Abstract
Objective
A missense mutation in the Microtubule Associated Serine/Threonine Like kinase gene (MASTL, FLJ14813) on human chromosome 10 was previously linked to a novel form of autosomal dominant inherited thrombocytopenia in a single pedigree. The mutation results in an amino acid change from glutamic acid at position 167 to aspartic acid and segregates perfectly with thrombocytopenic individuals within this extended family. The phenotype is characterized by mild thrombocytopenia with an average platelet count of 60,000 platelets per microliter of blood. We wanted to determine the expression and localization of MASTL, as well as its role in developing thrombocytes using an in vivo model system.
Methods
Northern blot analysis allowed us to examine expression patterns. Morpholino knockdown assays in zebrafish (Danio rerio) were employed to determine in vivo contribution to thrombocyte development. Transient expression in BHK cells resulted in localization of both the wild type and E167D mutant forms of MASTL kinase to the nucleus.
Results
Northern blot analysis indicates that MASTL mRNA is restricted in its expression to hematopoietic and cancer cell lines. A transient knockdown of MASTL in zebrafish results in deficiency of circulating thrombocytes. Transient expression of recombinant MASTL kinase in vitro demonstrates localization to the nucleus. Conclusions: Functional studies presented here demonstrate a direct relationship between the transient knockdown of the MASTL kinase expression and the reduction of circulating thrombocytes in zebrafish. This transient knockdown of MASTL in zebrafish correlates with a decrease in the expression of the thrombopoietin receptor, c-mpl, and the CD41 platelet adhesion protein, GpIIb, but has no effect on essential housekeeping zebrafish gene, EF1α.
Introduction
Platelets are generated from large, mature polyploid precursor cells called megakaryocytes. Megakaryocytes (MKs) follow a highly specialized developmental process, which includes lineage commitment of pluripotent progenitors, proliferation and maturation within the bone marrow, and endoreduplication, resulting in large polyploid cells (16-128N) (reviewed in [1-4]). Each mature megakaryocyte is polyploid and releases hundreds of platelets through a process of cytoplasmic fragmentation that has been functionally described (review by [3]). These unusual cellular processes are not well understood. The examinations of inherited human genetic mutations that result in decreased platelet counts have led investigators to discover new molecular modulators for megakaryocyte development [5-8]. These studies of inherited human mutations have resulted in an appreciation of the role that MYH9, GATA1, and AML1 play in megakaryopoiesis. In this report, we present functional evidence of the role of MASTL (FLJ14813) in megakaryocyte biology, a gene originally identified by its association with an autosomal dominant thrombocytopenia.
Previously, our lab and Savoia et. al. described two distinct pedigrees, characterized by autosomal dominant thrombocytopenia that have normal-sized platelets without associated clinical findings and genetic linkage to the same region of chromosome 10p [9, 10]. Together, these reports implicated a monogenic disorder, mapping to a ∼5 million base locus on chromosome 10p11-12, defined by linkage analysis.
Clinically, affected individuals have mild-moderate thrombocytopenia, easy bruising, but few serious complications from bleeding. Bone marrow from affected individuals contains a decreased frequency of mature polyploid MKs but an increase in CFU-MK's as assessed by colony assays. The failure of immature MKs to undergo terminal differentiation suggests that the affected gene blocks MK maturation, potentially affecting polyploidization/endomitosis and MK development. In our lab, a missense mutation of the MASTL gene was identified that results in a predicted single amino acid change of glutamic acid to aspartic acid at position 167 (E167D) in all family members affected with thrombocytopenia. This mutation was not detected in spouses or healthy, unrelated individuals [11]. While this amino acid change is subtle in structure, it is found within the predicted N-terminal kinase domain, which contains the putative catalytic domain as determined by sequence homology. A similar conservative glutamic acid to aspartic acid change within the catalytic domain of Jak2 has been engineered and resulted in loss of kinase activity as determined by autophosphorylation assays [12]. Taken together, these results indicate that the E167D MASTL kinase mutation might be responsible for inherited thrombocytopenia in this family.
MASTL kinase contains a putative serine/threonine kinase domain that is organized with the catalytic domain at the amino-terminus and the regulatory domain at the carboxy-terminus interrupted by a unique middle sequence. Elegant studies of greatwall kinase, the Drosophila putative homologue, have demonstrated that mutations in the kinase domains result in a disruption of cell division [13]. Several mutations generated in the kinase domain of Drosophila greatwall kinase resulted in abnormal condensation of chromosomal DNA and cell cycle arrest. Recent studies in Xenopus have provided additional evidence that greatwall kinase is a regulator of mitotic initiation and maintenance through the Cdc2/cyclin B positive feedback loop [14, 15].
In the current studies, we provide additional evidence that MASTL kinase plays a specific role in the development of hematopoietic cells. Here, we utilize the zebrafish model system to examine the role of MASTL in the development of zebrafish thrombocytes, which are functionally equivalent to mammalian platelets [16].
Materials and Methods
Morpholino gene silencing in zebrafish
Transgenic zebrafish carrying a reporter eGFP (enhanced Green Fluorescent Protein) construct under regulation of the zebrafish thrombocyte-specific CD41 promoter were developed in the lab of Dr. Robert I. Handin at Brigham & Women's Hospital (Boston, MA) [17]. From the ENSEMBL (Sanger Centre, UK) and GenBank zebrafish MASTL information (GenBank Accession #BC133739), we were able to design antisense morpholino oligomers 18 (GeneTools, Ltd, Oregon) against two independent targets in the MASTL locus. One morpholino was directed against the ATG translational start site (5′ GAG ATG GAA GCT CGT GGA TTG GCA T 3′ in exon 1, and the second morpholino was directed against the exon 3 splice acceptor site for the zebrafish Mastl gene (5′ ACT CCA TCA CCT ATG AAA GTG TGA A 3′). A third inverted morpholino (based on the second morpholino sequence) (5′ AAG TGT GAA AGT ATC CAC TAC CTC A 3′) was designed and injected as a control for specificity. Morpholinos were diluted in nuclease free distilled water to obtain a stock solution of 25 mg/ml (3mM). For injections, the stock solutions were diluted in Danieau's solution (58mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, 5.0 mM HEPES, pH 7.6) at a final concentration of 20 mg/mL. A small amount of 2% phenol red was added to the solution as a tracer to aid in monitoring the injection volume. Zebrafish embryos were obtained by pair crossing of adult CD41:GFP transgenic fish (15) and were microinjected at the 1-4 cell stage with 1 nL of the morpholino stock solution. Injected embryos were incubated at 28.5° C, and staged as described [18] at 48, 72 and 96 hours post fertilization (hpf).
Hemoglobinized cells were detected by treating the embryos at 48-72 hfp with o-dianisidine (benzidine) as described[19]. The presence of GFP-expressing thrombocytes in the CD41-GFP transgenic fish at 96 hpf was captured on a Nikon microscope equipped with a 10× objective (NA 1.4). Fluorescence images were acquired with an Orca IIER CCD camera (Hamamatsu, Hamamatsu City, Japan). Electronic shutters and image acquisition were under the control of Metamorph software (Molecular Devices, Downington, PA).
Preparation of RNA from Zebrafish and Subsequent PCR/Clone Blot Analysis
Total RNA was isolated from 20 uninjected and morpholino injected embryos at 72 and 96 hpf using TRIZOL (Invitrogen). First strand cDNA was prepared using SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen) according to the manufacturer's recommendations. The following primers were used for PCR amplification:
CD41 Forward: 5′ GTTCTTCACTCCACGGCTCACTATG
CD41 Reverse: 5′ AGATTTGCAAGTCTGACTTCAGTCTC
c-Mpl Forward: 5′ GATACTTCCGAAAGGTCAAGAGGTC
c-Mpl Reverse: 5′ TTGCGCCATTTCCAAGTCCTCTCTG
efl1α Forward: 5′ TACGCCTGGGTGTTGGACAAA
efl1α Reverse: 5′ TCTTCTTGATGTATCCGCTGAC
All primers were designed against exon sequences of the target genes. The PCR conditions were as follows: 94°C for 3 minutes followed by 35 cycles of 94°C, 55°C and 72°C for 30 seconds each. For analysis of the RT-PCR products, about 5-10 μL of each PCR product was electrophoresed and either analyzed by gel electrophoresis (Eflα) or transferred to nylon membrane followed by hybridization to radiolabeled DNA probes derived from corresponding cDNA clones (MASTL and c-mpl).
Northern blot hybridization
The human MASTL coding DNA construct was generated as a probe for Northern blot analysis. Forward and reverse oligonucleotides were constructed with the unique 5′ and 3′ restriction enzyme sites BamHI and NotI for cloning purposes and ease of isolating the double-stranded DNA fragment. The cDNA sequence was produced by PCR reaction using first-strand cDNA generated from human UT-7/TPO cells under normal culture conditions (DMEM, 10% FBS, 10ng/ml recombinant human TPO (PeproTech, Rocky Hills, New Jersey). The oligonucleotide sequences were as follows: forward 5′ GGA TCC CTG AAA CAT CAC AGC TTT CTC AA 3′ and reverse primer 5′ GCG GCC GCT TCA TCA CTT TCC TGG CAA TC 3′. The cycle conditions for PCR were: 1 minute at 97°C, 2 minutes at 65°C, 3 minutes at 72°C for 30 cycles with a final extension at 72°C for 15 minutes. This probe is specific for the cDNA base pairs 783-1060 of the MASTL/FLJ14813 published sequence (GenBank Accession number NM_032844). The purified fragment was radiolabeled using random prime labeling (RadPrime Labeling Kit, Invitrogen) and alpha 32P dCTP (6000Ci/mmole) (Perkin Elmer). The probe was denatured at 100°C for 10 minutes and applied to either the pre-blocked multi-tissue Northern or a human cancer cell line Northern (BD Biosciences) overnight at 60°C in MiracleHyb hybridization buffer (Stratagene). Following hybridization, the blot was washed twice in 2× SSC (sodium chloride, sodium citrate)/0.1% SDS for 15 minutes at RT, washed once in 1× SSC/0.01% SDS for 30 minutes at 60°C, and finally exposed to film at -80°C.
Mammalian Expression Vector Cloning
The entire coding DNA for the human MASTL kinase was generated by PCR from the start methionine codon until the final amino acid (leucine at position 878). The stop codon was not amplified so that we could clone into the pDsRed1N1 (BD Biosciences, Clontech) mammalian expression plasmid. This plasmid places the Discosoma sp red fluorescent protein (RFP) at the C-termini of your coding DNA. The expression of the resultant fusion protein is driven by the CMV promoter. The RFP has an excitation maximum of 558 nm and an emission maximum of 583 nm.
Cell culture and fluorescent microscopy
Baby hamster kidney (BHK) cells were cultured in vitro in DMEM/10% heat inactivated FBS/10mM glutamine/penicillin/streptomycin/amphotericin B (GIBCO BRL) at 37°C/5%CO2. Cells were transfected with 1μg of plasmid containing the fusion RFP-MASTL kinase, the E167D mutant MASTL kinase or RFP alone using FuGene6 (Roche) at a ratio of 4:1 (volume transfection reagent:μg of DNA). BHK cells were plated one day prior to transfection at 40% confluence. FuGene6 directions were followed for transfection reaction mixtures. The transient transfections were allowed to incubate at 37°C/5% CO2 for 72 hours before fluorescence became apparent in those cells engineered to express MASTL kinase (WT or E167D mutant). Cells were fixed with 4% paraformaldehyde/0.1% Triton X-100 in PBS containing Ca2+Mg2+ for 10 minutes at RT. Cells were then rinsed three times in PBS/ Ca2+Mg2+ at RT for 5 minutes each wash. Cell nuclei were counterstained with TO-PRO-3 iodide (Invitrogen) at 1μm for 30 minutes at RT before being washed twice in PBS/ Ca2+Mg2+. Finally, antifade gold (Invitrogen) was applied, and the slides were allowed to cure overnight at room temperature. Images were observed under a BioRad MRC 1020 confocal microscope with Nikon DIAPHOT 200 camera optics under 60× oil (BioRad). Tag Image File Format (TIFF) images were saved and imported into PowerPoint.
Results
Amino Acid Homology
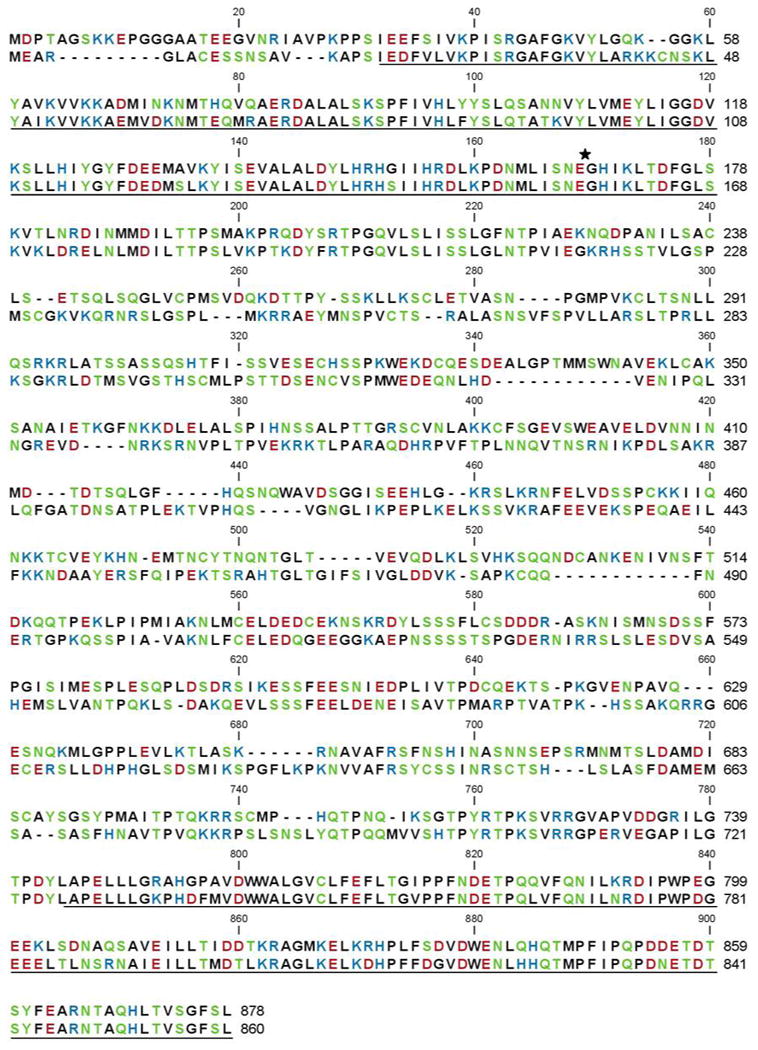
The amino acid similarity between the human and zebrafish MASTL kinase paralogous protein sequences is shown in Figure 1. The Danio rerio sequence is shown with the human amino acid sequence and aligned for greatest homology. We found that the primary sequence shared 48% amino acid identity and 63% similarity between these two species. Notably, the glutamic acid at position 167 in the human sequence that is associated with familial thrombocytopenia is conserved in the zebrafish amino acid sequence at position 157. The regions that share the greatest homology, perhaps not surprisingly, are within the putative kinase domains at either end of the protein.
Figure 1. Primary sequence alignment of human and zebrafish MASTL kinase.
The amino acid sequence homology is 63% similar and 48% identical between the human (top) and zebrafish (bottom) sequences. The putative kinase domains at the N- and C-termini are underlined, and the E167D point mutation is marked (★). Amino acids are colored according to polarity classification.
Transient Knockdown in vivo
Transient gene inactivation in the developing zebrafish embryo can be achieved by injection of antisense morpholino oligomers against the mRNA of active genes[20]. We have generated two independent morpholinos against the zebrafish paralog of the MASTL kinase (Figure 2) [21]. These morpholinos are directed against the ATG start site or the exon 3 splice acceptor junction, respectively. Injection of either morpholino results in a significant reduction in the number of circulating GFP+ thrombocytes in a transgenic zebrafish that has GFP expression under regulation by the CD41 promoter 16 (Figure 3) and in the concurrent ablation of zebrafish Mastl and CD41 mRNA (Figure 4). The morpholinos were injected at the 1-4 cell stage of zebrafish embryo development. Circulating blood thrombocytes are distinguishable at 3 days post fertilization in development [17]. In embryos that were injected with the antisense morpholinos, circulating thrombocytes were substantially decreased as determined by circulating GFP+ cells in which GFP expression is regulated by the CD41 promoter. At 48, 72, and 96 hours post fertilization (hpf), the injected embryos were examined visually for the presence of GFP+ cells in circulation and in the tail region between the dorsal aorta and the caudal vein. We observed significant reduction in the number of GFP-positive thrombocytes in MASTL morphants (77%, n=85/110), compared to the uninjected and control morphants. As shown in Figure 5, the number of circulating CD41+/GFP+ cells were completely ablated when compared to uninjected CD41:GFP transgenic zebrafish.
Figure 2. Antisense oligomer morpholinos against zebrafish MASTL.
Morpholino antisense oligonucleotides were generated against the ATG initiation start site as well as the exon 3 splice acceptor site (grey boxes) of the zebrafish MASTL gene.
Figure 3. Transient knockdown of MASTL with morpholinos results in thrombocytopenia in zebrafish.
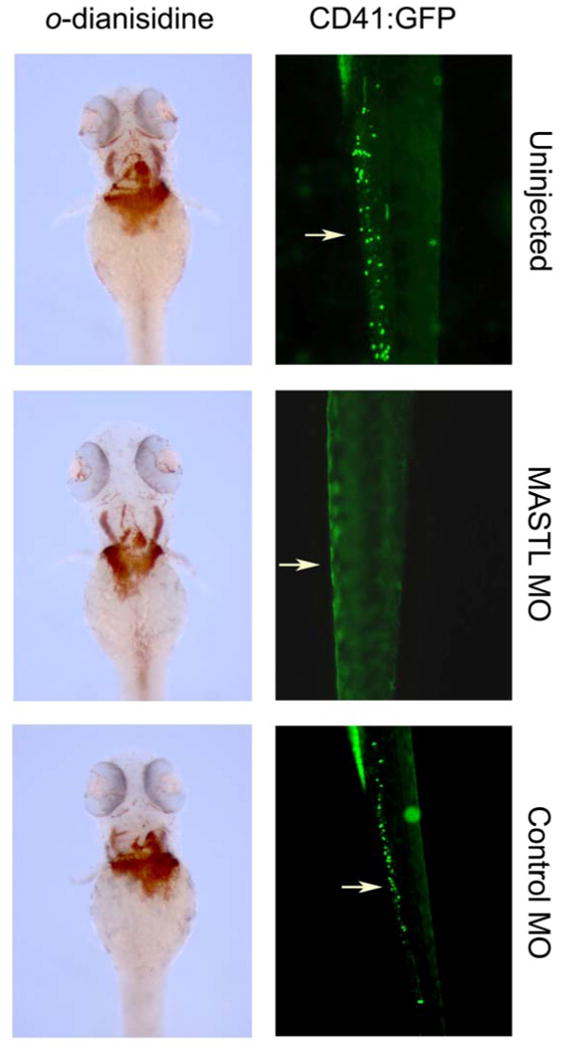
CD41:eGFP transgenic zebrafish were targeted for zebrafish MASTL ‘knockdown’ using morpholinos. Circulating thrombocytes expressing GFP were visualized in embryos injected with MASTL morpholino (MASTL-MO), compared to wild type (uninjected) and the inverted control morpholino (CONTROL-MO). Erythropoiesis was assessed by staining with o-dianisidine (lower three panels).
Figure 4. Transient knockdown of MASTL results in decreased expression of thrombocyte specific mRNA, c-mpl and CD41.
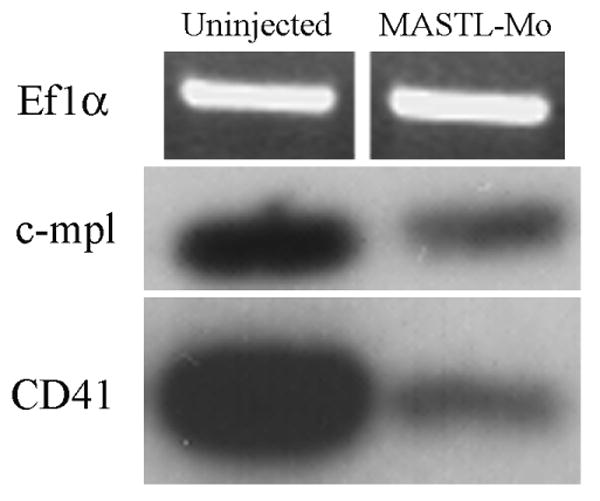
RT-PCR and clone blot analysis was performed from control and morphant zebrafish embryos. Specific primer sets to c-mpl, CD41, and the EFl-α genes were used in RT-PCR from either zebrafish MASTL morphants or uninjected embryos, followed by hybridization with c-mpl or CD41. EF1-α was visualized on a standard DNA agarose gel with ethidium bromide stain.
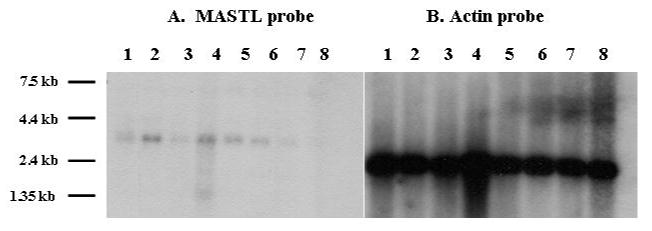
Figure 5. MASTL mRNA is expressed in human hematopoietic and cancer cell lines.

Northern blot from human cancer cell lines are hybridized with probes from human MASTL (Panel A) and Actin (Panel B) cDNA. The MASTL transcript size is 3 kb as expected from the published cDNA. From left to right, lanes are 1) Promyelocytic Leukemia HL-60, 2) HeLa Cell S3, 3) Chronic Myelogeous Leukemia K-562, 4) Lymphoblastic Leukemia MOLT-4, 5) Burkitt's Lymphoma Raji, 6) Colorectal Adenocarcinomas SW480, 7) Lung Carcinoma A549, and 8) Melanoma G361.
There was no effect on zebrafish erythropoiesis, as determined by staining with o-dianisidine, which stains primitive erythrocytes, indicating the specificity of the role of MASTL in thrombocyte production (Fig. 3).
The reduction of CD41 and c-mpl mRNA expression in the MASTL kinase morphants is also indicative of the reduction of key cell surface markers for zebrafish thrombocytes in vivo (Figure 4). These markers indicate that not only the presence of CD41-driven GFP-positive cells decreased, but other biological markers for thrombocyte development are markedly reduced while control EF1-α mRNA is unaffected.
Northern Blot Expression
The tissue expression of human MASTL kinase mRNA appears to be limited to megakaryocytes and transformed leukemic cell lines. We probed a multi-tissue Northern dot blot and a Northern blot with RNA from human cancer cell lines (BD Biosciences Clontech) with the 3′ probe for human MASTL kinase. The multi-tissue northern contained RNA from brain, thalamus, pituitary gland, spinal cord, heart, stomach, kidney, lung, liver, testis, and ovary which were all negative for MASTL kinase mRNA expression (data not shown). The human cancer cell Northern blot demonstrated a single transcript at the expected 3 kilobase size in the following cell lines: HL-60 (promyelocytic leukemia), HeLa S3, MOLT-4 (lymphoblastic lymphoma), Raji (Burkitt's lymphoma), and various myeloid and lymphoid leukemic cell lines (Figure 5).
Cellular Localization
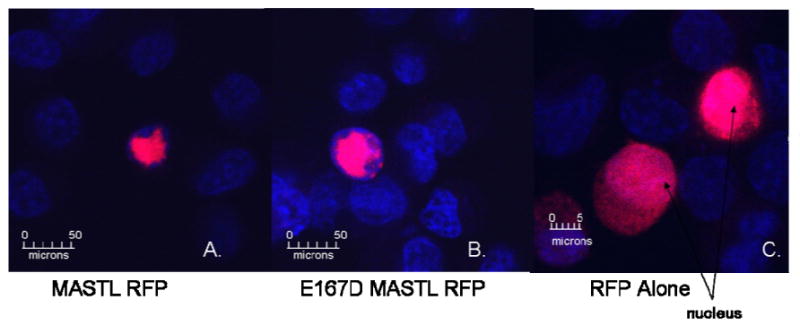
Transient expression of recombinant MASTL kinase or the E167D mutant MASTL kinase fused to red fluorescent protein (RFP) at its C-terminus in BHK cells demonstrates an alternate localization pattern from that of red fluorescent protein alone. The MASTL kinase protein, both wildtype and mutant forms, appears to localize within the nucleus of these BHK cells (Figure 6, Panels A & B) as determined by co-localization with the DNA counterstain, TO-PRO-3. The RFP when expressed alone (Figure 6, Panel C) is dispersed homogeneously throughout the nucleus and cytoplasm while the MASTL kinase RFP fusion seems to have very restricted localization within the cell nucleus.
Figure 6. Normal and mutant MASTL kinase localize to the nucleus in transiently transfected BHK cells.
Red fluorescent protein localized in BHK cells as shown by confocal microscopy. BHK cells were transiently transfected with either a human MASTL-RFP, E167D MASTL-RFP fusion expression vector (Panels A and B) or the RFP expression vector alone (Panel C). Cell nuclei were counterstained with TO-PRO-3 iodide (blue). Cells were analyzed by confocal microscopy under 60× oil magnification.
Discussion
MASTL kinase is the putative mammalian ortholog of the Drosophila greatwall kinase [13]. Greatwall kinase has been identified as a regulator of the cell cycle in Drosophila neuroblasts; its knockdown in these cells results in abnormal chromosomal condensation and cell cycle disruption, specifically mitotic arrest. Recent studies have demonstrated that greatwall kinase is involved in the positive feedback loop regulating the cell cycle regulator, Cdc2 [14]. Knockdown of greatwall kinase in Xenopus extracts led to increased phosphorylation of Cdc2, an inhibitor of cell cycle progression. These data suggest that greatwall kinase is required for mitotic entry and maintenance.
In megakaryocytes, mitosis occurs without concomitant nuclear or cell division, a specialized process called endomitosis. The possibility that MASTL kinase regulates endomitosis is suggested by the phenotype of humans with the E167D mutation, who have fewer polyploid megakaryocytes and much lower platelet counts than unaffected individuals. Consistent with this finding, we found that transient knockdown of the MASTL kinase in zebrafish decreased the number of circulating thrombocytes. However, neither of these findings proves that a mitotic defect is responsible for the thrombocytopenia in humans or fish with deficient or defective MASTL kinase, but they do make it much more likely that the thrombocytopenia is due to defective production and not reduced survival of the platelets (thrombocytes) in the circulation. The differences in thrombocytopoeisis in fish and mammals render it difficult to pinpoint whether the defect lies in proliferation of MK progenitors, MK maturation, or cytoplasmic fragmentation. In spite of the differences between fish and mammals, however, the cells produced (thrombocytes and platelets, respectively) have essentially the same functions and express a virtually identical repertoire of proteins. Thus, it seems reasonable to assume that the factors regulating the production of these cells are also conserved.
We have yet to elucidate the mechanism by which the missense mutation in the putative kinase domain of MASTL kinase produces thrombocytopenia. Of interest, no other mutations of MASTL kinase have been described, suggesting at least two possible general mechanisms. The first is that a more disruptive mutation may be incompatible with life, or so severe that the platelet phenotype is overlooked. Alternatively, the mutation could produce a gain-of-function phenotype, which could only result from one or a very limited set of mutations. Establishing the effect of the E167 mutation on MASTL functions is the goal of current investigation in our laboratory.
We also sought to gain insight into MASTL kinase biology by examining its tissue expression and cellular localization of MASTL kinase. MASTL kinase mRNA was not expressed in several tissues studied, but was expressed in transformed leukemic cell lines, suggesting that expression of the kinase may be restricted to hematopoietic cells.
Intracellular localization of human MASTL kinase has been difficult due to a lack of reagents to detect the protein. We therefore expressed human MASTL kinase fused with red fluorescent protein (RFP) to determine the subcellular compartments in which the protein usually resides. Both the recombinant wild-type fusion protein and a fusion protein carrying the E167D mutation localized to the nucleus of transfected BHK cells, consistent with the reported nuclear localization of greatwall kinase [13]. This finding suggests that the defect associated with the E167D mutation does not result from synthesis of an unstable protein or defective nuclear localization. Although MASTL kinase contains no obvious nuclear localization signal or DNA binding motifs, there are many examples of kinases involved in cell cycle control that are found in the nucleus. One such kinase, Polo-like kinase 1 (Plk1), has been shown to localize to the nucleus; a mutant Plk1 that cannot localize to the nucleus induces cell cycle arrest in G2 phase [22].
To date, we have not identified another thrombocytopenic family that has mutations in the MASTL gene. Given that the family we previously described had a relatively mild form of thrombocytopenia, such mutations may not come to medical attention, and the inherited nature of thrombocytopenia may not be readily identified. Another family harboring a mutation on chromosome 10 presented with a similar thrombocytopenic phenotype [10]. To our knowledge, the mutant gene has not been identified. It is clear from our experiments in zebrafish that a knockdown of MASTL kinase expression causes thrombocytopenia, suggesting that mutations within the MASTL kinase promoter region could also present with thrombocytopenia. The MASTL kinase promoter has not been characterized, but we suggest that the regulated expression levels have a direct effect on blood thrombocyte levels
We have shown that MASTL kinase has a role in MK development and ultimately platelet/thrombocytes production, although the mechanisms are not understood. We are currently examining how MASTL kinase regulates platelet production, as well as how the E167D mutation disrupts this regulation. Better understanding of MK regulation may give rise to novel treatments of platelet disorders.
Acknowledgments
This work was partially supported by grants from the National Institutes of Health to H.J.J. (K01-DK073136), J.G.D. and D.M.G. (RO1-HL074396-04), E.S. (T32-HL07623), B.H.P. (R01-DK070838, P01-HL032262), and the March of Dimes Foundation (1-FY06-365) to B.H.P. We thank Drs. Robert Handin and Hui-Feng Lin for providing us the CD41-GFP transgenic zebrafish line. We thank Dr. Joseph Italiano for access to the Orca IIER CCD camera and fluorescence imaging software. No financial interest/relationships with financial interest relating to the topic of this article have been declared.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ellis MH, Avraham H, Groopman JE. The regulation of megakaryocytopoiesis. Blood Rev. 1995;9:1–6. doi: 10.1016/0268-960x(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 2.Avraham H. Regulation of megakaryocytopoiesis. Stem Cells. 1993;11:499–510. doi: 10.1002/stem.5530110619. [DOI] [PubMed] [Google Scholar]
- 3.Schulze H, Shivdasani RA. Molecular mechanisms of megakaryocyte differentiation. Semin Thromb Hemost. 2004;30:389–398. doi: 10.1055/s-2004-833474. [DOI] [PubMed] [Google Scholar]
- 4.Matsumura I, Kanakura Y. Molecular control of megakaryopoiesis and thrombopoiesis. Int J Hematol. 2002;75:473–483. doi: 10.1007/BF02982109. [DOI] [PubMed] [Google Scholar]
- 5.Drachman JG. Inherited thrombocytopenia: when a low platelet count does not mean ITP. Blood. 2004;103:390–398. doi: 10.1182/blood-2003-05-1742. [DOI] [PubMed] [Google Scholar]
- 6.Geddis AE, Kaushansky K. Inherited thrombocytopenias: toward a molecular understanding of disorders of platelet production. Curr Opin Pediatr. 2004;16:15–22. doi: 10.1097/00008480-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Bellucci S. Megakaryocytes and inherited thrombocytopenias. Baillieres Clin Haematol. 1997;10:149–162. doi: 10.1016/s0950-3536(97)80055-8. [DOI] [PubMed] [Google Scholar]
- 8.Cines DB, Bussel JB, McMillan RB, Zehnder JL. Congenital and acquired thrombocytopenia. Hematology (Am Soc Hematol Educ Program) 2004:390–406. doi: 10.1182/asheducation-2004.1.390. [DOI] [PubMed] [Google Scholar]
- 9.Drachman JG, Jarvik GP, Mehaffey MG. Autosomal dominant thrombocytopenia: incomplete megakaryocyte differentiation and linkage to human chromosome 10. Blood. 2000;96:118–125. [PubMed] [Google Scholar]
- 10.Savoia A, Del Vecchio M, Totaro A, et al. An autosomal dominant thrombocytopenia gene maps to chromosomal region 10p. Am J Hum Genet. 1999;65:1401–1405. doi: 10.1086/302637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi MJ, Cummings CL, Drachman JG. FLJ14813 missense mutation: a candidate for autosomal dominant thrombocytopenia on human chromosome 10. Hum Hered. 2003;55:66–70. doi: 10.1159/000071812. [DOI] [PubMed] [Google Scholar]
- 12.VonDerLinden D, Ma X, Sandberg EM, Gernert K, Bernstein KE, Sayeski PP. Mutation of glutamic acid residue 1046 abolishes Jak2 tyrosine kinase activity. Mol Cell Biochem. 2002;241:87–94. doi: 10.1023/a:1020829617779. [DOI] [PubMed] [Google Scholar]
- 13.Yu J, Fleming SL, Williams B, et al. Greatwall kinase: a nuclear protein required for proper chromosome condensation and mitotic progression in Drosophila. J Cell Biol. 2004;164:487–492. doi: 10.1083/jcb.200310059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J, Zhao Y, Li Z, Galas S, Goldberg ML. Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol Cell. 2006;22:83–91. doi: 10.1016/j.molcel.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Haccard O, Wang R, et al. Roles of Greatwall kinase in the regulation of cdc25 phosphatase. Mol Biol Cell. 2008;19:1317–1327. doi: 10.1091/mbc.E07-11-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jagadeeswaran P, Sheehan JP, Craig FE, Troyer D. Identification and characterization of zebrafish thrombocytes. Br J Haematol. 1999;107:731–738. doi: 10.1046/j.1365-2141.1999.01763.x. [DOI] [PubMed] [Google Scholar]
- 17.Lin HF, Traver D, Zhu H, et al. Analysis of thrombocyte development in CD41-GFP transgenic zebrafish. Blood. 2005 doi: 10.1182/blood-2005-01-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 19.Iuchi I, Yamamoto M. Erythropoiesis in the developing rainbow trout, Salmo gairdneri irideus: histochemical and immunochemical detection of erythropoietic organs. J Exp Zool. 1983;226:409–417. doi: 10.1002/jez.1402260311. [DOI] [PubMed] [Google Scholar]
- 20.Nasevicius A, Ekker SC. The zebrafish as a novel system for functional genomics and therapeutic development applications. Curr Opin Mol Ther. 2001;3:224–228. [PubMed] [Google Scholar]
- 21.Woods IG, Wilson C, Friedlander B, et al. The zebrafish gene map defines ancestral vertebrate chromosomes. Genome Res. 2005;15:1307–1314. doi: 10.1101/gr.4134305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taniguchi E, Toyoshima-Morimoto F, Nishida E. Nuclear translocation of plk1 mediated by its bipartite nuclear localization signal. J Biol Chem. 2002;277:48884–48888. doi: 10.1074/jbc.M206307200. [DOI] [PubMed] [Google Scholar]


