Abstract
BACKGROUND
Anal cancer is an uncommon malignancy in the US; up to 93% of anal cancers are associated with human papillomavirus.
METHODS
Cases diagnosed between 1998 and 2003 from 39 population-based cancer registries were analyzed. The following anal cancer histologies were included in the analysis: squamous cell, adenocarcinoma, and small cell/neuroendocrine carcinomas. Incidence rates were age-adjusted to the 2000 US standard population.
RESULTS
From 1998 through 2003, the annual age-adjusted invasive anal cancer incidence rate was 1.5 per 100,000 persons. Squamous cell carcinoma (SCC) was the most common histology overall, accounting for 18,105 of 21,395 (84.6%) cases of anal cancer. Women had a higher rate of SCC (1.5 per 100,000) than men (1.0). Whites and blacks had the highest incidence rate (1.3), whereas Asians/Pacific Islanders (API) had the lowest rate (0.3). Incidence rates of anal SCC increased 2.6% per year on average. The majority of SCC cases were diagnosed at the in situ or localized stage (58.1%). API were more likely to be diagnosed with regional or distant stage disease than were other racial/ethnic groups (27.5% and 11.8%, respectively). Males had lower 5-year relative survival than females for all stages of disease.
CONCLUSIONS
Rates of anal SCC varied by sex, race, and ethnicity. A higher proportion of API were diagnosed at regional/distant stage. Men had lower 5-year survival rates than women. Continued surveillance and additional research are needed to assess the potential impact of the HPV vaccine on the anal cancer burden in the US.
Keywords: anal cancer, human papillomavirus, incidence rates, squamous cell carcinoma, vaccine
Anal cancer is an uncommon malignancy, accounting for only 1.5% of all gastrointestinal cancers and 4% of cancers involving the lower gastrointestinal tract.1 Although the rate of anal cancer is higher among women than among men, the annual incidence rate has increased for both sexes during the past 3 decades, with men having a higher rate of increase.2,3
Epidemiologic studies using state-of-the-art detection techniques have shown that up to 93% of anal cancers are associated with human papillomavirus (HPV) infection, predominantly oncogenic types 16 (HPV-16) and 18 (HPV-18). This association is specific to squamous cell cancers, including basaloid, cloacogenic, and transitional cell types that account for 70% of all anal cancers in the US.4 Women are more likely to have HPV-positive anal cancer than men.5 Furthermore, the risk of anal cancer is elevated among women with cervical and vulvar cancers, presumably because of a shared exposure to oncogenic HPV infections.6,7
Prophylactic HPV vaccines for the prevention of cervical cancer have the potential to protect against HPV-associated anal cancers. Although such protection is uncertain, prophylactic HPV vaccines against HPV-16 and HPV-18 may prevent squamous cell anal cancers in 86% to 89% of women, 40% to 53% of heterosexual men, and 95% of young, human immunodeficiency virus (HIV)-positive men who have sex with men (MSM).8 Defining the current burden of anal cancer in the US is key to the precise estimation of the potential impact of the HPV vaccine on anal cancer incidence. In this analysis, we examine anal cancer incidence and survival in the US population, with a focus on HPV-associated (squamous cell, transitional cell, basaloid, and cloacogenic) histologic types.
MATERIALS AND METHODS
Included in the analysis were anal cancer data collected between 1998 and 2003 from population-based cancer registries in 39 states that participate in the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR), the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program, or both, covering 83% of the US population. A detailed description of registries included is provided elsewhere.9
Anal cancer cases included in this analysis were limited to those with microscopically confirmed squamous cell carcinoma (SCC) and transitional cell carcinomas (International Classification of Diseases [ICD]-O-3 codes 8050–8084, 8120–8122, and 8125–8131), basaloid and cloacogenic carcinomas (8123–8124), small cell carcinomas and neuroendocrine tumors (8013, 8041–8045, and 8240–8246), and adenocarcinomas (8140–8149, 8160–8162, 8190–8221, 8260–8337, 8350–8551, 8570–8576, and 8940–8941) of the anus, anal canal, and anorectum (C21.0–C21.8), together with squamous cell, transitional cell, basaloid, and cloacogenic carcinomas of the rectum (C20.9). True rectal SCCs are rare but may be associated with HPV,10 and overlapping SCCs of the anus may be misclassified as rectal SCCs; therefore, they were included in the analysis.4,11 All other histologies were excluded from the analysis.
For the purpose of this analysis, we combined SCCs with basaloid, cloacogenic, and transitional cell carcinomas. These histologies are all considered to be HPV-associated malignancies. Analyses of in situ carcinomas were limited to HPV-associated histologic types. Anal intraepithelial neoplasia grade III (AIN III; ICD-O-3 code 8077) cases were not included in analyses of in situ carcinomas, because they were not available in the analytic data file for most cancer registries. Cancers diagnosed before 2001 were staged by use of SEER Summary Stage 1977, and cancers diagnosed during 2001 to 2003 were staged by use of SEER Summary Stage 2000.12,13 These 2 systems were combined to produce a single SEER summary staging variable with the following categories: localized (confined to the anus, anal canal, anorectum, or rectum), regional (extension to adjacent tissues or regional lymph nodes), distant (metastasis to other areas of the body), and unknown stage.
SEER*Stat statistical software (version 6.2.4) was used to calculate both annual and average annual age-adjusted incidence rates expressed per 100,000 population.14 All rates were age-adjusted by 5-year age groups for the purpose of comparing anal cancer cases by demographic, pathologic, and clinical variables of interest. Confidence intervals (95%) for the rates were calculated by the Gamma method using the Tiwari modification.15 The 2000 US standard population was used for age standardization. For most analyses, rates were suppressed when the category had <16 cases. Analyses were conducted by tumor behavior (in situ and invasive), histology, tumor stage (in situ, localized, regional, distant, and unstaged), sex, age at diagnosis (<50 years, 50–64 years, and ≥65 years), race (white, black, and Asian/Pacific Islander), ethnicity (Hispanic and non-Hispanic), and geographic region. Race and ethnicity were not mutually exclusive. The population coverage was 98 % for the Northeast and the Midwest, 63% for the South, and 88% for the West.
Temporal trends in the incidence of anal SCC were evaluated by use of the SEER 13 registries database, which contains incident cases from 1992 through 2004.16 Trends in rates were measured by use of the estimated annual percentage change (APC), which assumes that rates change by a constant percentage each year during a time period. SEER JoinPoint regression software (version 3.0) was used to examine changes in trend.17
A relative survival analysis was conducted through use of the SEER 17 registries database.18 Only patients who had microscopically confirmed anal cancer as the first primary tumor were included. Cases ascertained based on a death certificate only or through an autopsy were excluded. Survival was analyzed by tumor stage, age, sex, and race.
RESULTS
Incidence Rates of Invasive Anal Cancer by Histology
A total of 21,395 cases of invasive anal cancer (average annual count, 3566) and 2573 cases of in situ anal SCC (average annual count, 429) were diagnosed during 1998 through 2003. SCC was the most common histologic type, accounting for 85% of all anal malignancies studied, followed by adenocarcinomas (14%) and small cell/neuroendocrine cancers (1%) (Table 1). The rate of invasive anal cancer increased with increasing age for all histologic types studied, although the distribution of each histology varied by age group. SCC accounted for 93% of anal malignancies diagnosed among patients aged <50 years at diagnosis, but comprised only 78% of anal cancers among patients diagnosed at ≥65 years of age. By contrast, adenocarcinomas accounted for 6% of anal cancers among patients aged <50 years, but 21.2% of anal cancers among patients aged ≥65 years.
TABLE 1.
Count and Incidence Rates of Invasive Anal Cancer by Histology and by Age, Race, Ethnicity, Sex, and Region—US, 1998 to 2003*
| Total |
Squamous† |
Adenocarcinoma |
Small Cell/Neuroendocrine |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Count (% of Total) | Rate‡ | 95% CI | Count | Rate | 95% CI | Count | Rate | 95% CI | Count | Rate | 95% CI | |
| Total | 21,395 (100) | 1.52 | 1.50–1.54 | 18,105 | 1.28 | 1.27–1.30 | 3080 | 0.22 | 0.21–0.23 | 210 | 0.02 | 0.01–0.02 |
| Age, y | ||||||||||||
| <50 | 5077 (23.7) | 0.50 | 0.49–0.52 | 4716 | 0.47 | 0.45–0.48 | 305 | 0.03 | 0.03–0.03 | 56 | 0.01 | 0.00–0.01 |
| 50–64 | 6723 (31.4) | 3.15 | 3.07–3.22 | 5921 | 2.77 | 2.70–2.84 | 732 | 0.34 | 0.32–0.37 | 70 | 0.03 | 0.03–0.04 |
| ≥65 | 9595 (44.8) | 5.38 | 5.28–5.49 | 7468 | 4.19 | 4.10–4.29 | 2043 | 1.14 | 1.09–1.19 | 84 | 0.05 | 0.04–0.06 |
| Sex | ||||||||||||
| Male | 8233 (38.5) | 1.29 | 1.26–1.31 | 6497 | 1.00 | 0.98–1.03 | 1645 | 0.27 | 0.26–0.29 | 91 | 0.01 | 0.01–0.02 |
| Female | 13,162 (61.5) | 1.70 | 1.67–1.73 | 11,607 | 1.51 | 1.48–1.54 | 1435 | 0.18 | 0.17–0.19 | 119 | 0.02 | 0.01–0.02 |
| Race§ | ||||||||||||
| White | 18,805 (87.9) | 1.53 | 1.51–1.55 | 15,995 | 1.31 | 1.29–1.33 | 2637 | 0.21 | 0.20–0.22 | 173 | 0.01 | 0.01–0.02 |
| Black | 2009 (9.4) | 1.58 | 1.51–1.66 | 1654 | 1.27 | 1.21–1.34 | 325 | 0.29 | 0.26–0.32 | 30 | 0.02 | 0.02–0.03 |
| API | 237 (1.1) | 0.51 | 0.45–0.59 | 157 | 0.33 | 0.28–0.39 | 78 | 0.18 | 0.14–0.22 | ‖ | ‖ | ‖ |
| Ethnicity | ||||||||||||
| Non-Hispanic | 19,895 (93.0) | 1.53 | 1.51–1.55 | 16,844 | 1.30 | 1.28–1.32 | 2,863 | 0.22 | 0.21–0.23 | 188 | 0.02 | 0.01–0.02 |
| Hispanic¶ | 1500 (7.0) | 1.43 | 1.36–1.51 | 1261 | 1.19 | 1.12–1.26 | 217 | 0.22 | 0.19–0.25 | 22 | 0.02 | 0.01–0.03 |
| Region | ||||||||||||
| Northeast | 4832 (22.6) | 1.45 | 1.40–1.49 | 4088 | 1.23 | 1.19–1.27 | 698 | 0.20 | 0.19–0.22 | 46 | 0.01 | 0.01–0.02 |
| Midwest | 5186 (24.2) | 1.36 | 1.32–1.39 | 4324 | 1.13 | 1.10–1.17 | 814 | 0.21 | 0.20–0.23 | 48 | 0.01 | 0.01–0.02 |
| South | 6418 (30.0) | 1.67 | 1.63–1.71 | 5495 | 1.43 | 1.39–1.47 | 857 | 0.22 | 0.21–0.24 | 66 | 0.02 | 0.01–0.02 |
| West | 4959 (23.2) | 1.61 | 1.57–1.66 | 4198 | 1.36 | 1.32–1.40 | 711 | 0.24 | 0.22–0.26 | 50 | 0.02 | 0.01–0.02 |
95% CI indicates 95% confidence interval; API, Asian/Pacific Islander.
Data are from 39 population-based cancer registries that participate in the National Program of Cancer Registries and/or the National Cancer Institute’s Surveillance, Epidemiology, and End Results program and meet the high-quality data criteria.10 These registries cover 83% of the US population.
Includes squamous cell carcinoma of the anus, anal canal, and rectum and the squamous cell variants classified as transitional cell, basaloid, and cloacogenic.
Rates are per 100,000 population and are age-adjusted to the 2000 US standard population (19 age groups, Census P25-1130).
Race counts do not sum to total because cases in other race categories were excluded.
Rates were suppressed if <16 cases were reported.
Hispanic origin is not mutually exclusive of race categories (white, black, Asian/Pacific Islander).
The rate of invasive anal cancer was higher among women than among men for all anal cancers combined and for SCCs. There was no difference observed in the rate of all anal cancers combined and of anal SCC between blacks and whites. Blacks had a significantly higher rate of small cell/neuroendocrine cancers than did whites (rate ratio [RR] of 1.68; P<.05, data not shown). The rate of adenocarcinoma was significantly higher among blacks than among whites (RR of 1.36; P<.05; data not shown). Asians/Pacific Islanders had a significantly lower rate of all anal cancers combined and of each histologic subtype studied, although the number of Asians/Pacific Islanders with small cell/neuroendocrine cancers was too small to allow calculation of rates for that histologic subtype. Non-Hispanics had a significantly higher rate of anal SCC than Hispanics, but the rate of adenocarcinomas and small cell/neuroendocrine cancers was equivalent between the 2 groups. The rate of all invasive anal cancers combined and of invasive anal SCC was highest in the South. There was no regional variation in the rate of adenocarcinomas and small cell/neuroendocrine tumors.
Incidence Rates of Invasive Anal SCC
For invasive anal SCCs, the rate increased with age among both men and women (Table 2). Among men, the rate of SCC was significantly higher among blacks (RR of 1.23; P < .05) than among whites. By contrast, black women had a significantly lower rate (RR of 0.81; P < .05) of SCC than did white women. Asian/Pacific Islander men (RR of 0.20; P < .05) and women (RR of 0.28; P <.05) had a significantly lower rate of SCC than did whites. Hispanic men had a significantly lower rate of SCC than non-Hispanic men (RR of 0.76; P < .05), whereas there was no statistically significant difference in the rate of SCC between Hispanic and non-Hispanic women.
TABLE 2.
Count and Rates of Invasive Squamous Cell* Anal Cancer by Sex andby Age, Race, Ethnicity, and Region—US, 1998 to 2003†
| Male |
Female |
|||||||
|---|---|---|---|---|---|---|---|---|
| Count | Rate‡ | 95% CI | RR | Count | Rate | 95% CI | RR | |
| Total | 6497 | 1.00 | 0.98–1.03 | 11,608 | 1.51 | 1.48–1.54 | ||
| Age, y | ||||||||
| <50 | 2185 | 0.44 | 0.42–0.45 | 1.00 | 2531 | 0.50 | 0.48–0.52 | 1.00 |
| 50–64 | 2098 | 2.03 | 1.95–2.12 | 4.66§ | 3823 | 3.46 | 3.35–3.57 | 6.97§ |
| ≥65 | 2214 | 3.01 | 2.88–3.14 | 6.90§ | 5254 | 5.01 | 4.88–5.15 | 10.09§ |
| Race | ||||||||
| White | 5559 | 0.99 | 0.96–1.01 | 1.00 | 10,436 | 1.58 | 1.54–1.61 | 1.00 |
| Black | 743 | 1.21 | 1.12–1.31 | 1.23§ | 911 | 1.28 | 1.20–1.37 | 0.81§ |
| API | 45 | 0.20 | 0.14–0.27 | 0.20§ | 112 | 0.43 | 0.36–0.52 | 0.28§ |
| Ethnicity | ||||||||
| Non-Hispanic | 6067 | 1.02 | 1.00–1.05 | 1.00 | 10,777 | 1.53 | 1.50–1.56 | 1.00 |
| Hispanic‖ | 430 | 0.78 | 0.70–0.86 | 0.76§ | 831 | 1.50 | 1.40–1.61 | 0.98 |
| Region | ||||||||
| Northeast | 1545 | 1.03 | 0.98–1.08 | 1.00 | 2543 | 1.38 | 1.33–1.44 | 1.00 |
| Midwest | 1438 | 0.82 | 0.78–0.86 | 0.80§ | 2886 | 1.39 | 1.34–1.44 | 1.01 |
| South | 1907 | 1.08 | 1.03–1.13 | 1.05 | 3588 | 1.72 | 1.66–1.77 | 1.24§ |
| West | 1607 | 1.10 | 1.05–1.16 | 1.07 | 2591 | 1.57 | 1.51–1.63 | 1.14§ |
95% CI indicates 95% confidence interval; RR, rate ratio; API, Asian/Pacific Islander.
Includes squamous cell carcinoma of the anus, anal canal, and rectum and the squamous cell variants classified as transitional cell, basaloid, and cloacogenic.
Data are from 39 population-based cancer registries that participate in the National Program of Cancer Registries and/or the National Cancer Institute’s Surveillance, Epidemiology, and End Results program and meet the high-quality data criteria.10 These registries cover 83% of the US population.
Rates are per 100,000 population and are age-adjusted to the 2000 US standard population (19 age groups, Census P25-1130).
Significant at P ≤.05.
Hispanic origin is not mutually exclusive of race categories (white, black, API).
Among men, the rate of invasive anal SCC was significantly lower in the Midwest (RR of 0.80; P < .05) than in the Northeast (Table 2). Among women, the rate was significantly higher in the South (RR of 1.24; P <.05) and the West (RR of 1.14; P <.05) compared with the Northeast. Compared with the rate for males, the rate of anal SCC was higher among females in all age, race, ethnic, and geographic categories studied, with the exception of blacks, among whom the rate was equivalent between men and women.
Age-specific Incidence Rates of Invasive Anal SCC
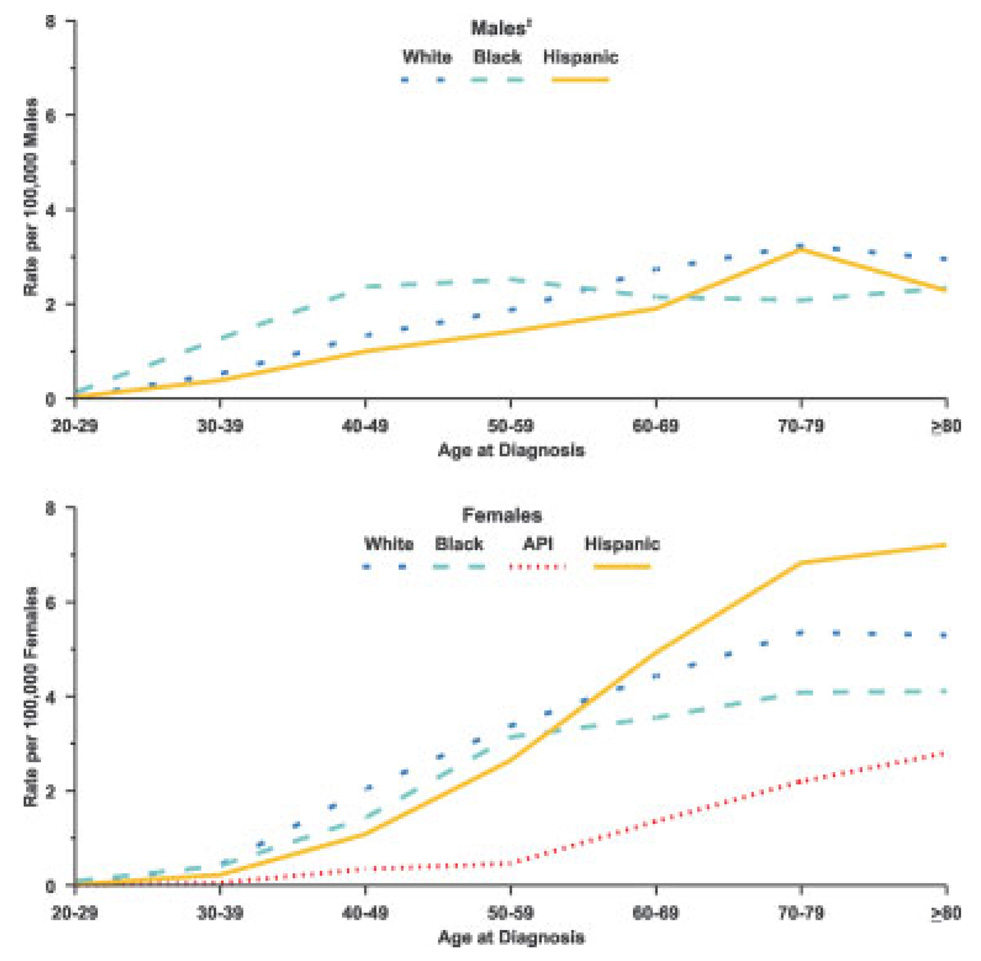
Among women, whites had the highest age-specific rate of invasive anal SCC between the ages of 20 and 60 years (Fig. 1), whereas Hispanic women had the highest rate above age 60 years. The rate of anal SCC was low among Asian/Pacific Islander women, but it increased markedly after age 55 years. Among men, blacks had the highest rate of SCC to approximately age 60 years, but black men had the lowest rate beyond age 70 years. White men had the highest rate beyond age 70 years.
FIGURE 1.
Age-specific incidence of invasive squamous cell anal cancer by sex and by race and ethnicity according to the National Program of Cancer Registries and Surveillance, Epidemiology, and End Results Program Registries, US, 1998 through 2003, are shown. Invasive squamous cell anal cancer includes squamous cell carcinoma of the anus, anal canal, and rectum and the squamous cell variants classified as transitional cell, basaloid, and cloacogenic. Hispanic origin is not mutually exclusive of race categories (white, black, and Asian/Pacific Islander [API]). Age is given in years. ‡Asian/Pacific Islander category was excluded for males because of insufficient sample size.
Time-trends of Invasive Anal SCC
Invasive anal SCC rates in the 13 SEER regions increased significantly from 1992 through 2004, by 2.7% per year among males and by 2.8% per year among females (Table 3). During the same time period, a marked annual increase in the rate of in situ tumors was observed among females (4.0% per year); among males the rate was level. Rates of invasive tumors increased by an estimated 3.0% per year among whites and 3.8% per year among blacks during the 13-year period of observation. The APC was particularly sharp among black females, among whom rates increased by 4.3% per year. In contrast, rates among Hispanics did not change significantly from 1992 through 2004, although rates in this ethnic group were based on fewer than 16 cases annually. Among women who were aged <50 years at the time of diagnosis, the rate of SCC increased sharply during the time period between 1992 and 1998 (APC of 14.3%), but it plateaued thereafter. Among men who were <50 years of age, rates increased during the entire study period (APC of 4.5%). Rates among men aged ≥65 years increased by 2.9% per year, whereas the increase in rates among men ages 50 to 64 years was smaller and nonsignificant. In contrast, rates for women ages 50 to 64 years increased by a substantial 4.7% per year since 1992, whereas rates among women aged ≥65 years remained unchanged.
TABLE 3.
APC for Squamous Cell* Anal Cancer and Incidence by Sex, Age at Diagnosis, Race, and Ethnicity—13 SEER Areas, 1992 to 2004†
| Total |
Males |
Females |
||||
|---|---|---|---|---|---|---|
| APC | 95% CI | APC | 95% CI | APC | 95% CI | |
| Tumor behavior | ||||||
| In situ | 1.0 | −1.8–3.9 | 0.0 | −3.6–3.8 | 4.0‡ | 0.6–7.5 |
| Invasive | 2.6§ | 2.0–3.3 | 2.7§ | 1.3–4.2 | 2.8‡ | 1.8–3.7 |
| Invasive Tumors | ||||||
| Age, y | ||||||
| <50 | 11.8‡ | 5.8–18.1 | 4.5‡ | 1.4–7.7 | 14.3‡ | 5.9–23.3 |
| (1992–1998) | (1992–2004) | (1992–1998) | ||||
| 0.0 | −4.5–4.6 | −0.7 | −6.1–5.1 | |||
| (1998–2004) | (1998–2004) | |||||
| 50–64 | 3.2‡ | 1.7–4.8 | 0.9 | −1.7–3.6 | 4.7‡ | 2.6–6.9 |
| ≥65 | 1.0 | −0.1–2.2 | 2.9‡ | 1.1–4.9 | 0.3 | −1.3–1.9 |
| Race§ | ||||||
| White | 3.0‡ | 2.0–4.0 | 3.0‡ | 1.1–4.9 | 3.2‡ | 2.0–4.3 |
| Black | 3.8‡ | 1.2–6.4 | 2.9 | −1.6–7.6 | 4.3‡ | 1.7–6.9 |
| Ethnicity | ||||||
| Hispanic‖ | 0.4 | −3.7–4.7 | −0.9¶ | −8.0–6.7 | 1.8¶ | −3.4–7.2 |
| Non-Hispanic | 3.0§ | 2.4–3.6 | 3.4‡ | 1.8–5.0 | 3.0‡ | 2.2–3.8 |
APC indicates annual percentage change; SEER, Surveillance, Epidemiology, and End Results program; 95% CI, 95% confidence interval.
Includes squamous cell carcinoma of the anus, anal canal, and rectum and the squamous cell variants classified as transitional cell, basaloid, and cloacogenic.
Data are from 13 area cancer registries that participated in the SEER program and met high-quality data criteria (San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta, San Jose-Monterey, Los Angeles, Alaska Native Registry, and rural Georgia).10 These registries cover 14% of the US population.
The estimated APC is significantly different from 0 (P ≤.05).
The Asian/Pacific Islander category was excluded due to small sample size.
Hispanic origin is not mutually exclusive from race categories (white, black, Asian/Pacific Islander).
Some of the annual age-adjusted rates used for calculating trends were based on <16 cases and should be interpreted with caution.
Stage Distribution of Anal SCC
The majority (58%) of anal SCCs were diagnosed at an early (in situ or localized) stage independent of age (Table 4). A greater proportion of those patients aged <50 years at diagnosis were found to have in situ SCC (23%) than did patients ages 50 to 64 years (10%) and those aged ≥65 years (6%). Asians/Pacific Islanders were more often initially diagnosed with regional or distant stage disease (28% and 12%, respectively) than were blacks (24% and 7%, respectively) or whites (22% and 7%, respectively). A higher proportion of men were initially diagnosed with in situ stage disease (17%) than were women (10%).
TABLE 4.
Stage* Distribution of Squamous Cell† Anal Cancer by Age, Race, Ethnicity, Sex, and Region—US, 1998 to 2003‡
| In Situ |
Localized |
Regional |
Distant |
Unstaged |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Count | %§ | Rate‖ | 95% CI | Count | % | Rate | 95% CI | Count | % | Rate | 95% CI | Count | % | Rate | 95% CI | Count | % | Rate | 95% CI | |
| Total | 2573 | 12.4 | 0.18 | 0.18–0.19 | 9434 | 45.6 | 0.67 | 0.66–0.68 | 4595 | 22.2 | 0.33 | 0.32–0.34 | 1377 | 6.7 | 0.10 | 0.09–0.10 | 2699 | 13.1 | 0.19 | 0.18–0.20 |
| Age, y | ||||||||||||||||||||
| <50 | 1373 | 22.5 | 0.14 | 0.13–0.14 | 2581 | 42.4 | 0.26 | 0.25–0.27 | 1197 | 19.7 | 0.12 | 0.11–0.13 | 323 | 5.3 | 0.03 | 0.03–0.04 | 615 | 10.1 | 0.06 | 0.06–0.07 |
| 50–64 | 689 | 10.4 | 0.32 | 0.30–0.35 | 3104 | 47.0 | 1.45 | 1.40–1.50 | 1539 | 23.3 | 0.72 | 0.68–0.76 | 458 | 6.9 | 0.21 | 0.20–0.24 | 820 | 12.4 | 0.38 | 0.36–0.41 |
| ≥65 | 511 | 6.4 | 0.29 | 0.26–0.31 | 3749 | 47.0 | 2.11 | 2.04–2.18 | 1859 | 23.3 | 1.04 | 1.00–1.09 | 596 | 7.5 | 0.34 | 0.31–0.36 | 1264 | 15.8 | 0.71 | 0.67–0.75 |
| Race | ||||||||||||||||||||
| White | 2161 | 11.9 | 0.18 | 0.17–0.19 | 8404 | 46.3 | 0.69 | 0.67–0.70 | 4036 | 22.2 | 0.33 | 0.32–0.34 | 1200 | 6.6 | 0.10 | 0.09–0.10 | 2355 | 13.0 | 0.19 | 0.18–0.20 |
| Black | 287 | 14.8 | 0.20 | 0.18–0.23 | 804 | 41.4 | 0.61 | 0.57–0.66 | 456 | 23.5 | 0.35 | 0.32–0.38 | 141 | 7.3 | 0.11 | 0.09–0.13 | 253 | 13.0 | 0.20 | 0.18–0.23 |
| API | 21 | 11.8 | 0.03 | 0.02–0.05 | 69 | 38.8 | 0.15 | 0.12–0.19 | 49 | 27.5 | 0.10 | 0.07–0.13 | 21 | 11.8 | 0.04 | 0.03–0.06 | 18 | 10.1 | 0.04 | 0.03–0.07 |
| Ethnicity | ||||||||||||||||||||
| Non-Hispanic | 2375 | 12.4 | 0.19 | 0.18–0.20 | 8820 | 45.9 | 0.68 | 0.67–0.70 | 4281 | 22.3 | 0.33 | 0.32–0.34 | 1267 | 6.6 | 0.10 | 0.09–0.10 | 2476 | 12.9 | 0.19 | 0.18–0.20 |
| Hispanic¶ | 198 | 13.6 | 0.14 | 0.12–0.16 | 614 | 42.1 | 0.57 | 0.52–0.62 | 314 | 21.5 | 0.30 | 0.26–0.34 | 110 | 7.5 | 0.11 | 0.09–0.13 | 223 | 15.3 | 0.21 | 0.18–0.24 |
| Sex | ||||||||||||||||||||
| Male | 1294 | 16.6 | 0.19 | 0.18–0.20 | 3487 | 44.8 | 0.53 | 0.52–0.55 | 1628 | 20.9 | 0.25 | 0.24–0.26 | 425 | 5.5 | 0.07 | 0.06–0.07 | 957 | 12.3 | 0.15 | 0.14–0.16 |
| Female | 1279 | 9.9 | 0.17 | 0.16–0.18 | 5946 | 46.1 | 0.78 | 0.76–0.80 | 2967 | 23.0 | 0.39 | 0.37–0.40 | 952 | 7.4 | 0.12 | 0.12–0.13 | 1742 | 13.5 | 0.22 | 0.21–0.23 |
| Region | ||||||||||||||||||||
| Northeast | 575 | 12.3 | 0.18 | 0.16–0.19 | 2157 | 46.3 | 0.65 | 0.62–0.68 | 1048 | 22.5 | 0.32 | 0.30–0.34 | 297 | 6.4 | 0.09 | 0.08–0.10 | 586 | 12.6 | 0.17 | 0.16–0.19 |
| Midwest | 553 | 11.3 | 0.15 | 0.13–0.16 | 2404 | 49.3 | 0.63 | 0.61–0.66 | 1117 | 22.9 | 0.29 | 0.28–0.31 | 301 | 6.2 | 0.08 | 0.07–0.09 | 502 | 10.3 | 0.13 | 0.12–0.14 |
| South | 736 | 11.8 | 0.20 | 0.18–0.21 | 2776 | 44.6 | 0.72 | 0.70–0.75 | 1252 | 20.1 | 0.33 | 0.31–0.35 | 347 | 5.6 | 0.09 | 0.08–0.10 | 1120 | 18.0 | 0.29 | 0.28–0.31 |
| West | 709 | 14.4 | 0.22 | 0.20–0.24 | 2097 | 42.7 | 0.68 | 0.65–0.71 | 1178 | 24.0 | 0.38 | 0.36–0.40 | 432 | 8.8 | 0.14 | 0.13–0.15 | 491 | 10.0 | 0.16 | 0.15–0.18 |
95% CI indicates 95% confidence interval; API, Asian/Pacific Islander.
For 1998 to 2000, the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Summary Stage 1977 was used; for 2001 to 2003, SEER Summary Stage 2000 was used. Data for the 2 staging systems are combined because the differences observed in comparative analysis were minimal.13, 14
Includes squamous cell carcinoma of the anus, anal canal, and rectum and the squamous cell variants classified as transitional cell, basaloid, and cloacogenic.
Data are from 39 population-based cancer registries that participate in the National Program of Cancer Registries and/or the SEER program and meet the high-quality data criteria.10 These registries cover 83% of the US population for 1998–2003.
Proportion of each category by stage of cancer.
Rates are per 100,000 population and are age–adjusted to the 2000 US standard population (19 age groups, Census P25-1130).
Hispanic origin is not mutually exclusive of race categories (white, black, API).
Five-year Relative Survival of Anal SCC
Overall, the 5-year relative survival among persons with anal SCC decreased with advancing stage at diagnosis (Table 5). Individuals aged <50 years had lower 5-year relative survival for in situ disease than did those diagnosed at older ages. Individuals aged ≥65 years had lower 5-year relative survival when diagnosed with regional and distant stage disease than did younger individuals. Blacks had a lower 5-year relative survival for in situ and localized disease than did whites. Males had lower 5-year relative survival than did females for all stages of disease.
TABLE 5.
Five-Year Relative Survival Among Patients With Squamous Cell* Anal Cancer, by Stage† and by Age, Race, and Sex—US, 1998 to 2003‡
| In Situ |
Localized |
Regional |
Distant |
Unstaged |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Count | Survival, % | Count | Survival, % | Count | Survival, % | Count | Survival, % | Count | Survival, % | |
| Total | 572 | 81.2 | 1875 | 81.8 | 1014 | 59.2 | 391 | 35.7 | 397 | 57.4 |
| Age, y | ||||||||||
| <50 | 335 | 74.8 | 575 | 79.4 | 258 | 66.7 | 101 | 39.3 | 90 | 60.0 |
| 50–64 | 131 | 93.1 | 630 | 80.7 | 390 | 66.9 | 145 | 36.9 | 130 | 65.0 |
| ≥65 | 106 | 83.4 | 670 | 84.0 | 366 | 44.1 | 145 | 26.1 | 177 | 49.6 |
| Race | ||||||||||
| White | 481 | 80.8 | 1641 | 82.6 | 878 | 58.9 | 335 | 35.9 | 339 | 58.1 |
| Black | 79 | 77.9 | 184 | 73.1 | 110 | 59.0 | 41 | 35.2 | 50 | 55.9 |
| API | 9 | § | 42 | 86.1 | 20 | 49.9 | 11 | § | 8 | 46.1 |
| Sex | ||||||||||
| Male | 320 | 73.7 | 732 | 75.5 | 403 | 51.9 | 124 | 23.9 | 157 | 50.6 |
| Female | 252 | 92.9 | 1143 | 85.6 | 611 | 63.9 | 267 | 41.1 | 240 | 62.5 |
API indicates Asian/Pacific Islander.
Includes squamous cell carcinoma of the anus, anal canal, and rectum and the squamous cell variants classified as transitional cell, basaloid, and cloacogenic.
For 1998 to 2000, the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Summary Stage 1977 was used; for 2001 to 2003, SEER Summary Stage 2000 was used. Data for the 2 staging systems are combined, because the differences observed in comparative analysis were minimal.13,14
Data are from17 area cancer registries that participated in the SEER program and met high-quality data criteria (San Francisco [SF], Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta, San Jose-Monterey [SJM], Los Angeles [LA], Alaska Native Registry and rural Georgia, California (excluding SF/SJM/LA), Kentucky, Louisiana, and New Jersey).
Intervals were insufficient to produce the statistic.
DISCUSSION
A key finding in this and other studies is the higher incidence of HPV-associated anal cancer among women than among men.2,3,19 Risk factors for anal cancer among women include an increasing number of sexual partners, a history of anogenital warts, a previous history of cervical intraepithelial neoplasia of type 3 or of cervical cancer, a history of current smoking at the time of diagnosis, a history of anal intercourse, HIV-positive status, and immunosuppression. 8,20–27
HPV is known to be a necessary cause for 100% of cervical cancers,28 and HPV is the presumed shared etiologic agent that leads to the subsequent increased risk for anal cancer. Anal intercourse is among the presumed mechanisms by which HPV is introduced into the anal canal, although the percentage of women with anal HPV infections or with anal cancer who engage in anal intercourse has been found to be low in some studies.8,23 The low percentage may be because of underreporting of anal intercourse by study participants, or there may be other means by which HPV is introduced to the anal canal (ie, by the use of sex toys or auto inoculation via the fingers or vaginal discharge).8,29
Men share many of the same risk factors for anal cancer as women. In addition, MSM have been found to be at high risk for anal cancer.20,22,23 The incidence of anal cancer among MSM has been estimated to be 35 per 100,000.27 Men who practice receptive anal intercourse are generally at higher risk.20,22,23 Men with HIVare also at increased risk for anal cancer.27
To our knowledge, few studies to date have described the burden of anal cancer among racial and ethnic minorities in detail. In accord with our findings, Frisch and Goodman30 reported that Asians/Pacific Islanders had a significantly lower incidence of anal SCCs than did Hawaiian whites and US mainland whites. Cress and Holly11 also found that the incidence of anal SCCs was lower among Asians/Pacific Islanders than among other racial/ethnic groups in California. Asians/Pacific Islanders have a low incidence of HIV31 and sexually transmitted diseases (STDs) such as chlamydia and syphilis,32 as well as a lower prevalence of smoking than among other racial and ethnic groups.33 The lower incidence of STDs and the lower prevalence of these risk factors may contribute to the lower incidence of anal cancer in this population. The increased incidence of anal SCC after the age of 50 to 59 years among Asian/Pacific Islander women may be attributable to delayed onset of sexual activity and acquisition of HPV in this population or to viral latency.30
Hispanic men had a lower incidence of anal SCC than did non-Hispanic men, but a similar difference was not observed between Hispanic and non-Hispanic women. Cress and Holly also found that Hispanic women had a higher rate of anal cancer than Hispanic men, and that Hispanics overall had a lower incidence of anal cancer than whites and blacks. Overall, Hispanics have a lower incidence of STDs, such as chlamydia,32 and a lower prevalence of smoking than other racial/ethnic groups.33 However, both Hispanic men and women have a higher incidence of HIV than do whites. A lower percentage of Hispanic than non-Hispanic men report acquisition of HIV through male-to-male sexual contact, suggesting a lower prevalence of receptive anal intercourse in the Hispanic population, perhaps leading to a reduced risk of anal squamous cell carcinoma.34 Hispanic women have a higher incidence of cervical cancer in all age groups than do whites, blacks, and Asians/Pacific Islanders.35 Because a history of cervical cancer is a known risk factor for the later development of anal cancer, cervical cancer may have contributed to the higher incidence of anal cancer in Hispanic women aged >60 years in this study.6,7
We found that black men had a significantly higher incidence of anal SCC than did white men, and black women had a significantly lower incidence than did white women, similar to findings in other studies.2,3 The higher rate of SCC among black men was limited to black men aged <60 years, suggesting a cohort effect. Black men have the highest incidence of HIV/acquired immunodeficiency syndrome (AIDS) (124.8 per 100,000) compared with all other races/ethnicities, perhaps contributing to the higher incidence of anal cancer among younger age groups in this population. Interestingly, black women have a higher incidence of HIV/AIDS and STDs such as chlamydia and syphilis than white women.32,34 Although rates of anal cancer are currently lower among black women than among white women, we found that rates of anal cancer among black women have been increasing since 1973.
Previous trend analyses have noted that the incidence of invasive anal cancer increased from 1973 to 2000 in both men and women.2,3,19 This analysis shows that rates have continued to increase among men and women through 2004. Young (aged <50 years) and middle-aged women (ages 50–64 years) were found to have a significant increase in anal SCC rates from 1992 through 2004. Young (aged <50 years) and older (aged ≥65 years) men were found to have a significant increase in rates during the same time period. Black and white women and white men also had a significant increase in rates during the time period studied, in contrast to black men and Hispanics of both sexes. These differences may be attributable to differences in sexual behavior or other risk factors by age, sex, and race/ethnicity, and to MSM and HIV/AIDS in men.
A higher proportion of men and those aged <50 years were diagnosed with in situ anal SCC. This is consistent with findings from a previous study.2 Young men who have risk factors for anal cancer, such as HIV-positive status, may be under closer medical surveillance and may be screened for anal cancer, resulting in a higher proportion with in situ disease.36 A higher proportion of Asians/Pacific Islanders were diagnosed with regional and distant disease. The incidence of anal cancer among Asians/Pacific Islanders is low; therefore, the reduced index of suspicion in this population may result in a later stage at diagnosis.
Men had a lower 5-year relative survival than women for all stages of anal SCC. This observation may be associated with the higher mortality among men with HIV/AIDS37 or to premature death from heart disease and other causes. Blacks had lower 5-year survival rates than did whites for in situ, localized, and regional disease, perhaps because of the higher burden of HIV/AIDS in this population or differences in access to care.
The current analysis is limited by the absence of information regarding HPV status or indicators of HPV exposure, such as sexual orientation, HIV status, or exposure to STDs. Furthermore, cases of AIN III were excluded from the analysis because cancer registries were not required to submit these cases to the NPCR during the study period. Because some pathologists equate AIN III with in situ disease, the incidence of in situ anal carcinoma may have been underestimated. Other limitations include the small number of cases within some subgroups of interest and the wide confidence intervals. We were unable to examine trends for Asian and Hispanic subpopulations because both designations are comprised of persons from many different countries. Anal cancer incidence, survival, and risk factors for these groups are likely to vary considerably. Although the registry coverage for the South region was estimated to be 63% of the population, potentially biasing regional comparisons, the data covered 83% of the US, a substantial strength of this analysis.
The quadrivalent HPV vaccine is currently approved for use in young women. The vaccine provides coverage for HPV types 6, 11, 16, and 18. A bivalent vaccine, covering HPV-16 and HPV-18, may receive US Food and Drug Administration approval soon. Given that 85% of anal cancers are of a histology known to be associated with HPV, a substantial portion of all anal cancers are theoretically preventable with the HPV vaccine. Research is currently underway to determine the efficacy of the vaccine in men. Further studies regarding the safety and effectiveness of the vaccine in high-risk populations such as MSM and HIV-positive men and women are needed. Screening for anal cancer is not currently recommended by the United States Preventive Services Task Force. Some researchers have advocated screening for anal cancer through use of anal Papanicolaou (Pap) smears to detect AIN in high-risk populations, particularly HIV-positive men.38 To our knowledge, no randomized clinical trials currently exist that determine whether screening for anal cancer decreases mortality or improves outcomes.39 There is little information regarding the natural history of AIN and the effectiveness and tolerability of treatment for anal dysplasia.39 Further research is needed to determine the risks and benefits of screening for anal cancer, particularly in high-risk populations. Continued surveillance of anal cancers will also be necessary to determine the impact of the HPV vaccine on anal cancer incidence among women as use of the vaccine becomes widespread.
Acknowledgments
This supplement to Cancer was supported by Cooperative Agreement Number U50 DP424071-04 from the Centers for Disease Control and Prevention.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
This article is a US Government work and, as such, is in the public domain in the United States of America.
REFERENCES
- 1.Clark MA, Hartley A, Geh JI. Cancer of the anal canal. Lancet Oncol. 2004;5:149–157. doi: 10.1016/S1470-2045(04)01410-X. [DOI] [PubMed] [Google Scholar]
- 2.Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the Surveillance, Epidemiology, and End Results experience, 1973–2000. Cancer. 2004;101:281–288. doi: 10.1002/cncr.20364. [DOI] [PubMed] [Google Scholar]
- 3.Melbye M, Rabkin C, Frisch M, Biggar RJ. Changing patterns of anal cancer incidence in the United States, 1940–1989. Am J Epidemiol. 1994;139:772–780. doi: 10.1093/oxfordjournals.aje.a117073. [DOI] [PubMed] [Google Scholar]
- 4.Frisch M, Melbye M. Anal cancer. In: Schottenfeld D, editor. Cancer Epidemiology and Prevention. 3rd ed. New York, NY: Oxford University Press; 2006. pp. 830–840. [Google Scholar]
- 5.Frisch M, Fenger C, van den Brule JC, et al. Variants of squamous cell carcinoma of the anal canal and perianal skin and their relation to human papillomaviruses. Cancer Res. 1999;59:753–757. [PubMed] [Google Scholar]
- 6.Rabkin C, Biggar R, Melbye M, Curtis R. Second primary cancers following anal and cervical carcinoma: evidence of shared etiologic factors. Am J Epidemiol. 1992;136:54–58. doi: 10.1093/oxfordjournals.aje.a116420. [DOI] [PubMed] [Google Scholar]
- 7.Frisch M, Olsen J, Melbye M. Malignancies that occur before and after anal cancer: clues to their etiology. Am J Epidemiol. 1994;140:12–19. doi: 10.1093/oxfordjournals.aje.a117154. [DOI] [PubMed] [Google Scholar]
- 8.Frisch M. On the etiology of anal squamous carcinoma. Br Med Bull. 2002;49:194–209. [PubMed] [Google Scholar]
- 9.Watson M, Saraiya M, Ahmed F, et al. Using population-based cancer registry data to assess the burden of human papillomavirus-associated cancers in the United States: overview of method. Cancer. 2008;113(10 suppl):2841–2854. doi: 10.1002/cncr.23758. [DOI] [PubMed] [Google Scholar]
- 10.Kong C, Welton M, Longacre T. Role of human papillomavirus in squamous cell metaplasia-dysplasia-carcinoma of the rectum. Am J Surg Pathol. 2007;31:919–925. doi: 10.1097/01.pas.0000213441.86030.fc. [DOI] [PubMed] [Google Scholar]
- 11.Cress RD, Holly EA. Incidence of anal cancer in California: increased incidence among men in San Francisco, 1973–1999. Prev Med. 2003;36:555–560. doi: 10.1016/s0091-7435(03)00013-6. [DOI] [PubMed] [Google Scholar]
- 12.Howe H, Jamison M, Havener L, Chen V, Ries L. Site-Specific Comparison of Summary Stage 1977 and Summary Stage 2000 Coding. Springfield, IL: The North America Association of Central Cancer Registries; 2005. [Google Scholar]
- 13.Phillips J. [Accessed on June 7, 2007];Summary stage: data effects of the changes in 2000. Available at: URL: http://www.naaccr.org/flesystem/pdf/Summary%20Stage%20Report%201-21-04b.pdf.
- 14.SEER*Stat Software. [Accessed on March 19, 2007];Surveillance Research Program, National Cancer Institute. Available at: URL: http://seer. cancer.gov/seerstat.
- 15.Tiwari R, Clegg L, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15:547–569. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 16.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence. Total U.S., 1969–2004 counties. Bethesda, MD: National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Cancer Statistics Branch; SEER 13 Registries limited-use, November 2006 Submission (1992–2004). Linked to county attributes. released April 2007, based on the November 2006 submission. [Google Scholar]
- 17.Joinpoint Regression Program, Version 3.0. [Accessed on May 1, 2007];Statistical Research and Applications Branch, National Cancer Institute. Available at: http://srab.cancer.gov/joinpoint.
- 18.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence. Total U.S., 1969–2004 counties. Bethesda, MD: National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Cancer Statistics Branch; SEER 17 Regs limited-use, November 2006 Submision (1973–2004 varying). Linked to county attributes. released April 2007, based on the November 2006 submission. [Google Scholar]
- 19.Maggard MA, Beanes SR, Ko CY. Anal canal cancer: a population-based reappraisal. Dis Colon Rectum. 2003;46:1517–1524. doi: 10.1097/01.DCR.0000093722.63657.B4. [DOI] [PubMed] [Google Scholar]
- 20.Daling J, Weiss N, Hislop T, et al. Sexual practices, sexually transmitted diseases, and the incidence of anal cancer. N Engl J Med. 1987;317:973–977. doi: 10.1056/NEJM198710153171601. [DOI] [PubMed] [Google Scholar]
- 21.Daling J, Sherman K, Hislop T, et al. Cigarette smoking and the risk of anogenital cancer. Am J Epidemiol. 1992;135:180–189. doi: 10.1093/oxfordjournals.aje.a116270. [DOI] [PubMed] [Google Scholar]
- 22.Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101:270–280. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 23.Frisch M, Glimelius B, van den Brule AJC, et al. Sexually transmitted infection as a cause of anal cancer. N Engl J Med. 1997;337:1350–1358. doi: 10.1056/NEJM199711063371904. [DOI] [PubMed] [Google Scholar]
- 24.Holmes F, Borek D, Owen-Kummer M, et al. Anal cancer in women. Gastroenterology. 1988;95:107–111. doi: 10.1016/0016-5085(88)90297-1. [DOI] [PubMed] [Google Scholar]
- 25.Tseng H-F, Morgenstern H, Mack T, Peters R. Risk factors for anal cancer: results of a population-based case-control study. Cancer Causes Control. 2003;14:837–846. doi: 10.1023/b:caco.0000003837.10664.7f. [DOI] [PubMed] [Google Scholar]
- 26.Edgren G, Sparen P. Risk of anogenital cancer after diagnosis of cervical intraepithelial neoplasia: a prospective population-based study. Lancet Oncol. 2007;8:311–316. doi: 10.1016/S1470-2045(07)70043-8. [DOI] [PubMed] [Google Scholar]
- 27.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000;92:1500–1510. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 28.Melbye M, Frisch M. The role of human papillomaviruses in anogenital cancers. Semin Cancer Biol. 1998;8:307–313. doi: 10.1006/scbi.1998.0081. [DOI] [PubMed] [Google Scholar]
- 29.Moscicki A-B, Durako SJ, Houser J, et al. Human papillomavirus infection and abnormal cytology of the anus in HIV-infected and uninfected adolescents. AIDS. 2003;17:311–320. doi: 10.1097/00002030-200302140-00004. [DOI] [PubMed] [Google Scholar]
- 30.Frisch M, Goodman MT. Human papillomavirus-associated carcinomas in Hawaii and the mainland U.S. Cancer. 2000;88:1464–1469. doi: 10.1002/(sici)1097-0142(20000315)88:6<1464::aid-cncr26>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 31.Hariri S, McKenna MT. Epidemiology of human immunodeficiency virus in the United States. Clin Microbiol Rev. 2007;20:478–488. doi: 10.1128/CMR.00006-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aral SO, Fenton KA, Holmes KK. Sexually transmitted diseases in the USA: temporal trends. Sex Transm Infect. 2007;83:257–266. doi: 10.1136/sti.2007.026245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Tobacco use among adults—United States, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:1145–1148. [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report. 2005 ed. Revised. Vol. 17. Atlanta: U.S: Department of Health and Human Services; 2007. pp. 1–54. [Google Scholar]
- 35.McDougall J, Madeleine M, Daling J, Li C. Racial and ethnic disparities in cervical cancer incidence rates in the United States, 1992–2003. Cancer Causes Control. 2007;18:1175–1186. doi: 10.1007/s10552-007-9056-y. [DOI] [PubMed] [Google Scholar]
- 36.Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Welton ML, Palefsky JM. The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV-positive men. JAMA. 1999;281:1822–1829. doi: 10.1001/jama.281.19.1822. [DOI] [PubMed] [Google Scholar]
- 37.Chin-Hong PV, Palefsky JM. Human papillomavirus anogenital disease in HIV-infected individuals. Dermatol Ther. 2005;18:67–76. doi: 10.1111/j.1529-8019.2005.05009.x. [DOI] [PubMed] [Google Scholar]
- 38.Chin-Hong PV, Palefsky J. Natural history and clinical management of anal human papillomavirus disease in men and women infected with human immunodeficiency virus. Clin Infect Dis. 2002;35:1127–1134. doi: 10.1086/344057. [DOI] [PubMed] [Google Scholar]
- 39.Chiao E, Giordano T, Palefsky J, Tyring S, Hashem E. Screening HIV-infected individuals for anal cancer precursor lesions: a systematic review. Clin Infect Dis. 2006;43:223–233. doi: 10.1086/505219. [DOI] [PubMed] [Google Scholar]