Abstract
Background
Diabetic nephropathy, a major complication of diabetes, is characterized by progressive renal injury and increased cardiovascular mortality. An increased urinary albumin excretion due dysfunction of the glomerular barrier is an early sign of diabetic nephropathy. An increased urinary excretion of higher molecular weight proteins such as IgM appears with progression of glomerular injury. We aim here to study the prognostic significance of urine IgM excretion in patients with type 1 diabetes mellitus (type 1 diabetic nephropathy).
Methods
This is an observational study of 139 patients with type1 diabetes mellitus (79 males and 60 females) under routine care at the diabetic outpatient clinic at the Lund University Hospital. The median follow-up time was 18 years (1 to 22) years. Urine albumin and urine IgM concentration were measured at time of recruitment.
Results
Overall 32 (14 male and 18 female) patients died in a cardiovascular event and 20 (11 male and 9 female) patients reached end-stage renal disease. Univariate analysis indicated that patient survival and renal survival were inversely associated with urine albumin excretion (RR = 2.9 and 5.8, respectively) and urine IgM excretion (RR = 4.6 and 5.7, respectively). Stratified analysis demonstrated that in patients with different degrees of albuminuria, the cardiovascular mortality rate and the incidence of end-stage renal disease was approximately three times higher in patients with increased urine IgM excretion.
Conclusion
An increase in urinary IgM excretion in patients with type 1 diabetes is associated with an increased risk for cardiovascular mortality and renal failure, regardless of the degree of albuminuria.
Background
Diabetic nephropathy (DN) develops in up to 30% of patients who have had diabetes for more than 20 years [1,2]. DN is characterized by persistent albuminuria, elevated blood pressure, and progressive decline in renal function [3]. Development of DN is associated with an increased risk of cardiovascular (CV) complications and mortality [4,5]. However, a large interindividual variation in the rate of decline in kidney function and mortality has been reported [3,6]. This highlights the need for identification of risk factors and early predictors of progression. An increased urinary albumin excretion is an early sign of DN. Impairment of the tubular protein reabsorption or in the charge-selectivity of the glomerular filtration barrier are probably the major causes of albuminuria in the early stages of type 1 DN [7]. An impairment of the glomerular size-selectivity and increased urine excretion of high molecular weight (HMW) proteins are seen in advanced stages of DN [8,9]. Increased urinary IgM excretion reflects an abundance of highly non-selective pore pathways in the glomerular filter [10]. Our studies on chronic glomerular disease generally show an association between increased urinary IgM excretion and poor kidney and patient survival [11,12]. The present study aims to evaluate the prognostic impact of increased urine IgM excretion in comparison to degree of albuminuria in an unselected population of patients with type 1 diabetes.
Methods
In this observational follow-up study, patients with type 1 diabetes mellitus regularly attending our out-patient clinic at the Lund University Hospital were identified and recruited prospectively between 1984 and 2003. Forty-six (25 male and 21 female) patients had an albumin excretion rate in the microalbuminuric range, 48 (25 male and 23 female) had a urinary albumin excretion rate in the macroalbuminuric range, and 45 (29 male and 16 female) patients had a urinary albumin excretion rate in the normal range. The level of albuminuria was confirmed in at least two out of three consecutive urine samples. A total of 139 patients with type 1 diabetes were followed prospectively until October 2007 or death. The study was approved by the Ethics Committee at Lund University Hospital, and all patients gave informed consent. The patient characteristics are shown in Table 1. The median age was 35 years (18 to 80), and the median serum creatinine was 85 μmol/l (42 to 486). Present medications and blood pressure were taken from the patient records. Causes of death were traced from the National Death Register at the Swedish Board of Health and Welfare, and the patients' hospital records [13], Table 2. CV death was classified as all deaths where unequivocal non-CV death was not established. End-stage renal disease (ESRD) was defined as start of renal replacement therapy (dialysis or kidney transplantation) or serum creatinine >500 μmol/l.
Table 1.
Characteristic of 139 patients with type 1 diabetes divided according to initial degree of albuminuria into normo (45), micro (46), and macro (48).
| Variable | Normal | Micro | Macro | P value |
| At baseline: | ||||
| Sex (Male/Female) | 44 (29/15) | 46 (25/21) | 49 (25/24) | 0.3, ns |
| Age (years) | 34 (20-72) | 35 (18-80) | 38 (21-79) | 0.09, ns |
| Duration of diabetes | 11 (1-54) | 18 (1-65) | 25 (1-67) | <0.001 |
| S. creatinine (μmol/l) | 74 (54-110) | 80 (42-175) | 103 (61-486) | <0.001 |
| GFR (ml/min/1.73 m2) | 91(45-141) | 78 (28-144) | 60(9-105) | <0.001 |
| Urine IgM (mg/mmol·10-3) | 6.7(1.7-31.8) | 8.7(2.5-40) | 11.5(2.8-363) | 0.009 |
| HbA1c % | 7.6(4.5-13.4) | 8.8(5.5-13.2) | 9.1(6.2-12.7) | 0.01 |
| ACEI/ARBs, n/n (%) | 0/0 (0%) | 3/3 (13%) | 15/5 (40.8%) | <0.001 |
| MAP, mmhg | 92(78-110) | 96(80-127) | 103(82-133) | <0.001 |
| During follow-up: | ||||
| Follow up time, years | 19(2-22) | 19(2-22) | 9(1-22) | 0.01 |
| MAP, mmhg | 93(73-127) | 99(78-125) | 103(73-147) | 0.005 |
| HbA1c % | 8.1(4.5-13.9) | 8.0(5.0-11.1) | 8.2(4.5-13.4) | 0.61 |
| ACEI/ARBs, n/n (%) | 5/0 (11.4%) | 9/15 (53.3%) | 20/16 (76.6%) | <0.001 |
| CV-Mortality rate per patient-year | 0.003994674 | 0.0110957 | 0.035836177 | <0.001 |
| Renal failure rate per patient-year | 0 | 0.007407 | 0.032895 | <0.001 |
Abbreviations: ACEI: Angiotensin converting enzyme inhibitors; ARBs: Angiotensin II receptor antagonists; CV: cardiovascular; ESRD: End stage renal disease; GFR: glomerular filtration rate; HbA1c: glycosylated hemoglobin; MAP: Mean arterial blood pressure.
Table 2.
Causes of death of 38 patients died during a median of 18 years follow-up time of 139 patients with type 1 diabetes mellitus.
| Cause | No. (M/F) | |
| Cardiovascular | 32 (14/18) | |
| Cardiac arrest | 8 (3/5) | |
| MI | 12 (5/7) | |
| Heart failure | 3 (2/1) | |
| Stroke | 9 (4/5) | |
| Malignancy | 2(0/2) | |
| Lung cancer | 1 (0/1) | |
| Uterus cancer | 1 (0/1) | |
| Sepsis | 2(0/2) | |
| Acute hypoglycemia | 1(1/0) | |
| Suicide | 1(0/1) | |
| Total | 38(15/23) | |
Abbreviations: MI = Myocardial Infarction.
Laboratory measurements
Glycosylated hemoglobin (HbA1c) was analyzed by high-performance liquid chromatography. Serum and urinary creatinine concentrations were analyzed by an enzymatic method (creatinine-amidino-hydrolase; KODAK EKTACHEM analyser, Instrument Kodak, NY, USA). Urinary albumin concentration was measured on fresh urine samples using turbidimetry (Cobas Mira S, Roche). The detection limits was 5 mg/l, and the coefficient of variation was between 1% and 8%. Urine samples were kept at -20°C until analysis for IgM in December 2006. Urine IgM concentrations were measured by ELISA as described elsewhere [10]. The lower detection limit for the urine IgM assay is 1 μg/l; the intra-assay and inter-assay variation is 4.6% and 10.9%, respectively. Glomerular filtration rate (GFR) was estimated using the MDRD formula [14,15], where eGFR = {186.3 × (serum creatinine mg/dl)-1.154 × (age)-0.203 (× 0.742 if female).
Statistical analysis
Data are presented as median (range) or mean ± SE. The patients groups were compared with non-parametric statistical tests Mann-Whitney U and Kruskal-Wallis H tests. The Fisher's exact test and Pearson Chi square (χ2) test were used when appropriate. Incidence rate of ESRD and mortality rate were calculated per person-year. Kaplan-Meier curves were used for survival analysis. Univariate Cox regression analysis was used to examine the baseline variables predictive of CV mortality and ESRD. Results described as relative ratios (RR) and the 95% confidence intervals (95% CI). Due to sample size restriction, subsequent stratified analysis was conducted only to compare the prognostic effect of albuminuria and IgM-uria. The patients were divided into low and high IgM excretion groups according to the median value (0.01 mg/mmol) of urine IgM excretion. Microalbuminuria was defined as a urinary albumin excretion rate of 3 to 20 mg/mmol; <3 mg/mmol was regarded as normal and >20 mg/mmol was regarded as macroalbuminuria [16]. Statistical analyses were performed using statistical package SPSS 13.0 (SPSS, Chicago Illinois, USA). The level of statistical significance was set at P values < 0.05.
Results
The median time to death or end of follow up by October 2007 was 18 years (1 to 22). During the follow up 38 patients died and 20 (11 male and 9 female) patients developed ESRD. Six (1 male and 5 female) patients died from non-CV events and 32 (14 male and 18 female) patients in a CV event (Table 2). These 32 (14 male and 18 female) patients were included in a further analysis of CV-mortality.
Univariate Cox-regression analysis identified the following predictors of CV mortality: age (HR = 11.5, 95% CI 3.5 to 38, P < 0.001), duration of diabetes (HR = 7.8, 95% CI 3 to 20, P < 0.001), mean arterial blood pressure (HR = 3.6, 95% CI 1.6 to 8.1, P = 0.002), serum creatinine (HR = 3.3, 95% CI 1.5 to 7.3, P = 0.004), eGFR (HR = 9.3, 95% CI 3.3 to 27.0, P < 0.001), urinary albumin excretion (HR = 2.9, 95% CI 1.7 to 5.0, P < 0.001) and urinary IgM excretion (HR = 4.6, 95% CI 2.0 to 10.7, P < 0.001). The predictors of ESRD were: duration of diabetes (HR = 4.3, 95% CI 1.6 to 12, P =0.005), mean arterial blood pressure (HR = 3.9, 95% CI 1.4 to 10.8, P = 0.009), serum creatinine (HR = 23.3, 95% CI 3.1 to 174, P = 0.002), GFR (HR = 12.4, 95% CI 2.9 to 54.0, P = 0.001), urinary albumin excretion (HR = 5.8, 95% CI 2.4 to 14, P < 0.001) and urinary IgM excretion (HR = 5.7, 95% CI 1.9 to 17, P = 0.002). Stepwise multivariate Cox regression analysis shows that urine IgM excretion, independently of the level of albuminuria, predicts CV mortality and renal failure (P = 0.004). There was no significant difference in CV mortality between those treated and those not treated by ACE inhibitors, (29.2% vs. 15.5%, P = 0.054) or between those treated by ACEi or ARBs (35.1% vs. 22.6, P = 0.26).
Patient and renal outcome by degree of albuminuria
Table 1, shows the baseline and follow-up data of the 139 patients studied divided according to the degree of baseline albuminuria. As expected, the duration of diabetes, serum creatinine and blood pressure were significantly higher in the macroalbuminuric group. During follow-up there was no difference in HbA1c, and more patients with macroalbuminuria received ACE inhibitors.
Cardiovascular mortality
Three patients in the normo-albuminuric and eight patients in the microalbuminuric group died of a CV event during the follow-up time, compared with 21 (36.8%) patients in the macroalbuminuric group (P < 0.001). The risk of CV mortality was 3.6% per patient-year in patients with macroalbuminuria compared with approximately 1.1% in patients with microalbuminuria; that is, the RR increment was 3.23 (Table 1).
Renal survival
Fifteen (30.6%) patients from the macroalbuminuric group developed ESRD compared with only five (5.6%) patients in the microalbuminuric group (P < 0.001). The risk of ESRD was approximately 3.3% per patient-year in patients with macroalbuminuria compared with approximately 0.7% in patients with microalbuminuria (RR 4.44, Table 1).
Patient and renal outcome by degree of IgM-uria
Table 3 shows the baseline and follow-up data of the 139 patients studied divided according to baseline urine IgM excretion. Patients with increased urine IgM excretion had significantly increased urine albumin excretion, higher blood pressure and higher serum creatinine compared with patients with low urine IgM excretion. The low IgM group had a mean urine IgM concentration of 0.0055 ± 0.0017 mg/mmol, and the high IgM group had a mean urine IgM concentration of 0.0258 ± 0.0457 mg/mmol. There was no difference in follow-up glycemic control between IgM groups, and patients with high IgM excretion had a higher percentage of renoprotective treatment with ACE inhibitors.
Table 3.
Characteristic of 139 patients with type 1 diabetes divided according to the median urine IgM level into low (<0.01 mg/mol) and high IgM (>0.01 mg/mol) groups.
| Variable | Low IgM group | High IgM group | P value |
| At baseline: | |||
| Sex (male/female) | 71 (49/22) | 68 (30/38) | 0.003 |
| Age (years) | 35(18-74) | 37(20-80) | 0.05 |
| Duration of diabetes (yr) | 15 (1-65) | 24 (1-67) | 0.001 |
| HbA1c % | 8.3 (4.5-13.4) | 9 (5.5-13.2) | 0.06, ns |
| Albuminuria (mg/mmol) | 3.6(0.23-268) | 21.3 (0.26-640) | <0.001 |
| S. Creatinine μmol/l | 78 (54-216) | 86 (42-486) | 0.009 |
| GFR (ml/min/1.73 m2) | 88 (27-141) | 67(9-144) | <0.001 |
| MAP mmhg | 93 (80-120) | 100 (78-133) | 0.004 |
| ACEI/ARBs, n/n (%) | 3/2 (7.1%) | 15/6 (31%) | <0.001 |
| During follow-up: | |||
| Follow up time (years) | 19 (2-22) | 14 (1-22) | <0.001 |
| MAP mmhg | 97 (73-119) | 103 (73-147) | 0.038 |
| HbA1c (%) | 8.25 (4.5-13.9) | 8 (4.5-13.4) | |
| 0.7, ns | |||
| Albuminuria (mg/mmol) | 1.3 (0.1-676) | 20.5 (0.1-1101) | <0.001 |
| ACEI/ARBs, n/n (%) | 12/14 (37%) | 22/17 (59%) | 0.008 |
| CV-Mortality rate per patient-year | 0.005838 | 0.029104 | <0.001 |
| Renal failure rate per patient-year | 0.003451 | 0.022161 | 0.003 |
Abbreviations: ACEI: Angiotensin converting enzyme inhibitors; ARBs: Angiotensin II receptor antagonists; CV: cardiovascular; ESRD: End stage renal disease; GFR: glomerular filtration rate; HbA1c: glycosylated hemoglobin; MAP: Mean arterial blood pressure.
Cardiovascular mortality
Seven (9.9%) patients with low urinary IgM excretion died from CV events during the follow-up time compared with 25 (36.8%) patients with high urinary IgM excretion (P < 0.001). The risk of CV mortality was 2.9% per patient-year in patients with high urine IgM excretion compared with approximately 0.6% in patients with low in IgM excretion (RR = 4.98, Table 3).
Renal survival
Sixteen (23.5%) patients from the high IgM group developed ESRD during the follow-up period compared with only four (5.6%) patients with low urinary IgM excretion (P = 0.003). The risk of ESRD was 2.2% per patient-year in patients with high urinary IgM excretion compared with approximately 0.35% in patients with low in IgM excretion (RR = 6.4, Table 3).
Patient and renal outcome by degree of albuminuria and IgM-uria
Cardiovascular mortality
Patients with microalbuminuria and high urine IgM excretion had 2.1% per patient-year risk for CV mortality, compared with approximately 0.46% in those with low IgM excretion (RR = 4.6, Table 4). Patients with macroalbuminuria and high urine IgM excretion had 4.8% per patient-year risk for CV mortality, compared with 1.7% in those with low IgM excretion, (RR = 2.8, Table 4). Even patients with normoalbuminuria and high urine IgM excretion had significantly higher risk for CV mortality compared with those with low IgM excretion (0.9% vs. 0.57% per patient-year, RR = 1.6, Table 4). The pattern of cumulative patient survival by degree of albuminuria and IgM-uria are show in Figure 1.
Table 4.
Patient and renal outcome of 139 patients with type 1 diabetes by degree of albuminuria and IgM-uria.
| Stage of DN | Baseline urine IgM excretion | Rate of renal failure per patient-year | CV-Mortality rate per patient-year | 10 year Patient survival | 10 year Renal survival |
| Normoalbuminuria (n = 44) | Low (n = 31) | 0 | 0.005671 | 96.5% | 100% |
| High (n = 13) | 0 | 0.009009 | 91.7% | 100% | |
| Microalbuminuria (n = 46) | Low (n = 24) | 0.004750594 | 0.004577 | 95.7% | 95.6% |
| High (n = 22) | 0.011811024 | 0.021127 | 84.9% | 87.5% | |
| Macroalbuminuria (n = 49) | Low(n = 16) | 0.014354067 | 0.017167 | 73.9% | 84% |
| High (n = 33) | 0.052631579 | 0.048159 | 59.5% | 53% |
Figure 1.
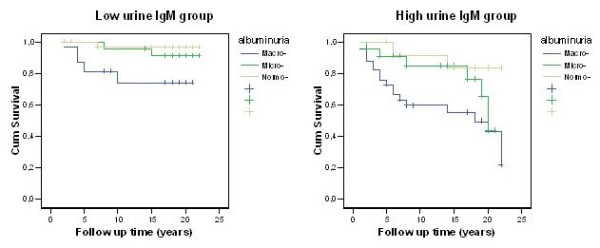
Cardiovascular mortality rate by albuminuria and urine IgM.
Renal survival
Patients with microalbuminuria and high urine IgM excretion had approximately 1.2% per patient-year risk for renal failure, compared with approximately 0.48 in those with low IgM excretion (RR = 2.4, Table 4). Patients with macroalbuminuria and high urine IgM excretion had 5.3% per patient-year risk for renal failure, compared with 1.4% in those with low IgM excretion, (RR = 3.7, Table 4). The pattern of cumulative renal survival by degree of albuminuria and IgM-uria is show in Figure 2.
Figure 2.
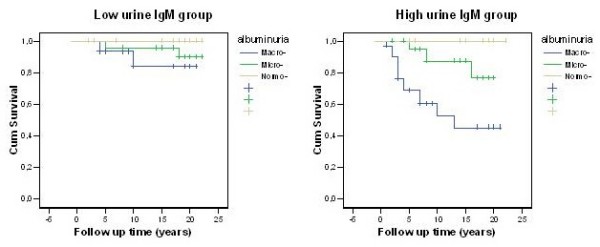
Renal survival rate by albuminuria and IgM-uria.
Discussion
In this cohort of long-time follow-up (median 18 years) of patients with type 1 diabetes, with and without diabetic nephropathy, we found that patients with an increased urinary IgM excretion had a higher mortality from CV causes, and higher disease progression rate to ESRD compared with patients with low urinary IgM excretion. In accordance with other reports, this study demonstrates that CV death due to atherosclerosis is the major cause of mortality in diabetic patients. Furthermore, the mortality was strongly associated with duration of diabetes mellitus and the degree of albuminuria [3,4]. To our knowledge our study is the first one investigating the impact of an increased urine IgM excretion on DN disease progression in type 1 diabetic patients.
We were able to show that the association of urine IgM excretion with a higher risk for CV mortality and ESRD is largely independent of the level of albuminuria. Patients with DN and increased urine IgM excretion, either in the micro or the macroalbuminuric stage, had at least a threefold higher risk of CV mortality and renal failure than those with low urinary IgM excretion, (Table 4, Figures 1 and 2).
Previous studies suggest that the development of DN in patients with type 1 diabetes is due to an early impairment of charge selectivity of the glomerular filter. The size selectivity dysfunction appears later, along with progression of the disease [17]. Thus, albuminuria per se could be due to impairment of glomerular charge or size selectivity, that is, could reflect minor or severe glomerular damage [18,19]. IgG-uria (molecular radius 55 Å), studied previously by others, is mainly due to an increased transport through the large pores [17]. However, the very large protein measured in this study, IgM (molecular radius 120 Å), is able to pass the glomerular filtration barrier only through very large defects (shunts) [10]. Thus, the occurrence of IgM in the final urine reflects a markedly increased population of highly unselective glomerular pathways and probably a more severe glomerular damage [11,12].
An increase in renal vascular resistance, due to atherosclerotic vascular disease, may induce ischemia and more severe structural changes in the glomeruli, and cause an increase in urinary excretion of HMW proteins through such shunt-like pathways [20-23]. This may explain why urinary IgM excretion correlates with CV events in this cohort of type 1 diabetic patients.
Conclusion
While albuminuria is an early predictor of DN progression, we were able in this study to show that an increased urinary IgM excretion in patients with DN was an independent predictor of CV complications. However, further studies are needed to evaluate the correlation between urine IgM excretion and surrogate markers of atherosclerosis in patients with CV disease and diabetes mellitus.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
RT undertook concept and design of the study, data collection, analysis and interpretation of data, and drafting of the manuscript. OT contributed to study design, data collection, interpretation of data, and critical review of intellectual content. BR contributed to interpretation of data, and critical review of intellectual content. OB contributed to the concept and design of the study, analysis and interpretation of data, drafting the manuscript, and critical review of intellectual content. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
This study was supported by generous grants from the research funds of the University Hospital in Lund, Swedish research council no: 08285 as well as Lund University research grants.
Contributor Information
Rafid Tofik, Email: Rafid.Tofik@med.lu.se.
Ole Torffvit, Email: ole.torffvit@med.lu.se.
Bengt Rippe, Email: bengt.rippe@med.lu.se.
Omran Bakoush, Email: omran.bakoush@med.lu.se.
References
- Dahlquist G, Stattin EL, Rudberg S. Urinary albumin excretion rate and glomerular filtration rate in the prediction of diabetic nephropathy; a long-term follow-up study of childhood onset type-1 diabetic patients. Nephrol Dial Transplant. 2001;16:1382–1386. doi: 10.1093/ndt/16.7.1382. [DOI] [PubMed] [Google Scholar]
- Raile K, Galler A, Hofer S, Herbst A, Dunstheimer D, Busch P, Holl RW. Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care. 2007;30:2523–2528. doi: 10.2337/dc07-0282. [DOI] [PubMed] [Google Scholar]
- Hovind P, Rossing P, Tarnow L, Smidt UM, Parving HH. Progression of diabetic nephropathy. Kidney Int. 2001;59:702–709. doi: 10.1046/j.1523-1755.2001.059002702.x. [DOI] [PubMed] [Google Scholar]
- Finne P, Reunanen A, Stenman S, Groop PH, Gronhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA. 2005;294:1782–1787. doi: 10.1001/jama.294.14.1782. [DOI] [PubMed] [Google Scholar]
- Borch-Johnsen K, Kreiner S. Proteinuria: value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed) 1987;294:1651–1654. doi: 10.1136/bmj.294.6588.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinke JM, Sinaiko AR, Kramer MS, Suissa S, Chavers BM, Mauer M. The early natural history of nephropathy in Type 1 Diabetes: III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes. 2005;54:2164–2171. doi: 10.2337/diabetes.54.7.2164. [DOI] [PubMed] [Google Scholar]
- Rippe C, Rippe A, Torffvit O, Rippe B. Size and charge selectivity of the glomerular filter in early experimental diabetes in rats. Am J Physiol Renal Physiol. 2007;293:F1533–1538. doi: 10.1152/ajprenal.00271.2007. [DOI] [PubMed] [Google Scholar]
- Bakoush O, Tencer J, Tapia J, Rippe B, Torffvit O. Higher urinary IgM excretion in type 2 diabetic nephropathy compared to type 1 diabetic nephropathy. Kidney Int. 2002;61:203–208. doi: 10.1046/j.1523-1755.2002.00108.x. [DOI] [PubMed] [Google Scholar]
- Torffvit O, Eriksson JW, Henricsson M, Sundkvist G, Arnqvist HJ, Blohme G, Bolinder J, Nystrom L, Ostman J, Svensson M. Early changes in glomerular size selectivity in young adults with type 1 diabetes and retinopathy. Results from the Diabetes Incidence Study in Sweden. J Diabetes Complicat. 2007;21:246–251. doi: 10.1016/j.jdiacomp.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Tencer J, Frick IM, Oquist BW, Alm P, Rippe B. Size-selectivity of the glomerular barrier to high molecular weight proteins: upper size limitations of shunt pathways. Kidney Int. 1998;53:709–715. doi: 10.1046/j.1523-1755.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- Bakoush O, Segelmark M, Torffvit O, Ohlsson S, Tencer J. Urine IgM excretion predicts outcome in ANCA-associated renal vasculitis. Nephrol Dial Transplant. 2006;21:1263–1269. doi: 10.1093/ndt/gfk074. [DOI] [PubMed] [Google Scholar]
- Bakoush O, Torffvit O, Rippe B, Tencer J. High proteinuria selectivity index based upon IgM is a strong predictor of poor renal survival in glomerular diseases. Nephrol Dial Transplant. 2001;16:1357–1363. doi: 10.1093/ndt/16.7.1357. [DOI] [PubMed] [Google Scholar]
- Hammar N, Alfredsson L, Rosen M, Spetz CL, Kahan T, Ysberg AS. A national record linkage to study acute myocardial infarction incidence and case fatality in Sweden. Int J Epidemiol. 2001;30:S30–34. doi: 10.1093/ije/30.suppl_1.s30. [DOI] [PubMed] [Google Scholar]
- Manjunath G, Sarnak MJ, Levey AS. Prediction equations to estimate glomerular filtration rate: an update. Curr Opin Nephrol Hypertens. 2001;10:785–792. doi: 10.1097/00041552-200111000-00009. [DOI] [PubMed] [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- Torffvit O, Lovestam-Adrian M, Agardh E, Agardh CD. Nephropathy, but not retinopathy, is associated with the development of heart disease in Type 1 diabetes: a 12-year observation study of 462 patients. Diabet Med. 2005;22:723–729. doi: 10.1111/j.1464-5491.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- Deckert T, Feldt-Rasmussen B, Djurup R, Deckert M. Glomerular size and charge selectivity in insulin-dependent diabetes mellitus. Kidney Int. 1988;33:100–106. doi: 10.1038/ki.1988.16. [DOI] [PubMed] [Google Scholar]
- Andersen S, Blouch K, Bialek J, Deckert M, Parving HH, Myers BD. Glomerular permselectivity in early stages of overt diabetic nephropathy. Kidney Int. 2000;58:2129–2137. doi: 10.1111/j.1523-1755.2000.00386.x. [DOI] [PubMed] [Google Scholar]
- Bakoush O, Torffvit O, Rippe B, Tencer J. Renal function in proteinuric glomerular diseases correlates to the changes in urine IgM excretion but not to the changes in the degree of albuminuria. Clin Nephrol. 2003;59:345–352. doi: 10.5414/cnp59345. [DOI] [PubMed] [Google Scholar]
- Bakoush O, Tencer J, Torffvit O, Tenstad O, Skogvall I, Rippe B. Increased glomerular albumin permeability in old spontaneously hypertensive rats. Nephrol Dial Transplant. 2004;19:1724–1731. doi: 10.1093/ndt/gfh276. [DOI] [PubMed] [Google Scholar]
- Diamond JR. Analogous pathobiologic mechanisms in glomerulosclerosis and atherosclerosis. Kidney Int Suppl. 1991;31:S29–34. [PubMed] [Google Scholar]
- Rippe C, Rippe A, Larsson A, Asgeirsson D, Rippe B. Nature of glomerular capillary permeability changes following acute renal ischemia-reperfusion injury in rats. Am J Physiol Renal Physiol. 2006;291:F1362–1368. doi: 10.1152/ajprenal.00123.2006. [DOI] [PubMed] [Google Scholar]
- Intensive therapy and progression to clinical albuminuria in patients with insulin dependent diabetes mellitus and microalbuminuria. Microalbuminuria Collaborative Study Group, United Kingdom. BMJ. 1995;311:973–977. [PMC free article] [PubMed] [Google Scholar]


