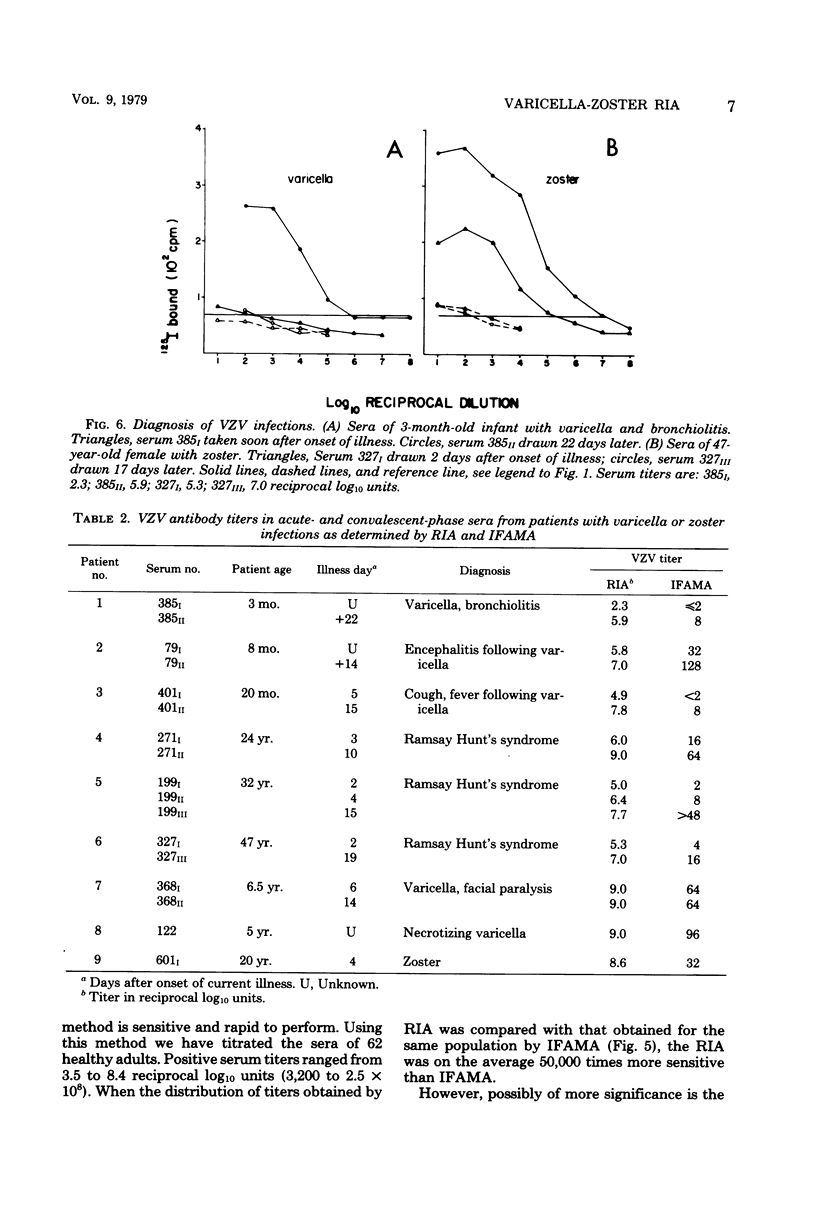
Abstract
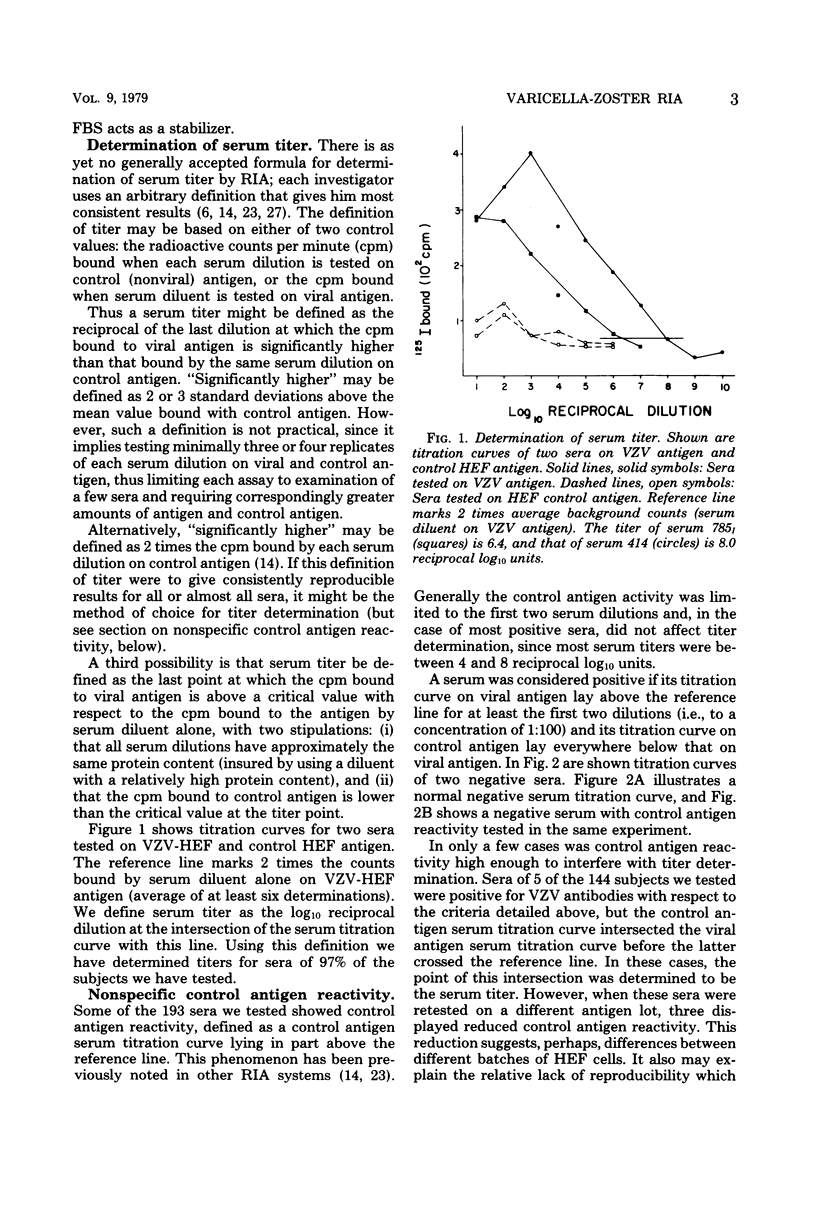
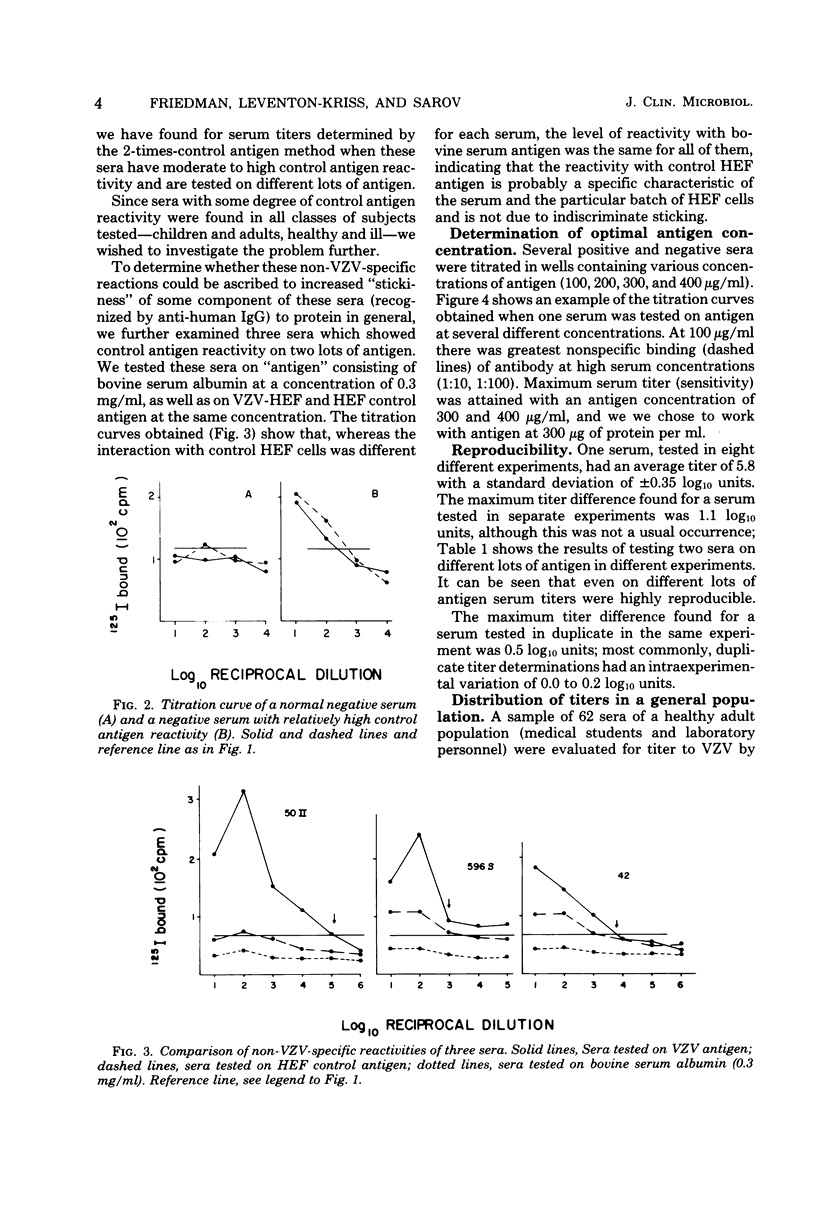
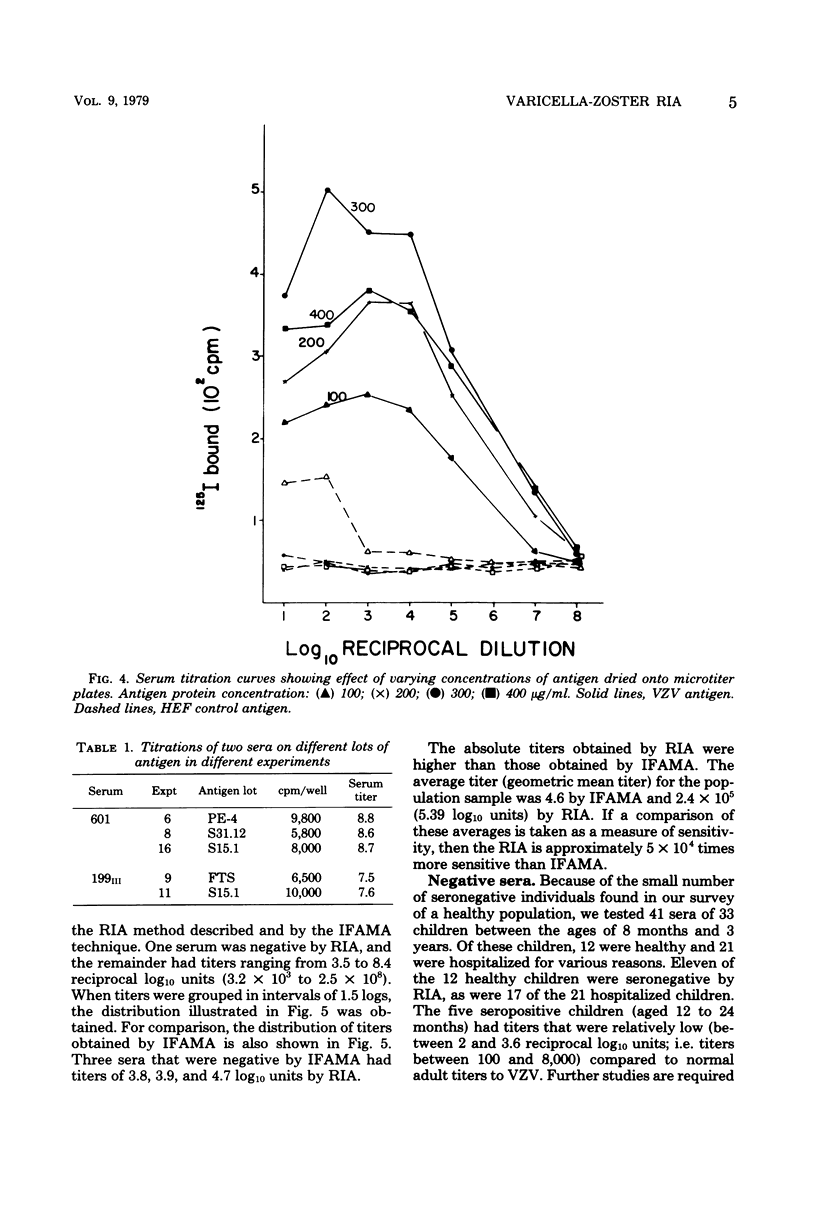
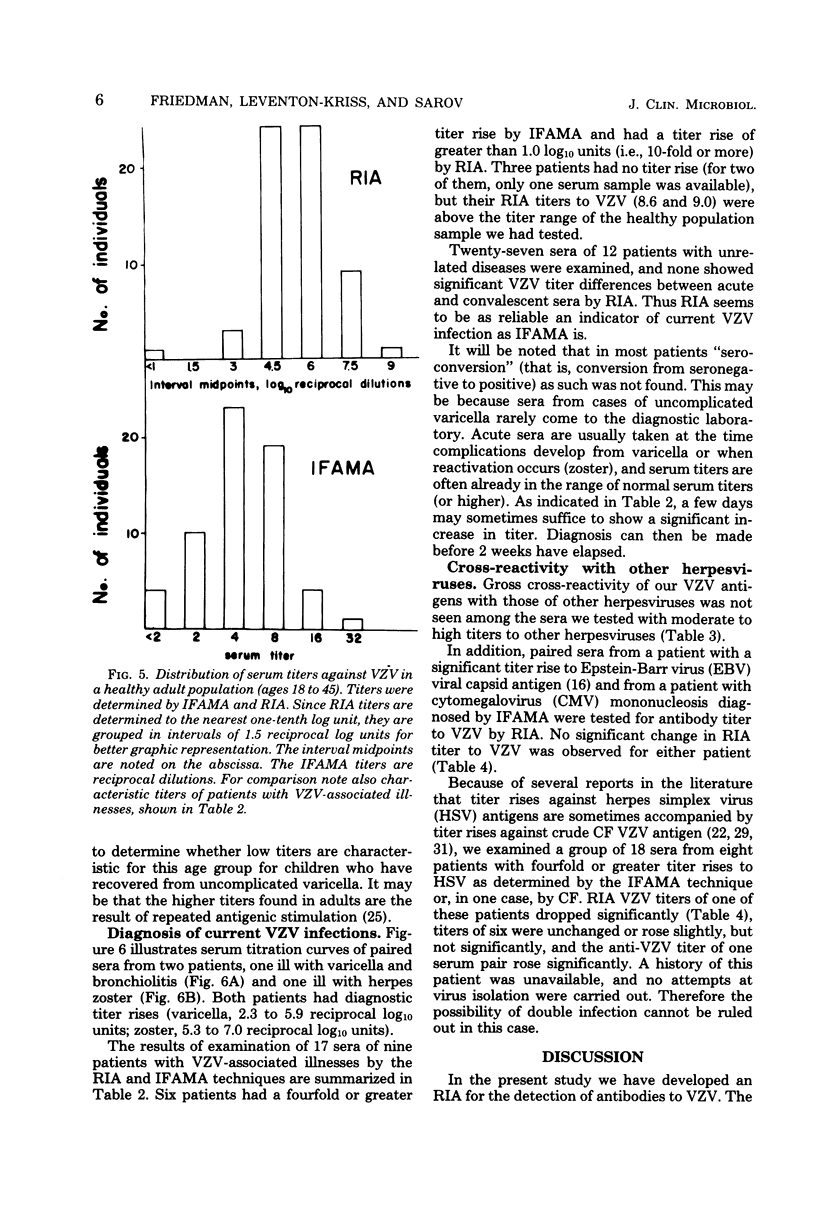
A sensitive solid-phase radioimmunoassay for detection of antibodies to varicella-zoster virus (VZV) is described. The antigen consisted of a sonically disrupted extract of VZV-infected human embryo cells. 125I-labeled rabbit anti-human immunoglobulin G (IgG) specific for the Fc portion of human IgG was used to detect human IgG bound to viral antigen. With this technique, 193 human sera were evaluated for their IgG antibody titer against ZVZ. Subjects included 62 healthy adults, 33 young children (12 healthy), and 49 patients. Titers obtained by the radioimmunoassay were compared with those obtained by indirect fluoresence antibody staining of membrane antigen. The radioimmunoassay technique described gave titers approximately 5 X 10(4) times higher than those shown by indirect fluorescence. It can be used for routine diagnosis, but is especially suited to determining immune status to VZV, as defined by presence or absence of antibodies to the virus; for epidemiological studies; or for determining patients at risk who are exposed to the virus. No heterotypic titer rises to VZV were observed in sera with fourfold or greater rises to Epstein-Barr virus or cytomegalovirus. Sera of eight subjects with fourfold or greater titer rises to herpes simplex virus reacted in various ways: in six cases no significant change occurred in titer to VZV; one had a significant decrease in titer by the radioimmunoassay; and one had a significant increase. Possible reasons for these titer changes are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein A. M., Donovan J. W. Malignant disease in children whose mothers had chickenpox, mumps, or rubella in pregnancy. Br Med J. 1972 Dec 16;4(5841):629–631. doi: 10.1136/bmj.4.5841.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arstila P., Vuorimaa T., Kalimo K., Halonen P., Viljanen M., Granfors K., Toivanen P. A solid-phase radioimmunoassay for IgG and IgM antibodies against measles virus. J Gen Virol. 1977 Jan;34(1):167–176. doi: 10.1099/0022-1317-34-1-167. [DOI] [PubMed] [Google Scholar]
- Asano Y., Nakayama H., Yazaki T., Ito S., Isomura S. Protective efficacy of vaccination in children in four episodes of natural varicella and zoster in the ward. Pediatrics. 1977 Jan;59(1):8–12. [PubMed] [Google Scholar]
- Babiuk L. A., Acres S. D., Rouse B. T. Solid-phase radioimmunoassay for detecting bovine (neonatal calf diarrhea) rotavirus antibody. J Clin Microbiol. 1977 Jul;6(1):10–15. doi: 10.1128/jcm.6.1.10-15.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bithell J. F., Draper G. J., Gorbach P. D. Association between malignant disease in children and maternal virus infections. Br Med J. 1973 Mar 24;1(5855):706–708. doi: 10.1136/bmj.1.5855.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechschmidt M., Gerlich W., Thomssen R. Radioimmunoassay for LCM virus antigens and anti-LCM virus antibodies and its application in an epidemiologic survey of people exposed to syrian hamsters. Med Microbiol Immunol. 1977 May 18;163(1):67–76. doi: 10.1007/BF02126711. [DOI] [PubMed] [Google Scholar]
- Bradley D. W., Maynard J. E., Hindman S. H., Hornbeck C. L., Fields H. A., McCaustland K. A., Cook E. H., Jr Serodiagnosis of viral hepatitis A: detection of acute-phase immunoglobulin M anti-hepatitis A virus by radioimmunoassay. J Clin Microbiol. 1977 May;5(5):521–530. doi: 10.1128/jcm.5.5.521-530.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunell P. A. Commentary: protection against varicella. Pediatrics. 1977 Jan;59(1):1–2. [PubMed] [Google Scholar]
- Brunell P. A., Gershon A. A. Passive immunization against varicella-zoster infections and other modes of therapy. J Infect Dis. 1973 Apr;127(4):415–423. doi: 10.1093/infdis/127.4.415. [DOI] [PubMed] [Google Scholar]
- Brunell P. A. Varicella-zoster virus vaccine. JAMA. 1978 Mar 13;239(11):1034–1035. [PubMed] [Google Scholar]
- Daugharty H., Warfield D. T., Davis M. L. Solid-phase radioimmunoassay of total and influenza-specific immunoglobulin G. Appl Microbiol. 1972 Feb;23(2):360–367. doi: 10.1128/am.23.2.360-367.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugharty H., Warfield D. T., Hemingway W. D., Casey H. L. Mumps class-specific immunoglobulins in radioimmunoassay and conventional serology. Infect Immun. 1973 Mar;7(3):380–385. doi: 10.1128/iai.7.3.380-385.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forghani B., Schmidt N. J., Lennette E. H. Sensitivity of a radioimmunoassay method for detection of certain viral antibodies in sera and cerebrospinal fluids. J Clin Microbiol. 1976 Dec;4(6):470–478. doi: 10.1128/jcm.4.6.470-478.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Achilli G., Chambers R. W. Determination of neutralizing antibody and IgG antibody to varicella-zoster virus and of IgG antibody to membrane antigens by the immunoperoxidase technique. J Infect Dis. 1977 Jun;135(6):975–979. doi: 10.1093/infdis/135.6.975. [DOI] [PubMed] [Google Scholar]
- Gotlieb-Stematsky T., Vonsover A., Modan M. Epstein-Barr virus and heterophile antibodies in infectious mononucleosis. Isr J Med Sci. 1974 Jul;10(7):731–737. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hutchinson H. D., Ziegler D. W., Feorino P. M. Radioimmunoassay for detection of antibodies to Epstein-Barr virus in human infectious mononucleosis serum specimens. J Clin Microbiol. 1975 May;1(5):429–433. doi: 10.1128/jcm.1.5.429-433.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPSENBERG J. G. POSSIBLE ANTIGENIC RELATIONSHIP BETWEEN VARICELLA ZOSTER VIRUS AND HERPES SIMPLEX VIRUS. Arch Gesamte Virusforsch. 1964 Sep 9;15:67–73. doi: 10.1007/BF01241422. [DOI] [PubMed] [Google Scholar]
- Kalimo K. O., Marttila R. J., Granfors K., Viljanen M. K. Solid-phase radioimmunoassay of human immunoglobulin M and immunoglobulin G antibodies against herpes simplex virus type 1 capsid, envelope, and excreted antigens. Infect Immun. 1977 Mar;15(3):883–889. doi: 10.1128/iai.15.3.883-889.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalimo K. O., Meurman O. H., Halonen P. E., Ziola B. R., Viljanen M. K., Granfors K., Toivanen P. Solid-phase radioimmunoassay of rubella virus immunoglobulin G and immunoglobulin M antibodies. J Clin Microbiol. 1976 Aug;4(2):117–123. doi: 10.1128/jcm.4.2.117-123.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalimo K. O., Ziola B. R., Viljanen M. K., Granfors K., Toivanen P. Solid-phase radioimmunoassay of herpes simplex virus IgG and IgM antibodies. J Immunol Methods. 1977;14(2):183–195. doi: 10.1016/0022-1759(77)90008-4. [DOI] [PubMed] [Google Scholar]
- Knez V., Stewart J. A., Ziegler D. W. Cytomegalovirus specific IgM and IgG response in humans studied by radioimmunoassay. J Immunol. 1976 Nov;117(5 PT2):2006–2013. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lander J. J., Alter H. J., Purcell R. H. Frequency of antibody to hepatitis-associated antigen as measured by a new radioimmunoassay technique. J Immunol. 1971 May;106(5):1166–1171. [PubMed] [Google Scholar]
- Leventon-Kriss S., Yoffe R., Rannon L., Modan M. Seroepidemiologic aspects of varicella zoster virus infections in an Israeli Jewish population. Isr J Med Sci. 1978 Jul;14(7):766–770. [PubMed] [Google Scholar]
- Parkinson A. J., Kalmakoff J. Detection of virus-specific immunoglobulins using a doubly labeled fluorescein-125I antibody. J Clin Microbiol. 1976 Jun;3(6):637–639. doi: 10.1128/jcm.3.6.637-639.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS A. H. Modification of chicken pox in family contacts by administration of gamma globulin. N Engl J Med. 1962 Aug 23;267:369–376. doi: 10.1056/NEJM196208232670801. [DOI] [PubMed] [Google Scholar]
- Ross C. A., Subak Sharpe J. H., Ferry P. Antigenic relationship of varicella-zoster and herpes simplex. Lancet. 1965 Oct 9;2(7415):708–711. doi: 10.1016/s0140-6736(65)90452-6. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H., Magoffin R. L. Immunological relationship between herpes simplex and varicella-zoster viruses demonstrated by complement-fixation, neutralization and fluorescent antibody tests. J Gen Virol. 1969 Apr;4(3):321–328. doi: 10.1099/0022-1317-4-3-321. [DOI] [PubMed] [Google Scholar]
- Vianna N. J., Polan A. K. Childhood lymphatic leukemia: prenatal seasonality and possible association with congenital varicella. Am J Epidemiol. 1976 Mar;103(3):321–332. doi: 10.1093/oxfordjournals.aje.a112230. [DOI] [PubMed] [Google Scholar]
- Williams V., Gershon A., Brunell P. A. Serologic response to varicella-zoster membrane antigens measured by direct immunofluorescence. J Infect Dis. 1974 Dec;130(6):669–672. doi: 10.1093/infdis/130.6.669. [DOI] [PubMed] [Google Scholar]
- Williamson A. P. The varicella-zoster virus in the etiology of severe congenital defects. A survey of eleven reported instances. Clin Pediatr (Phila) 1975 Jun;14(6):553-5, 558-9. doi: 10.1177/000992287501400607. [DOI] [PubMed] [Google Scholar]
- Zaia J. A., Oxman M. N. Antibody to varicella-zoster virus-induced membrane antigen: immunofluorescence assay using monodisperse glutaraldehyde-fixed target cells. J Infect Dis. 1977 Oct;136(4):519–530. doi: 10.1093/infdis/136.4.519. [DOI] [PubMed] [Google Scholar]


