2004 –2007 remained significantly lower (P < 0.001) than house finch seroprevalence, and the few outlier months with seroprevalence >15% were based on low sample sizes (<10 birds).
Fig. 8.
Percentage of sera positive for flavivirus antibody by enzyme immunoassay and numbers of sera tested each month for (A) all bird species, (B) house finches, and (C) house sparrows.
Horse Cases
The number of equine cases in Kern County was low, because of an extensive vaccination program coupled with naturally acquired immunity after subclinical infection. The number of reported WNV cases decreased annually from 47 in 2004 and 26 in 2005 to 4 in 2006 to 4 in 2007. No WEEV cases were reported. Clearly, equine cases were no longer a sensitive surveillance measure of tangential transmission.
Human Cases
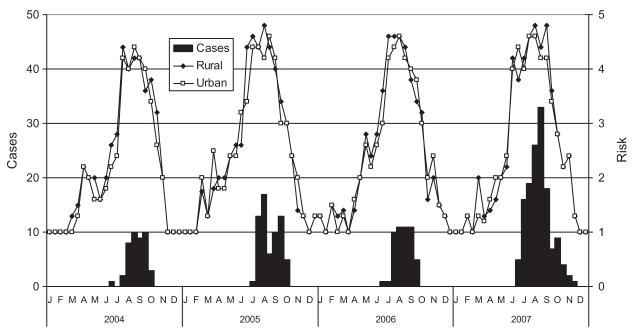
The number of reported and laboratory-confirmed human cases provided the only measure of tangential WNV transmission to humans (Fig. 9). From 2004 to 2007, 313 (annual range = 51 in 2006 to 138 in 2007) laboratory-confirmed human WNV cases were reported by the Kern County Health Department (Table 7), of which 250 (80%) were reported from the greater Bakersfield area. Based on the 2003 Kern County census of 801,648, annual incidence ranged from 6.4 to 17.2 per 100,000; for the greater Bakersfield population of 481,000, incidence was higher, ranging from 8.9 to 23.5 per 100,000. With an average number of 6.5 and 6.25 cases per year in towns located in the northwewst and southeast, incidence averaged 15.9 and 21.0 per 100,000 for the 4-yr period, increasing to a maximum of 22.0 and 47.1 per 100,000 during 2007. Case incidence for Kern County was consistently higher than the State of California. No cases of WEEV or SLEV were reported.
Fig. 9.
Number of laboratory-confirmed human cases of West Nile fever and neuroinvasive disease reported by the Kern County Department of Health and the relative average risk for rural and urban locations calculated from enzootic data.
Table 7.
Numbers of laboratory-confirmed human cases reported from each location by the Kern County Health Department
| Location | 2004 | 2005 | 2006 | 2007 | Total |
|---|---|---|---|---|---|
| NW | 8 | 5 | 4 | 9 | 26 |
| BK | 44 | 50 | 43 | 113 | 250 |
| SE | 6 | 3 | 2 | 14 | 25 |
| Othera | 8 | 2 | 2 | 12 | |
| Total | 58 | 66 | 51 | 138 | 313 |
| County incidence | 7.24 | 8.23 | 6.36 | 17.21 | |
| Bakersfield incidence | 9.15 | 10.40 | 8.94 | 23.49 | |
| California incidence | 2.07 | 2.34 | 0.74 | 1.01 |
Other communities within Kern County.
Most cases were reported during the months of July–October (Fig. 9), even though during 2007, enzootic amplification was detected a month earlier than during the 3 previous yr. Date of reporting provided a less informative statistic to generate a temporal pattern of infection, but these were the only data available from the Kern County Health Department. Delays in reporting date most likely included the variable time from infection to onset of symptoms, severity of disease, health care provider decision to request laboratory confirmation, serological testing, and reporting. Collectively, these factors could have caused delays from 2 to 6 wk, suggesting that human infection could have been offset by at least a month earlier than shown in Fig. 9 and concurrent with increases in enzootic amplification.
Human cases always occurred whenever rural or urban risk assessment scores exceeded our “epidemic condition” threshold of four (Fig. 9), and the numbers reported per half month were significantly correlated with both rural (r = 0.50, df = 30, P < 0.05) and urban (r = 0.46, df = 30, P < 0.05) risk scores. However, rural and urban risk as defined by Cx. tarsalis and Cx. quinquefasciatus abundance and infection were highly correlated (r = 0.97, n = 94, P < 0.001) and did not successfully delineate the differences between rural and urban transmission. This was unexpected because of differences in abundance and infection incidence described above for rural and urban areas of Kern County. Future risk assessment will be based spatially on human population density from census data.
Discussion
Outbreak Transmission of WNV
Unlike other urban outbreaks in California such as Los Angeles in 2004 (Wilson et al. 2006) or Sacramento in 2005 (Elnaien et al. 2006, Feiszli et al. 2007) that were confined to a single year, transmission of WNV to humans in Bakersfield continued at outbreak levels (9 –10 cases per 100,000) from 2004 to 2006 and more than doubled during 2007, a year that the overall California incidence dropped to 1 per 100,000 (http://westnile.ca.gov/). In contrast, enzootic activity remained relatively constant during these 4 yr, with similar mosquito infection incidence and sentinel chicken conversions each summer. Factors potentially enabling recurrent outbreak transmission in Bakersfield included (1) consistently warm climate, (2) abundant and competent vectors, (3) abundant and competent avian hosts, and (4) several anthropogenic factors.
Warm vernal temperature seemed critical for WNV amplification, with an early season temperature anomaly leading to increased amplification and human case incidence during 2007. The minimal developmental temperature for WNV in Cx. tarsalis was estimated experimentally to be 14.3°C (Reisen et al. 2006c), and mean ambient temperatures in Bakersfield consistently exceeded this thermal minimum for 6 – 8 mo each year. Maximum temperatures exceeded this value for multiple days during every month of the year, thereby allowing for a potentially long transmission season and virus amplification to outbreak levels during each summer. Temperature during the spring of 2007 remained 1–2°C above normal from March to May, followed by below normal temperatures during the remainder of the year. Above normal vernal temperatures undoubtedly enhanced WNV amplification by shortening the duration of the gonotrophic cycle (Reisen et al. 1992b) and the extrinsic incubation period (EIP) (Reisen et al. 2006c), thereby increasing host–vector contact and expediting transmission. Based on the mean ambient temperatures for March, April, and May 2007 (Fig. 2), the estimated durations of the EIP for WNV would be 52, 31, and 13 d, compared with 0 (below minimum—no viral replication), 38, and 15 d based on the 30-yr normals. Shortening the EIP would not only increase the rate, but also increase the probability, that an infected female would survive to transmit. Rapid vernal amplification was indicated by early season increases in WNV infection incidence in Cx. quinquefasciatus and in the percentage of WNV infection in dead birds.
Rainfall patterns were inconsistent; 2004 and 2007 were drought years, whereas 2005 and 2006 were years with above normal rainfall during spring and above normal discharge of the Kern River through Bakersfield. Greater discharge resulted in the intentional flooding of the ponds to recharge aquifers during 2005 and managed wetlands during 2006 producing above normal Cx. tarsalis abundance in Bakersfield and the northwest, respectively. Extreme drought conditions during the end of 2006 and most of 2007 undoubtedly reduced surface pool habitat for larvae but may have been offset by the large number of neglected swimming pools associated with the collapse of the Bakersfield housing market during the 2006 –2007 period. From April–June 2006 to July–September 2007, there was a 413% increase in notices of delinquency on home mortgages and a corresponding 305% increase in service requests to the Kern MVCD for treating neglected swimming pools (Carroll et al. 2008, Reisen et al. 2008d).
Although WNV amplified enzootically throughout Kern County during all years in both rural and urban transmission cycles, most tangential transmission to humans may have been in peridomestic environs by Cx. quinquefasciatus. Although originally thought to be a less competent vector for WNV (Goddard et al. 2002, Reisen et al. 2005a), we recently showed that Cx. quinquefasciatus from Bakersfield were similar in vector competence to Cx. tarsalis and that there was no significant change in susceptibility during 2007 in comparison to previous years (Reisen et al. 2008a). Host-seeking Cx. quinquefasciatus abundance measured by CO2 traps remained low in the northwest and within Bakersfield, rarely exceeded 10 females per CO2 trap night, and peaked during late summer. In contrast, abundance measured by gravid female traps in Bakersfield increased in May and peaked in July each summer. The latter data showed that CO2 traps were not a suitable measure of Cx. quinquefasciatus abundance especially in urban environments, that disparity in abundance patterns may have been related to a temporal change in blood-meal host selection patterns, and that catch may increase as a curvilinear function of population size (Reisen et al. 1991). Gravid female traps were considerably more sensitive, perhaps indicating that oviposition sites were limiting and that host-seeking females and therefore the rate of vector– host contact actually may have been greater and earlier in the year than indicated by CO2 traps. During the epidemic of 2007, the rapid early increase in infection incidence in Bakersfield in Cx. quinquefasciatus preceded increases in Cx. tarsalis abundance and infection incidence in rural northwest and southeast zones and urban Bakersfield, perhaps indicating that outbreak amplification started at periurban habits within Bakersfield.
Three competent avian hosts (western scrub-jay, house finch, and house sparrow) were abundant in peridomestic environments within Bakersfield, were infected repeatedly in both dead bird and live bird sampling programs, and were considered important in WNV amplification. Unlike other corvids such as American crows that roost communally after the nesting season, western scrub-jays remain territorial and therefore are distributed evenly within suburban environments (http://bna.birds.cornell.edu/bna/species/712/). They were highly competent hosts for WNV, with viremias peaking for several days at >9 log10 plaque forming units (PFUs) per milliliter (Reisen et al. 2005a) and >80% succumbing from experimental WNV infection. In agreement, this species has shown significant population declines within the Central Valley of California since the arrival of WNV (Wheeler et al. 2008). Similarly, house finches and house sparrows were widely distributed, competent hosts that succumb during infection (Reisen et al. 2005a, Fang and Reisen 2006) and frequently were fed on by Cx. tarsalis mosquitoes (Tempelis et al. 1976).
In the current study, it was difficult to resolve why house finches were more frequently infected than house sparrows. Parallel studies in Los Angeles have shown high seroprevalence rates for both species (Wilson et al. 2008), whereas in Coachella Valley, house finch exceeded house sparrow seorprevalence (Reisen et al. 2008c). House finch populations in the Central Valley have declined significantly since the arrival of WNV and an on-going avian pox epiornitic, allowing a dramatic rebound in house sparrow abundance (Wheeler et al. 2008). Although not evident from the ratio of HY to AHY birds in our current study, it could be that the low seroprevalence rates reflect this marked population increase. Large house sparrow populations with seemingly low infection rates were considered responsible for most WNV amplification during the initial outbreak of WNV in New York (Komar et al. 2001).
Other competent avian species observed in our study areas included mocking bird, American robin, and American crow (Komar et al. 2003, 2005); however, these species were much less abundant. Severe drought that dramatically reduced the abundance of rural bird species based on our trap success may have contributed to the 2007 epidemic by bringing surviving birds closer to irrigated areas around homes and by reducing the size of noncompetent avian host populations. Both California quail and mourning doves are low competent hosts for WNV (Komar et al. 2003, Reisen et al. 2005a, 2006d), but frequently were infected, thereby diverting infectious host-seeking vectors from competent host species. From the wet year of 2006 to the drought of 2007, our trapping success for these two species decreased 2022 and 149%, respectively, perhaps leading Culex vectors to feed more frequently on competent avian hosts and perhaps humans.
Anthropogenic factors may have played a significant role in the 2007 outbreak. Although the drought eliminated most surface water larval habitat, the large increase in the number of neglected swimming pools seemed to provide replacement habitat for Cx. quinquefasciatus and may even have allowed the production of Cx. tarsalis in urban settings (Reisen et al. 2008d). Similarly, we had found Cx. tarsalis exploiting neglected pools in Los Angeles (Reisen et al. 1990). Finding and treating neglected swimming pools, Jacuzzis, and ornamental ponds have provided a significant new challenge and extended the resources of the Kern MVCD. The decline in SLEV and WEEV cases historically also were attributed to changes in human behavior related to the use of air conditioning and TV viewing (Gahlinger et al. 1986). The downturn in the California economy stimulating residents to conserve electricity combined with the exceptionally cool summer of 2007 possibly could have stimulated more of the Bakersfield population to spend time outdoors in summer evenings, thereby increasing their risk of mosquito bites and WNV infection.
Failure of SLEV and WEEV Amplification in Kern County
Historically, SLEV was endemic in Kern County (Reeves and Hammon 1962) but recently has been detected only intermittently, with the last human cases associated with the 1989 outbreak (Tueller 1990, Reisen et al. 1992a). SLEV seems to have been displaced by WNV since its invasion into southern California in 2003 (Reisen et al. 2004b). Although antibody positive birds were found occasionally in Kern County during the current study and in Coachella Valley (Reisen et al. 2008c), all mosquito pools and sentinel chickens have remained negative for SLEV throughout California since 2004, supporting the notion that no two flaviviruses in same serogroup can amplify concurrently (Work 1971). Both SLEV and WNV seem to exploit the same niche, using the same mosquito and avian hosts. Both have similar temperature requirements for development within mosquitoes (Reisen et al. 2006c), leading to similar seasonality. In addition, Kern County and other California Culex mosquitoes have remained competent for SLEV as well as WNV (Reisen et al. 2008a). Both viruses are in the Japanese encephalitis virus serogroup and elicit cross protective immunity in vertebrates (Tesh et al. 2002, Fang and Reisen 2006); therefore, elevated herd immunity to WNV may impede the introduction and reestablishment of SLEV. WNV elicits more elevated viremias in birds and therefore may be able to more efficiently infect Culex mosquitoes and amplify more effectively than SLEV, even though the same Culex species require lower viremias for comparable infection patterns (Reisen et al. 2008a). Even with high mortality among competent hosts, WNV seroprevalence rates among surviving birds have been markedly greater than observed historically for either WEEV or SLEV (Reisen et al. 2000).
WEEV previously was endemic in Kern County (Reeves and Hammon 1962) but recently has declined as an equine and human health problem here and in most of North America (Reisen and Monath 1989). WEEV replicates more rapidly and at cooler temperatures within infected mosquitoes than does SLEV or WNV (Reisen et al. 2006c), which should provide WEEV a competitive advantage because it can be transmitted effectively over a longer part of the year than WNV. Although WEEV uses the same Cx. tarsalis and avian amplification hosts as does SLEV and WNV, WEEV is in the family Togaviridae, and previous exposure to WNV does not affect the viremia response of birds to WEEV infection (Reisen et al. 2008b). In agreement, WEEV has been detected intermittently in California since the arrival of WNV but has not amplified to levels within the Culex– bird cycle where transmission has spilled over to include Aedes and mammals (Hardy 1987). In addition, although Cx. quinquefasciatus occasionally have been found infected with WEEV in nature, this species cannot transmit WEEV (Hardy and Reeves 1990), and therefore, WEEV activity has been mostly confined to rural environments. WEEV does not seem to have become attenuated. A recent isolate from Cx. tarsalis collected in Imperial County during 2005 was found to be as equally infectious for Cx. tarsalis as the 1952 epidemic strain and produced a similar viremia response in white-crowned sparrows (Reisen et al. 2008b). Interestingly, the 2005 strain seemed less infectious for house sparrows, perhaps indicating lowered host competence in some birds. In contrast, a 1983 WEEV isolate from Kern County produced elevated viremias in a variety of bird species (Reisen et al. 2003b) and was more infectious than WNV for immature quail (Reisen et al. 2006d). Factors limiting the amplification of WEEV to outbreak levels remain under continued study.
Comparison of Kern County and Coachella Valley
Despite ecological similarities, the incidence of human infection in Coachella Valley has remained low (<2.5 per 100,000) compared with Kern County (7–17 per 100,000). These areas provide an excellent model to study arbovirus ecology, because both have the same Culex vectors, roughly the same size human population, and support extensive irrigated agriculture including grapes and citrus. However, Coachella Valley averages 3– 4°C warmer each month than Kern County, has significantly less precipitation during winter, and lacks a major river system. With warm winter temperatures, wetlands near the Salton Sea frequently have the earliest recorded arbovirus activity in California each year but rarely have human cases (Reisen et al. 2008c), despite low background antibody levels (Reisen et al. 1996). Factors that may have contributed to differences in human case incidence include (1) bimodal Cx. tarsalis seasonality, (2) spatial stratification of Culex vectors, (3) lack of “super spreader” bird species, (4) socioeconomic status of residents of the “hot zone,” and (5) human emigration and behavior during summer.
Elevated midsummer temperatures and the desiccation of larval habitats combine to create a bimodal seasonal abundance pattern for Cx. tarsalis in Coachella Valley (Reisen et al. 2008c), whereas in Kern County, abundance peaks in mid to late summer. A bimodal pattern results in a decrease in abundance after vernal amplification and during the hottest months most conducive to virus amplification and tangential transmission to humans. This scenario is compounded by the spatial segregation of the vector species, with Cx. tarsalis in the rural southeast and Cx. quinquefasciatus in the urban northwest, which may impede the dispersal of virus upland to more populated areas. In agreement, effective early season adult control in the southeast was found to interrupt transmission and impede dispersal of virus into the upper valley (Lothrop et al. 2008). By comparison, Bakersfield and smaller cities in Kern County are surrounded by agriculture allowing the amplification of virus to progress rapidly at rural/urban ecotones.
A major epidemiological difference between Kern and Coachella Valley was the widespread distribution and high abundance of the western scrub-jay in suburban Kern County but not Coachella Valley. Although present, the abundance and distribution of American crows in Kern County were fairly low and limited to the north near the town of Delano; American crows were almost absent from Coachella Valley. Although the Coachella Valley supports a highly diverse avifauna, large corvid and American robin populations are lacking, perhaps limiting infection in moderately susceptible mosquito populations (Reisen et al. 2006a). Our attempts to identify other communally nesting or roosting birds as a major source of virus amplification have not been successful (Reisen et al. 2005b, 2006d.
Two other factors may impact arbovirus epidemiology in Coachella Valley. In both areas, Hispanic agricultural workers frequently work at night to harvest time-sensitive crops and/or to avoid the heat of the day. Although both groups experience the same socioeconomic and health insurance hardships, workers in Coachella Valley live in the southeast and relatively near to Mexico. This results in an ethnic spatial segregation of the uninsured who are less prone to seek medical attention and of attending physicians who are less prone to recommend testing for pathogens such as WNV for which there are no cure. In addition, highly ill individuals can be transported rapidly to Mexico where care is available at lower cost. In combination, these socioeconomic factors lower the sensitivity of the passive case detection system and the perceived burden of disease within these communities. In support of this idea, most of the human cases reported from the Coachella Valley come from the upper valley where the age structure of the population is skewed toward the older age groups and the portion of the population with health insurance is relatively high. In addition, a large portion of the Palm Springs and upper valley community historically leaves the Coachella Valley during the hot summer months, thereby lowering the population base at risk from infection. Those that remain frequently remain indoors in air conditioning during the afternoon and evening when temperatures frequently exceed 40°C.
In summary, WNV is now endemic throughout the varied ecosystems of California and will remain an important public, veterinary, and wildlife health problem for the foreseeable future. Successful intervention and protection of the public health will depend on enhanced understanding of factors enabling virus persistence during winter and amplification during the late winter and early spring periods. Our on-going research examines the interplay among overwintering mechanisms, winter and vernal climate variation, passerine herd immunity, and avian species depopulation to develop models predictive of outbreaks. The current risk model ranking temperature, mosquito abundance and infection, and sentinel and dead bird data into quintiles and averaging these rankings per half month provided some emergency planning lead time and always increased to epidemic conditions coincidental with the onset of human cases. Risk ranked within this model is linked to an incremental response plan recommending escalating intervention methods. Future plans for this model include early winter forecasts of vernal and summer mosquito population abundance based on climate variation and modifying the current nowcasts to combine Cx. tarsalis and Cx. pipiens complex data and to segregate rural and urban risk spatially based on human population density.
Acknowledgments
We thank the Governing Board and staff of the Kern Mosquito and Vector Control District who provided technical and logistical support for this project. S. Hallam and the staff of the Arbovirus Field Station assisted with field collections. Mosquito pools and dead bird tissues were tested by R. E. Chiles, E. G. Green, B. C. Young, N. Kahl, M. Shaffii, S. Ashtari, M. Dannen, K. Simmons, A. Chow, and others at the Arbovirus Research Unit, Center for Vectorborne Diseases, U.C. Davis. Dead bird reports were provided by the Dead Bird Surveillance Program lead by S. Husted and staff of the Vectorborne Disease Section, California Department of Public Health. Tissues were collected at necropsy by the California Animal Health of Food Safety Laboratory at U.C. Davis. Reports of human cases were provided by the Kern Count Health Department. Sentinel chicken sera were tested by E. Baylis and staff at the Viral and Rickettsial Diseases Laboratory, California Department of Public Health. Selected data summaries were provided by B. Park and C. M. Barker, U.C. Davis. This research was funded, in part, by Grant RO1 AI55607 from the National Institute of Allergy and Infectious Diseases, NIH, special funds Mosquito Surveillance provided by the Centers for Disease Control and Prevention to the California Department of Public Health, NASA Earth-Sun Science Applied Sciences Program Research Opportunities in Space and Earth Science, Decision Support through Earth-Sun Science Research Results grant, and funds from the Mosquito Research Program allocated annually through the Division of Agriculture and Natural Resources, University of California.
References Cited
- American Ornithologists’ Union. Check-list of North American Birds. 7. American Ornithologists’ Union; Washington, DC: 1998. [DOI] [PubMed] [Google Scholar]
- Biggerstaff BJ. Pooled infection rate. Centers for Disease Control and Prevention; Ft. Collins, CO: 2003. [Google Scholar]
- Carroll BD, Takahashi RM, Reisen WK. West Nile Virus in Kern County: factors leading to the 2007 outbreak. Proc Mosq Vector Control Assoc Calif. 2008 (in press) [PMC free article] [PubMed] [Google Scholar]
- Chiles RE, Reisen WK. A new enzyme immunoassay to detect antibodies to arboviruses in the blood of wild birds. J Vector Ecol. 1998;23:123–135. [PubMed] [Google Scholar]
- Chiles RE, Green EN, Fang Y, Reisen WK, Edman JD, Brault AC. Surveillance for arboviruses in California mosquito pools: current and future protocols. Proc Mosq Vector Control Assoc Calif. 2004;72:15–17. [Google Scholar]
- Cornel AJ, Mcabee R, Rasgon J, Stanich MA, Scott TW, Coetzee M. Differences in extent of genetic introgression between sympatric Culex pipiens and Culex quinquefasciatus (Diptera: Culicidae) in California and South Africa. J Med Entomol. 2003;40:36 –51. doi: 10.1603/0022-2585-40.1.36. [DOI] [PubMed] [Google Scholar]
- Cummings RF. Design and use of a modified Reiter gravid mosquito trap for mosquito-borne encephalitis surveillance in Los Angeles County, California. Proc Mosq Vector Control Assoc Calif. 1992;60:170 –176. [Google Scholar]
- Davis CT, Ebel GD, Lanciotti RS, Brault AC, Guzman H, Siirin M, Lambert A, Parsons RE, Beasley DW, Novak RJ, Elizondo-Quiroga D, Green EN, Young DS, Stark LM, Drebot MA, Artsob H, Tesh RB, Kramer LD, Barrett AD. Phylogenetic analysis of North American West Nile virus isolates, 2001–2004: evidence for the emergence of a dominant genotype. Virology. 2005;342:252–265. doi: 10.1016/j.virol.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Ebel GD, DuPuis AP, Nicholas D, Young D, Maffei J, Kramer LD. Detection by enzyme-linked immunosorbent assay of antibodies to West Nile virus in birds. Emerg Infect Dis. 2002;8:979 –982. doi: 10.3201/eid0809.020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnaien DEA, Kelley K, Wright SA, Laffey R, Yoshimura G, Armijos V, Reed M, Goodman G, Reisen WK, Brown DA. Epidemic Amplification of West Nile Virus in Sacramento and Yolo Counties, June–September 2005. Proc Calif Mosq Vector Control Assoc. 2006;74:18 –20. [Google Scholar]
- Fang Y, Reisen WK. Previous infection with West Nile or St. Louis encephalitis viruses provides cross protection during reinfection in house finches. Am J Trop Med Hyg. 2006;75:480 – 485. [PubMed] [Google Scholar]
- Feiszli T, Park B, Kramer VL, Kjemtrup A, Eldridge BF, Fang Y, Reisen WK, Baylis E, Jean C, Glover J, Carney R, Padgett K, Erickson C, Husted S. Surveillance for mosquito-borne encephalitis virus activity in California, 2006. Proc Mosq Vector Control Assoc Calif. 2007;75:48 –59. [Google Scholar]
- Gahlinger PM, Reeves WC, Milby MM. Air conditioning and television as protective factors in arboviral encephalitis risk. Am J Trop Med Hyg. 1986;35:601– 610. doi: 10.4269/ajtmh.1986.35.601. [DOI] [PubMed] [Google Scholar]
- Goddard LB, Roth AE, Reisen WK, Scott TW. Vector competence of California mosquitoes for West Nile virus. Emerg Infect Dis. 2002;8:1385–1391. doi: 10.3201/eid0812.020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham RR, Hardy JL, Presser SB. Use of the in situ enzyme immunoassay for the rapid detection of arbovirus infections in mosquitoes in California. Proc Calif Mosq Vector Control Assoc. 1986;54:10. [Google Scholar]
- Hardy JL. The ecology of western equine encephalomyelitis virus in the Central Valley of California, 1945– 1985. Am J Trop Med Hyg. 1987;37:18s–32s. doi: 10.4269/ajtmh.1987.37.18s. [DOI] [PubMed] [Google Scholar]
- Hardy JL, Reeves WC. Experimental studies on infection in vectors. In: Reeves WC, editor. Epidemiology and control of mosquito-borne arboviruses in California, 1943–1987. Calif. Mosq. Vector Control Assoc; Sacramento, CA: 1990. pp. 145–250. [Google Scholar]
- Hayes EB, Komar N, Nasci RS, Montgomery SP, O’Leary DR, Campbell GL. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintze J. NCSS statistical software, version 2000. NCSS; Kaysville, UT: 1998. [Google Scholar]
- Hom A, Houchin A, McCaughey K, Kramer VL, Chiles RE, Reisen WK, Tu E, Glaser C, Cossen C, Baylis E, Eldridge BF, Sun B, Padgett K, Woods L, Marcus L, Hui LT, Castro M, Husted S. Surveillance for mosquito-borne encephalitis activity and human disease, including West Nile virus in California, 2003. Proc Mosq Vector Control Assoc Calif. 2004;72:48 –54. [Google Scholar]
- Hom A, Marcus L, Kramer VL, Cahoon B, Glaser C, Cossen C, Baylis E, Jean C, Tu E, Eldridge BF, Carney R, Padgett K, Sun B, Reisen WK, Woods L, Husted S. Surveillance for mosquito-borne encephalitis virus activity and human disease, including West Nile virus, in California, 2004. Proc Mosq Vector Control Assoc Calif. 2005;73:66 –77. [Google Scholar]
- Komar N, Panella NA, Burns JE, Dusza SW, Mascarenhas TM, Talbot TO. Serologic evidence for West Nile virus infection in birds in the New York City vicinity during an outbreak in 1999. Emerg Infect Dis. 2001;7:621– 625. doi: 10.3201/eid0704.010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N, Panella NA, Langevin SA, Brault AC, Amador M, Edwards E, Owen JC. Avian hosts for West Nile virus in St. Tammany Parish, Louisiana, 2002. Am J Trop Med Hyg. 2005;73:1031–1037. [PubMed] [Google Scholar]
- Kramer VL. California State Mosquito-borne Virus Surveillance and Response Plan. 2008 doi: 10.4269/ajtmh.2003.68.508. ( http://westnile.ca.gov/resources.php) [DOI] [PubMed]
- Lanciotti RS, Kerst AJ. Nucleic acid sequence-based amplification assays for rapid detection of West Nile and St. Louis encephalitis viruses. J Clin Microbiol. 2001;39:4506 – 4513. doi: 10.1128/JCM.39.12.4506-4513.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothrop HD, Lothrop BB, Gomsi DE, Reisen WK. Intensive early season adulticide applications decrease arbovirus transmission throughout the Coachella Valley, Riverside County, California. Vector Borne Zoonotic Dis. 2008;8:475– 489. doi: 10.1089/vbz.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaughey K, Miles SQ, Woods L, Chiles RE, Hom A, Kramer VL, Jay-Russel M, Sun B, Reisen WK, Scott TW, Hui LT, Steinlein DB, Castro M, Houchin A, Husted S. The California West Nile virus dead bird surveillance program. Proc Mosq Vector Control Assoc Calif. 2003;71:38 – 43. [Google Scholar]
- McClure HE, Reeves WC, Hammon WM. Ornithological investigations. In: Reeves WC, Hammon WM, editors. Epidemiology of the arthropod-borne encephalitides in Kern County, California, 1943–1952. Univ. Calif. Press; Berkeley, CA: 1962. pp. 109–154. [Google Scholar]
- Newhouse VF, Chamberlain RW, Johnston JG, Jr, Sudia WD. Use of dry ice to increase mosquito catches of the CDC miniature light trap. Mosq News. 1966;26:30 –35. [Google Scholar]
- Park B, Eldridge BF, Barker CM, Reisen WK. Building upon California’s surveillance gateway. Proc Mosq Vector Control Assoc Calif. 2008 (in press) [Google Scholar]
- Reeves WC. Clinical and subclinical disease in man. In: Reeves WC, editor. Epidemiology and control of mosquito-borne arboviruses in California, 1943– 1987. California Mosquito Vector Control Assoc; Sacramento, CA: 1990. pp. 1–25. [Google Scholar]
- Reeves WC, Hammon WM. Epidemiology of the arthropod-borne viral encephalitides in Kern County, California, 1943–1952. Univ Calif Berkeley Publ Publ Hlth. 1962;4:1–257. [PubMed] [Google Scholar]
- Reisen WK, Monath TP. Western equine encephalomyelitis. In: Monath TP, editor. The arboviruses: epidemiology and ecology. CRC; Boca Raton, FL: 1989. pp. 89–138. [Google Scholar]
- Reisen WK, Meyer RP, Milby MM. Overwintering studies on Culex tarsalis (Diptera:Culicidae) in Kern County, California: temporal changes in abundance and reproductive status with comparative observations on C. quinquefasciatus (Diptera:Culicidae) Ann Entomol Soc Am. 1986;79:677– 685. [Google Scholar]
- Reisen WK, Meyer RP, Tempelis CH, Spoehel JJ. Mosquito abundance and bionomics in residential communities in Orange and Los Angeles Counties, California. J Med Entomol. 1990;27:356 –367. doi: 10.1093/jmedent/27.3.356. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Milby MM, Meyer RP, Pfuntner AR, Spoehel J, Hazelrigg JE, Webb JP., Jr Mark-release-recapture studies with Culex mosquitoes (Diptera: Culicidae) in southern California. J Med Entomol. 1991;28:357–371. doi: 10.1093/jmedent/28.3.357. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Meyer RP, Milby MM, Presser SB, Emmons RW, Hardy JL, Reeves WC. Ecological observations on the 1989 outbreak of St. Louis encephalitis virus in the southern San Joaquin Valley of California. J Med Entomol. 1992a;29:472– 482. doi: 10.1093/jmedent/29.3.472. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Milby MM, Presser SB, Hardy JL. Ecology of mosquitoes and St. Louis encephalitis virus in the Los Angeles Basin of California, 1987–1990. J Med Entomol. 1992b;29:582–598. doi: 10.1093/jmedent/29.4.582. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Lin J, Presser SB, Enge B, Hardy JL. Evaluation of new methods for sampling sentinel chickens for antibodies to WEE and SLE viruses. Proc Calif Mosq Vector Control Assoc. 1993;61:33–36. [Google Scholar]
- Reisen WK, Presser SB, Lin J, Enge B, Hardy JL, Emmons RW. Viremia and serological responses in adult chickens infected with western equine encephalomyelitis and St. Louis encephalitis viruses. J Am Mosq Control Assoc. 1994;10:549 –555. [PubMed] [Google Scholar]
- Reisen WK, Chiles RE, Lothrop HD, Presser SB, Hardy JL. Prevalence of antibodies to mosquito-borne encephalitis viruses in residents of the Coachella Valley. Am J Trop Med Hyg. 1996;55:667– 671. doi: 10.4269/ajtmh.1996.55.667. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Lundstrom JO, Scott TW, Eldridge BF, Chiles RE, Cusack R, Martinez VM, Lothrop HD, Gutierrez D, Wright S, Boyce K, Hill BR. Patterns of avian seroprevalence to western equine encephalomyelitis and St. Louis encephalitis viruses in California, USA. J Med Entomol. 2000;37:507–527. doi: 10.1603/0022-2585-37.4.507. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Kramer LD, Chiles RE, Green EGN, Martinez VM. Encephalitis virus persistence in California birds: preliminary studies with house finches (Carpodacus mexicanus) J Med Entomol. 2001;38:393–399. doi: 10.1603/0022-2585-38.3.393. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Chiles RE, Green EN, Fang Y, Mahmood F. Previous infection protects finches from re-infection with St. Louis encephalitis virus. J Med Entomol. 2003a;40:300 –305. doi: 10.1603/0022-2585-40.3.300. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Chiles RE, Martinez VM, Fang Y, Green EN. Experimental infection of California birds with western equine encephalomyelitis and St. Louis encephalitis viruses. J Med Entomol. 2003b;40:968 –982. doi: 10.1603/0022-2585-40.6.968. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Chiles RE, Martinez VM, Fang Y, Green EN. Encephalitis virus persistence in California birds: experimental infections in mourning doves (Zenaidura macroura) J Med Entomol. 2004a;3:462– 466. doi: 10.1603/0022-2585-41.3.462. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Lothrop HD, Chiles RE, Madon MB, Cossen C, Woods L, Husted S, Kramer VL, Edman JD. West Nile virus in California. Emerg Infect Dis. 2004b;10:1369 –1378. doi: 10.3201/eid1008.040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK, Fang Y, Martinez VM. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission. J Med Entomol. 2005a;42:367– 375. doi: 10.1093/jmedent/42.3.367. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Wheeler SS, Yamamoto S, Fang Y, Garcia S. Nesting Ardeid colonies are not a focus of elevated West Nile virus activity in southern California. Vector Borne Zoonotic Dis. 2005b;5:258 –266. doi: 10.1089/vbz.2005.5.258. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Barker CM, Carney R, Lothrop HD, Wheeler SS, Wilson JL, Madon MB, Takahashi R, Carroll B, Garcia S, Fang Y, Shafii M, Kahl N, Ashtari S, Kramer V, Glaser C, Jean C. Role of corvids in epidemiology of West Nile virus in southern California. J Med Entomol. 2006a;43:356 –367. doi: 10.1603/0022-2585(2006)043[0356:rocieo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Fang Y, Lothrop HD, Martinez VM, Wilson J, OConnor P, Carney R, Cahoon-Young B, Shafii M, Brault AC. Overwintering of West Nile virus in Southern California. J Med Entomol. 2006b;43:344 –355. doi: 10.1603/0022-2585(2006)043[0344:oownvi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae) J Med Entomol. 2006c;43:309 –317. doi: 10.1603/0022-2585(2006)043[0309:EOTOTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK, V, Martinez M, Fang Y, Garcia S, Ashtari S, Wheeler SS, Carroll BD. Role of California (Callipepla californica) and Gambel’s (Callipepla gambelii) quail in the ecology of mosquito-borne encephalitis viruses in California, USA. Vector Borne Zoonotic Dis. 2006d;6:248 –260. doi: 10.1089/vbz.2006.6.248. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Barker CM, Fang Y, Martinez VM. Does variation in Culex vector competence enable outbreaks of West Nile virus in California? J Med Entomol. 2008a;45:1125–1138. doi: 10.1603/0022-2585(2008)45[1126:dvicdc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Fang Y, Brault AC. Limited interdecadal variation in mosquito (Diptera: Culicidae) and avian host competence for Western equine encephalomyelitis virus (Togaviridae: Alphavirus) Am J Trop Med Hyg. 2008b;78:681– 686. [PubMed] [Google Scholar]
- Reisen WK, Lothrop HD, Wheeler SS, Kensington M, Gutierrez A, Fang Y, Garcia S, Lothrop B. Persistent West Nile virus transmission and the displacement St Louis encephalitis virus in southeastern California, 2003–2006. J Med Entomol. 2008c;45:494 –508. doi: 10.1603/0022-2585(2008)45[494:pwnvta]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen WK, Takahashi RM, Carroll BD, Quiring R. Adjustable rate mortgages impact West Nile virus transmission. Emerg Infect Dis. 2008d;14:1747–1749. doi: 10.3201/eid1411.080719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PT, Reisen WK, Cowles DA. Effects of larval rearing density on interspecific competition between Culex tarsalis and Culex quinquefasciatus. J Soc Vector Ecol. 1995;20:139 –146. [Google Scholar]
- Tabachnick WJ, Powell JR. Genetic analysis of Culex pipiens populations in the central valley of California. Ann Entomol Soc Am. 1983;76:715–720. [Google Scholar]
- Tempelis CH, Reeves WC, Nelson RL. Species identification of blood meals from Culex tarsalis that had fed on passeriform birds. Am J Trop Med Hyg. 1976;25:744 –746. doi: 10.4269/ajtmh.1976.25.744. [DOI] [PubMed] [Google Scholar]
- Tesh RB, Travasos da Rosa AP, Guzman H, Araujo TP, Xiao SY. Immunization with heterologous flaviviruses protective against fatal West Nile encephalitis. Emerg Infect Dis. 2002;8:245–251. doi: 10.3201/eid0803.010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tueller JE. An outbreak of illness due to St. Louis encephalitis virus in the southern San Joaquin Valley, California, 1989. Proc Calif Mosq Vector Control Assoc. 1990;58:12. [Google Scholar]
- Urbanelli S, Silvestrini F, Reisen WK, deVito E, Bullini L. California hybrid zone between Culex pipiens pipiens and Cx. p quinquefasciatus revisited (Diptera: Culicidae) J Med Entomol. 1997;34:116 –127. doi: 10.1093/jmedent/34.2.116. [DOI] [PubMed] [Google Scholar]
- Wegbreit J, Reisen WK. Relationships among weather, mosquito abundance and encephalitis virus activity in California: Kern County 1990 –1998. J Am Mosq Control Assoc. 2000;16:22–27. [PubMed] [Google Scholar]
- Wheeler SS, Barker CM, Armijos MV, Carroll BD, Husted SR, Reisen WK. Differential impact of West Nile virus on California birds. The Condor. 2008 doi: 10.1525/cond.2009.080013. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J, Madon MB, Reisen WK. The overwintering and amplification of West Nile in the southern portion of Greater Los Angeles Vector Control District. Proc Mosq Vector Control Assoc Calif. 2005;73:12–13. [Google Scholar]
- Wilson JL, Reisen WK, Madon MB. Three years of West Nile virus in Greater Los Angeles County. Proc Mosq Vector Control Assoc Calif. 2006;74:9 –11. [Google Scholar]
- Wilson JL, Kluh S, Morales H, Madon MB, O’Connor P, Posey T, O’Connor J, Carney R, Fang Y, Garcia S, Reisen WK. Spatial and temporal ecology of West Nile virus in Los Angeles, California: implications for sporadic peristence. Proc Mosq Vector Control Assoc Calif. 2008 (in press) [Google Scholar]
- Work TH. On the Japanese B–West Nile virus complex or an arbovirus problem of six continents. Am J Trop Med Hyg. 1971;20:169 –186. doi: 10.4269/ajtmh.1971.20.169. [DOI] [PubMed] [Google Scholar]