Abstract
Information on the neurobiology of empathy and callousness provides clinicians an opportunity to develop sophisticated understanding of mechanisms underpinning antisocial behavior and its counterpart, moral decision making. This paper provides an integrated in-depth review of hormones (e.g., peripheral steroid hormones like cortisol) and brain structures (e.g., insula, anterior cingulate cortex, and amygdala) implicated in empathy, callousness and psychopathic-like behavior. The overarching goal of this paper is to relate these hormones and brain structures to moral decision-making. This review will begin in the brain, but will then integrate information about biological functioning in the body, specifically stress-reactivity. Our aim is to integrate understanding of neural processes with hormones like cortisol, both of which have demonstrated relationships to empathy, psychopathy, and antisocial behavior. The review proposes neurobiological impairments in individuals who display little empathy are not necessarily due to a reduced ability to understand the emotions of others. Instead, evidence suggests individuals who show little arousal to the distress of others likewise show decreased physiological arousal to their own distress; one manifestation of reduced stress reactivity may be a dysfunction in empathy which supports psychopathic-like constructs (e.g., callousness). This integration will assist in the development of objective methodologies that can inform and monitor treatment interventions focused on decreasing antisocial behavior.
Keywords: moral decision making, psychopathy, empathy, callousness
The purpose of this paper is to review and integrate the neurobiological underpinnings of empathy and callousness to promote understanding of mechanisms behind moral decision-making and, conversely, the development of antisocial behavior. This paper is divided into two main sections. We will begin in the brain, reviewing a triad of neurocircuitry involved in empathy as well as the unique neural signature of callousness and antisocial behavior. This section will highlight (a) brain areas that show overlapping activation across empathy- and callousness-focused investigations, and then (b) neural processes that are unique to callousness. Much of this circuitry has strong reciprocal connections with peripheral physiology, including stress-reactive hormones like cortisol. The second section will review evidence that cortisol and the hypothalamic-pituitary-adrenal (HPA) axis is (a) connected with the neurocircuitry involved with empathy and/or callousness and (b) correlated with empathy or prosocial behavior as well as callous or antisocial behavior. The point of combining neural and peripheral physiology is to suggest that impairments in moral decision-making in psychopathic individuals may not directly involve impairments in the ability to feel emotions of another. The mechanism may be more basic, involving general difficulties in responding to stressful or emotional stimuli, including social distress contexts. The neurocircuitry involved in promoting empathy and prosocial behavior needs to integrate stress signals from the periphery; empathic processes may be disrupted in the absence of that activation. The callous individual is hyporesponsive towards themselves as well as others.
Callousness / Unemotionality
Callousness and unemotional (CU) traits are related to maladaptive social information processing, even in children and adolescents (Frick & White, 2008; Pardini, Lochman, & Frick, 2003). While empathy may promote affiliation and prosocial behavior, CU traits have been associated with antisocial behavior and are a core feature of psychopathy (Enebrink, Andershed, & Langstrom, 2005; Frick, Bodin, & Barry, 2000). CU traits are particularly good in predicting which antisocial and violent youth will persist in their offending into adulthood (Frick, Cornell, Barry, Bodin, & Dane, 2003). When coupled with impulsivity, CU traits can provide a symptom picture of the syndrome of psychopathy across adolescents and adults (Hare, 2003; Pardini et al., 2003; Vitacco, Rogers, & Neumann, 2003; Vitacco, Salekin, & Rogers, in press). Although there are several important traits inherent in the psychopathy construct (Farrington, 2005; Lynam, 1998; Waschbusch, 2002), it is often deficits in emotionality and the failure to respond to the distress cues of others (i.e., callousness) which lie at the core of the impaired decision-making capabilities in these individuals (Kimonis, Frick, Fazekas, & Loney, 2006). Disruptions in empathy may also characterize these individuals. For example, children scoring high on the Antisocial Process Screening Device (ASPD, Frick & Hare, 2001) were more likely than low scorers to judge moral transgressions as acceptable. New evidence of empathy's grounding in the brain provides a convincing case to place emphasis on the relationship of callousness/unemotionality and deficient moral decision-making to the series of neurobiological variations exhibited by individuals with severe antisocial behavior, and psychopathy in particular.
Empathy
For centuries, the construct of empathy has held a foundation in neurobiology. Smith (1790) defined empathy as “the ability to understand another's perspective and to have a visceral or emotional reaction” (as cited by Hastings, Zahn-Waxler, & McShane, 2006). Operational definitions of empathy to permit empirical investigations have extended from a process-oriented definition (Preston & de Waal, 2002) to a clear separation of emotional form of empathy from the cognitive form of mentalizing (Knafo, Zahn-Waxler, Van Hulle, Robinson, & Rhee, 2008; Singer, 2006). Hastings, Zahn-Waxler and McShane (2006) also hone in their focus on emotions, and, like Preston and de Waal (2002) emphasize that empathy is a multistage process. They define empathy as “the recognition and sharing of another's emotional state” (Hastings et al., 2006). This last definition will generally guide our review.
While empathy may include both a cognitive and an emotion component, our focus will be on the emotion component for two reasons. First, it is the emotion process which appears to be most disrupted when moral decision-making is compromised in empathy-related disorders, like psychopathy (Blair, Jones, Clark, & Smith, 1997). This is not necessarily the case for all empathy-related disorders (e.g., autism) (Iacoboni & Dapretto, 2006). Second, extant literature on peripheral physiology including hormonal functioning is heavily influenced by emotion-related processes. The neural substrates of emotion also can be differentiated from cognition areas, and it is the emotion circuitry that is often dense with hormone receptors. Cognition is generally associated with hormones through a secondary or interactive mechanism with emotion processes (Dahl, 2004).
Before exploring neurobiological mechanisms for callousness or empathy, four caveats are warranted. First, it is not our intention to argue that someone has or does not have empathy. An understanding of neurobiological mechanisms requires an emphasis on individual differences in trait expression and the person-environment match (not on presence vs. absence of a response).
Second, this literature review and integration focuses on humans. As reviewed below, the neurocircuitry of pair-bonding - the ontogenetic basis for empathy - is very different across monogamous and polygamous species (Insel & Fernald, 2004). Most of the animal literature (including the hormonal literature) is only distally applicable to humans because most animal studies are conducted on polygamous species (i.e., the rat or rhesus macaque) while humans debatably tend to be more monogamous. Also, empathy is a recently evolved construct and is applicable to relatively few species (de Waal, Macedo, & Ober, 2006).
Third, although we emphasize the role of neurobiology in empathy and callousness, we recognize that they are proximal mechanisms and do not necessarily imply immutability. These traits are likely shaped by an interaction of environmental as well as biological processes over the course of development.
Fourth, despite its definition, “feeling or showing a lack of empathy or guilt” (Frick & White, 2008), our view is that callousness is not necessarily the precise converse of empathy at a behavioral level. Callousness largely refers to the omission of caring feelings or behaviors in contexts where others generally experience those feelings or behaviors. Empathy, on the other hand, involves a commission or expression of some feeling or behavior. It involves actively experiencing visceral emotion and understanding another's perspective. Measures of empathy typically assess whether the participant feels concern for the other person and sometimes by extension engages in caring, prosocial behaviors. If callousness were behaviorally the opposite of empathy, then its observation would merely involve the failure to observe prosocial behaviors in certain contexts. However, active disregard for others in distress (dismissiveness, enjoyment, condescension, hostility) is also seen (Hastings, Zahn-Waxler, Robinson, Usher, & Bridges, 2000). Disorders like psychopathy are defined by callous/antisocial behaviors, not merely the lack of empathic/prosocial behaviors (Blair, 2007a). This distinction, however, is largely heuristic; despite differences in study design, populations or theories, there is evidence of convergence across the empathy- and callous-focused perspectives, particularly at an emotional level. Our literature review which involves both literatures is predicated on the assumption that there are both similarities and differences in the neurobiological underpinnings of the empathy and callousness constructs. At an emotional level, they may be two ends of the same continuum but at a behavioral level, the model requires greater complexity because these behaviors are not precisely representative of these emotions. By exploring their similarities and differences, greater knowledge about the process of moral decision-making will be gained.
Neural underpinnings of empathy and callousness
Ontogeny of Neural System
The next few pages will review data that supports the idea that empathy is neurobiologically supported by a triangulation of neural circuitry. Empathy connects neurocircuitry for social behavior, physical pain, and the ability to represent both the self and another. Through these neurological connections, the brain promotes understanding of social distress or pain in others and experiences that distress very much as though the feeling was generated within the self. This neurobiological mechanism promotes social affiliation and behaviors, which seek to reduce the display of distress in others.
The ontogenetic roots of empathy likely arose within the mammalian brain to support social bonds between a mother and child (MacLean, 1985; Swain, Lorberbaum, Kose, & Strathearn, 2007). As reviewed by Hastings and colleagues (2006), the rudimentary appearance of affiliation and bonding first appeared with mammals, who provide nurturance and care for their offspring for an extended period of time. The neurobiological underpinnings of the mother-child bond, not surprisingly, involve the limbic system (i.e., the emotion circuitry). This circuitry was established to support bonding between a mother and child, with some components long believed to be active only around the time of pregnancy and lactation. Yet, a mother-child bond is not the only form of affiliation important for survival. The limbic system has been co-opted for other forms of affiliation as well, including pair bonding behaviors (Insel, 1997; Insel & Fernald, 2004) or affiliation among friends (Taylor et al., 2000). Insel and colleagues (1997; 2004) generalize the involvement of the limbic system with most forms of affiliation and a variety of social behaviors, including empathy. Nelson and colleagues (2005) also identify the limbic circuitry as one of three key components of social information processing. Neurohormones like oxytocin, vasopressin, and peripheral steroid hormones like cortisol are important modulators of limbic activity. Consistent with its ontogenetic roots, this affiliative circuitry is especially powerful in females (Swain et al., 2007; Taylor et al., 2000), but is functional in both genders (Geary & Flinn, 2002). The neurocircuitry for affiliation and bonding is especially important in modulating the response to social stress and, during difficult times, in promoting affiliative behavior towards offspring, mates and friends. Central and peripheral hormones, including cortisol, help modulate limbic activity during stress (Taylor et al., 2000). Thus, empathy involves many brain areas, but it may be largely instantiated in the limbic system as a function of its root in bonding and affiliation.
Not all bonding is warm and fuzzy. At the same ontogenetic time that mammals developed the neural structures necessary for bonding and attachment to support social behavior and affiliation, the nervous system was co-opted to encode the potential harmful consequences of social separation or distress. The social attachment system borrowed the physical pain neural circuitry to encode social pain (Eisenberger & Lieberman, 2004; Eisenberger, Lieberman, & Williams, 2003). Experiencing one's own social pain or feelings of rejection, however, does not necessarily indicate empathy. To do so would require a neural mechanism that connected personal emotions or distress with one's interpretation of those same feelings in another. Again, the brain likely co-opted existing neurocircuitry. As will be reviewed below, the representation of pain, distress, or emotions experienced by another is instantiated in the very same structures which encode the experience of pain, distress, or emotion in the self.
Mirror Neuron System
Interest in the neural underpinnings of empathy burgeoned over a decade ago when Di Pellegrino and colleagues accidentally discovered premotor neurons that fire when a primate performs goal-directed hand movements or when the primate merely observes these hand movements performed by the experimenter (di Pellegrino, Fadiga, Fogassi, Gallese, & Rizzolatti, 1992). This observation was influential because it provided a neural basis for imitation and shed light on how learning through observation could take place. These neurons were termed “mirror neurons” (Rizzolatti & Craighero, 2004).
The mirror neuron system is largely motoric, but it can help provide a plausible mechanism for certain social and emotional behaviors (e.g., imitation, Iacoboni & Dapretto, 2006). The mirror neuron system is fundamentally linked with emotion-related circuitry (Carr, Iacoboni, Dubeau, Mazziotta, & Lenzi, 2003). Individual differences in activity in the mirror neuron system is correlated with behavioral indices of children's empathic behavior and interpersonal skill (Pfeifer, Iacoboni, Mazziotta, & Dapretto, 2008; Schulte-Ruther, Markowitsch, Fink, & Piefke, 2007). This suggests that the mirror neuron system's connectivity to emotional and social behavior has mechanistic as well as practical significance.
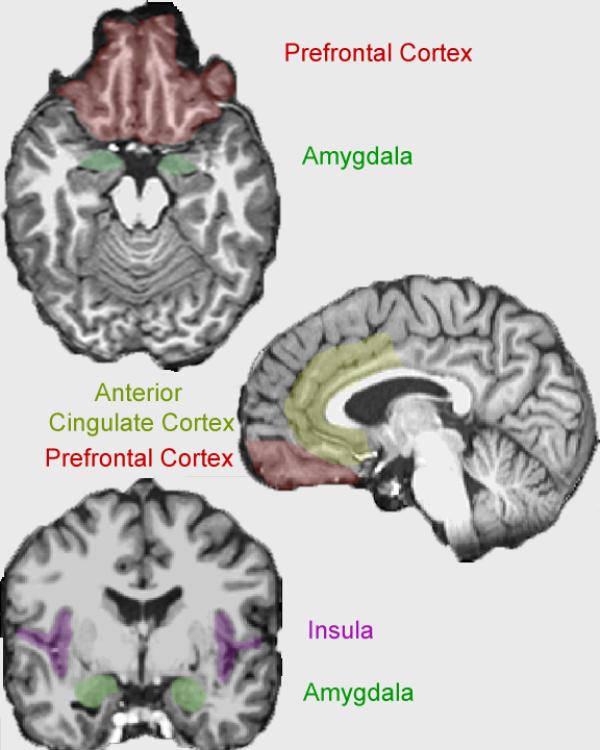
In addition to motor imitation, the mirror neuron mechanism is also implicated for pain. Much of the circuitry activated when one experiences pain is also activated when one imagines, anticipates, or observes others in pain (Craig, 2002, 2003). In those areas which are activated regardless of whether the self or another experience pain, the distinction between self and other is coded primarily by the degree of activation rather than having an anatomically distinct area of activation (Jackson, Brunet, Meltzoff, & Decety, 2006). For example, Singer and colleagues compared neural responses when an individual experienced a painful stimulus to when they observed a loved one receiving a similar painful stimulus. They found that much of the brain activity in pain pathways overlapped regardless of who received the painful stimulus (Singer et al., 2004). This study also highlighted at least two key brain areas that connect the mirror neuron system with the limbic system. These areas - the insula and the anterior cingulate cortex (ACC) - are ideally situated as pathways or relay stations for information connecting our experience of emotions with our understanding of the emotions of others.
Insula
The insula is generally implicated in negative emotional states like disgust, pain and hunger. The insula is a conduit for information to/from limbic and thalamic structures which convey arousal/ emotional/ homeostatic information from the periphery (Craig, 2002, 2003). One view of the insula is as a mechanism for mapping peripheral physiological responses or changes in arousal levels with top-down internal feedback signals about subjective feelings (Critchley, 2005). The insula is physically changed if signals from peripheral physiology are removed, demonstrating the importance of the connection with the body for this brain area (Critchley et al., 2003).
This is an important area for integrating emotional information with information from other cortical areas, including mirror areas. Carr and colleagues (2003) compared neural responses when participants imitated whole facial displays of emotion vs. observed the stimuli. They found premotor areas were activated during imitation of emotion expressions, and importantly, they found the insula and amygdala were preferentially activated during imitation. Another study found viewing faces of others' disgust triggered nearly as much insula activity as smelling a disgusting odor (Wicker et al., 2003). This evidence is consistent with a broader literature demonstrating insula activity during imitation or mental imagery (Phan, Wager, Taylor, & Liberzon, 2002).
Finally, the insula operates as a relay with the limbic system for pain, as evidenced by several studies that found insula activation during the experience, imitation, or imagination of pain. Singer et al (2004) identified insula activation when subjects received pain or when they observed their loved one experiencing pain. She interprets this as evidence that the neurocircuitry for empathizing with others is the same as the neurocircuitry for understanding feeling states of the self (Singer, 2006). The insula was also activated regardless of whether the participant imagined themselves or another person in painful situations (Jackson et al., 2006), and whether participants perceived or assessed painful stimuli in others (Jackson, Meltzoff, & Decety, 2005). The study of insula activation may help in understanding feeling states even in situations where there is no direct peripheral physiological input.
In general, the insula is activated across a broad range of contexts involving experiences of the self or another individual and regardless of whether the stimuli is motoric, pain, or emotion. The brain can distinguish at the cortical level whether the self or another individual is experiencing an emotion or pain or peripheral arousal; yet, at some core level, it is difficult to distinguish personal from socially relevant cues (Decety & Lamm, 2006).
Anterior Cingulate Cortex (ACC)
The functions of the ACC can generally be subsumed under the role of a neural alarm system (Eisenberger & Lieberman, 2004), signaling when something is wrong or when an automatic process should become effortful (Phan et al., 2002). The ACC is activated during error detection tasks, signaling the amount of distress associated with errors; it also becomes active during conflict detection tasks when performance is effortful or warrants an emotional evaluation. Consistent with this emotional distress role, the ACC is implicated in generating peripheral autonomic responses (Critchley, 2005); is morphometrically altered after autonomic denervation (Critchley et al., 2003); and is correlated with autonomic measures during stress (Critchley, Corfield, Chandler, Mathias, & Dolan, 2000). The neural alarm system function of the ACC is to receive this peripheral information and generate a neural signal when peripheral stress/distress cues are heightened enough to cross thresholds indicating conflict or error.
An emerging literature has shown how this brain area is particularly active during experiences of physical pain (Craig, 2002), and that this is very similar to neural signals when individuals experience social pain (such as rejection or exclusion) (Eisenberger & Lieberman, 2004). The ACC was activated when individuals experienced social rejection in the scanner; individual differences in ACC activation were correlated with self-reported distress (Eisenberger et al., 2003). Singer and colleagues (2004) have also shown involvement of the ACC during the experience of physical or social pain, illustrating that the ACC is preferentially indicating the emotional component of pain (i.e., distress) and not the sensory component. Further, this signal for emotional pain (whether physical or social) in the ACC is present regardless of whether the pain is experienced by the self or by another individual (Decety & Lamm, 2006; Jackson et al., 2006; Jackson et al., 2005).
Of note is that many of the studies which indicate involvement of the ACC also show preferential activation of the insula, suggesting that these structures frequently work together to support empathy-related functions (Decety & Lamm, 2006; Eisenberger et al., 2003; Jackson et al., 2006; Phan et al., 2002; Singer et al., 2004; Wicker et al., 2003). This observation led Critchley and colleagues (2005; 2003) to position the ACC and the insula at the center of a computational model for the integration of peripheral physiological processes with the expression of emotions and the context-specific change in that expression in order to reduce peripheral distress signals.
Alterations in Neural Circuitry related to Callousness, Antisocial Behavior and Psychopathy
Neurobiological studies which were specifically designed to understand callous individuals or behavior show striking parallels with the neurocircuitry implicated in empathizing. The key areas identified above with regard to empathy - the insula and the ACC - are often activated across a range of emotion-related tasks. Our perspective is that callousness and empathy may not only be at opposing ends of a broad prosocial spectrum (Murrie et al., 2007), but the neurobiology of psychopaths may be distinct. This section will begin by reviewing those studies which parallel the empathy literature with an emphasis on CU traits and then focus on studies that are specific to individual differences in psychopaths.
Parallels with Empathy: Studies implicating the Insula and ACC
Compared to studies that focus on empathy, studies that focus on callousness or individual differences in psychopathy often differ in both their choice of fMRI tasks as well as their populations of interest. This makes it even more noteworthy that there are frequently parallels between the two perspectives. Both the insula and the ACC are frequently implicated in empathy-tasks or tasks that probe callousness (e.g., fear conditioning, affective stimuli responses). Combining the callous and empathy perspectives has utility. Sterzer and colleagues (2007) found insula grey matter was reduced in children with conduct disorder, and that this reduction was greater in children with lower empathy and more aggressive behavior.
Rilling and colleagues (2007) examined performance in a Prisoner's dilemma task in individuals scoring high or low on psychopathy measures. Not only was there a behavioral difference in the task in high psychopathy scorers (i.e., a tendency toward defection rather than cooperation and less aversive conditioning), but the pattern of brain activation was also different. Participants scoring high on psychopathy had weaker activation in the ACC when choosing to defect (suggestive of less `conflict' detection) compared to low-psychopathy participants. Related tasks that tap into cooperation vs. defection also implicate the insula and ACC even in healthy controls (Fehr & Rockenbach, 2004). Sanfey and colleagues (2003) found insula and ACC activation in participants receiving unfair offers during a bargaining game, and the participants with the most insula activation were the most likely to reject unfair offers and experience the most negative emotion in response to getting unfair offers.
During fear conditioning, two studies have found that control participants activated the insula and the ACC as they paired neutral faces with pain, but psychopathic patients did not (Birbaumer et al., 2005; Veit et al., 2002). Finger and colleagues (2008) found diminished insula activation during reversal errors compared to correct responses in a learning task in adolescents, including those with psychopathic tendencies. Kiehl and colleagues (2001) found that criminal psychopaths (compared to nonpsychopathic criminals and noncriminal controls) had reduced activation in limbic and paralimbic structures (including the ACC) during an emotion memory task. Sterzer and colleagues (2005) found reduced ACC activation in conduct disorder boys in response to negative emotion images compared to healthy controls. Based on these and other lines of evidence, Kiehl (2006) concluded that the neural signature of the psychopath involves reduced activity in brain areas that transition from primary limbic regions to higher cortical areas; Kiehl (2006) collectively termed this the paralimbic system which includes the insula and the ACC. This terminology has been applied to the empathy circuitry as well (Singer, 2006).
Unique Signature of Callousness: Studies implicating the Limbic System
Despite the relatively consistent activation of the insula and the ACC in relation to empathy (or reduced activation in relation to callousness or in callous individuals), there are some important unique neural correlates of callousness or, more specifically, neurobiological differences in individuals high on CU traits or psychopathy.
One of these areas - the amygdala - may still be a critical component of callousness or empathy. The insula and ACC project to/from the amygdala and other limbic areas (Craig, 2002, 2003), suggesting that these structures may generally operate in conjunction with one another. This idea is supported by at least one study reviewed above (Carr et al., 2003). However, the amygdala may not necessarily be identified as a key structure of interest in empathy-related tasks because its activity is presumably subtracted out from the contrasts of interest (see discussion by Phan et al., 2002; Singer, 2006).
Amygdala
The amygdala is arguably the most important limbic area, particularly with reference to emotion. The amygdala receives information from the hypothalamus about the peripheral body states (e.g., fight or flight signals, stress). The amygdala is consistently activated in response to emotional and stressful stimuli, including expression, regulation, memory and learning of emotional stimuli, especially of fear (Johnstone et al., 2005; Kalin, Shelton, & Davidson, 2007; Phan et al., 2002).
The amygdala is critical for responses to emotion as it has been implicated in the mediation of arousal and vigilance, directs motivation toward relevant stimuli, and broadly responds to ambiguity (Davis & Whalen 2001), uncertainty (Hsu, Bhatt, Adolphs, Tranel, & Camerer, 2005) and emotional stimuli in general. It is notable that individuals with high CU traits often show reduced amygdala activation. First, most of the studies reviewed above which showed reduced insula or ACC activation in individuals high on psychopathy indicated reduced amygdala activation, suggesting that amygdala hyporesponsivity may generally correspond with the neurobiology of callousness. This extended across tasks involving cooperation (Rilling et al., 2007), fear conditioning (Birbaumer et al., 2005; Veit et al., 2002), emotional memory (Kiehl et al., 2001), emotion recognition (Sterzer et al., 2005), or even structural differences (Sterzer et al., 2007). Marsh and colleagues (2008) have extended this observation downward by studying youth with CU traits. They found youth with high levels of CU traits showed similar amygdala activation to fearful, angry or neutral faces while healthy comparison or youth with ADHD displayed the typical enhancement of amygdala activation in response to fear. In addition to reduced amygdala responsivity, youth with CU traits had reduced connectivity with regulatory brain areas.
These findings have led Blair (2007a) to posit that amygdala hyporesponsivity to emotional stimuli is a hallmark of the neurobiology of the psychopath. The amygdala enhances learning and memory for emotional events and consequently improves our ability to make decisions in similar future events. Healthy individuals are presumed to experience heightened amygdala activation in response to the distress of others, and consequently find that experience aversive. Healthy individuals learn to avoid the distress of others by either performing actions that reduce their distress (i.e., empathic or prosocial behaviors) or by learning to avoid performing actions associated with their distress (i.e., not engaging in antisocial behaviors). On the other hand, individuals with reduced amygdala activation to their own distress are predicted to have difficulty processing others' distress as well; consequently, they may show impairments in emotion-related decision-making because they have not benefited from the learning opportunities afforded with an active amygdala. This function of the amygdala would presumably extend to learning how to care about others, which also would include moral decision-making.
Consistent with this view is the observation that many tasks which trigger the amygdala do not necessarily involve the experience of fear as much as the observation or recognition of fear in others (i.e., fearful faces) (Blair et al., 1997). In combination with behavioral data indicating a reduction of fear recognition in psychopathic individuals (Blair, Budhani, Colledge, & Scott, 2005; Blair, Colledge, Murray, & Mitchell, 2001), Blair and colleagues (1997) concluded that it is fear recognition which is especially disrupted in psychopathy. This level of specificity may help explain why studies of emotion recognition implicate the amygdala whereas empathy-focused tasks do not necessarily converge. Studies that focus on empathy generally involve emotion induction (of the self or another) rather than recognition. Relatively passive stimuli (i.e., viewing contexts with or without pain) are akin to induction more so than recognition. It is important in the future to disentangle whether inconsistencies across studies are due to task or population differences between the types of studies that focus on empathy vs. callousness.
Orbitofrontal Cortex (OFC)
The OFC is a component of the prefrontal cortex (PFC) which is so closely connected to limbic structures that it is sometimes considered a limbic structure. Consistent with its anatomical duality, the OFC also has both limbic and prefrontal roles. The OFC is highly involved in emotion, mood, drives, and rewards (Cavada & Schultz, 2000; O'Doherty, Kringelbach, Rolls, Hornak, & Andrews, 2001). Yet, like other PFC structures (Dahl, 2004), the OFC primarily serves to regulate emotion, control mood, monitor rewards and punishments, and generally be engaged during planning and decision-making tasks (Kringelbach & Rolls, 2004). The OFC has extensive connections with the amygdala (Blair, 2007b; Kiehl et al., 2001). Together, the OFC and amygdala promote stimulus-reinforcement learning and decision making in healthy individuals, particularly during associative learning (Schoenbaum & Roesch, 2005; Schoenbaum, Saddoris, & Stalnaker, 2007). In support, Finger and colleagues (2008) found increased OFC (as well as the ACC) during successful operant extinction learning; these areas demonstrated functional connectivity with amygdala hypoactivity. In Blair's model (2007b), OFC dysfunctions in the psychopath result over time as a hypoactive amygdala fails to trigger a large enough response to emotional stimuli (including distress cues) to enhance emotional learning and memory instantiated in the OFC (Stalnaker, Franz, Singh, & Schoenbaum, 2007).
Ventromedial prefrontal cortex (vmPFC)
Relatedly, the vmPFC is implicated in relation to callousness through an interactive or secondary consequence of amygdala hypoactivity. Like other PFC structures, the vmPFC is highly implicated in a variety of decision-making contexts, in particular moral decision-making (Koenigs & Tranel, 2007; Young & Koenigs, 2007). ividuals with damage to the vmPFC tended to disregard the highly emotionally evocative component of a moral dilemma in favor of the `utilitarian' solution. King and colleagues (2006) also highlighted the activity of the vmPFC using and fMRI task which distinguished between context-appropriate behaviors vs. violent behavior. They found common activity in the amygdala and vmPFC when participants acted in a context-appropriate manner regardless of whether the appropriate behavior was violent. Marsh and colleagues (2008) found CU symptoms were most severe in youth with reduced functional connectivity between the amygdala and the vmPFC. Finally, Finger and colleagues (2008) found abnormal vmPFC activation in children with psychopathic traits during reversal learning; vmPFC responses were correlated with CU symptoms suggesting these youngsters may not have been processing the violation of reinforcement expectations when there was a contingency change. Nevertheless, as with the OFC, the function or dysfunction of the vmPFC in relation to empathic or callous behavior may be a consequence of alterations in limbic and paralimbic activity.
Summary and Integration
The neurocircuitry involved in empathic or callous behaviors involves several emotion-related and regulatory brain areas. The ontogenetic roots of empathy and callousness reflect both ends of the spectrum. This circuitry likely evolved from areas involved in promoting social and affiliative behavior in a variety of interpersonal relationships; at the same time, it also likely evolved from areas involved in signaling social distress in addition to physical distress. The key mechanism that allows one to experience the emotions or distress of another came with mirror neurons which fire regardless of whether the self or another experiences pain or distress. The insula and the ACC help promote empathy by connecting mirror areas with peripheral signals, and relaying this information with the limbic system regardless of whether the pain/distress signal originates in the self or another individual.
Psychopaths tend to show reduced insula and ACC activation across a broad range of tasks suggesting that their empathy-related neurocircuitry is hypoactive. Given that both the insula and ACC integrate information from the periphery, it may be that the dysfunctions in empathy-related neurocircuitry follow from overall reductions of stress or distress cues from the periphery (Critchley, 2005). For example, the ACC may be hypoactive because peripheral stress cues have not crossed a threshold that indicates conflict or error or the need to adjust behavior.
This hypoactivity may include other emotion-related areas like the amygdala (Blair, 2007a). The amygdala, insula and ACC often operate together. Blair further argues that the neurobiology that leads to deficits in moral decision-making in psychopathy is developmental when considering intercorrelations with prefrontal areas like the OFC and vmPFC. This developmental view is augmented by other research that shows the inverse association between empathy and antisocial behavior is not evident early in development, but rather increases in strength across development (Hastings, Zahn-Waxler, Robinson, Usher, & Bridges, 2000). Over time, limbic hypo-activity fails to trigger a large enough response to emotional stimuli like distress cues to enhance or permit emotional learning and memory. The limbic system in general, and the amygdala specifically, hold closer ties with stress-reactive hormones than the regulatory prefrontal areas as well (Kalin et al., 2007).
Peripheral Correlates of Neurocircuitry implicated in Empathy/Callousness
One of the most noteworthy functions of the neurocircuitry reviewed above is that these areas and pathways have been shown to have extensive connections with peripheral physiological functioning (Critchley, 2005; Levenson, 2003), and specifically to have strong reciprocal connections with the endocrine system (Liberzon et al., 2007). Though the hypothalamus is clearly important in connecting the brain with the periphery, most of the limbic structures receive peripheral inputs as well as centrally stimulating HPA axis activity (Herman & Cullinan, 1997; Herman et al., 2003; Herman, Prewitt, & Cullinan, 1996), including extensions from the insula and the amygdala to the nucleus of the hypothalamus responsible for triggering the cascade which will cause cortisol release (Risold, Thompson, & Swanson, 1997). Based on the strength of these connections and the review above which suggests that limbic and paralimbic structures are implicated in empathy-related processes or are altered in callous individuals, individuals who were particularly empathic or callous would be expected to have a corresponding physiological signature in their peripheral physiology.
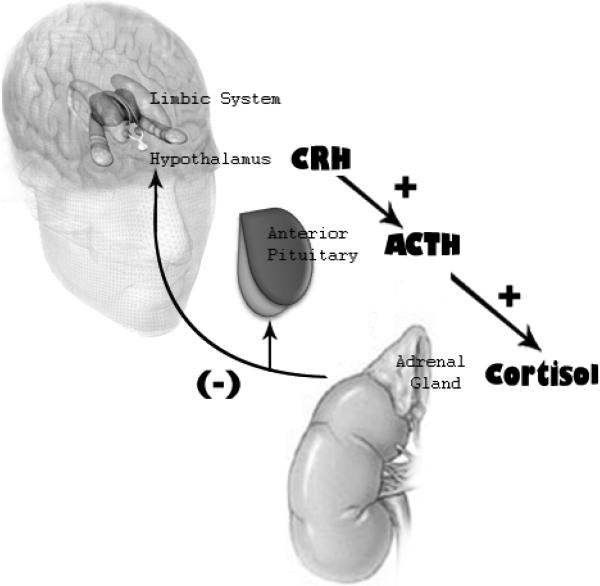
The limbic system sends and receives several types of peripheral input, including both branches of the stress response. The stress response (e.g., the fight or flight response) is characterized most immediately by sympathetic activity, including release of epinephrine (adrenaline) from the middle of the adrenal gland as a part of the autonomic nervous system (ANS); the release of the parasympathetic brake can also characterize ANS activity (Porges, 1995). The slower track involves the HPA axis which releases cortisol from the outside of the adrenal gland (Gunnar & Quevedo, 2007).
There is a longstanding literature demonstrating ANS associations (both sympathetic and parasympathetic measures) with empathy and related behaviors (see review by Hastings et al., 2006). Heightened ANS activity is often associated with the experience of personal distress and internalizing of emotions and consequently reduced expression of empathy and prosocial behaviors. Often, however, heightened ANS activity sets the stage for the experience of sufficient amounts of emotional distress to trigger prosocial behavior and caring for others. There is a parallel literature that focuses on the expression of callousness. Several studies from Raine's work highlights low ANS activity in children and adults with callous or antisocial behavior (Blair et al., 1997; Brennan & Raine, 1997; Raine, 2002; Raine, Lencz, Bihrle, LaCasse, & Colletti, 2000; Raine, Venables, & Mednick, 1997). A full review of the ANS correlates of empathy and callousness is beyond the scope of this review (and has been done by Hastings et al., 2006; Raine, 2002). This next section will focus on the stress hormone cortisol and the Hypothalamic-Pituitary-Adrenal (HPA) axis for several reasons. While cortisol is just one physiological marker, it is an important endpoint of the HPA axis and index of limbic activity.
Peripheral Physiological Signature: A focus on Cortisol
Our focus is on cortisol because the hormonal cascade of the HPA axis begins in the limbic system. While this may be obvious for limbic structures like the hypothalamus, connections of the HPA axis with limbic areas are inclusive and extensive. Vazquez (1998) and others (Gunnar & Vazquez, 2001) have changed the terminology to be LHPA (limbic-hypothalamus-pituitary-adrenal) to emphasize that this peripheral endproduct begins and ends largely in emotion-related neurocircuitry. While both the ANS and the HPA have reciprocal connections with the limbic systems, cortisol (much more easily than epinephrine) crosses the blood brain barrier and consequently the brain is a major target organ for cortisol (Gunnar & Quevedo, 2007). Moreover, cortisol has been shown to be a key modulator of several emotion-related neural functions, including empathy-related or prosocial behaviors as well as emotion-related learning and memory; cortisol has extensive connections with the social brain and those areas that relate to affiliation and social stress (Taylor et al., 2000). Cortisol maintains strong connections with limbic structures like the hippocampus which facilitates learning and memory, particularly emotion-related memory (Roozendaal, 2000, 2002). Taken together, this raises the possibility that cortisol may serve as a partial mechanism for the deficits in emotional learning and memory evidenced in developmental aspects of psychopathy.
Another reason our focus is on cortisol is because its response profile is slow compared to the nearly immediate reactivity of ANS measures. Likewise, it takes substantially more time for the HPA axis to return to baseline following a stressor, and this recovery is largely a result of negative feedback of peripheral cortisol release on limbic activity, including hypothalamic activity. Consequently, cortisol reactivity or hypoactivity has physiological implications (and by extension, brain activation patterns) across periods of minutes to hours, not milliseconds to seconds (Sapolsky, Romero, & Munck, 2000). On the other hand, circulating cortisol (i.e., basal levels) or fast-acting nongenomic stress-responsive cortisol levels can have nearly immediate implications for brain activation patterns by changing membrane excitability (Falkenstein, Tillmann, Christ, Feuring, & Wehling, 2000; Losel et al., 2003). Thus, cortisol levels are potentially important as both immediate modulators of brain activation as well as potentially responsible for mediating long-term genomic alterations (De Kloet, 2004; Liberzon et al., 2007). Cortisol's unique properties also enable it to directly change gene expression. Thus, it not only enters target cells more easily than other hormones but is also able to induce a more dramatic and longer-lasting effect when it arrives. Combined with the observation that cortisol activity and reactivity impact physiology for hours to days and that this impact is largely on limbic neurocircuitry, it is possible that HPA functioning is a major peripheral mechanism to explain how emotion-related neurocircuitry can get disrupted for long periods of time or is permanently altered across development. The long-term and possibly permanent duration of cortisol's effects is important to demonstrate (Gottlieb, 1991) because disorders of empathy (e.g., psychopathy) are developmental disorders in which symptoms generally persist throughout the life span (Blair, 1995; Hastings et al., 2000; Salekin & Frick, 2005).
Cortisol's Role in the Neurociruitry of Empathy and Callousness
Insula
There are relatively few studies which have directly linked insula activity with the HPA axis. Liberzon and colleagues (2007) found insula activity in response to traumatic stimuli was associated with adrenocorticotropic hormone (ACTH) responsivity. ACTH from the pituitary gland stimulates the release of cortisol, but this study did not observe direct associations of the insula with cortisol levels or responsivity.
ACC
There are several studies which have found associations between cortisol and ACC functioning. This literature is complicated because different indices of HPA activity are frequently employed. Cortisol Reactivity can be thought of as a consequence of brain activation starting in the limbic system, triggering the hypothalamus (Gunnar & Vazquez, 2006). Emotions should increase activity in the limbic system at the level of stress-appraisal (Eisenberger, Taylor, Gable, Hilmert, & Lieberman, 2007), while failing to appraise an event as stressful would trigger less HPA axis reactivity. In support, cortisol reactivity to a laboratory stressor has been associated with increased ACC activity later when participants were scanned during a social stressor (Eisenberger et al., 2007). In a subset of individuals with greater social support, diminished cortisol responses were also associated with reduced ACC activation. In studies by Wang and colleagues (2007; 2005), ACC responses to a laboratory stressor were positively correlated with cortisol reactivity, particularly in females. Electrical stimulation of the ACC results in cortisol increase (Eisenberger et al., 2007). Cortisol also enhanced ACC activity in response to pain in a fear conditioning task (Stark et al., 2006). Pretask laboratory cortisol levels (which likely reflect responsivity to laboratory arrival) and ACTH responsivity to traumatic stimuli were positively associated with ACC responsivity (Liberzon et al., 2007). These studies generally fit with the idea that ACC responsivity is associated positively with HPA responsivity.
A different pattern emerges if negative feedback functioning is analyzed. The administration of cortisol does not parallel a stress response as there is no activation of the HPA axis in the brain. Rather, it indexes the negative feedback of cortisol from the periphery back to the brain. This is parallel to HPA axis activity several minutes/hours after stress reactivity. As expected, these studies show that ACC activity is generally reduced when individuals display dysregulated negative feedback. Males receiving a placebo showed enhanced activity in the ACC during fear conditioning, but sensitivity to fear conditioning was absent when participants received cortisol (Stark et al., 2006). Another study found individuals who had high cortisol levels despite being given a potent synthetic cortisol (e.g., failed the dexamethasone suppression test) structurally had smaller ACCs than individuals who suppressed the dexamethasone (MacLullich et al., 2006).
Basal cortisol is also distinct from stress-reactive cortisol in its basic physiology (de Kloet, 2003), and the direction of effects on the emotion neurocircuitry (Gunnar & Quevedo, 2007). Circulating cortisol is more likely to have effects on the brain (i.e., bottom-up effects), whereas reactive cortisol indexes the downstream effects of limbic activation on the periphery. Two studies that examine basal HPA activity (integrated across several time points) show that basal cortisol is associated with reduced ACC functioning. Basal ACTH levels were associated with smaller ACCs in younger and older men (Wolf, Convit, de Leon, Caraos, & Qadri, 2002). Also, some of our work shows basal cortisol was associated with less ACC activity during emotion regulation in adolescents (Mazzulla et al., 2008).
In sum, basal and negative feedback functioning of HPA axis activation appears to reduce ACC activity whereas stress reactive cortisol is more frequently associated with enhanced ACC functioning. If reduced ACC activity is also associated with callousness, it would be expected that callous individuals would have low basal cortisol. Given that they generally have reduced ACC activity, we would in turn expect that their hypoactive ACC (and other limbic structures) would be less able to stimulate a stress response. Consequently, callous individuals would be expected to have reduced cortisol reactivity. Given the relative infrequency of a HPA response, it would further be expected callous individuals would have impaired negative feedback because the bottom-up component of the HPA axis is weakened and untested; negative feedback dysregulation would be further enhanced by hypoactivity of the ACC directly. In short, the expected HPA axis profile of the callous individual mirrors the profile of an individual with a hypoactive ACC.
Amygdala
Animal studies have demonstrated the importance of the amygdala for stimulating HPA axis activity (Hsu, Chen, Takahashi, & Kalin, 1998; Kalin, Shelton, & Davidson, 2004), particularly in reference to fear (Kalin, 1993). Like the ACC, the amygdala enhances HPA activity; it also has many cortisol receptors, suggesting that cortisol in turn helps regulate amygdala activity (Herman & Cullinan, 1997; Herman et al., 1996). The amygdala also connects with the hypothalamus, suggesting that its control over the HPA axis is direct (Risold et al., 1997).
The human literature reveals that greater amygdala functioning enhances the cortisol stress response. van Stegeren and colleagues (2008; 2007) found that viewing emotional pictures enhanced amygdala activity, and this heightened amygdala functioning was largest in participants with high cortisol levels. Drevets and colleagues (2002) found that heightened amygdala activity was associated with higher stressed cortisol levels. Finally, Urry and colleagues (2006) found individuals with a dysregulated diurnal rhythm (shallow declines across the day) had heightened amygdala activity when regulating their emotions. Taken together, these results suggest that stress enhances amygdala functioning which in turn enhances HPA functioning and elevates cortisol levels. Given that callous individuals are expected to have reduced amygdala functioning, it would be predicted that they would likewise show reduced stress responsivity. This prediction parallels that predicted by ACC functioning.
OFC and vmPFC
While the limbic structures like the insula, ACC and amygdala are thought to enhance HPA axis functioning (Herman & Cullinan, 1997; Herman et al., 1996), the PFC is more frequently implicated in the inhibition or regulation of the HPA axis (Liberzon et al., 2007). The distinction between stress reactivity and the bottom-up effects of cortisol on the brain again has bearing on the interpretation of the findings. The top-down role of cortisol is to index stress activation. Since the PFC generally inhibits limbic activity (Goldin, McRae, Ramel, & Gross, 2008) including hypothalamic release of hormones (Hoover & Vertes, 2007), one would expect that enhanced PFC activity would be associated with reduced cortisol reactivity. Yet cortisol also feeds back into the brain and has receptors on many key regulatory areas, including the PFC (Lupien & Lepage, 2001). This feedback is negative, so the anticipated direction of the effect of cortisol on regulatory areas is opposite that of stress reactive cortisol (Liberzon et al., 2007). If PFC activation reduces the stress response and consequently diminishes the availability of cortisol to effect the brain, then the long-term effects of enhanced PFC activation may lead to aberrant cortisol negative feedback and a reduction in the ability of circulating cortisol to reduce limbic activation. These two opposite predictions are not mutually exclusive because they are differentiated by the timing of the stress response.
There is some ambiguity in the literature about the role of the PFC in relation to cortisol. Negative associations have been reported with the vmPFC (Eisenberger et al., 2007). Stark and colleagues (2006) found that the administration of cortisol reduced fear conditioning responsivity in the mPFC and the OFC in males, a well as reduced habituation to the fear conditioned response in other prefrontal areas. Urry and colleagues (2006) reported greater vmPFC activation with concomitant reduced amygdala activation in individuals with normative declines in cortisol across the day. These studies support an inhibitory role of the PFC on the L-HPA axis.
Opposite findings are also reported. Kern et al (2008) found that heightened PFC functioning was associated with lower and higher cortisol responses to psychosocial stressors. Wang and colleagues (2007; 2005) found increases in responsivity of the PFC and OFC were positively associated with stress reactivity, particularly in males. ACTH response to traumatic stimuli was associated with mPFC activation in addition to the observed insula and ACC activation (Liberzon et al., 2007). This positive link between PFC functioning and stress reactivity may be due to anatomical distinctions between subareas of the PFC (ie., the OFC may be behaving as a part of the limbic system rather than a part of the PFC) or may be due to long-term implications of reduced negative feedback on PFC functioning.
Summary and Implications
Peripheral neurobiology is not anticipated to be as straightforward as “low cortisol relates to callousness”. This is due to the HPA axis interaction with neural functioning under basal, reactive and feedback states and these states differentially reflect top-down and bottom-up processes. Predictions for the neurobiology of empathic or callous individuals will focus on limbic activity (especially the predictions based on the ACC and amygdala). The PFC findings are more complex and generally make sense only in terms of the consequences of reduced HPA activation failing to feed back on the PFC.
The first implication of this model is that low basal cortisol may relate to CU traits primarily through bottom-up processes or a failure to prime limbic and paralimbic structures like the ACC or amygdala. The second implication is that hypoactivity in emotion-related neurocircuitry is expected to fail to trigger a stress response or cross a callous individual's threshold for stress activation, so the L-HPA axis (through top-down hypoactivity) produces a diminished stress response. The third expectation is that, over time, negative feedback functioning would be dysregulated, reflecting hypoactivity of the adrenal. Unfortunately, few empirical studies have examined negative feedback functioning.
The next section examines whether the peripheral physiology of empathy fits with a profile of high basal, highly reactive and well-regulated cortisol negative feedback. Conversely, it will be considered whether those with CU or psychopathic traits display HPA axis hypoarousal and, if cortisol reactivity is actually triggered, impaired negative feedback. This section will focus on the literature in children and adolescents because (a) psychopathy is considered a developmental disorder (Frick, 2006); (b) both empathy and callousness have their roots in early childhood (Frick, Cornell, Bodin et al., 2003; Zahn-Waxler, 2000); and (c) because the neurobiology model reviewed above sets forward different predictions for the development vs. the expression of adult psychopathy (Blair, 2007b). The HPA axis's utility as a peripheral marker depends on its ability to track the emergence of psychopathy and consequently must differentiate empathy or callousness relatively early in childhood.
Cortisol's Role in the Expression of Empathy
Theoretical implications for cortisol's modulatory role on the expression of social and prosocial behavior have been suggested (Swain et al., 2007; Taylor et al., 2000), and there is some empirical support. High cortisol reactivity to social novelty was associated with outgoing behavior in socially competent, well-liked preschoolers (Gunnar, Tout, de Haan, Pierce, & Stansbury, 1997). High cortisol was related to child-initiated social interaction, social competence, popularity, and social affiliation at school (Tennes & Kreye, 1985; Tennes, Kreye, Avitable, & Wells, 1986). Evidence for good social skills in high cortisol youth, especially girls, extends across family and peer domains (Booth, Granger, & Shirtcliff, 2008), and is especially true when adolescents are in social settings (Adam, 2006). This parallels findings in adult females (Adam & Gunnar, 2001). Another study highlighted gender differences in that empathic males and systematizing females had higher cortisol levels than those with typical cognitive styles (Nakayama, Takahashi, Wakabayashi, Oono, & Radford, 2007). Finally, Sethre-Hofstad and colleagues (2002) found mothers who were more attached with their children showed heightened cortisol responses to watching their child during a stressor, but only when their children also showed stress reactivity; when children were not especially challenged, neither mothers nor children exhibited cortisol reactivity. These findings indicate cortisol may promote social and prosocial behavior, as well as matched or attuned physiological functioning in stressful circumstances.
It is difficult to come to strong conclusions regarding the literature on cortisol and empathy because there is no definitive work on the topic. It is also complicated because cortisol is often associated with anxiety symptoms and other internalizing problems (Stansbury & Gunnar, 1994). Similar to the ANS literature, it may be that empathic/prosocial behaviors are supported by an optimal level of arousal reflected in moderately high cortisol levels and corresponding level of internal distress that facilitates empathy (Eisenberg, 2007).
Cortisol's Role in the Expression of Callousness or Antisocial Behavior
There is a fairly consistent literature that children and adolescents with low basal cortisol have more callous symptoms or antisocial behaviors. HPA hypoactivity extends across a broad range of symptom levels and types, suggesting that this physiological correlate is indexing a continuum of risk rather than a unique signature of psychopathy. Compared to healthy controls, clinic-referred disruptive children (Oosterlaan, Geurts, Knol, & Sergeant, 2005; Popma et al., 2007; Scerbo & Kolko, 1994; van de Wiel, van Goozen, Matthys, Snoek, & van Engeland, 2004), disruptive children with persistent and early onset aggression (McBurnett, Lahey, Rathouz, & Loeber, 2000), and children with oppositional defiant or conduct disorder (Kariyawasam, Zaw, & Handley, 2002; Pajer, Gardner, Rubin, Perel, & Neal, 2001) have all been found to have low cortisol levels; it is particularly the subgroup of disruptive children with callous symptoms (as opposed to the highly anxious children) who show the greatest evidence of hypoarousal. The link between CU symptoms and low basal cortisol extends to at-risk populations (Granger et al., 1998; Pajer, Gardner, Kirillova, & Vanyukov, 2001; Vanyukov et al., 1993). Low cortisol levels have also been correlated with antisocial behavior across the normal range of externalizing symptoms, especially in boys (Cicchetti & Rogosch, 2001; Flinn & England, 1995; Loney, Butler, Lima, Counts, & Eckel, 2006; Shirtcliff & Essex, 2008; Shirtcliff, Granger, Booth, & Johnson, 2005; Smider et al., 2002; Tennes & Kreye, 1985). The diurnal rhythm of children with antisocial symptoms may also be dysregulated or blunted, suggestive of an overall impairment in HPA functioning (Fairchild et al., 2008; Popma et al., 2007; Shirtcliff & Essex, 2008; Susman et al., 2007). While no studies claim a causal link between CU traits and HPA axis hypoactivity, some studies stress that the strong hormonal correlates of conduct or oppositional defiant disorder have clinical applications for use in the assessment of symptom severity and treatment effect in adolescents with externalizing behavior disorders (van de Wiel et al., 2004; van Goozen, Fairchild, Snoek, & Harold, 2007).
A few studies have not found low cortisol in individuals with more externalizing symptoms (van Bokhoven et al., 2005), but these studies had small sample sizes (Kruesi, Schmidt, Donnelly, Hibbs, & Hamburger, 1989), or focused on populations characterized by attention and inhibitory externalizing symptoms rather than CU traits or antisocial behavior (de Haan, Gunnar, Tout, Hart, & Stansbury, 1998; Gunnar et al., 1997; Sondeijker et al., 2007). While attention problems are within the disruptive behavior spectrum, they do not define CU traits as core symptoms and they do not necessarily show continuity with adult psychopathy. This gulf between subgroup criteria led some studies to compare subgroups within the disruptive behavior spectrum (McBurnett et al., 2005). In one such study only the oppositional defiant youth (with or without comorbid attention problems) showed weaker cortisol responsive relative to controls; the attention problem group paralleled control youth (van de Wiel et al., 2004). This implies that not only does low cortisol serve as a good predictor of externalizing behavior, but it can also help to distinguish between subtypes as well.
Attenuation in cortisol reactivity is also evident in children with CU traits or antisocial behavior (Susman, 2006). Children with oppositional defiant or conduct disorder had smaller cortisol responsivity to a frustration task (Fairchild et al., 2008; Snoek, Van Goozen, Matthys, Buitelaar, & van Engeland, 2004; Van Goozen, Matthys, Cohen-Kettenis, Buitelaar, & Van Engeland, 2000; Van Goozen et al., 1998); this was most evident when problems persisted during treatment (van de Wiel et al., 2004). Within at-risk youth, the magnitude of stress hyporesponsivity was associated with aggressive and impulsive symptoms (Moss, Vanyukov, & Martin, 1995). Cortisol reactivity was likewise blunted in normally developing youth with more concurrent and subsequent aggressive and disruptive behavior symptoms (Granger, Stansbury, & Henker, 1994). Interestingly, Brotman and colleagues (2007) found at-risk youth (who had an adjudicated sibling) also demonstrated an attenuated stress response, but this pattern normalized as they received a therapeutic family-based intervention. Contrarily, higher cortisol reactivity was associated with externalizing symptoms in normally developing youth in two studies (Susman, Dorn, Inoff-Germain, Nottelman, & Chrousos, 1997; Tout, de Haan, Campbell, & Gunnar, 1998), and one study involving youth with conduct problems (McBurnett et al., 2005). Nevertheless, the overall pattern is for low basal and blunted reactivity to stress in youth with CU symptoms.
Given that psychopathy is considered a developmental disorder, the above literature review on reduced HPA functioning in children and adolescents may have bearing on the development of psychopathy (van Honk & Schutter, 2006). Burke and colleagues (2007) found that cortisol in adolescents predicted callousness when the youth were young adults. Other studies have found basal cortisol was lower in individuals with more psychopathic traits (Holi, Auvinen-Lintunen, Lindberg, Tani, & Virkkunen, 2006; van Honk, Schutter, Hermans, & Putman, 2003). Relatedly, cortisol's diurnal rhythm was blunted within a subsample of psychopathic criminals compared to incarcerated non-psychopaths (Cima, Smeets, & Jelicic, 2008). Finally, individuals scoring higher on psychopathy measures show reduced cortisol responsivity to laboratory (Dishman, Wallace, Crawford, Grant, & Hinton, 1982; O'Leary, Loney, & Eckel, 2007) and pharmacological stressors (Netter, Hennig, & Rohrmann, 1999), suggesting that the overall pattern of blunted HPA levels and reactivity in children and adolescents with antisocial behavior has developmental extensions and unique predictive value with psychopathic characteristics in adults.
Summary and Integration: What does not stress me should not stress another
The neurocircuitry involved in both empathy and callousness and in their overlap promotes prosocial and empathic concern, and by extension moral decision-making, by co-opting brain areas that instantiate physical and social distress. Activation in response to distress extends to witnessing distress cues/contexts and the expression of distress in others. These brain areas receive substantial peripheral input and are responsible for integrating peripheral signals with concurrent neural processes. Cortisol levels and HPA responsivity are implicated in the functioning of these brain areas through bottom-up modulation and top-down activation, respectively. Peripheral signals like cortisol enhance activation in this neurocircuitry. Contrarily, diminished HPA activity reduces the potential degree of activation in empathy-related neurocircuitry, further reducing the potential for stress reactivity to begin in the L-HPA axis. The hypoactivity of the stress system is expected to perpetuate itself over time. The involvement of peripheral physiology at multiple levels suggests a basic mechanistic impairment in CU individuals.
Our suggestion is not that social information processing in CU individuals (or the heightened sociality in empathic individuals) is due to deficits in sociality or impairment in the representation of self. If it were, then callous individuals would have substantially greater difficulty in finding adequate alternative strategies, such as manipulating others' emotions (Pardini et al., 2003; Waschbusch, Walsh, Andrade, King, & Carrey, 2007). Also, it would not be expected that cortisol would relate to empathy or callousness because cortisol is associated with neural activation in areas that fire regardless of whether pain or distress is signaled by the self vs. other. Yet, HPA hypoactivity is not expected to be specific to the distress of another because cortisol modulates neural activity regardless of the object of distress. Individuals with reduced basal and reactive HPA axis are expected to fail to respond to emotional or stressful experiences that they themselves experience as well as fail to respond to similar stimuli experienced by another.
Especially with areas like the insula or ACC, this neural network illustrates how an individual feels the emotions/ stressors of another as though they experienced those emotions/ stressors themselves. The appearance of a lack of empathy is that individuals with CU traits consistently have a blunted stress response and hypo-responsive physiological input to the neurocircuitry described above. If the callous individual were in the same social context as a distressed conspecific, the callous individual would not trigger a stress response. Their representation of another's stress or emotion would be similarly blunted and they would fail to feel distress. The appearance of callousness is a consequence of a brain with a high threshold for detecting stress or registering arousal. It is not a mismatch between the representation of the self vs. other but rather a mismatch between what any two people experience as stressful.
Support for the hypoarousal model in populations with high thresholds
Is there evidence that people who under-represent emotion, pain or stress show impairments in empathy? A handful of studies have explored this question outside of populations defined as CU (so as to avoid a circular argument) (Hein & Singer, 2008). Individuals with a congenital insensitivity to pain (CIP), who presumably do not have a strong representation of pain in self, show reduced emotional responses to pain eliciting stimuli and impairments in inferences about the amount of pain experienced by others based on facial expressions or event descriptions (Danziger, Prkachin, & Willer, 2006). Underestimations were especially large when other emotional cues were lacking, suggesting CIP patients used alternative strategies to empathy. Individuals with lower physical pain thresholds also have lower sensitivity to social pain and distress (Eisenberger, Jarcho, Lieberman, & Naliboff, 2006). Individuals who have difficulty expressing their emotions (i.e., alexithymia patients) also under-estimate the experience of pain in themselves and others, and have low empathy scores (Moriguchi et al., 2007). Interestingly, individuals with alexithymia also showed reduced neural activation to painful situations in the ACC, suggesting this under-representation of distress may be instantiated in the empathy-related neurocircuitry as well as the periphery.
That individuals scoring higher on psychopathy scales likewise appear less responsive to stress or pain (Errico, Parsons, King, & Lovallo, 1993; O'Leary et al., 2007) raises the possibility that callous individuals display a similar mechanistic impairment in pain or emotion processing as opposed to a self vs. other impairment. That psychopaths also are reported to use alternative strategies to empathy (like the congenital insensitivity to pain patients) to process social information supports a mechanistic link 1. Hallmark symptoms of psychopathic individuals include the ability to manipulate others' emotions and are conscious of impression management (Forth, Kosson, & Hare, 2003). Psychopaths appear aware of the emotions of others without viscerally feeling the emotions of others.
Support for the hypoarousal model in contexts which are no longer stressful
Another way to distinguish whether the impairment in CU individuals is at the level of stress appraisal/activation as opposed to the self vs. other distinction is to ask whether individuals in specific contexts that they do not consider stressful show alterations in empathy-related processes. This context-specific perspective was initially criticized because empathy was considered a fast, automatic response (Preston & de Waal, 2002). It was difficult to theorize how something involuntary could be modulated by context, yet familiarity and fairness are frequent modulators of empathy-related neural processing and the personal distress one feels for another (Hein & Singer, 2008; Singer, 2007; Singer et al., 2006). Indeed, past experience with an unfair person can change neural activation patterns from an empathic distress signal to activation in reward areas in response to observing their pain (Fehr & Rockenbach, 2004). Thus, past experience with a person or event as well as stress appraisal modulates empathic neuronal responses (de Vignemont & Singer, 2006; Hein & Singer, 2008).
There has been additional support for the hypoarousal model from another perspective. Cheng and colleagues (2007) found that acupuncture physicians activated regulatory brain areas in response to viewing needles being inserted into body parts; nonphysicians activated the empathy-related neurocircuitry like the ACC and insula. Cheng (2007) and others (de Vignemont & Singer, 2006) suggest that behavioral responses in these types of contexts would be limited to cognitive forms of mentalizing rather than empathizing, again suggesting that alternative strategies are employed when empathizing is not supported by physiological arousal.
Conclusions and Caveats
The neurobiology of empathy and callousness share some common neurocircuitry involved in the shared representation of the emotions and distress. These brain areas integrate physiological input from the periphery. This neurocircuitry is less reactive in callous individuals. It is also less active in those with blunted HPA axis activity. This pattern of hypoactivation feeds in on itself in that blunted limbic activation in turn may fail to trigger a stress response in contexts that others' consider stressful.
The impairment in callous individuals suggests that their representation of stress or distress cues is dysfunctional as a consequence of a general pattern of hypo-arousal in stress-responsive systems. Since the representation of self vs. other is shared at a neural level, this hypoarousal manifests as impairment in the representation of the distress of others in contexts that are generally considered stressors for personal and social events.
A final caveat relates to the nature of this review paper. In this paper, evidence for how brain structures and hormones relate to various forms of moral behavior is presented. To that end, we erred on the side of highlighting consistencies in the data, parallels across a range of tasks, populations and findings. Other brain areas that are often related to these neural circuits have not been discussed at length, such as more cognitive areas such as the superior temporal cortex (Carr, et al, 2003; Kiehl, 2006), or the cerebellum (Decety & Lamm, 2006; Singer et al, 2004; Jackson, Meltzoff, & Decety, 2005). Many studies show a lateralization of effects and a slight preference for right-hemisphere activation (Decety & Lamm, 2007; Critchley, et al, 2000), yet bilateral activation is also common and it may be too early to tell if there is a functionally important lateralization to this neural circuitry and whether that preference would have peripheral physiological importance. Consistent studies were emphasized as opposed to the studies which do not necessarily find consistent activation of the brain areas discussed (e.g., the amygdala) or only show partial replication. It would be premature at this time to delve into the inconsistencies because they could easily be disregarded as byproducts of differences in study design, tasks, and populations and because it would be beyond the scope of this paper. Future studies should be better able to refine this broad literature with targeted empirical work.
What if it was stressful? Pessimistic and Optimistic Views
It remains to be seen whether individuals described as callous would behave empathically if the other person's distress crossed thresholds for stress reactivity. Two views are not optimistic. The shared neurobiological representation of the self vs. other is differentiated by level of activation rather than anatomy. Given that physiology is blunted for the self (which optimally activate this neurocircuitry), then the degree of distress that another individual would need to show to cross the threshold would be prohibitive. The second pessimistic view presupposes that stress reactivity is possible, yet infrequent. After a stress response, feedback from the periphery to the brain is negative, serving to return the system to basal levels. Negative feedback may be dysfunctional in individuals with blunted responsivity; consequently, stress recovery may be delayed. In this instance, the individual may be so overwhelmed by the rare occurrence of their own emotional arousal that they would be unable to assist the other individual. Though empathy-related neurocircuitry would be activated, their behavior would not necessarily be considered prosocial. There is evidence for this with ANS activity (Hastings et al., 2006).
An optimistic perspective, however, is that there are individual differences in callousness across a broad range of neural activation; added to individual differences is the observation that empathy can be context-specific. With appropriate contextual cues, this neurocircuitry would be activated and the individual would be motivated to show concern for others (de Vignemont & Singer, 2006; Hein & Singer, 2008). This stress threshold may be prohibitive for a very small subset of individuals, but the stress threshold is unique for different individuals and changes with experience and context. Many individuals may have high stress thresholds without necessarily being incapable of generating a stress response or experiencing social impairment as a function of reduced emotional empathy.
This is an important caveat regarding the distinction between psychopathy and callousness, especially if the impairment is in stress reactivity. Low cortisol does not necessarily mean the child will engage in antisocial behavior. Failure to share in the emotions of others implies a lack of empathy and reduced motivation to engage in prosocial behaviors, but it does not necessitate engaging in antisocial behaviors. Hurting others is different from disregarding their emotions. CU traits are expected to lead to antisocial behavior only in certain contexts or to emerge as a possible consequence of lowered stress thresholds over time (Frick, 2006; Hastings et al., 2000). The extension of hypo-responsivity to antisocial behavior requires motivation/rewards associated with those behaviors which are augmented by stress hypo-responsivity. This set of behaviors may perpetuate itself as the individual's hypo-responsivity fails to support feelings of guilt or remorse as a correlate of reduced activation in empathy-related neurocircuitry (Zahn-Waxler, 2000).
Gender Differences at Multiple Levels
This review has de-emphasized the importance of gender differences in the interest of space, but there are consistent differences between males and females at multiple levels (Zahn-Waxler, Crick, Shirtcliff, & Wall, 2005; Zahn-Waxler, Shirtcliff, & Marceau, 2008). Girls display more empathy than boys; this difference is magnified across childhood (Zahn-Waxler, 2000). Boys show more CU traits and have higher prevalence and earlier onset of conduct disorder and antisocial behavior (Maughan, Rowe, Messer, Goodman, & Meltzer, 2004; Moffitt, 1993a). The sex difference persists through adulthood and the emergence of psychopathy (Cale & Lilienfeld, 2002). The neurobiology involved in empathy and callousness is different in males and females, with empathy-related neurocircuitry being generally more active in females (Hein & Singer, 2008; Schulte-Ruther, Markowitsch, Shah, Fink, & Piefke, 2008; Singer et al., 2006). These gender differences extend to the stress response (Taylor et al., 2000); females are more reactive to social stressors and display a different biological and behavioral stress response that involves tending and befriending than males (Stark et al., 2006; Wang et al., 2007), further supporting a gender difference in central, peripheral and behavioral levels. Moreover, the association between HPA functioning and psychopathy traits is often moderated by gender (Loney et al., 2006; Nakayama et al., 2007; O'Leary et al., 2007; Popma et al., 2007; Shirtcliff et al., 2005), suggesting that the neurobiology of antisocial behavior may be fundamentally different in males and females.
Given the practical and theoretical significance of understanding the underpinnings of antisocial behavior, gender is a critical issue for behavioral scientists. Unfortunately, the study of females and psychopathy is lagging substantially behind their male counterparts in terms of quantity and sophistication. Future study must consider what empathy means in males. Generating an applicable biopsychosocial model of antisocial behavior to adolescent females will be a meaningful step in developing gender-specific interventions to alleviate antisocial behavior in both sexes. It is critical for improving and advancing the field to begin to systematically evaluate the relationship among cortisol, psychopathy, and antisocial behavior in referred and non-referred adolescent females.
Implications for Interventions
The motivation for understanding the neurobiology of empathy and callousness and the peripheral correlates of antisocial behavior is more than just by basic science. There are at least four possible policy implications or motivations for exploring biologically-motivated investigations on antisocial behavior. First, as emphasized above, neurobiologically informed methods may provide a window into the etiology of a disorder or mechanism behind a developmental phenomena. Second, biological forces may indicate who is the most vulnerable to a particular disorder (Moffitt, 1993b; van Honk & Schutter, 2006), or more interestingly, in what biological state an underlying vulnerability is most likely to be expressed (van de Wiel et al., 2004). Third, interventions may indicate when or whether initial biological vulnerability may be able to demonstrate changes in a relatively objective biological outcome in longitudinal studies (Brotman et al., 2007; Fisher, Gunnar, Chamberlain, & Reid, 2000; Fisher, Stoolmiller, Gunnar, & Burraston, 2007; van de Wiel et al., 2004). Fourth, when coupled with developmental studies that include measures of environmental influences on biological processes (and the interplay of nurture and nature), we will develop a better understanding of the neurobiology of empathy and callousness and their implications for the development of antisocial behavior (Knafo et al., 2008).
Early clinical viewpoints were of the belief that the treatment of psychopathy was considered to be a waste of clinical and financial resources because psychopathy was considered intractable; children manifesting psychopathic symptoms were considered to be on a trajectory of offending through adulthood (see Salekin, 2002 for a review of earlier positions). Concentrated research and clinical efforts have led to a change in the pendulum's swing; psychopathic traits, particularly in adolescents, may be amenable to treatment interventions. Both empathy and callousness are known to develop in the first years of life (Zahn-Waxler & Radke-Yarrow, 1990), Therefore, interventions with children at-risk for antisocial behavior may be particularly effective if they begin when children are young, more malleable, and without an entrenched history of indifference to the problems of others. Salekin's (2002) meta-analysis on the treatment of psychopathy and antisocial behavior in youth identified several treatment modalities that evidenced at least moderate success in decreasing antisocial behavior. Focused work by Caldwell and colleagues (Caldwell, Skeem, Salekin, & Van Rybroek, 2006; Caldwell & Van Rybroek, 2005; Caldwell, Vitacco, & Van Rybroek, 2006) demonstrated significant success in treating psychopathic traits in adolescents and reducing future antisocial behavior. Of note, the adolescents in Caldwell's treatment studies are especially violent and psychopathic. A promising finding is that even callous traits, often considered to be the core of psychopathy, decrease through treatment.
Early intervention treatment may prevent future offending behavior. While psychopathy may complicate treatment, the creation of specialized treatment program that focus on the core traits of psychopathy (e.g., callous and unremorseful behavior) offer incremental hope over previous generic treatment offered en masse to adolescent offenders (Vitacco et al., in press).This research suggests that antisocial behavior, including callous traits in psychopaths, may be amenable to treatment if treatment is focused, intensive, and serves as a mechanism to reduce the long-term risk for some of the most extreme adolescent offenders. Germane to the current paper is how biological indices of psychopathy can be effectively utilized in the development, implementation, and monitoring of treatment progress. This provocative extension helps to motivate theoretical and practical investigations of treatment-focused studies.
In sum, interest in understanding, treating and preventing antisocial behavior should be informed by neurobiological mechanisms of related behaviors and processes. Insights into moral decision-making will be gained by exploring the etiology of empathy and callousness and the interplay of peripheral physiological processes, including cortisol and HPA activity, with the neurocircuitry that promotes prosocial behavior and caring for others. In individuals described as callous, this literature indicated blunted cortisol activity and responses to stress regardless of whether the context involves distress in oneself or another. Attenuated reactivity was mirrored in empathy-related neurocircuitry.
Failing to experience extant physiological arousal in the face of danger or stress or emotions has a certain appeal in a world that seems stressed and afraid all the time (Sapolsky, 1998). Yet the stress response is adaptive, helping an individual anticipate, cope and respond to salient social contexts (McEwen, 1998). Hypoarousal may buffer the individual from some negative consequences of stress, but it may be a two-edged sword. It may render the individual less susceptible to pair-bonding and affiliation as a consequence of hyporesponsivity of empathy-related neurocircuitry to social cues (Taylor et al., 2000). Just as the callous individual may be less responsive to social distress, they may also be less responsive to the warmth and rewards of social affiliation (Gunnar & Vazquez, 2001; Heim, Ehlert, & Hellhammer, 2000; Shirtcliff et al., 2005).
Figure 2.
The L-HPA axis as a feedback loop. The top-down mechanism begins with signals in the emotion-related neurocircuitry in the brain (limbic system) triggering corticotropin-releasing hormone (CRH) from the hypothalamus. CRH then travels through the hypophyseal portal system, a small limited blood supply allowing communication between the hypothalamus and anterior pituitary. The anterior pituitary (located just above the soft palate in the mouth and very near the hypothalamus) releases several hormones, notably adrenocorticotropic hormone (ACTH). ACTH then travels through the blood to the adrenal gland, where the end-product cortisol is released. The bottom-up effects are also illustrated, in that cortisol also then feeds back to the pituitary and the brain, acting to inhibit neural activity and (by acting on receptors in the hypothalamus), to reduce further cortisol release.
Acknowledgements
Special thanks are due Paul Frick and Jamie L. Hanson for providing helpful advice, thought-provoking discussions, and careful review of the manuscript. Figure 1's images are courtesy of the Waisman Center for Brain Imaging at the University of Wisconsin-Madison and were coordinated with the expert assistance of Jamie L. Hanson.
Figure 1.
TOP: A low transverse MRI image of the prefrontal cortex (in red) and the amygdala (in green). MIDDLE: A mid-sagittal MRI image of the anterior cingulate cortex (in yellow) and the prefrontal cortex (in red). BOTTOM: A mid-coronal MRI image of the insula (in purple) and the amygdala (in green). It should be noted that the neuroanatomical distinction between the ventromedial prefrontal cortex and the orbitofrontal cortex is not well-delineated. Though the orbitofrontal cortex may be closer to the eyes than the ventromedial prefrontal cortex, the terms are sometimes used interchangeably. Consequently, the prefrontal cortex is illustrated, including both the vmPFC and the OFC.
Footnotes
This argument is not meant to imply that CIP or alexithymia patients are psychopathic. It only illustrates the importance of peripheral physiological activation for representing others' emotions.
References
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK, Gunnar M. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2001;26(2):189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, Flor H. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Archives of General Psychiatry. 2005;62(7):799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Blair RJ. A cognitive developmental approach to mortality: investigating the psychopath. Cognition. 1995;57(1):1–29. doi: 10.1016/0010-0277(95)00676-p. [DOI] [PubMed] [Google Scholar]
- Blair RJ. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Science. 2007a;11(9):387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Dysfunctions of medial and lateral orbitofrontal cortex in psychopathy. Annals of the New York Academy of Science. 2007b;1121:461–479. doi: 10.1196/annals.1401.017. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Budhani S, Colledge E, Scott S. Deafness to fear in boys with psychopathic tendencies. Journal of Child Psychology and Psychiatry. 2005;46(3):327–336. doi: 10.1111/j.1469-7610.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Colledge E, Murray L, Mitchell DG. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. Journal of Abnormal Child Psychology. 2001;29(6):491–498. doi: 10.1023/a:1012225108281. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Jones L, Clark F, Smith M. The psychopathic individual: a lack of responsiveness to distress cues? Psychophysiology. 1997;34(2):192–198. doi: 10.1111/j.1469-8986.1997.tb02131.x. [DOI] [PubMed] [Google Scholar]
- Booth A, Granger DA, Shirtcliff EA. Gender- and age-related differences in the association between social relationship quality and trait levels of salivary cortisol. Journal of Research on Adolescence. 2008;18(2):239–260. [Google Scholar]
- Brennan P, Raine A. Biosocial bases of antisocial behavior: Psychophysiological, neurological, and cognitive factors. Clinical Psychology Review. 1997;17:589–604. doi: 10.1016/s0272-7358(97)00036-6. [DOI] [PubMed] [Google Scholar]
- Brotman LM, Gouley KK, Huang KY, Kamboukos D, Fratto C, Pine DS. Effects of a psychosocial family-based preventive intervention on cortisol response to a social challenge in preschoolers at high risk for antisocial behavior. Archives of General Psychiatry. 2007;64(10):1172–1179. doi: 10.1001/archpsyc.64.10.1172. [DOI] [PubMed] [Google Scholar]
- Burke JD, Loeber R, Lahey BB. Adolescent conduct disorder and interpersonal callousness as predictors of psychopathy in young adults. Journal of Clinical Child Adolescent Psychology. 2007;36(3):334–346. doi: 10.1080/15374410701444223. [DOI] [PubMed] [Google Scholar]
- Caldwell MF, Skeem J, Salekin R, Van Rybroek GJ. Treatment response of adolescent offenders with psychopathic features: A two-year follow-up. Criminal Justice and Behavior. 2006;33:571–596. [Google Scholar]
- Caldwell MF, Van Rybroek GJ. Reducing violence in serious juvenile offenders using intensive treatment. International Journal of Law and Psychiatry. 2005;28:622–636. doi: 10.1016/j.ijlp.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Caldwell MF, Vitacco M, Van Rybroek GJ. Are violent delinquents worth treating? A cost-benefit analysis. Journal of Research in Crime and Delinquency. 2006;43:148–168. [Google Scholar]
- Cale EM, Lilienfeld SO. Sex differences in psychopathy and antisocial personality disorder. A review and integration. Clinical Psychology Review. 2002;22(8):1179–1207. doi: 10.1016/s0272-7358(01)00125-8. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of the Sciences of the United States of America. 2003;100(9):5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Schultz W. The mysterious orbitofrontal cortex. foreword. Cerebral Cortex. 2000;10(3):205. doi: 10.1093/cercor/10.3.205. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Lin CP, Liu HL, Hsu YY, Lim KE, Hung D, et al. Expertise modulates the perception of pain in others. Current Biology. 2007;17(19):1708–1713. doi: 10.1016/j.cub.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001;13:783–804. [PubMed] [Google Scholar]
- Cima M, Smeets T, Jelicic M. Self-reported trauma, cortisol levels, and aggression in psychopathic and non-psychopathic prison inmates. Biological Psychology. 2008;78(1):75–86. doi: 10.1016/j.biopsycho.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. A new view of pain as a homeostatic emotion. Trends in Neuroscience. 2003;26(6):303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology. 2005;493(1):154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. Journal of Physiology. 2000;523(Pt 1):259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Good CD, Ashburner J, Frackowiak RS, Mathias CJ, Dolan RJ. Changes in cerebral morphology consequent to peripheral autonomic denervation. Neuroimage. 2003;18(4):908–916. doi: 10.1016/s1053-8119(03)00011-9. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Danziger N, Prkachin KM, Willer JC. Is pain the price of empathy? The perception of others' pain in patients with congenital insensitivity to pain. Brain. 2006;129(Pt 9):2494–2507. doi: 10.1093/brain/awl155. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen P. The amygdala: Vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- de Haan M, Gunnar M, Tout K, Hart J, Stansbury K. Familiar and novel contexts yield different associations between cortisol and behavior among 2-year-old children. Developmental Psychobiology. 1998;33(1):93–101. doi: 10.1002/(sici)1098-2302(199807)33:1<93::aid-dev8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- De Kloet ER. Hormones, brain and stress. Endocrine Regulation. 2003;37(2):51–68. [PubMed] [Google Scholar]
- De Kloet ER. Hormones and the stressed brain. Annals of the New York Academy of Sciences. 2004;1018:1–15. doi: 10.1196/annals.1296.001. [DOI] [PubMed] [Google Scholar]
- de Vignemont F, Singer T. The empathic brain: how, when and why? Trends in Cognitive Science. 2006;10(10):435–441. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- de Waal F, Macedo S, Ober J. Primates and Philosophers: How Morality Evolved. Princeton University Press; Princeton, NJ: 2006. [Google Scholar]
- Decety J, Lamm C. Human empathy through the lens of social neuroscience. Scientific World Journal. 2006;6:1146–1163. doi: 10.1100/tsw.2006.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13:580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Experimental Brain Research. 1992;91(1):176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Dienstbier RA. Arousal and physiological toughness: implications for mental and physical health. Psychological Review. 1989;96(1):84–100. doi: 10.1037/0033-295x.96.1.84. [DOI] [PubMed] [Google Scholar]
- Dishman DJ, Wallace AM, Crawford A, Grant JK, Hinton JW. Unusual hormonal “stress relaxation” response in prisoners with convictions for assault with robbery. Journal of Psychosomatic Research. 1982;26(3):341–344. doi: 10.1016/0022-3999(82)90006-x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacology Biochemistry and Behavior. 2002;71(3):431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Eisenberg N. Empathy-related responding and prosocial behaviour. Novartis Foundation Symposium. 2007;278:71–80. discussion 80-96, 216-221. [PubMed] [Google Scholar]
- Eisenberger NI, Jarcho JM, Lieberman MD, Naliboff BD. An experimental study of shared sensitivity to physical pain and social rejection. Pain. 2006;126(13):132–138. doi: 10.1016/j.pain.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends in Cognitive Science. 2004;8(7):294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302(5643):290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35(4):1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enebrink P, Andershed H, Langstrom N. Callous-unemotional traits are associated with clinical severity in referred boys with conduct problems. Nordic Journal of Psychiatry. 2005;59(6):431–440. doi: 10.1080/08039480500360690. [DOI] [PubMed] [Google Scholar]
- Errico AL, Parsons OA, King AC, Lovallo WR. Attenuated cortisol response to biobehavioral stressors in sober alcoholics. Journal of Studies on Alcohol and Drugs. 1993;54(4):393–398. doi: 10.15288/jsa.1993.54.393. [DOI] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SH, Stollery SJ, Brown J, Gardiner J, Herbert J, et al. Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescence-onset conduct disorder. Biological Psychiatry. 2008;64(7):599–606. doi: 10.1016/j.biopsych.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones--a focus on rapid, nongenomic effects. Pharmacological Reviews. 2000;52(4):513–556. [PubMed] [Google Scholar]
- Farrington DP. The importance of child and adolescent psychopathy. Journal of Abnormal Child Psychology. 2005;33(4):489–497. doi: 10.1007/s10802-005-5729-8. [DOI] [PubMed] [Google Scholar]
- Fehr E, Rockenbach B. Human altruism: economic, neural, and evolutionary perspectives. Current Opinion in Neurobiology. 2004;14(6):784–790. doi: 10.1016/j.conb.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, Kosson DS, Chen G, Towbin KE, Leibenluft E, Pine DS, Blair RJ. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Archives of General Psychiatry. 2008;65(5):586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Mitchell DG, Jones M, Blair RJ. Dissociable roles of medial orbitofrontal cortex in human operant extinction learning. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Gunnar MR, Chamberlain P, Reid JB. Preventive intervention for maltreated preschool children: impact on children's behavior, neuroendocrine activity, and foster parent functioning. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(11):1356–1364. doi: 10.1097/00004583-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32(810):892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinn MV, England BG. Childhood stress and family environment. Current Anthropology. 1995;36:854–866. [Google Scholar]
- Forth AE, Kosson DS, Hare RD. Hare Psychopathy Checklist: Youth Version Technical Manual. Multi-health Systems; Toronto: 2003. [Google Scholar]
- Frick PJ. Developmental pathways to conduct disorder. Child and Adolescent Psychiatric Clinics of North America. 2006;15(2):311–331. vii. doi: 10.1016/j.chc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Bodin SD, Barry CT. Psychopathic traits and conduct problems in community and clinic-referred samples of children: further development of the psychopathy screening device. Psychological Assessment. 2000;12(4):382–393. [PubMed] [Google Scholar]
- Frick PJ, Cornell AH, Barry CT, Bodin SD, Dane HE. Callous-unemotional traits and conduct problems in the prediction of conduct problem severity, aggression, and self-report of delinquency. Journal of Abnormal Child Psychology. 2003;31(4):457–470. doi: 10.1023/a:1023899703866. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Cornell AH, Bodin SD, Dane HE, Barry CT, Loney BR. Callous-unemotional traits and developmental pathways to severe conduct problems. Developmental Psychology. 2003;39(2):246–260. doi: 10.1037//0012-1649.39.2.246. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Hare RD. Manual for the antisocial process screening device. Multi-health Systems; Toronto: 2001. [Google Scholar]
- Frick PJ, White SF. Research review: the importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. Journal of Child Psychology and Psychiatry. 2008;49(4):359–375. doi: 10.1111/j.1469-7610.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- Geary DC, Flinn MV. Sex differences in behavioral and hormonal response to social threat: commentary on Taylor et al. (2000) Psychological Review. 2002;109(4):745–750. doi: 10.1037/0033-295x.109.4.745. discussion 751-743. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. Experiential canalization of behavioral development: Theory. Developmental Psychology. 1991;27:4–13. [Google Scholar]
- Granger DA, Serbin LA, Schwartzman AE, Lehoux PM, Cooperman JM, Ikeda S. Children's salivary cortisol, internalizing behavior problems, and family environment: Results from the Concordia Longitudinal Risk Project. International Journal of Behavioral Development. 1998;22:707–728. [Google Scholar]
- Granger DA, Stansbury K, Henker B. Preschooler's behavioral and neuroendocrine responses to social challenge. Merrill-Palmer Quarterly. 1994;40:20–41. [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Tout K, de Haan M, Pierce S, Stansbury K. Temperament, social competence, and adrenocortical activity in preschoolers. Developmental Psychobiology. 1997;31(1):65–85. doi: 10.1002/(sici)1098-2302(199707)31:1<65::aid-dev6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Vazquez D. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology. 2nd ed. Vol. 2. Wiley; New York: 2006. pp. 533–577. [Google Scholar]
- Gunnar M, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Developmental Psychopathology. 2001;13(3):515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hare RD. Hare psychopathy checklist-revised (PCL-R): Second edition, technical manual. Multi-Health Systems. 2003 [Google Scholar]
- Hastings PD, Zahn-Waxler C, McShane K. We are, by nature, moral creatures: Biological bases of concern for others. In: Killen M, Smetana J, editors. Handbook of moral development. Lawrence Erlbaum Associates; Mahwah, NJ: 2006. pp. 483–516. [Google Scholar]
- Hastings PD, Zahn-Waxler C, Robinson J, Usher B, Bridges D. The development of concern for others in children with behavior problems. Developmental Psychology. 2000;36(5):531–546. doi: 10.1037/0012-1649.36.5.531. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress- related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hein G, Singer T. I feel how you feel but not always: the empathic brain and its modulation. Current Opinion in Neurobiology. 2008 doi: 10.1016/j.conb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neuroscience. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinology. 2003;24(3):151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Critical Reviews in Neurobiology. 1996;10(34):371–394. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- Holi M, Auvinen-Lintunen L, Lindberg N, Tani P, Virkkunen M. Inverse correlation between severity of psychopathic traits and serum cortisol levels in young adult violent male offenders. Psychopathology. 2006;39(2):102–104. doi: 10.1159/000091021. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Structure and Function. 2007;212(2):149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer C. Neural Systems Responding to Degrees of Uncertainty in Human Decision-Making. Science. 2005;310:1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Chen FL, Takahashi LK, Kalin NH. Rapid stress-induced elevations in corticotropin-releasing hormone mRNA in rat central amygdala nucleus and hypothalamic paraventricular nucleus: an in situ hybridization analysis. Brain Research. 1998;788(12):305–310. doi: 10.1016/s0006-8993(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nature Reviews Neuroscience. 2006;7(12):942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Insel TR. A neurobiological basis of social attachment. American Journal of Psychiatry. 1997;154(6):726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: Searching for the Social Brain. Annual Review of Neuroscience. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44(5):752–761. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24(3):771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Somerville LH, Alexander AL, Oakes TR, Davidson RJ, Kalin NH, Whalen PJ. Stability of amygdala BOLD response to fearful faces over multiple scan sessions. Neuroimage. 2005;25(4):1112–1123. doi: 10.1016/j.neuroimage.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Kalin NH. The neurobiology of fear. Scientific American. 1993;268(5):94–101. doi: 10.1038/scientificamerican0593-94. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience. 2004;24(24):5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biological Psychiatry. 2007;62(10):1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariyawasam SH, Zaw F, Handley SL. Reduced salivary cortisol in children with comorbid attention deficit hyperactivity disorder and oppositional defiant disorder. Neuroendocrinology Letters. 2002;23:45–48. [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33(4):517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: evidence for paralimbic system dysfunction. Psychiatry Research. 2006;142(23):107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, Liddle PF. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological Psychiatry. 2001;50(9):677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Fazekas H, Loney BR. Psychopathy, aggression, and the processing of emotional stimuli in non-referred girls and boys. Behavioral Sciences and the Law. 2006;24(1):21–37. doi: 10.1002/bsl.668. [DOI] [PubMed] [Google Scholar]
- King JA, Blair RJ, Mitchell DG, Dolan RJ, Burgess N. Doing the right thing: a common neural circuit for appropriate violent or compassionate behavior. Neuroimage. 2006;30(3):1069–1076. doi: 10.1016/j.neuroimage.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Knafo A, Zahn-Waxler C, Van Hulle CA, Robinson JL, Rhee SH. The developmental origins of a disposition toward empathy: Genetic and environmental contributions. Emotion. 2008;8:737–752. doi: 10.1037/a0014179. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the Ultimatum Game. Journal of Neuroscience. 2007;27(4):951–956. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, Damasio A. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature. 2007;446(7138):908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kruesi MJP, Schmidt ME, Donnelly H, Hibbs ED, Hamburger SP. Urinary free cortisol output and disruptive behavior in children. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28:441–443. doi: 10.1097/00004583-198905000-00024. [DOI] [PubMed] [Google Scholar]
- Levenson RW. Blood, sweat, and fears: the autonomic architecture of emotion. Annals of the New York Academy of Sciences. 2003;1000:348–366. doi: 10.1196/annals.1280.016. [DOI] [PubMed] [Google Scholar]
- Liberzon I, King AP, Britton JC, Phan KL, Abelson JL, Taylor SF. Paralimbic and medial prefrontal cortical involvement in neuroendocrine responses to traumatic stimuli. American Journal of Psychiatry. 2007;164(8):1250–1258. doi: 10.1176/appi.ajp.2007.06081367. [DOI] [PubMed] [Google Scholar]
- Loney BR, Butler MA, Lima EN, Counts CA, Eckel LA. The relation between salivary cortisol, callous-unemotional traits, and conduct problems in an adolescent non-referred sample. Journal of Child Psychology and Psychiatry. 2006;47(1):30–36. doi: 10.1111/j.1469-7610.2005.01444.x. [DOI] [PubMed] [Google Scholar]
- Losel RM, Falkenstein E, Feuring M, Schultz A, Tillmann HC, Rossol-Haseroth K, Wehling M. Nongenomic steroid action: controversies, questions, and answers. Physiological Reviews. 2003;83(3):965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: can't live with it, can't live without it. Behavioral Brain Research. 2001;127(12):137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Lynam DR. Early identification of the fledgling psychopath: locating the psychopathic child in the current nomenclature. Journal of Abnormal Psychology. 1998;107(4):566–575. doi: 10.1037//0021-843x.107.4.566. [DOI] [PubMed] [Google Scholar]
- MacLean PD. Brain evolution relating to family, play, and the separation call. Archives of General Psychiatry. 1985;42(4):405–417. doi: 10.1001/archpsyc.1985.01790270095011. [DOI] [PubMed] [Google Scholar]
- MacLullich AM, Ferguson KJ, Wardlaw JM, Starr JM, Deary IJ, Seckl JR. Smaller left anterior cingulate cortex volumes are associated with impaired hypothalamic-pituitary-adrenal axis regulation in healthy elderly men. Journal of Clinical Endocrinology and Metabolism. 2006;91(4):1591–1594. doi: 10.1210/jc.2005-2610. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, Blair RJ. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165(6):712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Maughan B, Rowe R, Messer J, Goodman R, Meltzer H. Conduct disorder and oppositional defiant disorder in a national sample: developmental epidemiology. Journal of Child Psychology and Psychiatry. 2004;45(3):609–621. doi: 10.1111/j.1469-7610.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- Mazzulla EC, Oler JA, Johnstone T, Kirkland JZ, Shirtcliff EA, Armstrong JM, Davidson RJ, Essex MJ. The effect of cortisol on fMRI signal during emotion-regulation in adolescent subjects. the Society for Neuroscience; Washington DC. November 15-17.2008. [Google Scholar]
- McBurnett K, Raine A, Stouthamer-Loeber M, Loeber R, Kumar AM, Kumar M, et al. Mood and hormone responses to psychological challenge in adolescent males with conduct problems. Biological Psychiatry. 2005;57(10):1109–1116. doi: 10.1016/j.biopsych.2005.01.041. [DOI] [PubMed] [Google Scholar]
- McBurnett KM, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Archives of General Psychiatry. 2000;57:38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course -persistent antisocial behavior: A developmental taxonomy. Psychological Review. 1993a;100:674–701. [PubMed] [Google Scholar]
- Moffitt TE. The neuropsychology of conduct disorder. Development and Psychopathology. 1993b;5:135–151. [Google Scholar]
- Moriguchi Y, Decety J, Ohnishi T, Maeda M, Mori T, Nemoto K, Matsuda H. Empathy and judging other's pain: an fMRI study of alexithymia. Cerebral Cortex. 2007;17(9):2223–2234. doi: 10.1093/cercor/bhl130. [DOI] [PubMed] [Google Scholar]
- Moss HB, Vanyukov MM, Martin CS. Salivary cortisol responses and the risk for substance abuse in prepubertal boys. Biological Psychiatry. 1995;38:547–555. doi: 10.1016/0006-3223(94)00382-D. [DOI] [PubMed] [Google Scholar]
- Murrie DC, Marcus DK, Douglas KS, Lee Z, Salekin RT, Vincent G. Youth with psychopathy features are not a discrete class: a taxometric analysis. Journal of Child Psychology and Psychiatry. 2007;48(7):714–723. doi: 10.1111/j.1469-7610.2007.01734.x. [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Takahashi T, Wakabayashi A, Oono H, Radford MH. Sex differences in the relationship between cortisol levels and the Empathy and Systemizing Quotients in humans. Neuroendocrinology Letters. 2007;28(4):445–448. [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Netter P, Hennig J, Rohrmann S. Psychobiological differences between the aggression and psychoticism dimension. Pharmacopsychiatry. 1999;32(1):5–12. doi: 10.1055/s-2007-979182. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O'Leary MM, Loney BR, Eckel LA. Gender differences in the association between psychopathic personality traits and cortisol response to induced stress. Psychoneuroendocrinology. 2007;32(2):183–191. doi: 10.1016/j.psyneuen.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Geurts HM, Knol DL, Sergeant JA. Low basal salivary cortisol is associated with teacher-reported symptoms of conduct disorder. Psychiatry Research. 2005;134(1):1–10. doi: 10.1016/j.psychres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Pajer K, Gardner W, Kirillova GP, Vanyukov M. Sex differences in cortisol levels and neurobehavioral disinhibition in children of substance abusers. Journal of Child and Adolescent Substance Abuse. 2001;10(4):65–76. [Google Scholar]
- Pajer K, Gardner W, Rubin RT, Perel J, Neal S. Decreased cortisol levels in adolescent girls with conduct disorder. Archives of General Psychiatry. 2001;58(3):297–302. doi: 10.1001/archpsyc.58.3.297. [DOI] [PubMed] [Google Scholar]
- Pardini DA, Lochman JE, Frick PJ. Callous/unemotional traits and social-cognitive processes in adjudicated youths. Journal of the American Academy of Child Adolescent Psychiatry. 2003;42(3):364–371. doi: 10.1097/00004583-200303000-00018. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Iacoboni M, Mazziotta JC, Dapretto M. Mirroring others' emotions relates to empathy and interpersonal competence in children. Neuroimage. 2008;39(4):2076–2085. doi: 10.1016/j.neuroimage.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Popma A, Doreleijers TA, Jansen LM, Van Goozen SH, Van Engeland H, Vermeiren R. The diurnal cortisol cycle in delinquent male adolescents and normal controls. Neuropsychopharmacology. 2007;32(7):1622–1628. doi: 10.1038/sj.npp.1301289. [DOI] [PubMed] [Google Scholar]
- Porges SW. Cardiac vagal tone: a physiological index of stress. Neuroscience and Biobehavioral Reviews. 1995;19(2):225–233. doi: 10.1016/0149-7634(94)00066-a. [DOI] [PubMed] [Google Scholar]
- Preston SD, de Waal FB. Empathy: Its ultimate and proximate bases. Behavioral and Brain Sciences. 2002;25(1):1–20. doi: 10.1017/s0140525x02000018. discussion 20-71. [DOI] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: A review. Journal of Abnormal Child Psychology. 2002;30(4):311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Mednick SA. Low resting heart rate at age 3 years predisposes to aggression at age 11 years: Evidence from the Mauritius Child Health Project. Journal of the American Academy of Child. 1997;36:1457–1464. doi: 10.1097/00004583-199710000-00029. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Glenn AL, Jairam MR, Pagnoni G, Goldsmith DR, Elfenbein HA, Lilienfeld SO. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biological Psychiatry. 2007;61(11):1260–1271. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Risold PY, Thompson RH, Swanson LW. The structural organization of connections between hypothalamus and cerebral cortex. Brain Research Reviews. 1997;24(23):197–254. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. 1999 Curt P. Richter award. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25(3):213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiology of Learning and Memory. 2002;78(3):578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Salekin RT. Psychopathy and therapeutic pessimism. Clinical lore or clinical reality? Clinical Psychology Review. 2002;22(1):79–112. doi: 10.1016/s0272-7358(01)00083-6. [DOI] [PubMed] [Google Scholar]
- Salekin RT, Frick PJ. Psychopathy in children and adolescents: the need for a developmental perspective. Journal of Abnormal Child Psychology. 2005;33(4):403–409. doi: 10.1007/s10802-005-5722-2. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300(5626):1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why zebras don't get ulcers : an updated guide to stress, stress-related diseases, and coping. W.H. Freeman and Co.; New York: 1998. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Scerbo SA, Kolko DJ. Salivary testosterone and cortisol in disruptive children: Relationship to aggressive, hyperactive, and internalizing behaviors. Journal of the American Academy of Child Psychiatry. 1994;33:1174–1184. doi: 10.1097/00004583-199410000-00013. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47(5):633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Stalnaker TA. Reconciling the roles of orbitofrontal cortex in reversal learning and the encoding of outcome expectancies. Annals of the New York Academy of Sciences. 2007;1121:320–335. doi: 10.1196/annals.1401.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Ruther M, Markowitsch HJ, Fink GR, Piefke M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: a functional magnetic resonance imaging approach to empathy. Journal of Cognitive Neuroscience. 2007;19(8):1354–1372. doi: 10.1162/jocn.2007.19.8.1354. [DOI] [PubMed] [Google Scholar]
- Schulte-Ruther M, Markowitsch HJ, Shah NJ, Fink GR, Piefke M. Gender differences in brain networks supporting empathy. Neuroimage. 2008;42(1):393–403. doi: 10.1016/j.neuroimage.2008.04.180. [DOI] [PubMed] [Google Scholar]
- Sethre-Hofstad L, Stansbury K, Rice MA. Attunement of maternal and child adrenocortical response to child challenge. Psychoneuroendocrinology. 2002;27(6):731–747. doi: 10.1016/s0306-4530(01)00077-4. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Developmental Psychobiology. 2008;50:691–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Development and Psychopathology. 2005;17:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Singer T. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neuroscience and Biobehavioral Reviews. 2006;30(6):855–863. doi: 10.1016/j.neubiorev.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Singer T. The neuronal basis of empathy and fairness. Novartis Foundation Symposium. 2007;278:20–30. discussion 30-40, 89-96, 216-221. [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439(7075):466–469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smider NA, Essex MJ, Kalin NH, Buss KA, Klein MH, Davidson RJ, Goldsmith HH. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: A prospective study. Child Development. 2002;73(1):75–92. doi: 10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- Smith A. The theory of moral sentiments. A. Millar; London: 1790. [Google Scholar]
- Snoek H, Van Goozen SH, Matthys W, Buitelaar JK, van Engeland H. Stress responsivity in children with externalizing behavior disorders. Developmental Psychopathology. 2004;16(2):389–406. doi: 10.1017/s0954579404044578. [DOI] [PubMed] [Google Scholar]
- Sondeijker FE, Ferdinand RF, Oldehinkel AJ, Veenstra R, Tiemeier H, Ormel J, Verhulst FC. Disruptive behaviors and HPA-axis activity in young adolescent boys and girls from the general population. Journal of Psychiatric Research. 2007;41(7):570–578. doi: 10.1016/j.jpsychires.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54(1):51–58. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Stansbury K, Gunnar MR. Adrenocortical activity and emotion regulation. Monographs of the Society for Research in Child Development. 1994;59(23):108–134. [PubMed] [Google Scholar]
- Stark R, Wolf OT, Tabbert K, Kagerer S, Zimmermann M, Kirsch P, Schienle A, Vaitl D. Influence of the stress hormone cortisol on fear conditioning in humans: evidence for sex differences in the response of the prefrontal cortex. Neuroimage. 2006;32(3):1290–1298. doi: 10.1016/j.neuroimage.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biological Psychiatry. 2005;57(1):7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37(1):335–342. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: stress, early vulnerabilities and the attenuation hypothesis. Neuroscience Biobehavioral Reviews. 2006;30(3):376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Schiefelbein VL, Herwehe S, Heaton JA, Dorn LD. Morningness/eveningness, morning-to-afternoon cortisol ratio, and antisocial behavior problems during puberty. Developmental Psychology. 2007;43(4):811–822. doi: 10.1037/0012-1649.43.4.811. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dorn LD, Inoff-Germain G, Nottelman ED, Chrousos GP. Cortisol reactivity, distress behavior, behavior problems, and emotionality in young adolescents: A longitudinal perspective. Journal of Research on Adolescence. 1997;7:81–105. [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. Journal of Child Psychology and Psychiatry. 2007;48(34):262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA. Biobehavioral response to stress in females: Tend and befriend, not fight-or-flight. Psychological Review. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Tennes K, Kreye M. Children's adrenocortical responses to classroom activities and tests in elementary school. Psychosomatic Medicine. 1985;47:451–460. doi: 10.1097/00006842-198509000-00005. [DOI] [PubMed] [Google Scholar]
- Tennes K, Kreye M, Avitable N, Wells R. Behavioral correlations of excreted catecholamines and cortisol in second-grade children. Journal of the American Academy of Child and Adolescent Psychiatry. 1986;25:764–770. doi: 10.1016/s0002-7138(09)60193-x. [DOI] [PubMed] [Google Scholar]
- Tout K, de Haan M, Campbell EK, Gunnar MR. Social behavior correlates of cortisol activity in child care: gender differences and time-of-day effects. Child Development. 1998;69(5):1247–1262. [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26(16):4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bokhoven I, Van Goozen SH, van Engeland H, Schaal B, Arseneault L, Seguin JR, Nagin DS, Vitaro F, Tremblay RE. Salivary cortisol and aggression in a population-based longitudinal study of adolescent males. Journal of Neural Transmission. 2005;112(8):1083–1096. doi: 10.1007/s00702-004-0253-5. [DOI] [PubMed] [Google Scholar]
- van de Wiel NM, van Goozen SH, Matthys W, Snoek H, van Engeland H. Cortisol and treatment effect in children with disruptive behavior disorders: a preliminary study. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(8):1011–1018. doi: 10.1097/01.chi.0000126976.56955.43. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychological Bulletin. 2007;133(1):149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- Van Goozen SHM, Matthys W, Cohen-Kettenis PT, Buitelaar JK, Van Engeland H. Hypothalamic-pituitary-adrenal axis and autonomic nervous system activity in disruptive children and matched controls. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1438–1445. doi: 10.1097/00004583-200011000-00019. [DOI] [PubMed] [Google Scholar]
- Van Goozen SHM, Matthys W, Cohen-Kettenis PT, Gispen-de Weid C, Wiegant VM, Van Engeland H. Salivary cortisol and cardiovascular activity during stress in oppositional defiant disorder boys and normal controls. Biological Psychiatry. 1998;43:531–539. doi: 10.1016/S0006-3223(97)00253-9. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJ. Unmasking feigned sanity: a neurobiological model of emotion processing in primary psychopathy. Cognitive Neuropsychiatry. 2006;11(3):285–306. doi: 10.1080/13546800500233728. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJ, Hermans EJ, Putman P. Low cortisol levels and the balance between punishment sensitivity and reward dependency. Neuroreport. 2003;14(15):1993–1996. doi: 10.1097/00001756-200310270-00023. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Wolf OT, Everaerd W, Rombouts SA. Interaction of endogenous cortisol and noradrenaline in the human amygdala. Progress in Brain Research. 2008;167:263–268. doi: 10.1016/S0079-6123(07)67020-4. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Wolf OT, Everaerd W, Scheltens P, Barkhof F, Rombouts SA. Endogenous cortisol level interacts with noradrenergic activation in the human amygdala. Neurobiology of Learning and Memory. 2007;87(1):57–66. doi: 10.1016/j.nlm.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Moss HB, Plail JA, Blackson T, Mezzich AC, Tarter RE. Antisocial symptoms in preadolescent boys and in their parents: Associations with cortisol. Psychiatry Research. 1993;46:9–17. doi: 10.1016/0165-1781(93)90003-y. [DOI] [PubMed] [Google Scholar]
- Vazquez DM. Stress and the developing limbic-hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 1998;23(7):663–700. doi: 10.1016/s0306-4530(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Veit R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, et al. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neuroscience Letters. 2002;328(3):233–236. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- Vitacco M, Rogers R, Neumann C. The Antisocial Process Screening Device: An examination of its construct and criterion-related validity. Assessment. 2003;10:143–150. doi: 10.1177/1073191103010002005. [DOI] [PubMed] [Google Scholar]
- Vitacco MJ, Salekin RT, Rogers R. Forensic issues and adolescent psychopathy. In: Salekin RT, Lynam DR, editors. Handbook of Adolescent Psychopathy. in press. [Google Scholar]
- Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, McEwen BS, Detre JA. Gender Difference in Neural Response to Psychological Stress. Social Cognitive and Affective Neuroscience. 2007;2(3):227–239. doi: 10.1093/scan/nsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(49):17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschbusch DA. A meta-analytic examination of comorbid hyperactive-impulsive-attention problems and conduct problems. Psychological Bulletin. 2002;128(1):118–150. doi: 10.1037/0033-2909.128.1.118. [DOI] [PubMed] [Google Scholar]
- Waschbusch DA, Walsh TM, Andrade BF, King S, Carrey NJ. Social problem solving, conduct problems, and callous-unemotional traits in children. Child Psychiatry and Human Development. 2007;37(4):293–305. doi: 10.1007/s10578-006-0033-6. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Convit A, de Leon MJ, Caraos C, Qadri SF. Basal hypothalamo-pituitary-adrenal axis activity and corticotropin feedback in young and older men: relationships to magnetic resonance imaging-derived hippocampus and cingulate gyrus volumes. Neuroendocrinology. 2002;75(4):241–249. doi: 10.1159/000054715. [DOI] [PubMed] [Google Scholar]
- Young L, Koenigs M. Investigating emotion in moral cognition: a review of evidence from functional neuroimaging and neuropsychology. British Medical Bulletin. 2007;84:69–79. doi: 10.1093/bmb/ldm031. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C. The development of empathy. guilt, and internalization of distress: Implications for gender differences in internalizing and externalizing problems. In: Davidson RJ, editor. Anxiety, depression, and emotion: Wisconsin symposium on emotion. Vol. 1. Oxford University Press; New York, NY: 2000. pp. 222–265. [Google Scholar]
- Zahn-Waxler C, Crick NR, Shirtcliff EA, Wall K. The origins and development of psychopathology in females and males. In: Cicchetti D, Cohen D, editors. Handbook of developmental psychopathology. 2nd ed. Wiley; New York: 2005. [Google Scholar]
- Zahn-Waxler C, Radke-Yarrow M. The origins of empathic concern. Motivation and Emotion. 1990;14(2):107–130. [Google Scholar]; Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: gender and psychopathology. Annual Review of Clinical Psychology. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: gender and psychopathology. Annual Review of Clinical Psychology. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]