Abstract
Objective
To assess the efficacy of two psychosocial interventions for caregivers of older persons with spinal cord injury (SCI).
Design
A multisite, three-group, randomized controlled trial comparing two active intervention conditions with each other and to an information-only control group. One hundred seventy-three caregiver and care-recipient dyads were randomly assigned to one of three conditions: a caregiver-only treatment condition in which caregivers received a multicomponent intervention based on their risk profile; a dual-target condition in which the caregiver intervention was complemented by a treatment targeting the care recipient, designed to address both caregiver and care recipient risk factors; and an information-only control condition in which the caregiver received standard printed information about caregiving, SCI, and aging.
Outcome Measures
A multivariate outcome comprised of six indicators linked to the goals of the interventions was the primary outcome of the study. The multivariate outcome included measures of depressive symptoms, burden, social support and integration, self-care problems, and physical health symptoms.
Results
At 12 months, caregivers in the dual-target condition had improved quality of life as measured by our multivariate outcome when compared to the control condition. Using the dyad as the unit of analysis, the dual-target condition was superior to both the control condition and the caregiver-only condition in our multivariate outcomes analysis. Dyads enrolled in the dual-target condition had significantly fewer health symptoms than control condition and caregiver-only condition participants and were less depressed when compared to participants in the caregiver-only condition. In follow-up analyses we found that a higher proportion of caregivers in the dual-target condition had clinically significant improvements in depression, burden, and health symptoms when compared with the caregiver-only condition.
Conclusion
Caregivers are in need of and can benefit from interventions that help them manage the medical and functional limitations of the care recipient. Intervention strategies that target both the caregiver and care recipient are particularly promising strategies for improving the quality of life of caregivers.
Keywords: caregiving, spinal cord injury, intervention
Persons with spinal cord injury (SCI) are typically cared for at home by family members who are responsible for providing a wide range of services, some of which were formerly delivered by traditional health care providers. Although it is generally recognized that caregivers of individuals with chronic illnesses and disabilities experience high levels of psychiatric and physical morbidity (Schulz, O’Brien, Bookwala, & Fleissner, 1995; Schulz & Martire, 2004; Schulz, Newsom, Fleissner, deCamp, & Nieboer, 1997; Schulz et al., 1995; Schulz & Quittner, 1998), including increased risk of mortality (Schulz & Beach, 1999), caregivers of individuals with SCI may be at even greater risk of negative outcomes because of the unique challenges of caring for an individual with SCI and the long duration of the caregiving career. Researchers consistently report elevated levels of physical stress, emotional stress, burnout, fatigue, anger and resentment, and depression among caregivers of persons with SCI (Boschen, Tonack, & Gargaro, 2005; Dreer, Elliott, Shewchuk, Berry, & Rivera, 2007; Elliott & Shewchuk, 1998; Elliott, Shewchuk, & Richards, 2001; Lucke, Coccia, Goode, & Lucke, 2004; Shewchuk, Richards, & Elliott, 1998; Weitzenkamp, Gerhart, Charlifue, Whiteneck, & Savic, 1997). Caregivers providing support to older adults with SCI may experience particularly acute negative health effects because the older individual with SCI poses unique challenges brought about by the combined effects of aging and injury.
The overwhelming evidence on the health effects of caregiving and the high costs of institutional care have spawned numerous studies aimed at testing interventions to improve health outcomes of caregivers and help them maintain their relative at home. The vast majority of this literature has focused on caregivers providing support to older adults with chronic disease-related disability, particularly those suffering from Alzheimer’s disease (Pinquart & Sorenson, 2003; Schulz, Martire, & Klinger, 2005; Selwood, Johnston, Katona, Lyketsos, & Livingston, 2007). With the exception of one recently published randomized trial (Elliott & Berry, in press), there exist no other well-controlled intervention studies for caregivers of individuals with SCI, even though researchers have been calling for such studies for more than a decade (Boschen et al., 2005; Dreer et al., 2007; Elliott & Shewchuck, 1998; Lucke et al., 2004; Rodgers et al., 2007; Sheija & Manigandan, 2005). The study by Elliott and Berry (in press) showed that brief problem-solving training significantly decreased dysfunctional problem solving among caregivers but had little impact on other outcomes, possibly due to the brevity of the intervention and/or the small sample size. The primary purpose of this article is to report the results of a randomized clinical trial designed to improve the quality of life of caregivers of individuals with SCI. We compare two active intervention conditions with each other (caregiver-only intervention vs. a dual-target intervention) and to an information-only control group. The caregiver-only intervention provided caregivers with enhanced access to resources, education and skills training, strategies for improving self-care and emotional well-being, and social support. The dual-target intervention provided support to both the caregiver and individual with SCI.
Our approach to developing the interventions for this study was guided by both theory and extant research on caregiver interventions. Modeled after the successful REACH intervention trial for caregivers of patients with dementia (Belle et al., 2006), our starting point was the assumption that caregiving presents multiple challenges at varying levels of intensity that are not easily addressed. As a result, there is no single, easily implemented, and consistently effective method for achieving clinically significant effects among caregivers or care recipients (Schulz et al., 2002; Selwood et al., 2007). Most existing studies target a single problem such as depression or burden or use a multicomponent approach that combines educational components with strategies for enhancing problem-solving skills and/or managing emotional responses to caregiving challenges (Schulz, 2000; Sorensen, Pinquart, & Duberstein, 2002).
Although we subscribe to the multicomponent approach to caregiver interventions, we diverge from the existing literature in several respects. First, we believe a “one size fits all” approach to caregiver interventions is likely to be ineffective. Because of the diversity of challenges inherent in the caregiving situation, interventions need to allow for some degree of tailoring to meet the specific needs of the individual. Thus, we subscribe to a structured—but at the same time, tailored—approach to delivering interventions that are responsive to individual needs. Second, we believe that there are important synergies to be attained by simultaneously treating the caregiver and care recipient, a relatively unexplored area in the caregiving intervention literature (Martire, Lustig, Schulz, Miller, & Helgeson, 2004). For individuals with severe disabilities embedded in supportive personal relationships, it is important to consider the reciprocal impact that providers and receivers of care have on each other (Bookwala & Schulz, 1996). Psychosocial interventions targeting both patient and family caregiver may have added benefit as compared to interventions aimed at only one of these individuals. While both types of interventions may improve the emotional well-being and health behaviors of either individual, dyadic intervention has greater potential due to its effects on factors such as effective support seeking and support provision within the dyad (Martire & Schulz, 2007).
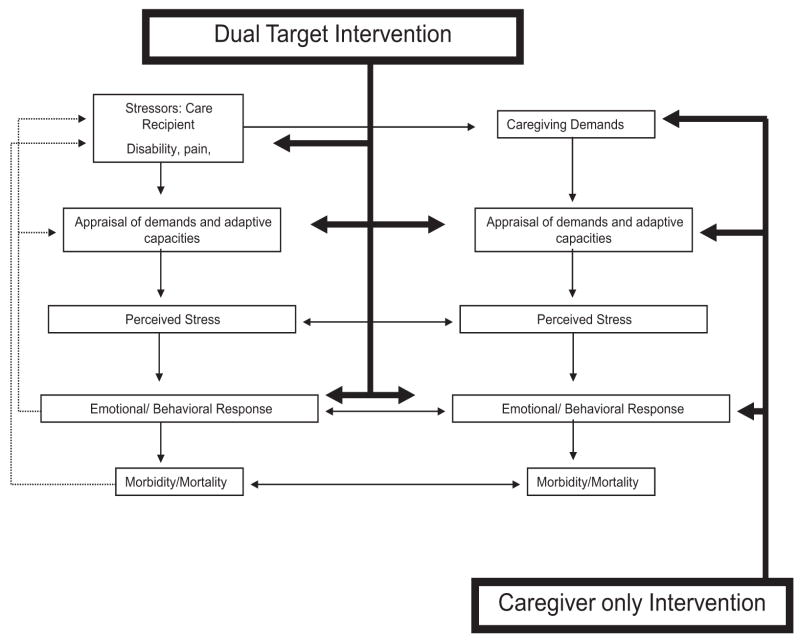
A more specific conceptual model illustrating our approach to treatment in the current study is illustrated in Figure 1. The left half of the panel describes a stress-health model applied to the care recipient, while the right half illustrates how recipient disability affects caregiver outcomes (Schulz & Williamson, 1993; Schulz, 2000). In contrast to most caregiving analyses, this model specifies important reciprocal relations between care recipient and caregiver in terms of the perception of and the emotional and behavioral response to stress, as well as reciprocal effects of illness. The bolded arrows in Figure 1 contrast the hypothesized impact of dual-target versus caregiver-only interventions. One of our central predictions is that caregiver outcomes will be enhanced in the dual-target treatment condition when compared to a traditional caregiver-only treatment condition and that both active treatments will be superior to the control condition. The synergistic effects of the dual-target condition should also be evident in analyses that focus on the dyad as the unit of analysis (i.e., a composite measure that reflects the impact of the intervention on the dyad as a unit).
Figure 1.
Conceptual model for stress-health process applied to care recipients and caregivers; hypothesized intervention targets of dual-target and caregiver-only interventions.
Methods
Study Design Overview
The study design was a multisite, three-group, randomized clinical trial comparing two active intervention conditions with each other and to an information-only control group. Caregiver and care recipient (individual with SCI) were randomly assigned to one of three conditions: a caregiver-only intervention condition; a dual-target intervention condition that targeted both the caregiver and the care recipient; or an information-only control condition in which the caregiver received standard printed information about caregiving, SCI, and aging typically available from social service and health agencies. Both intervention conditions involved the use of a computer-telephone technology system that was proven to be effective in caregiver intervention studies (e.g., Eisdorfer et al., 2003). The interventions were delivered at the caregiver’s/care-recipient’s home and via a computer telephone technology system over a 6-month period. All participants were assessed at home by certified assessors at baseline and at 6 and 12 months after baseline. Assessors were blinded to treatment condition at the follow-up assessments.
Eligibility Criteria, Recruitment, and Retention
The caregiver was self-identified. Eligibility criteria for caregivers included provision of instrumental or emotional support for a spouse, relative, partner, or friend with SCI for at least the past 6 months; having regular contact with the individual with SCI (at least a minimum of face-to-face contact one time per month and telephone contact on a weekly basis); living with or near the individual with SCI; being over the age of 18; having a telephone; planning to remain in the geographic area for at least 6 months; and competency in English.
Eligibility criteria for the individual with SCI included having an SCI (quadriplegia or paraplegia) due to an acquired injury or disease with complete or incomplete lesion as defined by the American Spinal Cord Injury Association; being age 35 years or older; having a mobility impairment as the result of the SCI (needing some form of assistance to ambulate); living in the community in a nongroup setting for a minimum of 1 year after injury; having a telephone; planning to remain in the geographic area for at least 6 months; and competency in English. We included age as an exclusion criterion for the care recipients as one of our focuses was on adults aging with SCI.
Dyads were excluded if the caregiver or care recipient (a) had a terminal illness with life expectancy < 6 months; (b) was in active treatment for cancer other than maintenance use of tamoxifen or lupron; (c) was blind or deaf; or (d) had a cognitive impairment (defined at screening as a score on the Short Portable Mental Status Questionnaire ≥ 4 errors).
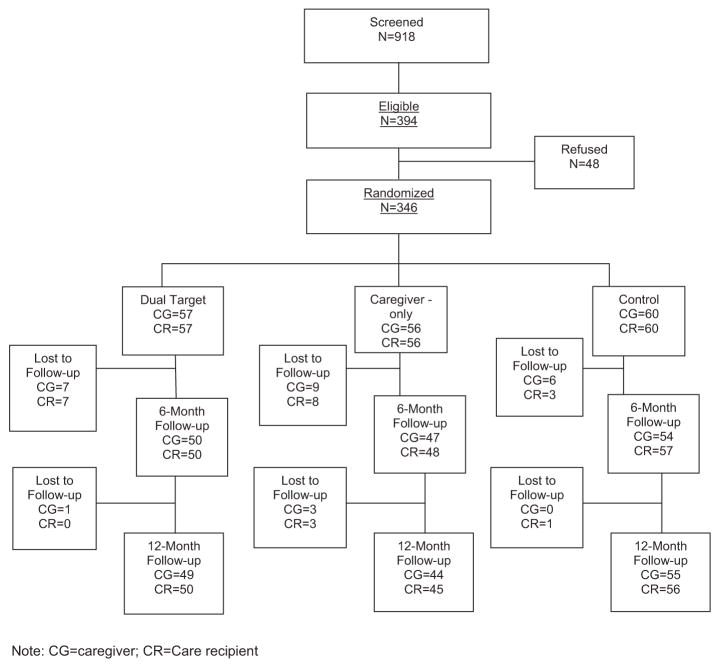
Dyads were recruited from multiple community sites and health and social service agencies in two cities, Pittsburgh, PA and Miami, FL. Extensive outreach efforts to the communities at both sites included radio and television announcements, newspaper articles and advertisements, targeted newsletters, and community presentations. Recruitment and retention results are summarized in Figure 2.
Figure 2.
Recruitment flowchart.
Procedures
Potential study participants were initially screened via telephone. If both members of the dyad were eligible for the project and expressed interest in participating, an in-home baseline assessment was conducted separately for the caregiver and the care recipient by certified assessors. Following completion of the baseline battery, participants were randomly assigned to one of the three conditions. Randomization was performed at the Miami site using a computer-generated algorithm and transmitted to the Pittsburgh site using a standard protocol. In-home follow-up assessments occurred at 6 and 12 months after baseline by certified assessors who were masked to treatment condition. Each assessment required about 90 min to complete. Participants were compensated $25 upon the completion of each assessment.
Each site obtained local Institutional Review Board approval, and both members of the dyad provided written informed consent. A Data Safety Monitoring Board met at regular intervals throughout the study to monitor adverse events associated with study participation.
Caregiver-Only Intervention
This intervention condition was designed to provide caregivers with knowledge and cognitive/behavioral skills to reduce environmental and personal stress, improve health and self-care, enhance access to formal and informal support, and improve emotional well-being. The intervention targeted five known areas of caregiver risk: lack of knowledge about caregiving and caregiver burden, social support and integration, emotional well-being, communication, self-care and physical health. Because of the variability inherent in the caregiving situation, the intervention allowed for some tailoring to meet the specific needs of the individual. The tailoring of the intervention was based on individual risk profiles obtained from the baseline assessment. All caregivers received standard information on all of the five treatment modules, but the emphasis on a particular topic varied as a function of the needs of the caregiver. For example, individuals who had high levels of depressive symptomatology (i.e., high scores on the Center for Epidemiologic Studies Depression Scale [CESD]) received a stronger dose of the emotional well-being module than individuals with few depressive symptoms. In addition, individuals had the option of selecting specific topics within modules (e.g., exercise or sleep hygiene in the self-care and physical health module). The intervention involved a range of strategies including provision of information, didactic instruction, problem solving, skills training, stress management techniques, and telephone support groups (see Table 1). These strategies were adapted from the REACH intervention program (Belle et al., 2006).
Table 1.
Summary of Intervention Goals, Content, and Activities: Dual-Target Condition
| Target area and objectives | Baseline needs assessment and intervention checklist | Exemplar topics: Caregiver | Exemplar topics: Individual with SCI | Example of intervention activities |
|---|---|---|---|---|
| Burden/knowledge and skills | Indicates a lack of knowledge about SCI or aging with SCI; providing support to someone with SCI, community resources; safety issues (care recipient— e.g., concerns about bathing/showering) | Understanding SCI and the effects of SCI | Understand SCI and the emotional effects of SCI | Provision of educational materials |
| Increase the knowledge about SCI, available resources, process of aging, caregiving, the impact of SCI on friends and family, providing support to someone with SCI | Indicates stress or difficulty with caregiving tasks or with completion of ADL/IADL tasks | Issues related to providing support for someone with SCI | Issues related to aging with SCI | Referral to menus and resource guide on screen telephone system |
| Issues related to aging with SCI | Available community resources and how to access these resources | |||
| Available community resources and how to access these resources | Understanding the impact of SCI on friends and family members | |||
|
| ||||
| Self-care and preventative health behaviors | Indicates problems with sleeping, missing medical appointments, weight gain or loss, lack of exercise, drugs/alcohol use problems | Health effects of caregiving | Health issues associated with SCI (e.g., pain, skin integrity) | Provision of educational materials such as nutrition and exercise guidelines |
| Enhance caregiver’s and the individual with SCI physical well-being and self-care behaviors | Items selected on the intervention checklist pertaining to this topic (e.g., sexuality, pain management, fatigue/weakness) | Health issues associated with SCI (e.g., pain, skin integrity) | Nutrition | Didactic instruction on the importance of nutrition and exercise |
| Nutrition | Exercise | Review of medical appointment schedule reminder (tool) | ||
| Exercise | Pain management | |||
| Medical appointments | Respiratory problems | |||
| Sleep | Sexuality | |||
| Impact of nonadherence to self-health on the caregiver | ||||
|
| ||||
| Emotional well-being | Score ≥ 8 on the CESD; indicating feeling depressed angry, stressed or not having time for engaging in pleasant activities | Health effects of stress | Health effects of stress | Provision of educational materials |
| Enhance the caregiver’s/individual with SCI emotional well-being and skills for mood and stress management | Items selected on the intervention checklist pertaining to this topic (e.g., depression and SCI; grieving; social effects of stress) | Emotional effects of stress | Depression and SCI | Didactic instruction |
| Caregiving and stress | Aging and SCI | Demonstration of strategies for engaging in pleasant events | ||
| Strategies for mood management | Grieving and SCI | Practice of strategies for relaxation (e.g., breathing exercises) | ||
| Importance of engaging in pleasant events | Strategies for mood management | |||
| Strategies for mood management | Importance of engaging in pleasant events | |||
| Strategies for relaxation | ||||
|
| ||||
| Communication | Indicates difficulty communicating with individual with SCI/caregiver; family/friends, or with health care professionals | Definition of effective communication | Definition of effective communication | Provision of educational materials |
| Enhance the caregiver’s ability to communicate with the individual with SCI, family/friends, health care professionals and ability of the individual with SCI to communication with family/friends, health care professionals | Items selected on the intervention checklist pertaining to this topic (e.g., expressing appreciation, family adjustment to SCI; how to select a personal care assistant) | Importance of effective communication | Importance of effective communication | Didactic instruction |
| Strategies for communicating with health care professionals | Good listening skills | Role playing of strategies for effective communication | ||
| Strategies for communicating with family and friends | Disability and communication | Practice of communication tips | ||
| Strategies for communicating with health care professionals | ||||
| Strategies for communicating with family and friends | ||||
|
| ||||
| Social support/integration | Indicates lack of satisfaction with instrumental, emotional or informational support or a lack of social engagement | Importance of socialization | Importance of socialization | Provision of educational materials |
| Enhance the caregivers ability to connect with others for ideas and emotional support and remain integrated with the community | Items selected on the intervention checklist pertaining to this topic (e.g., continuing education, employment assistance, volunteering) | Strategies for connecting with family/friends | Strategies for connecting with family/friends | Didactic instruction |
| Enhance the individual with SCI ability to integrate into the community, engage with family and friends | Strategies for community involvement | Strategies for community involvement | Support groups | |
| Value of support groups | Vocational rehabilitation | |||
| Job training | ||||
Note. SCI = spinal cord injury; ADL = Activities of Daily Living; IADL = Instrumental Activities of Daily Living; CESD = Center for Epidemiologic Studies Depression Scale.
Examples of specific areas covered by the caregiver intervention included management of medical (e.g., pressure sores) and behavioral issues (e.g. Activities of Daily Living dependencies); coordination of care with health care providers and service systems (e.g., rehabilitation services); enhancing support from formal and informal resources such as other family members; social isolation and relationship problems (e.g. sexuality and SCI); communication with health care providers and family/friends; stress management and relaxation techniques; and strategies to enhance caregiver health and well-being as well as that of the care recipient (e.g. exercise, making time for oneself).
The intervention was delivered over a 6-month period and consisted of seven 60- to 90-min individual sessions delivered at home (five sessions) or by telephone (two sessions) and five structured telephone-based support group sessions. Certified interventionists who had at least a bachelor’s degree in psychology or a related field conducted all sessions. The support group sessions included a facilitator and up to six caregivers and were interspersed with the intervention sessions.
Caregivers were also provided with a screen phone and a notebook that contained standard information on SCI, the aging process, caregiving and community resources, and instructions on how to use the screen phone. During the initial session, caregivers were given an introduction to the intervention, provided with the standardized packet of information, and received training on the use of intervention materials and the screen telephone system. The screen telephone system was menu driven (text and speech) and was used to facilitate the telephone support groups. The system also provided information on the intervention topics and community resources.
During the second session, interventionists reviewed caregiver responses to the baseline assessment, negotiated with the caregiver the areas of concentration for subsequent sessions, and reviewed use of the telephone system. This session also focused on issues of health and well-being in the caregiver and care recipient. Subsequent sessions were devoted to communication problems and strategies to address them, emotional well-being and stress management, and enhancing social support, respectively. Each session concluded with a review of strategies to practice and tasks to be completed in preparation for the next session. The final intervention session provided a review of the major topics that were discussed throughout the intervention program and encouraged the participants to use the skills they had learned.
Dual-Target Intervention
The dual-target intervention condition was designed to complement the caregiver-only intervention and targeted both the caregiver and the individual with SCI (care recipient, see Table 1). The caregiver component intervention was identical to that of the caregiver-only condition.
The care recipient intervention was designed to provide the individual with SCI knowledge and cognitive and behavioral skills to improve management of environmental and emotional stress, improve their health and self-care, enhance their access to formal and informal support, and improve their emotional well-being. Procedures and intervention strategies used with the caregiver were adapted for the care recipient. In addition, care recipients were taught about the potential impact of providing care to someone with SCI on the health and well-being of the caregiver. For example, the module on self-care and preventive health behaviors not only emphasized the importance of these strategies for the care recipient’s health but also ways in which they might benefit the caregiver by reducing the caregiver’s level of burden. As was the case for the caregiver, the emphasis on a particular topic was tailored to the needs of the care recipient on the basis of the baseline assessment. The intervention was delivered over 6 months and involved seven individual intervention sessions at home (five sessions) or via telephone (two sessions) using the same time schedule as outlined above. The caregiver was not present at these sessions. Five telephone support group sessions were also provided and followed the same format as the caregiver groups; they were comprised exclusively of the individuals with SCI. The care recipients also received a screen phone (if they lived in a different residence from the caregiver) and were provided with a notebook of information relevant to SCI, aging, and community resources.
Information-Only Control Group
Caregivers who were randomly assigned to the control condition received the same standardized packet of written information on SCI, the aging process, community resources and programs, and caregiving as participants randomly assigned to the active intervention conditions. In addition, three “check-in” telephone contacts were made at months 3, 5, and 9 following randomization by the certified interventionists.
Treatment Fidelity and Quality Control of Assessments
All interventionists had a bachelor’s/master’s degree in psychology, counseling, social work, or related discipline. Interventionists received intensive training that included reading materials, structured role play and practice opportunities, and three cross-site training conference calls. They were also required to submit a practice audiotape of the first intervention session and the session on emotional health for review and feedback from the site principal investigators prior to certification. Treatment implementation was monitored and maintained by weekly supervision meetings at each site and monthly cross-site conference calls. Interventionists also submitted an audiotape of an initial session and one other session for review 6 months after their initial certification. In addition, a delivery assessment form was completed after each contact with a caregiver. These forms were reviewed by the site coordinator to ensure compliance with the intervention protocol.
All assessors were required to complete a standard training protocol that included background readings, attendance at a training session, and role play of a baseline interview, which was reviewed by the site PI or project coordinator. An individual could be certified as both an assessor and interventionist; however, they were not permitted to conduct a follow-up assessments with persons to whom they were delivering the intervention.
Measures
Standard demographic data were collected on all study participants. These data along with characteristics of the spinal cord injury are reported in Table 2.
Table 2.
Caregiver and Care-Recipient Characteristics by Treatment Group
| Caregivers (n = 173) |
Care recipients (n = 173) |
|||||
|---|---|---|---|---|---|---|
| Sample characteristic (N = 346) | Dual treatment (n = 57) | Caregiver only (n = 56) | Control (n = 60) | Dual treatment (n = 57) | Caregiver only (n = 56) | Control (n = 60) |
| Age | ||||||
| M (SD) | 50.7 (14.3) | 53.7 (14.3) | 53.4 (15.8) | 53.4 (12.7) | 57.7 (12.5) | 54.4 (13.2) |
| Mdn (IQR) | 52 (41.5–61.5) | 52 (42–67.7) | 52 (43.2–61.2) | 52 (42.5–59.5) | 57.5 (47–70.2) | 53 (43.6) |
| Gender (N, %) | ||||||
| Women | 48 (84.2) | 42 (75) | 41 (68.3) | 16 (28.1) | 18 (32.1) | 27 (45.0) |
| Men | 9 (15.8) | 14 (25) | 19 (31.7) | 41 (71.9) | 38 (67.9) | 33 (55.0) |
| Education | ||||||
| Less than high school | 3 (5.3) | 2 (3.6) | 0 (0) | 3 (5.3) | 2 (3.6) | 4 (6.7) |
| High school | 13 (22.8) | 17 (30.4) | 20 (33.3) | 15 (26.3) | 10 (17.9) | 12 (20.0) |
| More than high school | 41 (71.9) | 37 (66.1) | 40 (66.7) | 39 (68.4) | 44 (78.6) | 44 (73.3) |
| Employment | ||||||
| Employed | 32 (56.1) | 23 (41.1) | 32 (53.3) | 15 (26.3) | 13 (23.2) | 13 (21.7) |
| Homemaker | 6 (10.5) | 5 (8.9) | 6 (10.0) | 1 (1.8) | 1 (1.8) | 5 (8.3) |
| Retired | 12 (21.1) | 16 (28.6) | 17 (28.3) | 15 (26.3) | 24 (42.9) | 24 (40.0) |
| Unemployed | 7 (12.3) | 12 (21.4) | 5 (8.3) | 26 (45.6) | 18 (32.1) | 18 (30.0) |
| Race | ||||||
| White | 39 (68.4) | 43 (76.8) | 46 (76.7) | 43 (75.4) | 40 (71.4) | 48 (80.0) |
| African American | 9 (15.8) | 8 (14.3) | 6 (10.0) | 6 (10.5) | 8 (14.3) | 5 (8.3) |
| Hispanic/Latino | 7 (12.3) | 5 (8.9) | 6 (10.0) | 6 (10.5) | 6 (10.7) | 5 (8.3) |
| Other | 2 (3.5) | 0 (.0) | 2 (3.3) | 2 (3.5) | 2 (3.6) | 2 (3.3) |
| Incomea | ||||||
| < 20,000 | 5 (9.3) | 9 (17.0) | 11 (19.3) | 11 (19.6) | 9 (17.6) | 16 (27.6) |
| 20,000–39,999 | 16 (29.6) | 10 (18.9) | 14 (24.6) | 14 (25.0) | 13 (25.5) | 10 (17.2) |
| 40,000–59,999 | 14 (25.9) | 14 (26.4) | 12 (21.1) | 9 (16.1) | 10 (19.6) | 9 (15.5) |
| > 60,000 | 19 (35.2) | 20 (37.7) | 20 (35.1) | 22 (39.3) | 19 (37.3) | 23 (39.7) |
| Living with care recipient | 46 (80.7) | 49 (87.5) | 45 (75) | |||
| Length of caregiving relationship in years | ||||||
| M (SD) | 8.4 (8.4) | 7.7 (7.8) | 9.0 (10.6) | |||
| Mdn (IQR) | 5.0 (2.0–12.5) | 6 (2–11) | 5.0 (2.0–13) | |||
| Relationship to participant with SCI (N, %) | ||||||
| Spouse | 38 (66.7) | 43 (76.8) | 39 (66.1) | |||
| Parent | 2 (3.5) | 1 (1.8) | 1 (1.7) | |||
| Child | 3 (5.3) | 5 (8.9) | 6 (10.2) | |||
| Other | 14 (24.6) | 7 (12.5) | 13 (21.7) | |||
| Location of SCI (N, %) (missing, n = 25) | ||||||
| Lumbar | 3 (6.5) | 2 (4.2) | 4 (7.4) | |||
| Thoracic | 14 (30.4) | 16 (33.3) | 21 (38.9) | |||
| Cervical | 29 (63.0) | 30 (62.5) | 29 (53.7) | |||
| Completeness of injury (missing, n = 26) | ||||||
| Complete | 18 (32.1) | 22 (39.3) | 23 (38.3) | |||
| Incomplete | 32 (57.1) | 26 (46.4) | 26 (43.3) | |||
| Number of ADLs caregiver helps with (range, 0–16) | ||||||
| M (SD) | 5.1 (4.2) | 4.1 (3.1) | 5.1 (4.2) | |||
| Mdn (IQR) | 5 (1–8.7) | 4 (1.2–6) | 5 (1–8.7) | |||
| Number of IADLs caregiver helps with (range, 0–8) | ||||||
| M (SD) | 3.5 (2.4) | 3.9 (2.2) | 3.5 (2.4) | |||
| Mdn (IQR) | 4 (1–5) | 4 (2–5) | 4 (1–5) | |||
Note. SCI = spinal cord injury; ADL = Activities of Daily Living; IADL = Instrumental Activities of Daily Living; IQR = interquartile range.
Sample sizes for each group reflect missing data.
Primary Outcome Measures: Caregiver and Care Recipient
A multivariate outcome comprised of six measures linked to the five components of the intervention was identified a priori as the primary outcome of the study. The multivariate outcome included measures of depressive symptoms, burden, social support, social integration, self-care, and physical health symptoms. Each of these measures is described below. With the exception of the caregiver burden measure, identical outcome measures were administered to the care recipient.
Depression
The 10-item version of the CESD (Irwin, Artin, & Oxman, 1999; Radloff, 1977) was used to assess depression in both caregivers and care recipients. Using the previous week as a reference, respondents rated each item on a scale from 0 (experienced rarely or none of the time) to 3 (experienced most or all of the time). Scores range from 0 through 30, with higher scores indicating increased presence of depressive symptoms; a score of 8 or higher (equivalent to 16 or higher on the 20-item scale) is widely interpreted as being at risk for clinical depression (Andresen, Malmgren, Carter, & Patrick, 1994; Irwin et al., 1999). Cronbach’s alpha at baseline was .76.
Caregiver burden
The brief (12-item) version of the Caregiver Burden Interview (Bedard et al., 2001; Zarit, Orr, & Zarit, 1985) was used to assess burden. Each item was rated on a 5-point scale from 0 (never) through 4 (nearly always), yielding a possible range of 0 to 48. Higher values indicate greater levels of caregiver burden. This measure was administered to caregivers only. Cronbach’s alpha at baseline was .85.
Social support and social integration
Satisfaction with social support was assessed with three items assessing instrumental, emotional, and informational support (Belle et al., 2006). For each item respondents indicated their level of satisfaction—0 (not at all), 1 (a little), 2 (moderately), or 3 (very). Scores for the three items were summed to yield a total score ranging from 0 to 9. Cronbach’s alpha at baseline was .73. Social integration was assessed with three items assessing the number of relatives, friends, neighbors, and others that “you see or hear from at least once a month, feel close to, feel you can call on for help with chores, transportation, etc.” Scores could range from 0 to 15, with higher scores reflecting greater social integration. Cronbach’s alpha at baseline was .76. We used two separate measures of social relationships because measures of support have been shown to buffer the effects of stress while measures of social integration have main effects on health outcomes (Cohen & Wills, 1985).
Self-care problems
Based on a measure used in other caregiving studies (Burton, Schulz, Jackson, Hirsch, & Zdaniuk, 2000; Wisniewski et al., 2003), self-care problems were assessed with seven items asking respondents the extent to which they had been drinking more than usual in the past month, smoking more than usual, exercising regularly, eating healthy or well-balanced meals, seeing primary care physician for routine checkups, seeing a dentist for routine checkups, and taking prescription medications appropriately. Each item had a yes or no (1, 0) response, and items were summed (and reverse scored as appropriate) such that a higher score indicated poor self-care. Cronbach’s alpha was .48, indicating that these self-care behaviors are only moderately correlated.
Physical health symptoms
Physical health symptoms were assessed with four items asking respondents how frequently (0 [never], 1 [sometimes], 2 [often]) they experienced sore throats, colds or headaches; stomach or intestinal problems; back or other muscle or joint pain; and sleeping problems. Higher scores indicate more health problems. This measure was developed and used in the REACH caregiving trial (Belle et al., 2006). We expected low to moderate associations between symptoms on this scale, and this is reflected in the Cronbach’s alpha of .54.
Statistical Analysis
We first calculated change scores for each of the outcome measures by subtracting baseline values from the 6-month and 12-month follow-up values. We examined change scores separately for caregivers, care recipients, and for the two combined (mean dyad score). Change scores were then analyzed using multivariate ANOVAs to assess differences between dual-target and caregiver-only conditions and between each of the two treatment groups and the control condition. The multivariate outcomes analyses which produced a significant omnibus F-test were followed with univariate tests examining each of the outcome variables separately. These procedures were used to test group differences among caregivers, care recipients, and caregiver–care-recipient dyads.
Methods developed for the REACH II caregiver intervention trial were also used to assess clinical significance within each outcome domain for caregivers (Belle et al., 2006). A criterion of 0.5-SD improvement from baseline to 6- and 12-month follow-ups was used to represent a clinically significant change as it represents the upper end of the distribution of effect sizes reported in the caregiving literature (Brodaty, Green, & Koschera, 2003; Schulz et al., 2002; Sorensen et al., 2002). Within each treatment group for each outcome measure, we calculated the number of individuals who improved by at least 0.5 SD from baseline to follow-up and subtracted the number of individuals who worsened by at least 0.5 SD, yielding a net improvement score expressed as the number and percent of individuals who fell into this category. By using the bootstrap method (Davison & Hinkley, 1997), we estimated the 95% confidence intervals (CIs) of the differences in number (n) and percentage (%) net improvement scores between the two treatment groups and between treatment groups and control. We used selection with equal probability and with replacement to create 1,000 bootstrap samples, from which we obtained the 95% CIs based on the percentile of distributions of n and %.
Multiple imputation methods were used to handle missing data in the analyses (Rubin, 1987). Multiple imputation is the preferred method for dealing with data that are not missing at random and avoids many of the conceptual and statistical problems of single imputation methods such as carrying forward the last observation (Schafer & Graham, 2002). Using the NORM statistical program (Schafer, 1999), we predicted missing outcome values for each participant (e.g., depressive symptoms at 12 months) from observed values on a set of background variables correlated with the outcome and the baseline value of that outcome. The program used a Markov Chain Monte Carlo technique to create five simulated data sets, which were then used to run five sets of multivariate ANOVAs. Thus, for each multivariate ANOVA, we obtained five omnibus test F-ratios. We selected the median of the five values to report and on which to base our conclusions regarding statistical significance. NORM was also used to combine the parameter estimates and standard errors from the univariate follow-up analyses using Rubin’s (1987) rules, in order to obtain significance levels for each of the three a priori contrasts (dual target vs. control, caregiver only vs. control, and dual target vs. caregiver only).
Results
Of the 918 individuals screened, 394 were eligible for participation. Forty-eight eligible participants refused participation, leaving 346 participants (173 dyads) randomized to three conditions (Figure 2). Eighty-eight percent of participants (n = 306) completed the 6-month follow-up, and 86% of participants (n = 299) completed the 12-month follow-up. Primary reasons for dropping out of the study were refusal (44%), unable to contact (29%), moving out of the area (13%), too ill (9%), and death or no longer providing support (5%). There were no statistically significant differences in dropout rates across the three groups. No significant differences were found in demographic characteristics or baseline status on outcome variables between those who completed the study and those who did not.
Demographic Characteristics and Baseline Scores
Caregivers were predominantly White women with a median age of 52 years who were often the spouse of the person with SCI. They had been providing care to the care recipient for a median of 5 years and helped with 5 of 16 Basic Activities of Daily Living and 4 of 8 Instrumental Activities of Daily Living (Katz, Ford, Moskowitz, Jackson, & Jaffe, 1963; Lawton & Brody, 1969). Persons with SCI were predominantly White men with a median age of 54. The location of SCI was lumbar (5%, with 11% complete lesions), thoracic (30%, with 53% complete lesions), or cervical (51%, with 35% complete lesions). Time since injury varied from 1 to 63 years with a median duration of 8 years (Table 2).
Within each treatment group, caregivers and SCI participants had similar demographic characteristics (Table 2) and baseline scores on the primary outcome measures (Table 3). We also compared demographic characteristics across sites and found that caregivers from the Miami site were ethnically more diverse, such that a greater number of participants from the Miami site were Hispanic. Depression scores among caregivers were generally high (Table 3) with mean scores above the risk threshold for clinical depression (8 on the 10-item CESD). For both burden and depression, caregivers had baseline values slightly higher than those observed among caregivers of Alzheimer’s patients (Belle et al., 2006). Depression levels of persons with SCI were also high with more than half of the sample reporting symptoms above the threshold for being at risk for clinical depression.
Table 3.
Mean Baseline, 12-month, and Change Scores Based on Intent-to-Treat Sample (Imputed Missing Values Included) for Caregivers, Care Recipients, and Caregiver–Care-Recipient Dyad, by Treatment Group
| Variable | Dual target (12 men, n = 49), M (SD) | Caregiver only (12 men, n = 44), M (SD) | Control (12 men, n = 55), M (SD) |
|---|---|---|---|
| Caregiver outcomes | |||
| CES-D (range 0–30) | |||
| Baseline | 9.19 (5.97) | 7.93 (4.73) | 8.94 (5.77) |
| 12 month | 7.79 (5.44) | 8.29 (4.99) | 8.53 (5.01) |
| 12 month-baselinea | −1.40 (4.48) | .36 (3.94) | −.40 (4.53) |
| Burden (range 0–48) | |||
| Baseline | 12.54 (8.74) | 11.79 (6.66) | 11.23 (7.78) |
| 12 month | 9.54 (8.95) | 11.09 (7.46) | 10.07 (7.96) |
| 12 month-baselinea | −3.01 (7.02) | −.70 (5.14) | −1.17 (5.23) |
| Self-care problems (range 0–10) | |||
| Baseline | 1.28 (1.30) | 1.56 (1.19) | 1.60 (1.34) |
| 12 month | 1.55 (1.39) | 1.54 (1.48) | 1.80 (1.44) |
| 12 month-baselinea | .27 (1.33) | −.02 (1.12) | 20 (1.21) |
| Health symptoms (range 0–8) | |||
| Baseline | 3.50 (1.81) | 3.07 (1.85) | 3.31 (1.77) |
| 12 month | 2.72 (1.87) | 2.86 (1.61) | 3.17 (1.82) |
| 12 month – baselinea | −.78 (1.39) | −.21 (1.29) | −.14 (1.29) |
| Sat with soc supp (range 0–9) | |||
| Baseline | 5.47 (2.76) | 5.46 (2.47) | 5.73 (2.46) |
| 12 month | 5.77 (2.93) | 6.29 (2.97) | 5.48 (2.89) |
| 12 month – baselinea | .30 (3.33) | .84 (2.77) | −.25 (3.03) |
| Social integration (range 0–15) | |||
| Baseline | 8.23 (3.07) | 9.30 (3.17) | 9.67 (3.14) |
| 12 month | 8.63 (2.92) | 9.09 (3.08) | 8.70 (3.07) |
| 12 month – baselinea | .40 (2.57) | −.21 (2.42) | −.97 (2.65) |
| Care-recipient outcomes | |||
| CESD (range 0–30) | |||
| Baseline | 9.84 (6.14) | 8.82 (5.65) | 9.75 (5.91) |
| 12 month | 9.35 (6.07) | 9.55 (6.63) | 10.28 (6.19) |
| 12 month – baselinea | −.49 (4.79) | .73 (4.93) | .53 (6.20) |
| Self-care problems (range 0–10) | |||
| Baseline | 1.61 (1.29) | 1.34 (1.04) | 1.45 (1.13) |
| 12 month | 1.70 (1.47) | 1.31 (1.32) | 1.54 (1.26) |
| 12 month – baselinea | .08 (1.40) | −.03 (1.07) | .10 (1.06) |
| Health symptoms (range 0–8) | |||
| Baseline | 4.16 (1.62) | 3.96 (1.64) | 4.33 (1.98) |
| 12 month | 3.84 (1.97) | 4.32 (1.96) | 4.82 (1.71) |
| 12 month – baselinea | −.32 (1.56) | .36 (1.76) | .48 (1.80) |
| Sat with soc supp (range 0–9) | |||
| Baseline | 6.72 (2.12) | 6.69 (2.12) | 6.70 (2.31) |
| 12 month | 6.38 (2.94) | 6.74 (2.25) | 6.72 (2.90) |
| 12 month – baseline | −.34 (2.71) | .05 (2.75) | .02 (3.46) |
| Social integration (range 0–15) | |||
| Baseline | 9.09 (3.69) | 9.59 (3.13) | 9.90 (2.97) |
| 12 month | 8.51 (3.62) | 9.38 (2.91) | 10.02 (2.75) |
| 12 month – baseline | −.58 (2.26) | −.21 (2.63) | .12 (2.62) |
| Caregiver– care-recipient mean dyad outcomes | |||
| CESD (range 0–30) | |||
| Baseline | 9.52 (4.71) | 8.38 (3.98) | 9.34 (4.81) |
| 12 month | 8.57 (4.69) | 8.92 (4.72) | 9.41 (4.15) |
| 12 month – baselinea | −.95 (3.40) | .54 (3.53) | .06 (4.17) |
| Self-care problems (range 0–10) | |||
| Baseline | 1.45 (1.04) | 1.45 (.83) | 1.52 (.99) |
| 12 month | 1.62 (1.14) | 1.42 (1.13) | 1.67 (1.09) |
| 12 month – baselinea | .18 (.97) | −.02 (.80) | .15 (.84) |
| Health symptoms (range 0–8) | |||
| Baseline | 3.83 (1.29) | 3.52 (1.31) | 3.82 (1.30) |
| 12 month | 3.28 (1.54) | 3.59 (1.24) | 3.99 (1.31) |
| 12 month – baselinea | −.55 (1.04) | .07 (1.19) | .17 (1.20) |
| Sat with soc supp (range 0–9) | |||
| Baseline | 6.10 (1.85) | 6.07 (1.59) | 6.21 (1.88) |
| 12 month | 6.07 (2.51) | 6.52 (2.09) | 6.10 (2.53) |
| 12 month – baselinea | −.02 (2.46) | .44 (2.15) | −.12 (2.65) |
| Social integration (range 0–15) | |||
| Baseline | 7.28 (2.43) | 7.52 (2.32) | 7.81 (2.01) |
| 12 month | 7.14 (2.55) | 7.83 (2.36) | 7.75 (2.18) |
| 12 month – baseline | −.14 (2.00) | .31 (1.99) | −.07 (2.06) |
Note. CESD = Center for Epidemiologic Studies Depression Scale; Sat with soc supp = satisfaction with social support.
Negative change scores = improvement.
Primary Outcomes
Mean baseline, 12-month follow-up, and change score values for caregivers, SCI participants, and caregiver-SCI participant dyads are reported by treatment group in Table 3. Because the rates of change at 6 months were relatively small, we do not show 6-month values.
Baseline scores
All outcomes were tested for group differences at baseline. Three multivariate ANOVA analyses were conducted comparing all three groups against each other on all caregiver, care-recipient, and mean dyad outcomes. None of the omnibus tests reached significance, indicating that the groups were not different on the multivariate outcome at baseline. This analysis was followed with closer examination of the number of individuals with baseline outcome levels above clinical threshold in each group. The proportions of individuals with outcome scores above clinical threshold were calculated for caregiver, care-recipient, and mean dyad outcomes. For the depressive symptoms measure, CESD, a cut-off of 8 points or higher was used as the clinical threshold. For other outcomes, a score more than half SD above the total sample mean (i.e., burden, self-care, and health symptoms) or below the sample mean (i.e., satisfaction with support and social integration) was used as a clinical threshold. Proportions of participants who met these criteria were compared among the three groups using the chi-square test. The dual-target group had significantly more people who met threshold criteria for mean dyad social integration than the control group (20% vs. 10%, χ2 = 5.20, p = .023) and had a higher proportion of individuals with depressive symptom scores above clinical threshold than the caregiver-only group (38% vs. 25%, χ2 = 5.55, p = .018). Baseline values for these two variables were included as covariates in the analyses of dyadic outcome changes. Inclusion of these variables as covariates did not change the pattern of significant results. Thus, for the sake of consistency, the analyses without these covariates are reported below.
Change scores
A summary of the 12-month change score analyses are reported in Table 4. All analyses of change were conducted with the intent-to-treat sample and multiple imputation. The omnibus multivariate ANOVA of caregiver outcomes yielded significant effects for 12 months when comparing the dual-target condition with the control condition, F(6, 110) = 2.48, p = .027, and univariate follow-up analysis showed that at 12 months, outcomes for dual-target condition were superior to control condition for health symptoms (p = .012) and social integration (p = .007).
Table 4.
Multivariate ANOVA and Significant Univariate F-Tests for 12-Month Outcomes by Treatment Group
| Outcomes | Dual target vs. control | Dual target vs. caregiver only | Caregiver only vs. control |
|---|---|---|---|
| Caregiver outcomes | |||
| Omnibus F-test | F6,110 =2.48; p = .027 | F6,108 = 1.34; p = .248 | F6,109 = 1.66; p = .136 |
| CESD | NS | NT | NT |
| Burden | NS | NT | NT |
| Self-care problems | NS | NT | NT |
| Health symptoms | DT > C (p = .012) | NT | NT |
| Satisfaction with support | NS | NT | NT |
| Social integration | D > C (p = .007) | NT | NT |
| Mean caregiver—care-recipient dyad outcomes | |||
| Omnibus F-test | F5,111 = 2.30; p = .049 | F5,107 = 2.53; p = .033 | F5,110 = 1.24; p = .294 |
| CESD | NS | DT > CG only (p = .014) | NT |
| Self-care problems | NS | NS | NT |
| Health symptoms | DT > C (p = .002) | DT > CG only (p = .031) | NT |
| Satisfaction with support | NS | NS | NT |
| Social integration | NS | NS | NT |
Note. NS = Not significant; NT = Not tested because omnibus ANOVA was not significant; CESD = Center for Epidemiologic Studies Depression Scale; DT > C = dual-target condition improved more than control condition; DT > CG only = dual-target condition improved more than caregiver-only condition.
We also explored outcomes for the care recipients and for caregiver-care recipient dyads as a unit. No statistically significant omnibus treatment effects were found for the care recipients, although they trended in the predicted direction; however, several statistically significant effects were found when mean values of caregiver and care recipient outcomes were calculated and used as an outcome measure. Omnibus ANOVAs were significant when the dual-target condition was compared with the control condition on 12-month changes, F(5, 111) = 2.30 p = .049. The dual-target condition was also superior to the caregiver-only condition on 12-month changes, F(5, 107) = 2.53, p = .033. Univariate follow-up analyses showed that the dual-target condition was superior to the control condition for health symptoms (p = .002), and the dual-target condition was superior to the caregiver-only condition for CESD and health symptoms (p = .014; p = .031, respectively). The effect sizes associated with omnibus ANOVAs were evaluated by partial eta-square and ranged from .12 to .14, indicating that treatment group membership explained 12–14% of the overall variance in the multivariate outcome. The effect sizes for univariate effects were evaluated using Cohen’s d and ranged from .4 to .6, indicating a medium effect size (Cohen, 1988).
Clinically Significant Effects for Caregivers Within Each Outcome Domain
Table 5 shows the numbers and proportions of caregivers with clinically significant changes of 0.5 SD or more for each of the outcome measures. Net improvement at 12 months favored the dual-target condition when compared with the caregiver-only condition for CESD (26.4% vs. −7.2%, CI = 6.5% to 57.1%), burden (36.8% vs. 5.3%, CI = 1.9% to 61.6%), and health symptoms (42.1% vs. 7.1%, CI = 7.2% to 62.7%). The dual-target condition was also superior to the control condition for burden (36.8% vs. 6.7%, CI = 2.3% to 58.7%) and health symptoms (42.1% vs. 8.3%, CI = 7.0% to 62.5).
Table 5.
Summary of Clinically Significant Caregiver Changes in Primary Outcome Measure for Caregivers by Treatment Group Between Baseline and 12-Month Follow-up
| Dual target, n = 57 |
Caregiver only, n = 56 |
Difference |
Control, n= 60 |
Differences |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diff in net impr dual target vs. caregiver-only Δnb |
Diff in net impr dual target vs. controlb |
Diff in net impr caregiver-only vs. controlb |
||||||||||
| Outcome | Improved n (%) |
Got worse n (%) |
Net impra n (%) |
Improved | Got Worse | Net impra n (%) |
Δ%; Δn (95% CI); Δ% (95% CI) |
Improved n (%) |
Got worse n (%) |
Net impra n (%) |
Δn (Δ%); Δn (95% CI); Δ% (95% CI) |
Δn (Δ%); Δn (95% CI); Δ% (95% CI) |
| CESD | 23 (40.4) | 8 (14.0) | 15 (26.4) | 13 (23.2) | 17 (30.4) | −4 (−7.2) | 19 (33.6); (3 to 33); (6.5 to 57.1) | 17 (28.3) | 15 (25.0) | 2 (3.3) | 13 (25.1); (−2 to 28); (−3 to 48.7) | −6 (−10.5); (−21 to 9); (−35.7 to 16.1) |
| Burden | 30 (52.6) | 9 (15.8) | 21 (36.8) | 20 (35.7) | 17 (30.4) | 3 (5.3) | 18 (31.5); (0 to 35); (1.9 to 61.6) | 19 (31.7) | 15 (25.0) | 4 (6.7) | 17 (30.1); (0 to 33); (2.3 to 58.7) | −1 (−1.4); (−17 to 13); (−28.3 to 24.7) |
| Self-care | 16 (28.1) | 20 (35.1) | −4 (−7.0) | 18 (32.1) | 13 (23.2) | 5 (8.9) | −9 (−15.9); (−26 to 7); (−43.5 to 13.2) | 12 (20.0) | 21 (35.0) | −9 (−15.0) | 5 (8.0); (−12 to 21); (−20.3 to 36.5) | 14 (23.9); (−2 to 29); (−2.7 to 51.7) |
| Health symptoms | 31 (54.4) | 7 (12.3) | 24 (42.1) | 19 (33.9) | 15 (26.8) | 4 (7.1) | 20 (35.0); (2 to 33); (7.2 to 62.7) | 20 (33.3) | 15 (25.0) | 5 (8.3) | 19 (35.8)*; (2 to 35); (7.0 to 62.5) | −1 (−1.2); (−17 to 15); (−26.9 to 28.7) |
| Sat with support | 20 (35.1) | 14 (24.6) | 6 (10.5) | 19 (33.9) | 10 (17.9) | 9 (16.0) | −3 (−5.5); (−19 to 12); (−31.3 to 21.9) | 13 (21.7) | 17 (28.5) | −4 (−6.8) | 10 (17.3); (−6 to 25); (−9.1 to 43.9) | 13 (22.8); (−3 to 27); (−3.1 to 48.3) |
| Soc net density | 16 (28.1) | 17 (29.8) | −1 (−1.7) | 13 (23.2) | 14 (25.0) | −1 (−1.8) | 0 (0.1); (−22 to 5); (−46.2 to 11.3) | 10 (16.7) | 22 (36.7) | −12 (−20.0) | 11 (18.3); (−5 to 26); (−7.6 to 44.4) | 11 (18.2); (−4 to 26); (−5.8 to 43.8) |
Note. Statistically significant effects are in bold. CESD = Center for Epidemiologic Studies Depression Scale; Sat with support = satisfaction with social support; Soc net density = social network density; CI = confidence interval. Clinically significant improvement and worsening was defined as an unadjusted standardized change of ± 0.5 SD or more from baseline to follow-up.
Net impr = participants who improved − participants who worsened.
Diff in net impr = net improvement in intervention group − net improvement in comparison group. Δn = difference in number; Δ% = difference in percentage.
Site Effects
We also assessed differences in outcomes among participants recruited in Pittsburgh, PA, versus those recruited in the Miami, FL, area. We tested for main effects in outcomes by site and the interactions between treatment and site. We found one significant site by treatment interaction showing that 12-month difference in the multivariate outcome between the caregiver-only versus control condition was greater in Miami than in Pittsburgh, F(6, 107) = 2.49, p < .027. Univariate analyses showed that this effect was due to greater improvements in self-care and in depressive symptoms in Miami—F(1,112) = 5.94, p < .016; F(1, 112) = 4.23, p < .042, respectively—when compared to Pittsburgh.
Gender and Interventionist Effects
We tested for differences in gender distributions across the three groups and found no significant group effect.
In order to check for potential interventionist effects, we tested for both main effects and interaction effects for interventionists using a procedure in which each interventionist was dummy coded and then entered as a covariate in multivariate ANOVAs comparing treatment groups on primary outcomes. There were eight interventionists (four at each site) delivering intervention sessions. None of the covariate main effects or interactions with treatment was significant.
Exposure to Treatment Effects
Overall, there was little variability in exposure to treatment. A large majority of participants randomized to intervention conditions participated in all seven intervention sessions (80% of caregivers and 86% of persons with SCI). Among caregivers, 5% missed three or fewer sessions and 15% missed four or more sessions. Among care recipients, 5% missed three or fewer and 9% missed four or more sessions. Comparing participants who completed all sessions with those who did not yielded no statistically significant differences in outcomes for caregivers or persons with SCI.
Discussion
The unique challenges and health effects of caring for an individual with SCI have been documented in numerous studies, and our findings are consistent with those of earlier studies showing that SCI caregivers report high levels of depression and burden. To our knowledge, this is the first large-scale randomized clinical trial designed to improve the health and well-being of SCI caregivers. Two multicomponent interventions were compared with each other and with a control condition. The interventions were designed to maximize outcomes in multiple domains and allowed for tailoring to respond to individual variation in need. The caregiver-only intervention focused solely on the caregiver whereas the dual-target intervention focused on both the caregiver and the person with SCI. We predicted that both interventions would be superior to the control condition and that the dual-target condition would be superior to the caregiver-only condition. The pattern of results only partially supported our predictions. Overall, the dual-target condition clearly improved the quality of life of the caregiver as well as the dyad, but no significant effects were found for the caregiver-only condition. We focused on 12-month outcomes because few changes were observed at 6 months. The latter finding is consistent with other studies demonstrating that intervention effects sometimes grow stronger over time (Scheier et al., 2005).
At 12 months, the dual-target condition was superior to the control condition on our multivariate outcome. Follow-up univariate analyses showed that caregivers in the dual-target condition reported significantly fewer health symptoms and were more socially integrated than control caregivers. Although the direction of effects favored the caregiver-only condition when compared to the control condition, the multivariate outcome for this comparison was not statistically significant. Similarly, the comparison between dual-target and caregiver-only did not yield a statistically significant difference on our multivariate outcome, although the direction of effects favored the dual-target condition.
To further test the hypothesis that the dual-target intervention is superior to the caregiver-only condition, we performed additional analyses in which the dyad was the unit of analysis. We reasoned that complementary treatments targeting both the caregiver and the individual with SCI should have synergistic effects resulting in more positive outcomes for the dyad as a whole. The findings from these analyses supported this prediction. The dual-target condition was superior to both the control condition and the caregiver-only condition in our multivariate outcome analysis. Dyads enrolled in the dual-target condition had significantly fewer health symptoms than control condition participants and were less depressed and had fewer health symptoms when compared to participants in the caregiver-only condition. The consistent positive impact of the intervention on health symptoms suggests that this component of our intervention which provided instruction and tools to enhance self-care and preventive health behaviors was particularly effective.
To our knowledge, this is the first caregiver intervention study in which dyads are used as the unit of analysis. We believe this analytic approach provides compelling evidence for observing the beneficial effects of the intervention on the unit as a whole as opposed to a single individual. This approach also helps to account for—and in this case, rule out— the possibility that an intervention benefiting the caregiver may at the same time be detrimental to the care recipient.
The benefits of the dual-target condition were also demonstrated when clinical significance was used as the outcome criterion. A higher proportion of caregivers in the dual-target condition had clinically significant improvements in depression, burden, and health symptoms when compared with the caregiver-only condition. Similar, although slightly weaker, effects were found for the comparison between dual-target and control condition.
Taken together, these findings suggest three conclusions. First, the dual-target condition was an effective strategy for improving the quality of lives of the caregiver and the dyad as a whole. This is an encouraging finding as it opens the door to new treatment strategies that can be applied to caregivers and care recipients simultaneously. By definition, caregiving studies focus on the caregiver; the dual-target approach reminds us that there may be important benefits to including the care recipient as well. The dual-target approach acknowledges the interactive and reciprocal nature of caregiver and care recipient outcomes and encourages us to develop treatment strategies that acknowledge the important influence of dyadic interactions on both caregiver and patient outcomes. Second, the fact that we obtained no significant effects for the caregiver-only condition raises questions about the efficacy of this approach for this caregiver population. In part, these negative findings may be due to issues of power as most of the effects trended in the predicted direction but did not reach statistical significance. Because of the long duration of their caregiving careers and the unique challenges they face, caregivers of persons with spinal cord injury may require more intensive and extraordinary interventions than caregivers of older individuals with more common age-related disabilities. Third, as we move toward more complex intervention strategies that involve multiple targets, we will need to expand our strategies for outcomes assessment. Caregiver intervention studies assume but rarely test the hypotheses that the intervention benefits accrue to both members of the dyad. Examining care recipient outcomes separately from caregiver outcomes is not the same as examining linked outcomes at the dyad level. For example, in this study we found virtually no impact of the interventions on care recipients as a group; however, we found multiple effects of treatment when we combined SCI participant scores with those of their caregiver. The pattern of results were consistent with those observed for caregivers only but were generally stronger, suggesting that both members of the dyad were contributing to the positive outcomes.
There are several clinical implications of this study. Our results clearly show that caregivers of persons with SCI are in need of and can benefit from interventions that help them manage the medical and functional limitations of the care recipient, enhance support from formal and informal resources, reduce social isolation, and encourage them to monitor and improve their own health and well-being. Inasmuch as caregiving is a highly interactive endeavor in which caregivers and care recipients mutually affect each other, it is critical that both members of the dyad be involved in clinical interventions. This study provides intervention strategies that can be useful in achieving this goal, but even these methods can be improved upon. Our recent work on the effects of suffering in the caregiving context suggests that clinical interventions that provide the caregiver with tools for addressing care recipient suffering may be particularly effective in achieving positive outcomes for both caregivers and care recipients (Schulz, Boerner, Shear, Zhang, & Gitlin, 2006; Schulz et al., 2008; Schulz et al., in press).
The materials and protocols developed for our study can feasibly be adapted for wide-scale implementation in community settings by individuals with a background in psychology, rehabilitation counseling, social work, nursing, occupational therapy, or other related disciplines. Future research should focus on identifying strategies for adapting our intervention for implementation at the community level within the existing health, rehabilitation, and aging services networks. Future availability of community-based intervention services modeled after this one would be a valuable referral resource for family caregivers and their care recipients.
Our study has several limitations. The fact that intervention effects increased over time suggests that it takes time for their impact to incubate. It would have been useful to follow study participants beyond the 1-year follow-up to assess long-term effects of treatment. Also, although more than 20% of our sample was comprised of minority individuals, their number was not large enough to enable analyses by subgroups. Other caregiver studies (Belle et al., 2006) have shown that intervention effects vary by the race/ethnicity of the target individuals. An important follow up to this study should assess treatment effects in different racial/ethnic groups. It is important to remember as well that our findings apply to individuals with SCI who are on average 8 years after injury; our results do not necessarily generalize to the acute phase aftermath of SCI. Finally, some researchers might question our choice of the control condition. The most appropriate control condition for the type of intervention that we used is under considerable debate. Options include treatment as usual, minimal support, or attention control. For ethical reasons and to motivate control participants to remain in the study, we offered participants something minimal but meaningful, and thus opted for a minimal support control condition in which caregivers received basic written educational material and three brief telephone calls. Future studies might evaluate our intervention with larger samples and some form of attention control group. Despite these limitations, this study is an important first step in advancing the science of intervention research with caregivers of persons with SCI.
Acknowledgments
Preparation of this manuscript was in part supported by grants from NINR (NR08272, NR09573), NIA (AG15321, AG026010), NIMH (MH071944), NCMHD (MD000207), NHLBI (HL076852, HL076858), and the NSF (EEEC-0540856).
Contributor Information
Richard Schulz, Department of Psychiatry and University Center for Social and Urban Research, University of Pittsburgh.
Sara J. Czaja, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine
Amy Lustig, University Center for Social and Urban Research, University of Pittsburgh.
Bozena Zdaniuk, University Center for Social and Urban Research, University of Pittsburgh.
Lynn M. Martire, Department of Psychiatry and University Center for Social and Urban Research, University of Pittsburgh
Dolores Perdomo, Department of Psychiatry and Behavioral Sciences, University of Miami Miller School of Medicine.
References
- Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) American Journal of Preventive Medicine. 1994;10:77–84. [PubMed] [Google Scholar]
- Bedard M, Molloy DW, Squire L, Dubois S, Lever JA, O’Donnell M. The Zarit Burden Interview: A new short version and screening version. The Gerontologist. 2001;41:652–657. doi: 10.1093/geront/41.5.652. [DOI] [PubMed] [Google Scholar]
- Belle SH, Burgio L, Burns R, Coon D, Czaja SJ, Gallagher-Thompson D, et al. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups. Annals of Internal Medicine. 2006;145:727–738. doi: 10.7326/0003-4819-145-10-200611210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookwala J, Schulz R. Spousal similarity in subjective well-being. The cardiovascular health study. Psychology and Aging. 1996;11:582–591. doi: 10.1037//0882-7974.11.4.582. [DOI] [PubMed] [Google Scholar]
- Boschen KA, Tonack M, Gargaro J. The impact of being a support provider to a person living in the community with a spinal cord injury. Rehabilitation Psychology. 2005;50:397–407. [Google Scholar]
- Brodaty H, Green A, Koschera A. Meta-analysis of psychosocial interventions for caregivers of people with dementia. Journal of the American Geriatrics Society. 2003;51:657–664. doi: 10.1034/j.1600-0579.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- Burton L, Schulz R, Jackson S, Hirsch C, Zdaniuk B. Transitions in spousal caregiving: Experience from the Caregiver Health Effects Study (CHES) The Gerontologist. 2000;40:73. doi: 10.1093/geront/43.2.230. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum.; 1988. [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98:310–357. [PubMed] [Google Scholar]
- Davison AC, Hinkley DV. Bootstrap methods and their application. United Kingdom: Cambridge University Press.; 1997. [Google Scholar]
- Dreer LE, Elliott TR, Shewchuk R, Berry JW, Rivera P. Family caregivers of persons with spinal cord injury: Predicting caregivers at risk for probable depression. Rehabilitation Psychology. 2007;52:351–357. doi: 10.1037/0090-5550.52.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisdorfer CE, Czaja SJ, Loewenstein DL, Rubert MP, Arguelles S, Mitrani V, et al. The effect of a family therapy and technology-based intervention on caregiver depression. The Gerontologist. 2003;43:521–531. doi: 10.1093/geront/43.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T, Berry JW. Brief problem-solving training for family caregivers of persons with recent-onset spinal cord injuries: A randomized controlled trial. Journal of Clinical Psychology. doi: 10.1002/jclp.20527. (in press) [DOI] [PubMed] [Google Scholar]
- Elliott T, Shewchuk R. Recognizing the family caregiver: Integral and formal members of the rehabilitation process. Journal of Vocational Rehabilitation. 1998;10:123–132. [Google Scholar]
- Elliott T, Shewchuk R, Richards J. Family caregiver social problem-solving abilities and adjustment during the initial year of the caregiving role. Journal of Counseling Psychology. 2001;48:223–232. [Google Scholar]
- Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: Criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D) Archives of Internal Medicine. 1999;159:1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. Journal of the American Medical Association. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- Lucke KT, Coccia H, Goode JS, Lucke JF. Quality of life in spinal cord injured individuals and their caregivers during the initial 6 months following rehabilitation. Quality of Life Research. 2004;13:97–110. doi: 10.1023/B:QURE.0000015284.95515.17. [DOI] [PubMed] [Google Scholar]
- Martire LM, Lustig AP, Schulz R, Miller GE, Helgeson VS. Is it beneficial to involve a family member? A meta-analytic review of psychosocial interventions for chronic illness. Health Psychology. 2004;23:599–611. doi: 10.1037/0278-6133.23.6.599. [DOI] [PubMed] [Google Scholar]
- Martire LM, Schulz R. Involving family in psychosocial interventions for chronic illness. Current Directions in Psychological Science. 2007;16:90–94. [Google Scholar]
- Pinquart M, Sorenson S. Differences between caregivers and non-caregivers in psychological health and physical health: A meta-analysis. Psychology and Aging. 2003;18:250–267. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rodgers ML, Strode AD, Norell DM, Short RA, Dyck DG, Becker B. Adapting multiple-family group treatment for brain and spinal cord injury intervention development and preliminary outcomes. American Journal of Physical Medicine and Rehabilitation. 2007;86:482–492. doi: 10.1097/PHM.0b013e31805c00a1. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Multiple imputation for nonresponse in surveys. New York, NY: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- Schafer JL. Multiple imputation: A primer. Statistical Methods in Medical Research. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Scheier MF, Helgeson VS, Schulz R, Colvin S, Berga S, Bridges MW, et al. Interventions to enhance physical and psychological functioning among younger women who are ending nonhormonal adjuvant treatment for early stage breast cancer. Journal of Clinical Oncology. 2005;23:4298–4311. doi: 10.1200/JCO.2005.05.362. [DOI] [PubMed] [Google Scholar]
- Schulz R. Handbook on dementia caregiving: Evidence-based interventions for family caregivers. New York, NY: Springer.; 2000. [Google Scholar]
- Schulz R, Beach S. Caregiving as a risk factor for mortality. The caregiver health effects study. Journal of the American Medical Association. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- Schulz R, Beach SR, Hebert R, Martire LM, Monin JK, Tompkins CA, et al. Spousal suffering and partner’s depression and cardiovascular disease: The Cardiovascular Health Study. American Journal of Geriatric Psychiatry. doi: 10.1097/JGP.0b013e318198775b. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Boerner K, Shear K, Zhang S, Gitlin LN. Predictors of complicated grief among dementia caregivers: A prospective study of bereavement. American Journal of Geriatric Psychiatry. 2006;14:650–658. doi: 10.1097/01.JGP.0000203178.44894.db. [DOI] [PubMed] [Google Scholar]
- Schulz R, Martire L, Klinger J. Evidence-based caregiver interventions in geriatric psychiatry. Psychiatric Clinics of North America. 2005;28:1007–1038. doi: 10.1016/j.psc.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Schulz R, Martire LM. Family caregiving in persons with dementia: Prevalence, health effects, and support strategies. American Journal of Geriatric Psychiatry. 2004;12:240–249. [PubMed] [Google Scholar]
- Schulz R, McGinnis KA, Zhang S, Martire LM, Hebert RS, Beach SR, et al. Dementia patient suffering and caregiver depression. Alzheimer Disease and Associated Disorders. 2008;22:170–176. doi: 10.1097/WAD.0b013e31816653cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Newsom JT, Fleissner K, deCamp AR, Nieboer AP. The effects of bereavement after family caregiving. Aging and Mental Health. 1997;1:269–282. [Google Scholar]
- Schulz R, O’Brien AT, Bookwala J, Fleissner K. Psychiatric and physical morbidity effects of Alzheimer’s Disease caregiving: Prevalence, correlates, and causes. The Gerontologist. 1995;35:771–791. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- Schulz R, O’Brien A, Czaja S, Ory M, Norris R, Martire LM, et al. Dementia caregiver intervention research: in search of clinical significance. The Gerontologist. 2002;42:589–602. doi: 10.1093/geront/42.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Quittner AL. Caregiving through the life-span: Overview and Future Directions. Health Psychology. 1998;17:107–111. [PubMed] [Google Scholar]
- Schulz R, Williamson GM. Psychosocial and behavioral dimensions of physical frailty. Journals of Gerontology (Special Issue on Physical Frailty: A Treatable Cause of Dependence in Old Age) 1993;48:39 – 43. doi: 10.1093/geronj/48.special_issue.39. [DOI] [PubMed] [Google Scholar]
- Selwood A, Johnston K, Katona C, Lyketsos C, Livingston G. Systematic review of the effect of psychological interventions on family caregivers of people with dementia. Journal of Affective Disorders. 2007;101:75–89. doi: 10.1016/j.jad.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Sheija A, Manigandan C. Efficacy of support groups for spouses of patients with spinal cord injury and its impact on their quality of life. International Journal of Rehabilitation Research. 2005;28:379–383. doi: 10.1097/00004356-200512000-00015. [DOI] [PubMed] [Google Scholar]
- Shewchuk RM, Richards JS, Elliott TR. Dynamic processes in health outcomes among caregivers of patients with spinal cord injuries. Health Psychology. 1998;17:125–129. doi: 10.1037//0278-6133.17.2.125. [DOI] [PubMed] [Google Scholar]
- Sorensen S, Pinquart M, Duberstein P. How effective are interventions with caregivers? An updated meta-analysis. The Gerontologist. 2002;42:356–372. doi: 10.1093/geront/42.3.356. [DOI] [PubMed] [Google Scholar]
- Weitzenkamp DA, Gerhart KA, Charlifue SW, Whiteneck GG, Savic G. Spouses of spinal cord injury survivors: The added impact of caregiving. Archives of Physical Medicine and Rehabilitation. 1997;78:822–827. doi: 10.1016/s0003-9993(97)90194-5. [DOI] [PubMed] [Google Scholar]
- Wisniewski S, Belle SH, Coon DW, Marcus SM, Ory MG, Burgio L, et al. The Resources for Enhancing Alzheimer’s Caregiver Health (REACH): Project design and baseline characteristics. Psychology and Aging. 2003;18:375–384. doi: 10.1037/0882-7974.18.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarit SH, Orr NK, Zarit JM. The hidden victims of Alzheimer’s disease: Families under stress. New York, NY: University Press; 1985. [Google Scholar]