Abstract
Background
Inflammatory bowel disease (IBD) is a risk factor for developing colorectal cancer but the mechanisms are poorly characterized. Mice lacking the G-protein alpha subunit Gi2-alpha spontaneously develop colitis and colon cancer with high penetrance. Compared to canonical Wnt/APC signaling-based animal models of colon cancer, the tumors in Gi2-alpha−/− mice more closely recapitulate the features of IBD-associated cancers seen in humans. They are predominantly right-sided, multifocal, mucinous, and arise from areas of flat dysplasia.
Methods
In evaluating the potential contribution of epithelial Gi2-alpha signaling to this phenotype, we found that Gi2-alpha−/− colonic epithelium is hyperproliferative even before the onset of colitis, and resistant to the induction of apoptosis. We generated colon cancer cell lines overexpressing dominant-negative Gi2-alpha.
Results
Like other cells lacking Gi2-alpha, these cells release less arachidonic acid, an important antiinflammatory and epithelial growth regulator. They are also hyperproliferative and resistant to camptothecin-induced apoptosis and caspase-3 activation.
Conclusions
The colitis-associated cancers in Gi2-alpha−/− mice appear very similar to those seen in human IBD patients, and Gi2-alpha is a direct negative regulator of colonic epithelial cell growth.
Keywords: colitis associated colon cancer, inflammatory bowel disease, animal models of colon cancer, heterotrimeric G-protein signaling
Colorectal cancer (CRC) is a feared complication of chronic inflammatory bowel disease (IBD), a condition that affects nearly half a million patients in the U.S. Although IBD-associated CRC shares some of the same genetic alterations seen in sporadic CRCs, there are differences in the timing and nature of these changes, including earlier loss of heterozygosity at the p53 locus and a lower incidence of ras mutations (reviewed in Ref. 1). There are important clinical and histopathological differences between sporadic and IBD-associated CRC as well (reviewed in Ref. 2). In the latter there is a greater incidence of right-sided/proximal bowel cancers, particularly in Crohn’s disease (CD).3 The incidence of tumors with mucinous differentiation is also higher in IBD patients, as is the likelihood of multiple synchronous malignancies4 and the presence of areas of flat dysplasia. On these grounds, IBD-associated colon cancer appears to be a distinct entity from sporadic colon cancer.
Of the mediators that have been shown to be important in the regulation of mucosal immunity as well as carcinogenesis, the arachidonic acid (AA)-COX-PGE2 axis is particularly well studied. The role of PGE2 is controversial in IBD; PGE2 levels are commonly upregulated in diseased colonic mucosa, yet COX inhibition (and thus PGE2 production) following nonsteroidal antiinflammatory (NSAID) use is strongly associated with relapse and worsening of symptoms.5 While there is a great deal of evidence that COX inhibitors inhibit polyp growth and decrease the relative risk of developing sporadic colon cancers,6 the data are considerably less clear in IBD-associated carcinogenesis. Some studies have shown a benefit in ulcerative colitis (UC) patients,7 while other studies show no preventative benefit of NSAID use by patients.8–10 We have previously shown that Gi2-alpha−/− mice develop cancer despite lower colonic mucosal PGE2 levels, even in the setting of inflammation.11 IBD-associated carcinogenesis therefore appears to be less dependent on the COX-PGE2 pathway.
Although there is great interest in the mechanisms underlying IBD-associated colon cancer, efforts have been impeded by a relative lack of suitable animal models. Mice on a 129Sv/C57 background that lack the G-protein alpha subunit Gi2-alpha spontaneously develop colitis starting at 6–8 weeks of age. During the initial characterization of Gi2-alpha−/− mice,12 it was noted that a subset of animals (8/26, or 31%) developed adenocarcinomas of the colon. However, the natural history and histopathologic features of cancer in Gi2-alpha−/− mice have not been completely enumerated, and it is not clear whether loss of Gi2-alpha signaling has a direct or indirect effect on the epithelium that may contribute to carcinogenesis. We tested the hypothesis that the loss of Gi2-alpha signaling would directly affect the balance of growth and apoptosis in the colonic epithelium, independent of the influence of other cell types or mediators. In this study we evaluated the natural history of colitis and colon cancer and studied whether known defects in arachidonic acid metabolism in the absence of Gi2-alpha are conserved in colonic epithelia, and whether the lack of Gi2-alpha signaling directly affects the balance of growth and apoptosis in colon cancer cells.
MATERIALS AND METHODS
Mice
Wildtype (WT) and Gi2-alpha−/− mice on a crossbred 129Sv/C57BL6 background were housed in a pathogen-free facility at the University of California, Irvine, vivarium. Male Gi2-alpha−/− mice were bred with heterozygous females in order to maximize fertility and the yield of Gia2-alpha-knockout animals. Genotyping was performed on genomic tail DNA using a multiplex polymerase chain reaction (PCR) reaction that generates an 805 bp product for the WT allele and a 509 bp product for the mutant allele.
Ethical Considerations
All experiments were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of California, Irvine (IACUC Protocol #2002-2357-1) and were consistent with Federal guidelines.
Histopathologic Assessment of Colitis and Colon Cancer
In all, 123 Gi2-alpha−/− mice ranging in age from 4–53 weeks of age were sacrificed and their colons removed. The lumens were rinsed thoroughly with phosphate-buffered saline (PBS) and the serosal surfaces inked with 4 colors of surgical marking ink to denote cecal, proximal, mid, and distal colonic segments. The tissues were submitted for fixation either as intact “jelly rolls” or as linear segments on edge. The degree of colitis (none, minimal, mild, moderate, or severe) was assessed based on the following criteria: 1) extent and severity of inflammatory infiltrates, 2) presence of mucosal ulceration, 3) depletion of goblet cells, 4) presence of transmural inflammation and/or serositis, and 5) presence of crypt ulcers. Colon cancers were defined as the presence of glands that had invaded into (at least) the submucosa and were scored for their location and presence or absence of mucinous differentiation.
Anti-BrdU Staining
For immunofluorescent staining of BrdU-labeled sections, age-matched, colitis-free 6-week-old (n = 8 each) WT and Gi2-alpha−/− mice were treated with 50 mg/kg BrdU 2 hours before sacrifice. Following formalin fixation / paraffin embedding, deparaffinized sections were stained using a Zymed anti-BrdU staining kit (Invitrogen, Carlsbad, CA) and counterstained with DAPI following the manufacturer’s instructions. For each age group the number of BrdU+ and DAPI+ epithelial nuclei from 10 well-oriented crypts per mouse were counted and the mean ± SEM percentage of BrdU+ cells was calculated. At least 5000 DAPI+ cells were counted in each cohort of young mice. The data presented are from 3 separate iterations of the same experimental procedure.
In Vivo Assessment of Apoptosis Induction
Groups of age-matched WT and Gi2-alpha−/− mice (n = 7 each) were injected i.p. with 15 mg/kg of azoxymethane (AOM) in sterile saline. Four hours later (2 hours before sacrifice) the animals were injected i.p. with 200 µL of 5 mg/mL BrdU in sterile saline. Following sacrifice, whole colons were removed, the tip of the cecum was snipped, and the fecal contents washed out via a 4″ gavage needle. Colonic tissues were pinned out and fixed overnight in 10% neutral buffered formalin and then submitted on edge for paraffin embedding. Then 5-µm-thick sections were stained for apoptotic cells using an anti-digoxigenin antibody-based Apoptag staining kit (Chemicon, Temecula, CA) and counter-stained with DAPI per the manufacturer’s instructions. For the evaluation of basal colonic epithelial apoptosis in vivo, untreated tissue sections were stained with 1:200 rabbit anti-cleaved caspase 3 (Cell Signaling #9661, Danvers, MA) and developed with DAB. Ten medium-power fields from cleaved caspase-3- and TUNEL-stained sections each colon were photographed and counted. Cleaved caspase-3+ and TUNEL+ cells were counted and expressed as a percentage of hematoxylin-counterstained or DAPI+ (respectively) epithelial cells. The data are expressed as the mean ± SEM of the normalized ratio of TUNEL+/PI+ cells and represent the results of 2 identical experiments.
Generation of Human Colon Cancer Cell Linesx Overexpressing G203T-Gi2-alpha
The human colon cancer cell lines T84 (ATCC #CCL-248) and RKO (ATCC #CRL-2577) were grown in DMEM/F12/5% FCS or RPMI/10% FCS, respectively. Cells were stably transfected with either a control “Neo” plasmid (pcDNA-Neo3.1(+) (Invitrogen, Carlsbad, CA), or a plasmid encoding the dominant-negative human Gi2-alpha cDNA,13 G203T-Gi2-alpha (from the University of Missouri, Rolla cDNA resource center). The transfections were performed using 4 µg of cDNA and an Amaxa nucleofector (Amaxa USA, Gaithersburg, MD) with their primary mammalian epithelial nucleofector kit per the manufacturer’s instructions. Forty-eight hours after nucleofection, G418 selection with 200 µg/mL (for T84 cells) or 700 µg/mL (for RKO cells) was initiated. These concentrations were determined by kill curves on untransfected cells. Following the establishment of resistant colonies, pooled clones were evaluated for the overexpression of human Gi2-alpha by semiquantitative real-time PCR (using primers hGi2-alpha-forward: 5′-GAACGACCTGGAGCGTATTG-3′; hGi2-alpha-reverse: 5′-GAGGATGATGGACGTGTCTG-3′), normalized to beta actin (actin-forward: 5′-CATGTACGTTGCTATCCAGGC-3′; actin-reverse: 5′-CTCCTTAATGTCACGCACGAT-3′). Overexpression of Gi2-alpha protein was evaluated by Western blotting using a 1:500 dilution of mouse monoclonal anti-Gi2-alpha antibody, clone L5 (Santa Cruz Biotechnology, Santa Cruz, CA).
Human Colon Cancer Cell Line 3H-Arachidonic Acid Labeling and Release
For experiments involving arachidonate release, 2 × 105 cells/well were plated in 12-well dishes, allowed to adhere in RPMI-5 for 8 hours, then loaded with 0.3 µCi/well 3H-arachid-onate (208 Ci/mmol, 1 mCi/mL; Amersham Biosciences, Buckinghamshire, UK) in RPMI-0.2% bovine serum albumin (BSA) overnight. Typical cellular uptake was >90% and was similar in WT and Gi2-alpha−/− cells. Each well was washed 3 times with RPMI-0.2% BSA, then treated with 500 µM adenosine triphosphate (ATP) or 0.1 U/mL thrombin (Sigma, St. Louis, Mo) in 500 µM for 60 minutes with or without 5 minutes of pretreatment with 200 nmol/L phorbol 12-myristate 12-acetate (PMA). Two hundred out of the 500 µL of supernatants was removed and the incorporated cellular arachidonate collected by lysing adherent cells in 10% sodium dodecylsulfate. Fractions were assayed for 3H-arachidonate release by scintillation counting and the percentage of ligand-stimulated arachidonate release calculated as [(2.5 × counts per minute in supernatant)/(2.5(counts per minute in supernatant)) plus counts per minute in lysate]*100.
Proliferation Assays of Human Colon Cancer Cell Lines
In all, 2.5 × 104 WT, Neo, or G203T-Gi2-alpha expressing RKO or T84 clones were plated in triplicate in 96-well plates and allowed to attach overnight. Four hours after the addition of fresh complete medium, 10 µL/well of WST-1 reagent (Roche Applied Science, Nutley, NJ) was added to each well, and the conversion to formazan monitored by measuring the OD450 at varying times up to 2 hours. The slope of the resulting curves is proportional to cell proliferation.
In Vitro Assessment of Apoptosis Induction and Caspase 3 Activity Assays
Basal and camptothecin-induced caspase 3 activities were determined in cultures of control and G203T-Gi2-alpha-expressing T84 and RKO cells. Briefly, subconfluent 100 mm dishes of cells were preincubated with 20 µM arachidonic acid overnight and then treated with 5 µM camptothecin for 6 hours. Trypsinized cells were resuspended at 5 × 107 cells/mL and lysed in 50 mM HEPES, pH 7.4,100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 1 mM EDTA, and 10% glycerol. Cleared lysates were assayed in triplicate for caspase 3 activity in 100 µL reactions containing 80 µL assay buffer, 10 µL lysate, and 10 µL of 500 µM DEVD-pNA (Biomol, Plymouth Meeting, PA), monitoring the OD405 of the reaction over time. Samples containing buffer and DEVD-pNA substrate alone were used as a negative control. The activity of caspase 3 in pmol/min/mg protein was calculated according to the manufacturer’s instructions.
For Western blot analysis of cleaved caspase-3 activity, RKO cells were treated with 5 µM camptothecin for 0, 3, 6, and 24 hours, lysed in basal lysis buffer (20 mM Tri pH 7.9, 137 mM NaCl, 5 mM EDTA, 10% glycerol, 1% Triton X-100, 1 mM EGTA 1 Roche complete-mini protease inhibitor tablet, 1 mM NaPPi, and 10 mM NaF), and analyzed for cleaved caspase-3 by Western blot using a 1:1000 dilution of anti-cleaved caspase-3 antibody.
Statistical Analyses
Statistical significance was assessed using Student’s paired t-test for linear data (epithelial cell proliferation in vivo and in vitro, arachidonic acid release, caspase-3 activity), and the Mann-Whitney U-test for nonlinear data (normalized TUNEL-staining data).
RESULTS
Spectrum of Colitis and Colon Cancer in Gi2-alpha−/− Mice
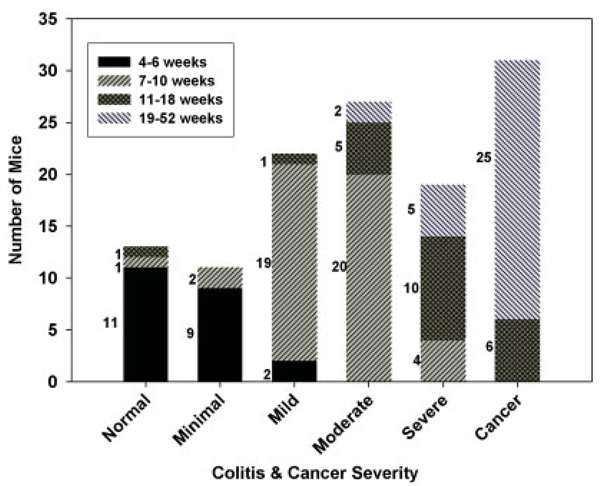
We summarize the prevalence of increasingly severe colitis and colon cancer in Gi2-alpha −/− mice, stratified into 4 age groups, in Figure 1. These age groupings correspond to the age where colitis begins (4–6 weeks), colitis but not cancer is present (7–10 weeks), cancer incidence is increasing (11–18 weeks), and cancer incidence is prevalent (19–52 weeks). In our breeding colony, nearly all Gi2-alpha−/− mice develop colitis by the seventh week of life (Fig. 1), consisting of an expansion of the lamina propria by mononuclear infiltrates. However, 2 mice sacrificed at 7.9 and 11.9 weeks were still considered to have a “normal” number of lamina propria mononuclear cells.
FIGURE 1.
Natural history of colitis and colon cancer in Gi2-alpha−/− mice. Whole colons from 123 Gi2-alpha−/− mice were stratified by age into 4 groups and analyzed for the presence and severity of colitis and colon cancer. The number to the left of each bar reflect the number of mice in each age group with a given degree of colitis. Nearly all Gi2-alpha−/− animals have at least mild colitis by 7 weeks of age and begin to develop colon cancer as early as 12 weeks of age. [Color figure can be viewed in the online issue, which is available at www.inter-science.wiley.com.]
In these mice the inflammatory infiltrates are often lymphoplasmacytic, although some animals with mild colitis have a predominance of neutrophils. As the degree of colitis progresses, the infiltrates expand into the submucosa and eventually through the muscularis propria and serosal layer (Fig. 2A). Some animals with severe colitis have significant numbers of mast cells, scattered within the muscularis propria (Fig. 2B). An additional curious feature in some cases is the presence of dilated serosal lymphatic vessels which have a large cuff of surrounding mononuclear cells around them (Fig. 2C). Importantly, the distribution of colitis along the length of the colon is patchy, with areas of involvement interspersed with “skip” areas. In our experience, large areas of ulcerated mucosa, crypt ulcers, strictures, and adhesions between adjacent loops of bowel are not prominent, nor have we have identified any granulomas in any colitic tissues.
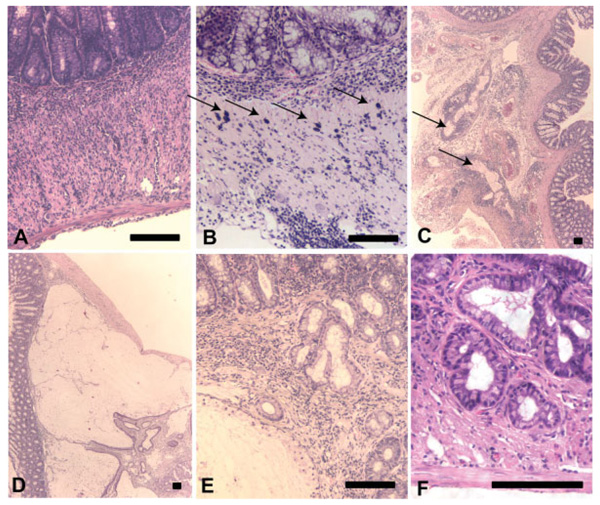
FIGURE 2.
Histopathologic features of colitis and cancer in Gi2-alpha−/− mice. Mice with moderate to severe colitis often develop transmural inflammation and serositis (A,C). Some colon specimens contain numerous intramuscular mast cells (B, arrows), and/or prominent lymphoplasmacytic cuffing of serosal lymphatic channels (C). Mucinous carcinomas are characterized by large mucin pools that dissect through the muscularis; note the overlying nonneoplastic mucosa at left (D). Many of the carcinomas are characterized by very well-differentiated glands with small basal nuclei and mucin vacuoles (shown invading into muscle in E,F.) Scale bars = 100 µm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Of 123 Gi2-alpha−/− mice whose whole colons were available for study, 31 mice were found to have at least 1 carcinoma, defined as the presence of glands invasive through (at least) the muscularis mucosa into the submucosa. Table 1 summarizes the distribution and multiplicity of tumors, divided into 2 age groups. In the 9 mice aged 12–24 weeks, 80% of the tumors (16/20) were either in the cecum or proximal colon, and 90% consisted of smaller, nonmucinous tumors. The earliest invasive cancer occurred at 11.9 weeks of age. Cancers in the older age group (25–53 weeks of age) showed a similar (76%) predilection for the cecum and proximal colon, but there were a greater proportion of tumors containing mucinous differentiation (17/48, or 35%). With respect to the issue of multifocality, there was an average of 2.2 ±1.3 (mean ± SD) malignancies per colon, indicating a high degree of multifocality; nearly half (14/31) of the mice had 3 or more separate cancers.
TABLE 1.
Location and Histologic Features of Carcinomas from Gi2-alpha−/− Mice
| 12–24 weeks of age (n = 9) |
25–53 weeks of age (n = 22) |
Overall: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Non-mucinous | Mucinous | Total No. |
% of total |
Non-mucinous | Mucinous | Total No. |
% of total |
Non-mucinous | Mucinous | Total No. |
% of total |
| Cecum | 13 | 0 | 13 | 65 | 22 | 5 | 27 | 56.3 | 35 | 5 | 40 | 58.8 |
| Proximal | 2 | 1 | 3 | 15 | 4 | 5 | 9 | 18.8 | 8 | 6 | 12 | 17.6 |
| Mid | 3 | 1 | 4 | 20 | 5 | 3 | 8 | 16.7 | 8 | 4 | 12 | 17.6 |
| Distal | 0 | 0 | 0 | 0 | 0 | 4 | 4 | 8.3 | 2 | 4 | 4 | 5.9 |
| Totals | 18 | 2 | 20 | 29.40% | 31 | 17 | 48 | 70.60% | 49 | 19 | 68 | 100% |
Whole colons (n = 123) from Gi2-alpha−/− mice were evaluated for the presence and severity of colitis and colon cancer, and the data grouped into young (12–24 weeks of age) and old (25–53 weeks) age groups as shown. All cancers arise in areas of mucosa that contain at least moderate levels of colitis.
We defined tumors as mucinous if they contained areas of mucin lakes lined at least in part by strips of epithelium; overall, 28% of the cancers showed mucinous differentiation (Fig. 2D). Other notable features of cancers in Gi2-alpha−/− mice include very low-grade histologic features, the absence of obvious polypoid structures, and their tendency to invade downwards into the underlying submucosa and muscularis propria. While inflamed colonic mucosa and sporadic colon cancers often display a loss of goblet cells and/or cytoplasmic mucin vacuoles, many of the nonmucinous tumors in Gi2-alpha−/− mice were composed of monotonous tubular glands containing some goblet cells and many secretory cells with prominent apical vacuoles. Notably, although there are areas of flat dysplasia overlying many of the invasive cancers, in many other examples the overlying epithelium is inflamed but appears to be otherwise uninvolved. Indeed, many of these cancers would not be identifiable at all if looked at endoscopically.
Colonic Epithelium in Gi2-alpha−/− Mice Is Hyperproliferative Before the Onset of Colitis and Is Resistant to Apoptosis
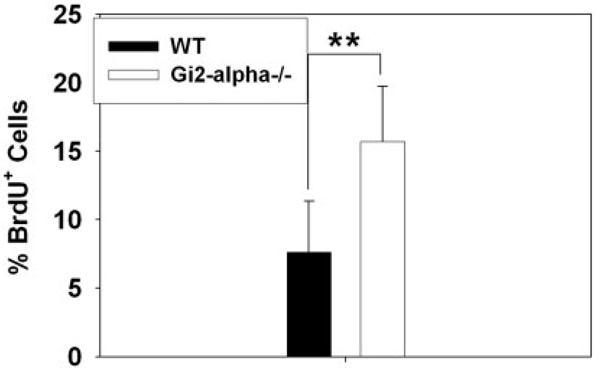
The development of invasive cancers so soon after the onset of colitis led us to test whether the loss of Gi2-alpha signaling in the colonic epithelium might have direct effects on the balance of growth and apoptosis. To that end, we evaluated basal proliferative indices in the colonic epithelium of WT and Gi2-alpha−/− before and after the onset of colitis. As shown in Figure 3, colitis-free 6-week Gi2-al-pha−/− mice exhibited a greater than 2-fold increase in the percentage of BrdU-labeled cells compared to WT littermates (15.68 ± 4.04% versus 7.63 ± 4.03% [mean ± SD], n = 8, P < 0.0001). A similar result was obtained from the inflamed epithelium of old Gi2-alpha−/− mice (mean age 40 weeks), with an average proliferative index of 20.21 ± 11.55% versus WT (7.03 ± 2.68%, n = 3), P < 0.0001, data not shown due to small sample size). The BrdU+ cells in the Gi2-alpha−/−crypts could be found extending as high as two-thirds of the way up the crypt base, in contrast to WT crypts, where the signal did not extend past the bottom 40% of the crypt.
FIGURE 3.
Gi2-alpha−/− colonic epithelium is hyperproliferative before the onset of colitis. Groups of age-matched colitis-free 6-week-old (n = 8) WT and Gi2-alpha−/− mice were injected i.p. with BrdU and the labeled tissues assessed for BrdU incorporation by immunofluorescent staining. The percentage of BrdU + colonic epithelial cells in 10 well-oriented crypts per mouse are expressed asa mean ± SD.**P< 0.01.The data from the 6-week-old experiments are pooled from 2 separate iterations of the experiment.
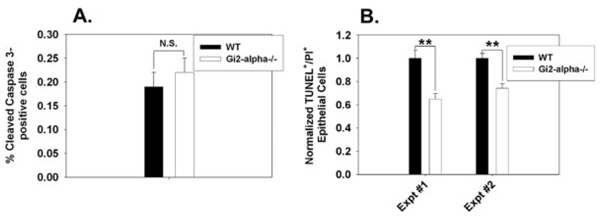
We next tested whether the increased colonic epithelial proliferation observed in colitis-free Gi2-alpha−/− mice might be accompanied by a decrease in apoptosis. We attempted to assess basal levels of apoptosis in WT and Gi2-alpha−/− tissues by performing immunofluorescent TUNEL staining but TUNEL + cells were identified too infrequently to perform meaningful analysis (fewer than 1 per crypt on average, data not shown). We then stained the same untreated tissues for cleaved caspase-3, which resulted in comparable levels of caspase-3 plus epithelial cells (40 medium power fields of crypt epithelium; WT: 0.19 ± 0.03% versus Gi2-alpha: 0.22 ± 0.03%, P = not significant), indicating no significant difference in basal apoptosis rates between WT and Gi2-alpha−/− crypt epithelium.
The natural history of colon cancer in IBD patients suggests that longstanding chronic inflammation predisposes to colon cancer via DNA damage due to oxidative stress.14 Because of the apparently extremely low basal levels of apoptosis in both WT and Gi2-alpha−/− colonic epithelium, we decided to experimentally induce DNA damage in an attempt to provoke either a reparative or apoptotic response in vivo. For in vivo studies we chose the alkylating agent azoxymethane. Intestinal epithelial apoptosis occurs following injection of a single dose of azoxymethane (AOM) in rodents,15 and peak levels of apoptosis have been reported at 6 hours after AOM injection in mice.16 We therefore treated groups of 12–20-week-old mice with 15 mg/kg AOM for 6 hours, performed TUNEL staining on colonic sections, and counted the number of TUNEL+ cells. In 2 separate experiments we consistently observed a significant decrease in the percentage of TUNEL+ cells in Gi2-alpha−/− epithelium compared to WT tissues (Experiment #1: 2-tailed P = 0.0003, Experiment #2: P = 0.00002) (Fig. 4B).
FIGURE 4.
Resistance to short-term azoxymethane-induced apoptosis in Gi2-alpha−/− colonic epithelium. WT and Gi2-alpha−/− mice(n = 3 each and 4 each, in replicate experiments) were left untreated (A), or given i.p. injections of 15 mg/kg azoxymethane and sacrificed 6 hours later. Untreated tissues were stained for cleaved-caspase-3 and azoxymethane-treated tissues were stained for TUNEL. The TUNEL results (normalized mean ± SEM) of 2 experiments are shown. **P < 0.001.
Generation of Stably Transfected Colon Cancer Cells Overexpressing Dominant-Negative (G203T)-Human Gi2-alpha
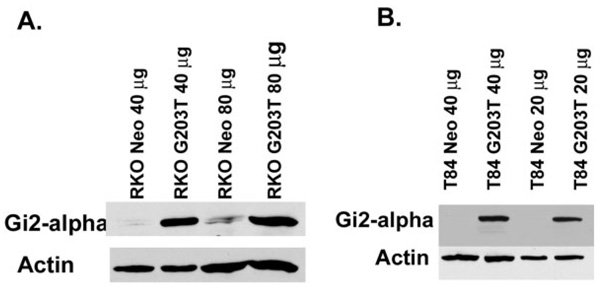
Taken together, these in vivo data suggest that the loss of Gi2-alpha signaling directly affects the balance of growth and apoptosis in the colonic epithelium. To rule out the possibility that the lack of Gi2-alpha indirectly affects epithelial growth via alterations in other adjoining cell types, we generated stably transfected lines of 2 separate human colon cancer cell lines (T84 and RKO) overexpressing the dominant-negative human Gi2-alpha2 point mutant, G203T-hGi2-alpha.17 The pooled G418-resistant clones were found to overexpress Gi2-alpha protein compared to cells stably transfected with the neomycin-resistance plasmid alone (Fig. 5).
FIGURE 5.
Validation of dominant-negative (203T-Gi2-alpha) expressing colon cancer cell lines. Total cellular protein from vector-transfected or G203T-Gi2-alpha-transfected RKO cells (A) or T84 cells (B) was immunoblotted with anti-Gi2-alpha antibodies, demonstrating overexpression of Gi2-alpha in the G203T-h Gi2-alpha-transfected cells
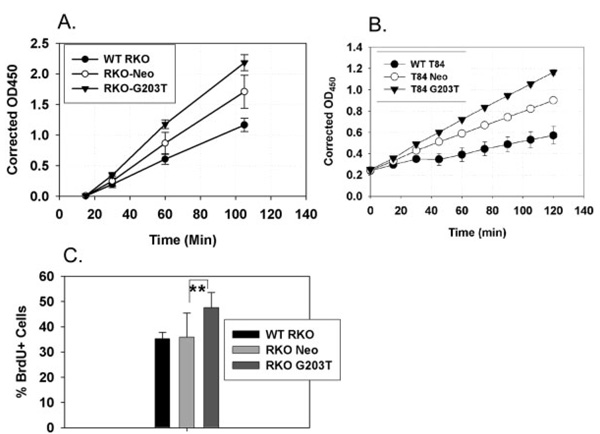
Increased In Vitro Proliferation of T84and RKO Clones
To test the effect of overexpressing dominant-negative Gi2-alpha13 on colonic epithelial cell growth, we first measured basal proliferation rates of wildtype, vector-control, and G203T-Gi2-alpha expressing RKO and T84 cells using the WST-1 tetrazolium salt proliferation assay. Both RKO (Fig. 6A) and T84 (Fig. 6B) cells overexpressing G203T-Gi2-alpha proliferated at a greater rate than either wildtype or vector-control cells. Overall, RKO clones proliferated at a much higher rate than the T84 cells. We corroborated the proliferation results by labeling RKO cells in culture with BrdU, counterstaining with DAPI, and counting BrdU+ cells. As shown in Figure 6C, G203T-Gi2-alpha-expressing RKO cells showed a significantly higher level of BrdU-labeled cells (G203T: 47.54 ± 5.99% versus Neo: 35.8 ± 9.5%, P = 0.004). Attempts to perform BrdU labeling experiments on T84 cultures were hampered by an inability to accurately perform cell counts due to the tightly clustered growth pattern that T84 cells display.
FIGURE 6.
Proliferation of G203T-h Gi2-alpha-expressing human colon cancer cells. Triplicate wells of 1*105 RKO (A) or T84 (B) cells expressing nothing (WT), empty neomycin resistance vector (Neo), or dominant-negative Gi2-alpha (G203T) were starved overnight then incubated with 10 µL/well of WST-1 reagent. Data shown represent the mean ± SD at each timepoint and are representative of at least 3 separate experiments. In C, 6 high-power fields containing at least 200 of each of the RKO clones were assessed for BrdU incorporation. G203T- Gi2-alpha-expressing RKO cells exhibited significantly (**P = 0.0004) higher levels of labeling compared to vector control orWT RKO cells.
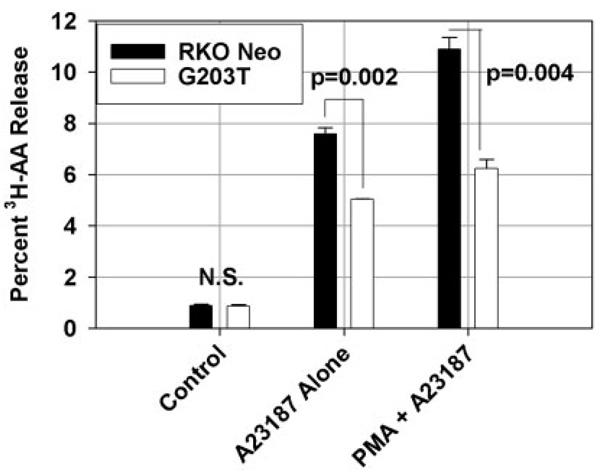
Decreased AA Release from G203T-Gi2-alpha-Expressing RKO Cells
Defects in arachidonic acid metabolism have been implicated in colon carcinogenesis.18,19 Cells lacking Gi2-alpha harbor defects in cPLA2 activation and arachidonate release,17,20 and we have previously shown that colonic stromal myofibroblasts release less AA and produce less PGE2.11 We therefore tested whether RKO cells expressing G203T-Gi2-alpha would also display the same general arachidonic acid release defect. As shown in Figure 7, while basal arachido-nate release was comparable between Neo and G203T trans-fectants, the addition of either 1 µM A23187 alone or in combination with 200 nM PMA resulted in significantly less arachidonate release from G203T-Gi2-alpha-expressing cells. The relative decrease in AA release was comparable to that seen previously in Gi2-alpha−/− myofibroblasts. Therefore, loss of Gi2-alpha signaling either by congenital absence or by overexpression of the dominant-negative mutant results in decreased arachidonic acid mobilization both in epithelial cells and subepithelial myofibroblasts.
FIGURE 7.
Loss of Gi2-alpha signaling in RKO cells results in decreased epithelial arachidonic acid release. Two × 105 RKO-Neo- or G203T-expressing cells were labeled overnight with 0.3 µCi/well 3H-arachidonic acid then treated for 30 minutes with 1 µM A23187 alone or in combination with 200 nM PMA. 3H-ara-chidonicacid release into the supernatant was assayed by scintillation counting. The data shown are means ± SD of duplicate measurements and are representative of 3 separate experiments.
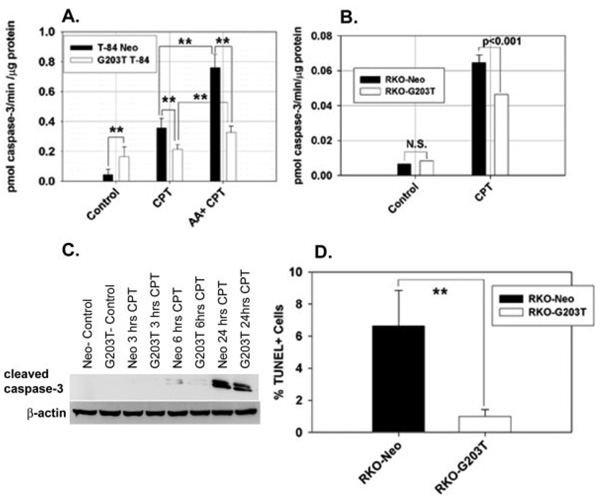
Resistance to Camptothecin-Induced Apoptosis in Cells Expressing Dominant-Negative Gi2-alpha
We noted resistance to the induction of apoptosis in Gi2-alpha−/− mouse colonic epithelium following treatment with azoxymethane in vivo (Fig. 4). To test whether the loss of Gi2-alpha signaling has a direct effect on colonic epithelial cell apoptosis in the absence of other cell types, we treated RKO and T84 cells with 5 µM camptothecin (CPT). CPT is the prototype of a family of chemotherapeutic agents used to treat colon cancer that induce apoptosis by inhibiting topo-isomerase I, which leads to activation of caspase cascades.21 We assessed apoptosis using 3 complementary assays. Surprisingly, the overall basal caspase-3 activity level was somewhat higher in the G203T-expressing T84 cells than in control lines, while there was minimal basal caspase-3 activity in the RKO cell lines. In the absence of Gi2-alpha signaling, alterations in cell death in the absence of DNA damage agents may be directly relevant to tumor formation in vivo. In the presence of 5 µM camptothecin, both T84 and RKO cells expressing G203T-Gi2-alpha had significantly lower caspase-3 activity than vector-control cells. The addition of 20 µM arachidonic acid 16 hours prior to CPT treatment potentiated the caspase-3 activity in both control and Gi2-alpha-expressing cells, consistent with a proapoptotic role for arachidonate in colonic epithelium.
To corroborate these findings, we also ran Western blots for cleaved caspase-3 on the camptothecin-treated RKO cells. A time-course study revealed that the onset of cleaved caspase-3 production in response to camptothecin treatment was ≍6 hours. The amount of cleaved caspase-3 species at 17 and 19 kDa was greater following camptothecin treatment in RKO-Neo cells than in RKO-G203T cells at both 6 and 24 hours (Fig. 8C). Similarly, treatment of RKO cells grown on coverslips and treated with 5 µM CPT led to a greater percentage of RKO-Neo cells that were immunoreactive by TUNEL staining compared to RKO-G023T (Fig. 8D). Collectively, the data indicate that the overexpression of dominant-negative Gi2-alpha renders colon cancer cells more resistant to the induction of apoptosis by camptothecin treatment.
FIGURE 8.
Resistance to campto-thecin-induced caspase-3 activation and apoptosis in cells expressing dominant-negative Gi2-alpha. T84 (A) and RKO (B) cells expressing control vector alone (Neo) or G203T- Gi2-alpha were treated with 5 µM camptothecin for 6 hours alone, or following overnight pretreatment with 20 µM ar-achidonicacid. The data shown are the mean ± SD of triplicate caspase-3 activity measurements and are representative of 3 separate experiments. C: 100 µg of the cell lysates from control and CPT-treated cells at 0,3,6, and 24 hours were separated and blotted with anti-activated caspase-3 Ab (recognizes 19 and 17 kDa fragments). D: CPT-treated Neo and Gi2-alpha-expressing cells were evaluated for apoptosis by TUNEL staining; the data represent the mean ± SD percentage of TUNEL+ cells in 10 medium-powerfields.**P < 0.001.
DISCUSSION
In our colony, we find that the penetrance of colitis is nearly 100% by 7 weeks of age. The original description likened the colitis to that seen in UC, noting the exclusively colonic localization of inflammation, and the worst colitis being present in the distal colon.12 We find that the histopathologic features are somewhat more CD-like, given the patchy distribution of inflammation that is often the most severe in the cecum, in addition to transmural disease and serositis. However, there are important features of CD that are not seen in Gia2−/− mice, which lack mucosal granulomas, prominent fissuring, and ileitis.
The most striking feature of our analysis is the incidence, location, histology, and multifocality of the colon cancers that arise in Gi2-alpha−/− mice. Of the 32 mice older than 6 months at the time of sacrifice, nearly 80% of the animals had colon cancer. More than 75% of the cancers were located in the cecum or ascending colon and were multifocal. Many of these cancers arose out of areas of nonpolypoid dysplasia, although some invasive lesions are overlaid by inflamed but nondysplastic epithelium. These tumors displayed mucinous differentiation nearly 30% of the time. Together these features are very reminiscent of those seen in human patients with IBD-associated colon cancer.22–24 Choi and Zelig3 reviewed the features of IBD-associated cancers in 80 patients (28 CD and 52 UC)—50% of the CD and 36% of the UC cancers were located in the right colon; 22% of the CD patients and 15% of the UC patients had mucinous cancers, and 12% of all patients had multiple synchronous tumors.
There are several animal models of colitis that also develop colon cancer. IL-10 KO mice, like Gi2-alpha−/− mice, develop transmural disease with a high incidence of colon cancer (60% at 6 months, n = 10),25 although the distribution within the colon was not described further. The mucinous differentiation and relatively low-grade features seen in some Gi2-alpha−/− tumors are also seen in IL-10−/− mice. In the initial description of colon cancers in IL-10−/− mice, lesions with these features were not counted as adenocarcinomas, but rather thought to represent colitis cystica profunda, an uncommon nonneoplastic lesion that can mimic colon cancer.26 It is unlikely that all of the mucinous tumors in Gi2-alpha−/− mice represent this benign entity, as several of these mucinous tumors are invasive into adjacent structures such as the pancreas. It should also be noted that the majority of adenocarcinomas that arise are in fact non-mucinous and are composed of irregular, poorly organized glands invading into an inflamed, desmoplastic stroma.
In all segments of the colon, 100% of Smad3-KO mice develop multifocal adenocarcinomas in a Helicobacter infection-dependent manner.27 Review of the histopathologic spectrum of tumors observed in Smad3 mutant mice28 reveals striking similarity to those seen in Gi2-alpha−/− mice. We have performed Helicobacter species-specific PCR on stool samples from our colony and found products specific for H. bilis, H. hepaticus, and H. rodentium (data not shown). We have also previously identified resistance to TGFβ signaling in T-cells from Gi2-alpha−/− mice,29 although there are no differences in Smad3 expression between WT and Gi2-alpha littermates. Because TGFβ is a negative regulator of colonic epithelial proliferation,28 it will be interesting to see whether there is decreased responsiveness to TGFβ in cells overex-pressing dominant-negative Gi2-alpha.
The features of colon cancer in Gi2-alpha−/− mice are also strongly reminiscent of those seen in patients with hereditary nonpolyposis colon cancer (HNPCC), who develop multifocal right-sided mucinous or medullary-type adenocarcinomas arising out of nonpolypoid (villous) adenomatous mucosa.30 All these features (lymphocytic infiltration, proximal location, mucinous differentiation) have been shown to be independent predictors of a tumor being MSI-H (defined as having microsatellite instability at ≥30% of the tested microsatellite markers).31 Although no direct link between Gi2-alpha signaling and mismatch repair has been identified, there is emerging evidence that microsatellite instability is commonly found not only in IBD-associated cancers14 but in the surrounding nondysplastic mucosa as well.32 If the tumors in Gi2-alpha−/− mice are found to exhibit MSI and whether MSI is a particular feature of this model or is a common feature in animal models of chronic colitis, remains to be seen.
A widely held principle in colitis-associated carcinogenesis is that carcinomas arise in areas of inflammation. Indeed, this is true in Gi2-alpha−/− mice. However, the rapid onset of dysplasia and cancer (as early as 12 weeks of age) so soon after the development of colitis prompted us to ask whether the loss of Gi2-alpha has a direct effect on epithelial proliferation and/or apoptosis, in the absence of an inflammatory milieu. We found that the Gi2-alpha−/− epithelium is hyperproliferative in the absence of colitis (Fig. 3). One limitation of this result is that there is evidence of widespread immune activation in Gi2-alpha−/− mice before the onset of histologically identifiable colitis,33 so inflammatory mediators might disrupt epithelial growth regulation in “precolitic” mice. We therefore evaluated proliferative and apoptotic indices in dominant-negative Gi2-alpha-expressing colon cancer cells in vitro and found comparable responses to those seen in vivo (Fig. 6, Fig 8). These data support the contention that Gi2-alpha signaling is a direct negative regulator of colonic epithelial cell growth.
The Gi2-alpha−/− model of colitis-associated colon cancer poses a paradox. The loss of Gi2-alpha results in decreased epithelial (Fig. 7) and stromal11 arachidonic acid release, leading to decreased mucosal PGE2 levels, yet the epithelium is hyperproliferative. How might decreased ara-chidonate be related to the inhibition of apoptosis? Arachidonate has been shown to enhance the activity of intestinal sphingomyelinases that produce proapoptotic ceramides,34 and inhibition of AA release has clear effects on apoptosis and carcinogenesis in vivo. Animals deficient in cPLA2 develop a 5.5-fold greater tumor burden in the azoxymethane model of induced colon carcinogenesis due to significantly decreased colonic epithelial cell apoptosis seen by TUNEL assay, despite lower tissue PGE2 levels.18 We are currently testing whether cell lines deficient in Gi2-alpha harbor decreased sphingomyelinase activity and ceramide generation which may underlie the observed antiapoptotic phenotype.
Acknowledgments
Supported by NIH grants K08 DK59816 and R21 DK071591 (to R.A.E.). L.B. is supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
REFERENCES
- 1.Itzkowitz SH. Molecular biology of dysplasia and cancer in inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35:553–571. doi: 10.1016/j.gtc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Odze RD. Pathology of dysplasia and cancer in inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35:533–552. doi: 10.1016/j.gtc.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Choi P, Zelig MP. Similarity of colorectal cancer in Crohn’s disease and ulcerative colitis: implications for carcinogenesis and prevention. Gut. 1994;35:950–954. doi: 10.1136/gut.35.7.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Itzkowitz SH. Inflammatory bowel disease and cancer. Gastroenterol Clin North Am. 1997;26:129–139. doi: 10.1016/s0889-8553(05)70287-9. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi K, Smale S, Premchand P, et al. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:196–202. doi: 10.1016/s1542-3565(05)00980-8. [DOI] [PubMed] [Google Scholar]
- 6.Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 7.Rubin D, LoSavio A, Yadron N, et al. Aminosalicylate therapy in the prevention of dysplasia and colorectal cancer in ulcerative colitis. Clin Gastroenterol Hepatol. 2006;4:1346–1350. doi: 10.1016/j.cgh.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Terdiman J, Steinbuch M, Blumentals WA, et al. 5-Aminosalicylic acid therapy and the risk of colorectal cancer among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:367–371. doi: 10.1002/ibd.20074. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein C, Nayar G, Hamel A, et al. Study of animal-borne infections in the mucosas of patients with inflammatory bowel disease and population-based controls. J Clin Microbiol. 2003;41:4986–4990. doi: 10.1128/JCM.41.11.4986-4990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan EP, Lichtenstein GR. Chemoprevention: risk reduction with medical therapy of inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35:675–712. doi: 10.1016/j.gtc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Edwards RA, Smock AZ. Defective arachidonate release and PGE2 production in Gia2-deficient intestinal and colonic subepithelial myofi-broblasts. Inflamm Bowel Dis. 2006;12:153–165. doi: 10.1097/01.MIB.0000201100.72191.19. [DOI] [PubMed] [Google Scholar]
- 12.Rudolph U, Finegold MJ, Rich SS, et al. Ulcerative colitis and adeno-carcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995;10:143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 13.Inoue S, Hoshino S, Kukimoto I, et al. Purification and characterization of the G203T mutant alpha i-2 subunit of GTP-binding protein expressed in baculovirus-infected Sf9 cells. J Biochem (Tokyo) 1995;118:650–657. doi: 10.1093/oxfordjournals.jbchem.a124959. [DOI] [PubMed] [Google Scholar]
- 14.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 15.Hirose Y, Yoshimi N, Makita H, et al. Early alterations of apoptosis and cell proliferation in azoxymethane-initiated rat colonic epithelium. Jpn J Cancer Res. 1996;87:575–582. doi: 10.1111/j.1349-7006.1996.tb00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aizu W, Guda K, Nambiar P, et al. p53 and its co-activator p300 are inversely regulated in the mouse colon in response to carcinogen. Toxicol Lett. 2003;144:213–224. doi: 10.1016/s0378-4274(03)00221-2. [DOI] [PubMed] [Google Scholar]
- 17.Winitz S, Gupta SK, Qian NX, et al. Expression of a mutant Gi2 alpha subunit inhibits ATP and thrombin stimulation of cytoplasmic phospho-lipase A2-mediated arachidonic acid release independent of Ca2+ and mitogen-activated protein kinase regulation. J Biol Chem. 1994;269:1889–1895. [PubMed] [Google Scholar]
- 18.Ilsley JN, Nakanishi M, Flynn C, et al. Cytoplasmic phospholipase A2 deletion enhances colon tumorigenesis. Cancer Res. 2005;65:2636–2643. doi: 10.1158/0008-5472.CAN-04-3446. [DOI] [PubMed] [Google Scholar]
- 19.Nakanishi M, Rosenberg DW. Roles of cPLA2alpha and arachidonic acid in cancer. Biochim Biophys Acta. 2006;1761:1335–1343. doi: 10.1016/j.bbalip.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray-Whelan R, Reid JD, Piuz I, et al. The guanine-nucleotide-binding protein subunit G alpha i2 is involved in calcium activation of phospholipase A2. Effects of the dominant negative G alpha i2 mutant, [G203T]G alpha i2, on activation of phospholipase A2 in Chinese hamster ovary cells. Eur J Biochem. 1995;230:164–169. doi: 10.1111/j.1432-1033.1995.tb20547.x. [DOI] [PubMed] [Google Scholar]
- 21.Sordet O, Khan QA, Kohn KW, et al. Apoptosis induced by topoisom-erase inhibitors. Curr Med Chem Anticancer Agents. 2003;3:271–290. doi: 10.2174/1568011033482378. [DOI] [PubMed] [Google Scholar]
- 22.Harpaz N, Talbot IC. Colorectal cancer in idiopathic inflammatory bowel disease. Semin Diagn Pathol. 1996;13:339–357. [PubMed] [Google Scholar]
- 23.Greenstein AJ, Slater G, Heimann TM, et al. A comparison of multiple synchronous colorectal cancer in ulcerative colitis, familial polyposis coli, and de novo cancer. Ann Surg. 1986;203:123–128. doi: 10.1097/00000658-198602000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delaunoit T, Limburg PJ, Goldberg RM, et al. Colorectal cancer prognosis among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:335–342. doi: 10.1016/j.cgh.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 25.Berg DJ, Davidson N, Kuhn R, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guest CB, Reznick RK. Colitis cystica profunda. Review of the literature. Dis Colon Rectum. 1989;32:983–988. doi: 10.1007/BF02552279. [DOI] [PubMed] [Google Scholar]
- 27.Maggio-Price L, Treuting P, Zeng W, et al. Helicobacter infection is required for inflammation and colon cancer in SMAD3-deficient mice. Cancer Res. 2006;66:828–838. doi: 10.1158/0008-5472.CAN-05-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu Y, Richardson JA, Parada LF, et al. Smad3 mutant mice develop metastatic colorectal cancer. Cell. 1998;94:703–714. doi: 10.1016/s0092-8674(00)81730-4. [DOI] [PubMed] [Google Scholar]
- 29.Wu JY, Jin Y, Edwards RA, et al. Impaired TGF-beta responses in peripheral T cells of Galphai2−/− mice. J Immunol. 2005;174:6122–6128. doi: 10.4049/jimmunol.174.10.6122. [DOI] [PubMed] [Google Scholar]
- 30.Gatalica Z, Torlakovic E. Pathology of the hereditary colorectal carcinoma. Fam Cancer. 2007 doi: 10.1007/s10689-007-9146-8. (in press). [DOI] [PubMed] [Google Scholar]
- 31.Jenkins MA, Hayashi S, O’Shea AM, et al. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: a population-based study. Gastroenterology. 2007;133:48–56. doi: 10.1053/j.gastro.2007.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishitsuka T, Kashiwagi H, Konishi F. Microsatellite instability in inflamed and neoplastic epithelium in ulcerative colitis. J Clin Pathol. 2001;54:526–532. doi: 10.1136/jcp.54.7.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohman L, Franzen L, Rudolph U, et al. Immune activation in the intestinal mucosa before the onset of colitis in Galphai2-deficient mice. Scand J Immunol. 2000;52:80–90. doi: 10.1046/j.1365-3083.2000.00759.x. [DOI] [PubMed] [Google Scholar]
- 34.Chan TA, Morin PJ, Vogelstein B, et al. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci USA. 1998;95:681–686. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]