Abstract
The impact of chronic rhinosinusitis (CRS) on the olfactory mucosa (OM) is dramatic. Cellular profiles and epithelial integrity in OM biopsies were evaluated using histological and immunohistochemical methods to define a strategy for future histological studies of CRS. We have examined nasal biopsies of 54 CRS patients (18 - 63 years old) and have defined specific histopathological patterns of the OM: normal pseudostratified, goblet cell hyperplasia, squamous metaplasia and erosion. Goblet cell hyperplasia was most similar to a normal pseudostratified OM pattern but with goblet cells intermixed in the apical layers. Squamous metaplasia exhibited an absence of olfactory supporting cells and had olfactory sensory neurons that were morphologically abnormal. It is unknown if these neurons would be functional in this type of tissue transformation. The pattern of erosion exhibited a severe loss of epithelial layers and a higher prevalence of infiltrating inflammatory cells within the olfactory epithelium when compared to the other OM patterns. Although it is not known if the OM remodeling patterns we have noted correspond to specific stages or distinct pathways of the disease, the template proposed here can be used in further studies to understand how the histopathological progression of CRS relates to olfactory loss and the response to treatment.
Keywords: olfactory epithelium, remodeling, histopathology
INTRODUCTION
Chronic rhinosinusitis (CRS) is a primary cause of olfactory loss among patients presenting to chemosensory clinics1, and is the most common chronic medical condition in the United States, affecting an estimated 33 million people/year.2 CRS is symptomatically defined as the presence of rhinosinusitis symptoms persisting for greater than 12 weeks as outlined by the Sinus and Allergy Health Partnership Task Force definition for adult CRS.3
The impact of this disease on the olfactory mucosa (OM), defined as both the olfactory epithelium and lamina propria, is dramatic and involves inflammation and tissue degradation that under normal circumstances would be followed by repair, regeneration and recovery. Instead, in chronic disease, tissue remodeling can persist and lead to significant loss of differentiated epithelium in both respiratory and olfactory mucosa. In approximately 25-30% of cases, this long term inflammatory process can result in partial or complete olfactory loss.2 While conductive impairment accounts for some cases of olfactory loss, particularly in the presence of polyposis or severe edema, obstruction of airflow often does not correlate with olfactory performance4. In these cases, damage to the sensorineural pathways in the epithelium has been proposed as an alternative cause of olfactory impairment. Several studies have reported histopathological alterations and the loss of olfactory sensory neurons in the nasal biopsies of patients with olfactory loss due to chronic sinusitis5-7, viral infection8, head trauma9-10, or allergic rhinitis.11 To better understand the processes underlying OM degradation and repair, we developed a panel of pathological stains and cellular biomarkers for characterizing olfactory sensory neurons, olfactory supporting cells, and immune cells to better understand the processes underlying OM degradation and repair. We have identified four OM patterns that were present to differing degrees in CRS biopsies.
HISTOPATHOLOGY OF THE OLFACTORY MUCOSA
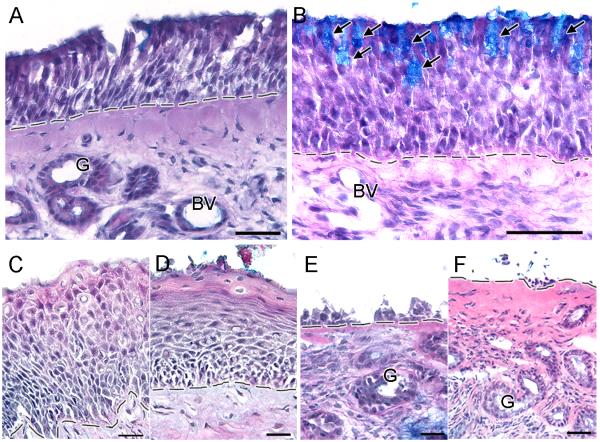
A 1-2 mm3 biopsy was taken from the superior aspect of the middle turbinate (n = 45) or the opposed septum (n=9) of 54 CRS patients (13M and 41F) ranging in age from 18-63 years old (mean, 42.6 ± 1.78). The precise region where the biopsies were taken was dependent on accessibility in the nasal cavity in each patient. We (E.A.P. and D.R.) have taken 500+ nasal biopsies over the past 10 years. Following informed consent, biopsies were taken under local anesthetic according to protocols approved by the Institutional Review Board of Thomas Jefferson University by experienced otorhinolaryngologists in the physician’s clinic. Approximately 63% of the biopsies of CRS patients had olfactory sensory neurons based on immunohistochemical analysis. We analyzed over 150 alcian blue/hematoxylin and eosin-stained sections and observed four distinctive OM patterns: normal pseudostratified, goblet cell hyperplasia, squamous metaplasia, and erosion. The human olfactory epithelium has a laminar organization composed of an apical layer of olfactory supporting cells, several layers of mature and immature olfactory sensory neurons underneath, and one or more lower layers composed of proliferating basal cells which sit on top of the basement membrane and can differentiate into olfactory neurons or supporting cells (Fig 1A). Beneath the basement membrane is the lamina propria which is composed of connective tissue, mucus glands, blood vessels, axonal bundles, ensheathing cells, trigeminal fibers and local immune cells.12
Figure 1.
Olfactory mucosa patterns observed in the biopsies of CRS patients. A. A normal pseudostratified human olfactory epithelium has a laminar organization consisting of an apical layer of olfactory supporting cells, several layers of immature and mature olfactory sensory neurons, and one or more layers of basal cells sitting on top of the basement membrane. Beneath the basement membrane is the lamina propria which is composed of connective tissue, mucus glands, blood vessels, olfactory axon bundles, trigeminal fibers and local immune cells. B. Goblet cell hyperplasia pattern is characterized by the presence of goblet cells intermixed with olfactory supporting cells in the olfactory sensory epithelium. C. Squamous metaplasia pattern is characterized by the transformation of the apical layers of cells into squamous-like layers. D. Flattening of squamous-like apical layers indicates severe squamous metaplasia pattern. E - F. Erosion pattern is characterized by various degrees of olfactory epithelium loss, leaving an exposed basement membrane. Arrows = goblet cells, G = glands, BV = blood vessel, dashed line = basement membrane. Scale bar: A and F = 40μm, B = 50μm, C-E = 20μm.
In goblet cell hyperplasia, goblet cells were intermixed with olfactory supporting cells, as revealed by Alcian blue staining, while the underlying neuronal layers in the olfactory epithelium remained unchanged (Fig 1B). Goblet cells are normally found only in respiratory epithelium13, and it has been reported that areas of damaged olfactory epithelium are often replaced with respiratory epithelium.12 However in most cases, biopsies with goblet cell hyperplasia contained neuronal cells, indicating the presence of sensory epithelium and not respiratory epithelium.
Squamous metaplasia was characterized by the transformation of the apical layers of the olfactory epithelium into thick squamous-like cellular layers, which may extend deep into the olfactory epithelium (Fig 1C). In more severe cases, the apical layers became flattened, sloughed off, and transformed into a continuous squamous layer (Fig 1D). Another characteristic of squamous metaplasia was the hyperproliferation of multiple layers of basal cells (data not shown). Squamous metaplasia could be a protective response of epithelia to injury, irritation, and chronic inflammation, but this “protection” may paradoxically impair recovery of sensory or respiratory epithelium after remodeling.
The degree of epithelial erosion varied from damage confined to the apical cell layers, coincident with the loss of olfactory supporting cells, to extensive erosion leaving only a few cell layers attached to the basement membrane (Fig 1E), to complete loss of all cell layers leaving an exposed basement membrane (Fig 1F). This could occur if the cellular matrix (e.g., matrix metalloproteinasess and their tissue inhibitors) in the olfactory epithelium was compromised by chronic inflammation. Degradative enzymes and acids released by inflammatory cells and activation of matrix metalloproteinases may directly alter the intracellular composition.14-15 This could lead to a greater susceptibility to damage from ordinary mechanical stresses as well as render the tissue more susceptible to damage during excision. In either case, altered tissue composition could result in the erosion pattern we observed most commonly among patients with the most severe clinical pathology.
These olfactory epithelial changes were accompanied by morphological alterations in the lamina propria, such as thickening of the basement membrane, invagination of the lamina propria into the olfactory epithelium, fibrosis of the connective tissue, and infiltration of inflammatory cells. Specific characteristics of the lamina propria were observed with each OM pattern of remodeling. There were fewer mucus glands but more vasculature in the lamina propria of squamous metaplasia compared to the two other OM patterns. Infiltration of immune cells (i.e., eosinophils, neutrophils, and macrophages) into the olfactory epithelium was most prevalent in the OM exhibiting a pattern of erosion.
CELLULAR CHARACTERISTICS OF OLFACTORY MUCOSA PATTERNS
Standard immunohistochemical methods were applied as previously described.16 Antibodies used in this study were mouse anti-cytochrome 2A5 (1:500, gift from Dr. Xin-Xin Ding), goat anti-olfactory marker protein (1:2000, cat. #54-1000, WAKO, Richmond, VA), rabbit polyclonal anti-PGP9.5 (1:1000, cat. #AB1761, Chemicon, Temecula CA), mouse anti-β-tubulin III (1:500, cat. #MMS-435P, Babco, Berkeley, CA), mouse anti-CD64 (1:50, cat. #555525. BD Biosciences, San Jose, CA), mouse anti-elastase (1:200, cat. #E2230, BD Biosciences), and mouse anti-major basic protein (1:50, cat. #550843, BD Biosciences).
Each OM pattern was characterized by distinctive cellular profiles. In the normal pseudostratified OM pattern, olfactory supporting cells and serous cells of the mucus glands were cytochrome 2A5-immunoreactive (cyp2A5-ir). Cyp2A5 plays an important role in the metabolism of air-borne compounds found in the environment and its localization in the olfactory supporting cells provides protection for the underlying olfactory sensory neurons.17-18 Olfactory supporting cells were absent in eroded olfactory epithelium and absent in squamous-like olfactory epithelium, potentially leaving the underlying neuronal cell layers and lamina propria vulnerable to air-borne toxicants and environmental insults. In addition, there is growing evidence that olfactory supporting cells play a role in the maintenance of the olfactory neurons. Hegg and her colleagues suggested that olfactory supporting cells play a role in olfactory sensory neuron proliferation by ATP-induced neutrotrophic factor mediation.19-20 Hence the loss of olfactory supporting cells in both erosion and squamous OM patterns could have significant implications for both tissue remodeling and normal regeneration processes. Olfactory marker protein-immunoreactive (OMP-ir) mature olfactory sensory neurons were observed in the normal OM pattern. These cells exhibited a typical morphology with dendritic processes, cell bodies located below the olfactory supporting cells and axonal processes projecting through the basement membrane and into the lamina propria. Similar cellular morphologies were observed in goblet cell hyperplasia. In squamous metaplasia, OMP-ir olfactory sensory neurons had abnormal morphology in which the olfactory sensory neurons lacked dendritic processes and were localized just above the basement membrane and/or in the apical squamous layers. The neuronal identity of these apically localized OMP-ir cells was confirmed with other neuronal markers including PGP9.5 and β-tubulin III, which co-localized with OMP. Thus, a subset of these squamous-like cells expressed neuronal proteins and perhaps was olfactory neurons before transformation. However, it seems unlikely that these abnormal OMP-ir olfactory sensory neurons are functional and even if some function remains, it is doubtful that odorants could penetrate through the thick, squamous-like layers. In erosion, the loss of OMP-ir olfactory sensory neurons varied with the degree of damage.
In CRS, the infiltration of immune cells was observed within the lamina propria, sometimes as dense clusters or scattered throughout, and also within the olfactory epithelium. As an initial analysis, we examined the infiltration of specific immune cells in the olfactory epithelium. Major basic protein-ir eosinophils, CD64-ir macrophages, and elastase-ir neutrophils were the most prevalent in the olfactory epithelium with erosion, followed by squamous metaplasia, and then goblet cell hyperplasia. This sequence suggests that the pattern of erosion may be the initial result of chronic inflammatory responses whereas squamous metaplasia may represent a state of protective OM repair.
SUMMARY
Our purpose was to characterize nasal tissue remodeling caused by CRS and to assist in generating hypotheses as to the possible cellular/inflammatory mechanisms contributing to the disease. The presence of all four OM patterns in CRS patients indicates that the OM undergoes continuous remodeling and repair. However, it is unknown if these patterns represent successive steps in the progression of the disease or result from different remodeling pathways involved in CRS, perhaps related to initial cause, history of therapy or medication, or genetics. It is notable that each of the OM patterns were detected in differing degrees in both diseased and healthy subject biopsy samples (data not shown). This suggests that the processes observed in CRS represent repair and remodeling processes that occur normally in response to the wear and tear of pollution, airborne infectious agents and other environmental challenges. Cytokines and other factors released both by dying cells and activated immune cells play critical roles in the processes of repair and regeneration. In the OM, several factors released by macrophages have been directly implicated in this process, including leukemia inhibitory factor (LIF), LIF receptor, STAT3, macrophage inflammatory protein-1α and moncyte chemoattractant protein-1.21-22
Indeed, a mild inflammatory state may be the ‘default’ condition of the nasal mucosa, and treatments designed to block that process may prove counterproductive. Histopathological and immunohistochemical data from this study also suggest that the chronic inflammatory process has a detrimental effect on the neuroepithelium that may exceed its ability to regenerate. In addition, the loss of olfactory supporting cells in both erosion and squamous metaplasia suggests that tissue repair and cellular recovery in the olfactory epithelium may depend on these cells. Future studies will examine the inflammatory mechanisms causing the loss of both the olfactory sensory neurons and the olfactory supporting cells, which could lead to insights into potential treatments for CRS-related smell loss.
ACKNOWLEDGEMENTS
The authors would like to thank Chris T. Klock and Aldona A. Vainius for their assistance. We greatly appreciate all of our subjects who volunteered for this study. This study was supported by the National Institute on Deafness and Other Communication Disorders DC006760.
REFERENCES
- 1.Cowart BJ, Young IM, Feldman RS, Lowry LD. Clinical disorders of smell and taste. Occupational Medicine: State of the Art Reviews. 12:465–483. [PubMed] [Google Scholar]
- 2.Blackwell DL, Collins JG, Colles R, National Center for Health Statistics Summary health statistics for U.S. adults: National Health Interview Survey, 1997. Vital Health Stat. 2002;10:15–16. [PubMed] [Google Scholar]
- 3.Benninger MS, Ferguson BJ, Hadley JA, Hamilo DL, Jacobs M, Kennedy DW, Lanza DC, Marple BF, Osgthorpe JD, Stankiewicz JA, Anon J, Denney J, Emanuel I, Levine H. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol-Head Neck Surg. 2003;129:S1–S32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 4.Doty RL, Mishra A. Olfaction and its alteration by nasal obstruction, rhinitis and rhinosinusitis. Laryngoscope. 2001;111:409–423. doi: 10.1097/00005537-200103000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kern RC. Chronic sinusitis and anosmia: pathologic changes in the olfactory mucosa. Laryngoscope. 2000;110:1071–1077. doi: 10.1097/00005537-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Malekzadeh S, Hamburger MD, Whelan PJ, Biedlingmaier JF, Baraniuk JN. Density of middle turbinate subepithelial mucous glands in patients with chronic rhinosinusitis. Otolaryngol. Head Neck Surg. 2002;127:190–195. doi: 10.1067/mhn.2002.126800. [DOI] [PubMed] [Google Scholar]
- 7.Rehl RM, Balla AA, Cabay RJ, Hearp ML, Pytynia KB, Joe SA. Mucosal remodeling in chronic rhinosinusitis. Am. J. Rhinol. 2007;21:65–657. doi: 10.2500/ajr.2007.21.3096. [DOI] [PubMed] [Google Scholar]
- 8.Yamagishi M, Fujiwara M, Nakamura H. Olfactory mucosal findings and clinical course in patients with olfactory disorders following upper respiratory viral infection. Rhinol. 1994;32:113–118. [PubMed] [Google Scholar]
- 9.Yamagishi M, Nakamura H, Suzuki S. Immunohistochemical examination of olfactory mucosa in patients with olfactory disturbance. Ann. Otol. Rhinol. Laryngol. 1990;99:205–210. [PubMed] [Google Scholar]
- 10.Yamagishi M, Nakano Y. A re-evaluation of the classification of olfactory epithelia in patients with olfactory disorders. Eur. Arch. Otorhinolaryngol. 1992;249:393–399. doi: 10.1007/BF00192261. [DOI] [PubMed] [Google Scholar]
- 11.Church JA, Bauer H, A J, Bellanti JA. Hyposmia associated with atopy. Ann Allergy. 1978;40:105–109. [PubMed] [Google Scholar]
- 12.Morrison EE, Costanzo RM. Morphology of the human olfactory epithelium. J. Comp. Neurol. 1990;297:1–13. doi: 10.1002/cne.902970102. [DOI] [PubMed] [Google Scholar]
- 13.Moran DT, Rowley JC, III, Jafek BW, Lovell MA. The fine structure of the olfactory mucosa in man. J. Neurocytol. 1982;11:721–746. doi: 10.1007/BF01153516. [DOI] [PubMed] [Google Scholar]
- 14.Kostamo K, Tervahartiala T, Sorsa T, Richardson M, Toskala E. Metalloproteinase function in chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2007;117:638–643. doi: 10.1097/MLG.0b013e318030aca6. [DOI] [PubMed] [Google Scholar]
- 15.Watelet JB, Bachert C, Claeys C, Van Cauwenberge P. Matrix metalloproteinases MMP-7, MMP-9 and their tissue inhibitor TIMP-1: expression in chronic sinusitis vs nasal polyposis. Allergy. 2004;59:54–60. doi: 10.1046/j.1398-9995.2003.00364.x. [DOI] [PubMed] [Google Scholar]
- 16.Yee KK, Rawson NE. Immunolocationization of retinoic acid receptors in the mammalian olfactory system and the effects of olfactory denervation on receptor distribution. Neurosci. 2005;131:733–743. doi: 10.1016/j.neuroscience.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Liu Y-Q, Su T, Ren X, Shi L, Liu D, Gu J, Zhang Q-Y, Ding X. Immunoblot analysis and immunohistochemical characterization of CYP2A expression in human olfactory mucosa. Biochem. Pharmacol. 2003;66:1245–1251. doi: 10.1016/s0006-2952(03)00476-3. [DOI] [PubMed] [Google Scholar]
- 18.Ling G, Gu J, Genter MB, Zhuo X, Ding X. Regulation of cytochrome P450 gene expression in the olfactory mucosa. Chemico-Biol. Interact. 2004;147:247–258. doi: 10.1016/j.cbi.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Hayoz S, Hegg CC. ATP-induced ATP release via purinergic receptors stimulation in mouse olfactory epithelium. Chem Senses. 2008;33:S118. [Google Scholar]
- 20.Jia C, Hegg CC. ATP-induced proliferation is mediated via neuropeptide Y (NPY) in adult mouse olfactory epithelium. Chem Senses. 2008;33:S77. [Google Scholar]
- 21.Getchell TV, Shah DS, Partin JV, Subhedar NK, Getchell ML. Leukemia inhibitory factor mRNA expression is upregulated in macrophages and olfactory receptor neurons after target ablation. J. Neurosci. Res. 2002;67:246–254. doi: 10.1002/jnr.10090. [DOI] [PubMed] [Google Scholar]
- 22.Getchell TV, Subhedar NK, Shah DS, Hackely G, Partin JV, Sen G, Getchell ML. Chemokine regulation of macrophage recruitment into the olfactory epithelium following target ablation: involvement of macrophage inflammatory proterin-alpha and monocyte chemoattractant protein-1. J. Neurosci. Res. 2002;70:784–793. doi: 10.1002/jnr.10432. [DOI] [PubMed] [Google Scholar]